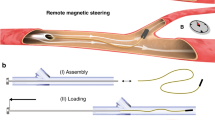
Abstract
Magnetic actuation techniques and microrobots have attracted considerable interest due to their potential applications in biomedicine. Interventional techniques have emerged as a minimally invasive approach to treat a wide range of vascular diseases. The current practice of interventional procedures is, however, limited by manual control of interventional devices and time-consuming procedures. Moreover, fluoroscopy is considered as an essential part of the procedure today despite posing many limitations for patients and physicians. Recently, various microrobotic solutions have been proposed for vascular interventions, including advances in magnetic navigation systems and magnetically steerable catheters and guidewires, which have shown potential benefits such as reduced radiation doses, improved access to difficult-to-reach and tortuous anatomy. This paper reviews the commercial magnetic actuation systems and magnetically actuated interventional microrobots that have been developed by academic research groups and medical companies worldwide, outlining their capability, applicability as well as limitations. We further address the challenges and future prospects of the research toward clinical acceptance of magnetic interventional technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The term “microrobot” typically refers to a device or a material with individual parts sized in the millimeter and submillimeter range [1]. One of the most promising scientific and societal impacts of the microrobots is in biomedical applications such as targeted therapy [2,3,4], cell delivery [5,6,7], bio-sensing [8] and treatment of vascular diseases [9], due to their small size and ability to access hard-to-reach regions in the human body in a minimally invasive way, which have not been easy to access with any of the currently existing medical devices [10,11,12]. Recently, such microrobots have shown their applications in the field of vascular interventions, including percutaneous coronary intervention (PCI) and atrial fibrillation (AF) ablation, where the procedures involve the use of interventional devices such as catheters, guidewires and endoscopes.
PCI is a minimally invasive medical technique that is performed to open up blood vessels in the heart that have been narrowed by plaques, a disease known as atherosclerosis [13,14,15]. During PCI, guidewires and catheters are inserted through the femoral or radial artery and moved up to the coronary arteries that have been occluded by the atherosclerotic plaques. An additional balloon catheter can also be introduced to the lesion to expand the narrowed arteries [16]. Interventional techniques similar to PCI can also be performed in cerebro-, neuro- and peripheral vascular networks [17,18,19,20].
AF ablation is an interventional procedure for treating abnormal, typically and/or irregular heart rhythms. During the procedure, a physician manually inserts a catheter into a blood vessel in the groin and slides it up to the heart. The physician then uses the catheter to scar a small section of the heart by making tiny burns using radiofrequency energy [21, 22]. Scarring helps to prevent the heart from sending abnormal electrical signals that cause atrial fibrillation. Although each type of intervention has a slightly different procedure, they all have similar limitations in common. First of all, as the procedure is performed manually by a physician, the outcome of the procedure is profoundly dependent on the skill and experience of the physician, resulting in the standard of medical care for patients being random and questionable [23, 24]. Second, fluoroscopy (i.e., X-ray imaging) is considered an essential part of the procedure to monitor the interventional devices despite the significant health risks for physicians, nurses and patients [25, 26]. As procedures become complicated and the clinical condition of the patient is serious, more X-ray radiation exposure can be incurred due to extended exposure times. The emission of ionizing X-ray radiation may cause skin injury [27], as well as increase the long-term risk of malignancy [28]. It has been reported that interventional procedures can result in a considerable radiation dose to the patient and physician, even when operated with state-of-the-art fluoroscopic units [29,30,31].
The integration of the conventional interventional device with a microrobot can transform the device into an innovative device that offers potential solutions to the aforementioned issues through remote control and more efficient steering of the device. For the manipulation of such small devices, different types of actuation methods have been studied and reported, powering energy from various sources such as piezoelectric [32,33,34], electrostatic [35], thermal [36,37,38], optical [39,40,41], optothermal [42], bacterial [43,44,45], chemical [46] and magnetic [47,48,49,50,51,52,53] techniques. The majority of the above methods have their own limitations, especially for in vivo or clinical applications; for example, piezoelectric method requires high voltages, chemical actuation uses toxic hydrogen peroxide as main fuel source, bacterial actuation requires a low level of cytotoxicity, and some methods are often limited to two-dimensional (2D) actuation, and some are not biocompatible. Of them, magnetic actuation, which is an off-board remote control method for powering the spatial mobility and maneuverability of a microrobot, has recently attracted considerable interest due to its good controllability and precision. In contrast to the other alternative methods, such as optical, thermal and chemical signals, an external magnetic field is capable of penetrating biological tissues safely, which makes it an excellent candidate for in vivo applications [54]. Based on the magnetic method, researchers have developed magnetically actuated interventional devices and magnetic actuation systems. Furthermore, a few companies have developed and attempted commercialization of their magnetic actuation systems on the market, mainly for catheter ablation [55,56,57].
In this paper, we present recent progress in the development of guidewire- and catheter-based microrobots for vascular interventions, also known as magnetic guidewires and catheters, with a special emphasis on magnetic actuation systems that have been developed to control the magnetic interventional devices. The contents are structured as follows: Sect. 2 provides basic theories on the magnetic manipulation by magnetic force and torque; Sect. 3 reviews some of the commercial magnetic actuation systems; Sect. 4 summarizes the magnetically actuated guidewires and catheters developed by academic and medical groups worldwide, and the challenges with the future prospects of the magnetic interventional technologies are discussed in Sect. 5, followed by conclusions in Sect. 6.
2 Magnetic manipulation: force and torque
Magnetic actuation systems are used for remote control and steering of magnetic catheters and guidewires. The mechanism of magnetic actuation or manipulation is based on the principles of magnetism. When a magnetized object such as a microrobot is exposed to an externally applied magnetic field, it can experience magnetic force Fm and torque Tm within the region of magnetic fields. The magnetic force and torque acting on a magnetized object can be mathematically expressed as follows [58]:
where m is the internal magnetization (A m−1), B is the magnetic flux density (T) and V is the volume of the magnetic material (m3). In free space, B can be further expressed as follows [59]:
where μ0 is the magnetic permeability of free space, which has a constant value of \( 4\pi \times 10^{ - 7} \,{\text{N}} \cdot {\text{A}}^{ - 2} \) and H is the magnetic field strength (A m−1).
Considering the geometry of the magnetic material is relatively small in comparison with the spatial changes in the applied field—which is realistic for medical microrobots—the magnetic field can be modeled to be uniform across the tiny microrobot. In addition, the internal magnetization of the material, which slightly varies across the body in reality, can be modeled to be constant throughout the magnetized object, and the above force and torque equations can be simplified in terms of the net magnetic moment M (A m2) of the body, which is the product of the average internal magnetization and the volume of the body as below:
The governing equations clearly indicate that the magnetic force is directly proportional to the gradient of the external magnetic field, and the magnetic torque is the cross-product of the two vectors: net magnetic moment vector and the magnetic flux density vector of the magnetized object. The magnetic force carries the object toward the region of stronger field strength. Rotational torques are developed between the external magnetic field and the magnetized object when the magnetic moment and the external field direction are misaligned. The magnetic torques tend to align the magnetic moment vectors of the object in the direction of the magnetic field (Fig. 1) [60].
Net magnetic forces and torques exerted on the magnetized objects under a uniform and b gradient external magnetic fields. Reproduced with permission [60]. Copyright 2012, Pearson Education
Applying this principle, magnetic catheters and guidewires can be steered by an external magnetic field generated from a set of electromagnets or permanents magnets (Fig. 2). Although they often experience both the magnetic force Fm and torque Tm from Eqs. (1) and (2) when placed in a magnetic field as the gradients of the magnetic field are rarely zero, it is the magnetic torque that plays the greater role in steering due to its ability to align the direction of the magnetic moment of the magnetic component with the direction of the applied field. The magnetic force is used when we need to further pull the object in the direction of the field gradient.
When deciding upon magnetic actuation techniques, electromagnets have often been preferred due to their flexibility in changing the magnetic field strength by adjusting the electric current input in the coils [61]. This has resulted in the same electromagnet being used for different levels of magnetic field strength, while one permanent magnet can only be demonstrated with one particular field strength, as a result of which electromagnetic actuation methods have a more extensive range of applications, although the continuous supply of electricity remains a downside. The advantage of permanent magnets is that they do not require electric currents to generate a magnetic field and follow a non-decaying magnetization profile [62]. This means that it is safe from failure arising from substantial thermal dissipation in the coil system as is the case with electromagnets. However, the magnetic field cannot be zero in the case of permanent magnets, which limits the usability of the space due to a permanent magnetic field in the given space, such as a procedure room.
3 Commercial magnetic actuation systems
The magnetic actuation technologies allow for remote control of interventional instruments through the use of magnetic fields. While the development of magnetically actuated microrobots has only been confined to a basic research extent, which will be explained in the subsequent sections, magnetically actuated interventional devices, including ablation catheters in particular, have been used since the early 2000s for treating cardiac arrhythmias. Furthermore, several magnetic actuation systems have been commercialized for the remote navigation of magnetic ablation catheters. Such systems differ from magnetic resonance imaging (MRI) systems in their ability to precisely manipulate the directionality of the generated magnetic fields and thus, precisely manipulate magnetic catheters. They have shown promising results in animal testing, as well as in clinical evaluation to some extent, though each system has revealed its own limitations.
In recent years, several academic research groups have also attempted to develop magnetic actuation systems for the control of microrobots [63, 64]. However, their application has often been limited to the wireless control of tiny untethered microrobots in vitro, mainly due to the restrictive size of the workspace, which is not ideal for in vivo or clinical applications of the magnetic interventional devices.
For the application of any magnetic actuation system in vascular interventions, the compatibility of the system with other surgical equipment such as the patient’s bed and the fluoroscopic unit is essential, as is already the case for the commercial magnetic actuation systems.
3.1 The Niobe (Stereotaxis)
The Niobe robotic magnetic actuation system (Stereotaxis Inc., MO, USA), which was launched in 2003 for treating cardiac arrhythmia, consists of two gigantic bodies with permanent magnets located on either side of the patient’s bed (Fig. 3a). The magnets are mechanically rotated by computer-aided motors, and the steering of magnetic guidewires or catheters is achieved through magnetic fields generated by the two bodies with permanent magnets (Fig. 3b). The Niobe system is equipped with a fluoroscopy system (Siemens AXIOM Artis dFC™, Siemens Medical, Germany) for imaging and also integrates with interfaces, such as a catheter advancer system (Cardiodrive, Stereotaxis) for remote control of catheter ablation, and the blood vessels can also be reconstructed in three-dimensional (3D) view during ablation. A number of in vivo and clinical studies in the field of catheter ablation treatment of cardiac arrhythmias using the Niobe system have been reported [65,66,67,68,69,70,71,72,73,74]. The major limitation of the Niobe system is its slow rotational speed of the magnets and limited response time.
The Niobe system. a The two permanent magnets are located on either side of the patient’s bed, with the Siemens AXIOM Artis dFC™ single-plane fluoroscopy system. Reproduced with permission [75]. Copyright 2006, John Wiley and Sons. b The permanent magnet (indicated by the blue color) is mechanically rotated by a computer-aided motor inside the fiber glass container. Reproduced with permission [76]. Copyright 2006, John Wiley and Sons
3.2 The Genesis (Stereotaxis)
An upgraded version of the Niobe system is the Genesis system (Stereotaxis Inc.) that has recently been introduced as a Stereotaxis’ next-generation robotic magnetic navigation system (Fig. 4a). As was the case in the Niobe system, the Genesis system also deploys two permanent magnets on both side of the patient’s bed, but it has differentiated benefits from its design such as smaller size, lighter weight, faster and more flexible movement of magnets than the Niobe system. According to the information provided by Stereotaxis, the Genesis system is about 90 kg lighter than the Niobe system, resulting in improved responsiveness and patient access for physicians, nursing team and anesthetists.
The Genesis system. a The two permanent magnets are located on either side of the patient’s bed, with a C-arm, vision and master–slave systems. b Comparison between the permanent magnets of Niobe and Genesis systems. The red bars represent the maximum displacements made by the rotation of the magnets. No published work is available on the Genesis system due to the system being recently released. Reproduced with permission from Stereotaxis, Inc. (Copyright 2019)
One of the most fundamental design improvements in the Genesis system is that the two permanent magnets are rotated about their center of mass, with the magnets and the mechanical motors that rotate them embedded together in the flexible robotic arms (Fig. 4b). This differs from the Niobe system, where magnets are held by the mechanical motors pivoted at the back and are swung about the pivot, resulting in a large displacement of space. In the case of the Genesis system, a smaller space is displaced as the magnets are rotated efficiently along their center of mass. A smaller displacement means that the magnets can be positioned closer to the inside of the housing, meaning a stronger magnetic field can be achieved by using the same magnets. The second advantage of rotating the magnet about its center of mass is the reduced momentum that needs to be overcome for any initiation or change of motion, which eventually can lead to faster speed and reduced response time. The Genesis system can adjust magnetic vectors 73% faster than the Niobe system. The significant reduction in magnet size and weight allowed Stereotaxis to build highly flexible robotic arms that provide an increased range of motion as opposed to the fixed arms in the Niobe system. This allows for a wider X-ray angulation and serves as a potential platform on which future applications in other anatomies might be possible. In addition to developing magnetic actuation systems, Stereotaxis has also started working on developing magnetically actuated catheters in collaboration with Osypka AG, a manufacturer of interventional medical devices based in Germany. The Genesis system is fully compatible with a C-arm (Model S, Stereotaxis), vision (Odyssey Vision™ System, Stereotaxis) and master–slave (Vdrive, Stereotaxis) systems during operation. As the Genesis system has just been launched in the USA, no published work is available on the system, and limited information was collected from the public domain and product launch presentations.
3.3 The Catheter Guidance Control and Imaging (CGCI) system (Magnetecs Corporation)
The CGCI system (Magnetecs Corporation, CA, USA), designed for use in catheter ablation, consists of a magnetic chamber of eight electromagnets, four of which are placed in the top plane of the patient’s bed in a semi-spherical arrangement and the other four placed symmetrically in the bottom plane (Fig. 5). Due to the spherical configuration of the eight electromagnets, the CGCI system can generate isotropic magnetic fields, where the magnetic fields can be stably modified almost in real time. However, these electromagnets are fixed around the 3D workspace, which means there is no rotational or translational motion of the magnets as in other magnetic actuation systems. The whole system is controlled from a remote master–slave control, which is also integrated with cardiac mapping and navigation equipment, including fluoroscopy, intra-cardiac echocardiography, etc. [77]. Magnetecs is planning on additional applications of the CGCI system in the areas of interventional cardiology, gastroenterology and neurology. Several human clinical trials carried out using the CGCI system have been reported [78,79,80]. The main limitations of the CGCI include fixed positions of the magnets (i.e., no rotational or translational movement) and limited access to the patient during operation.
The CGCI system incorporates eight electromagnets fixed in a spherical configuration. Reproduced with permission [81]. Copyright 2013, Elsevier
3.4 The Aeon Phocus (Aeon Scientific)
The Aeon Phocus (Aeon Scientific AG, Zurich, Switzerland) is a catheter steering system for the treatment of cardiac arrhythmias (Fig. 6a). The system consists of seven electromagnets arranged around the patient’s torso and is used in conjunction with an angiography system (Artis Zee, Siemens, Germany). The physician can remotely control the magnetic catheter with a joystick in a separate control room and can thus be protected from X-ray radiation. However, the main downside of the Aeon Phocus might be the limited access of medical staff to the patient as the patient’s upper body is likely to be surrounded and enclosed by the seven electromagnets during operation (Fig. 6b). It should be noted that the Aeon Scientific is no longer in business.
4 Magnetically actuated interventional devices
4.1 Guidewire-based microrobots
Soft materials, including elastomers and hydrogels, possess unique properties such as deformability and flexibility [84,85,86], which could be used to fabricate a soft microrobot that can improve the steerability of guidewires. The deformable and flexible structures of such microrobots make them ideal candidates for application in complex vascular networks. Taking advantage of these properties, Jeon et al. [52] developed a flexible guidewire-based microrobot, which was fabricated via replica-molding using polydimethylsiloxane (PDMS) incorporating a neodymium (NdFeB, N52) permanent magnet, to enhance the steerability of a conventional coronary guidewire (Fig. 7a). The fabricated microrobot was attached to the tip of the guidewire using a microspring. As the microrobot was composed of a deformable material, the tip of the guidewire could be steered using a low-intensity external magnetic field. They demonstrated the performance of the guidewire in a 2D blood vessel phantom, where a uniform external magnetic field was generated by an eight-coil electromagnetic system called MiniMag (MagnebotiX, Zurich, Switzerland) [87]. The guidewire was successfully guided to the desired locations and steered into branches at angles of 45° and 80°, which showed its potential for use in the coronary arteries. Subsequently, Jeon et al. [88] also introduced a second-generation of magnetically actuated guidewire-based microrobot where the soft body of the microrobot was composed of PDMS, two permanent magnets and a microspring (Fig. 7b). In this work, another eight-coil electromagnetic system called OctoMag (MagnebotiX, Zurich, Switzerland) [89] was used to generate a uniform external magnetic field for the manipulation of the microrobot. They measured the steering angles of the microrobot, which were observed to range from 21.1° to 132.7° at a magnetic field intensity of 15 mT. The steerability and trackability of the guidewire microrobot were demonstrated in 2D and 3D blood vessel phantoms, and the results showed that the guidewire was successfully guided into any targeted locations in the phantoms, suggesting a microrobotic approach in the field of vascular intervention for improving the navigation of a conventional coronary guidewire.
Magnetically actuated guidewires. a Schematic and actual images of magnetically actuated one-magnet flexible guidewire-based microrobot for coronary intervention. Reproduced under CC BY 4.0 (cropped) [52]. b Schematic illustration of magnetically controlled two-magnet microrobot and 3D coronary phantom in vitro tracking experiment. Reproduced under CC BY 4.0 (cropped) [88]. c Schematic illustration of ferromagnetic soft continuum robot and in vitro navigation in 3D cerebral phantom. Reproduced with permission [90]. Copyright 2019, American Association for the Advancement of Science. d Magnetically steerable micro-guidewire for neuroradiology. Reproduced with permission [91]. Copyright 2006, Springer Nature. e Schematic illustration and photograph of magnetic guidewire with a 0.9 mm magnetic bead, developed for arterial catheterization. Reproduced with permission [92]. Copyright 2015, John Wiley and Sons. f Overview of Stereotaxis’ magnetic guidewire, enlarged view of magnetic tip and angiographic images of coronary arteries during clinical trials. Reproduced with permission [76]. Copyright 2006, John Wiley and Sons
In 2019, Kim et al. [90] developed a ferromagnetic soft-robot for application in the cerebrovascular intervention (Fig. 7c). As the first step of the fabrication process, ferromagnetic composite ink was prepared by mixing non-magnetized NdFeB particles with an elastomer such as PDMS or thermoplastic polyurethane (TPU). The sample was then permanently magnetized by a strong impulse of magnetic fields, which turned the previously freely flowing mixture into a thixotropic paste with shear-yielding and shear-thinning properties due to strong interaction between the magnetized NdFeB microparticles. The main body of the robot was shaped by either printing or injection molding after it was thermally welded to a commercial guidewire with a TPU jacket. Hydrogel skin was grown on the outer surface of the robot to provide hydrophilicity to the robot, which helped to reduce the surface friction. The embedded NdFeB particles were coated with thin shells of silica to prevent corrosion of the particles at the hydrated interface with the water-containing hydrogel skin. In this study, the robot was manually manipulated using a cylindrical permanent magnet, which provided magnetic fields required for actuation at a distance. The direction and strength of the magnetic fields were varied by changing the position and orientation of the magnet. They demonstrated the steering and navigating capabilities of the robot in a tortuous environment as well as in a 3D cerebrovascular phantom network. In addition, an optical fiber was incorporated in the robot’s body and tested in the carotid artery section of the vascular phantom to demonstrate the concept of magnetically steerable laser delivery.
In 2006, Krings et al. [91] compared the in vitro navigation capabilities of a magnetic neurovascular guidewire to those with a standard, manually controlled guidewire (Fig. 7d). The magnetic guidewire was equipped with a 2-mm-long permanent magnet positioned at its distal end. The experiments were carried out by operators with different levels of training and experience in guidewire handling. Two different 2D glass phantoms and a 3D vessel phantom were used for the experiments. According to the results, the magnetic guidewire was faster and more accurate only with less-experienced operators and there were no significant differences between the magnetic and manual manipulation by the experienced operators, although their findings suggested the features of the magnetically actuated guidewire might improve the efficacy and safety of complex neuro-interventional procedures in the future.
In 2015, Lalande et al. [92] demonstrated the steerability of a magnetic guidewire in vivo using an MRI system (Fig. 7e). The magnetic guidewire was composed of a ferromagnetic bead, which was glued to its tip. Here, a magnetic gradient generated by the coils in the MRI system produced a force on the bead which steered the guidewire in the desired direction. The in vivo navigation and steering experiments were carried out with two rabbits. Although the success rate was higher in the renal arteries, the majority of attempts in the other territories failed. The main limitation in this study was the absence of real-time navigation due to hardware incapacity to steer while tracking. The presence of the steering gradient coils in the MRI system made imaging impossible. Thus, the guidewire was blindly controlled based on the previous tracking sequence.
In 2005, Tsuchida et al. [76] carried out clinical trials to investigate the efficacy of guidewire navigation across the human coronary artery using a magnetic navigation system versus conventional manual navigation (Fig. 7f). The magnetic guidewire used in this study was designed by Stereotaxis, featuring a gold-encapsulated NdFeB permanent magnet at its distal segment. The Niobe system was integrated with a C-arm single-planar digital angiography system for the magnetic guidewire navigation. A total of 53 patients underwent trials with the magnetic guidewire navigation for coronary artery disease. The results, however, suggested there was no significant advantage of magnetic navigation compared to manual navigation in terms of procedural time, contrast volume or fluoroscopy time in simple lesions. In fact, significantly shorter procedure and fluoroscopy time were observed in manual navigation.
4.2 Catheter-based microrobots
Catheters that can be controlled by an external magnetic field are referred to as magnetic catheters or magnetically actuated catheter-based microrobots. As was the case in the guidewires, permanent magnets appear to be popular magnetic materials used in the fabrication of magnetic catheters. The application area of the magnetic catheters is confined to catheter ablation in the complex cardiac anatomy.
In 2017, Chautems et al. [83] presented a catheter connected to a magnet at its distal end by a string-like tether (Fig. 8a). The proximal end of the tether was able to extend from the catheter’s distal end, and the length of the tether was adjustable to control the tension on the tether. During an ablation procedure, the distal end of the tethered magnet is positioned in contact with the heart wall and extending the tether after tip contact transfers the tension to the contact point. In this study, they modeled the kinematics of the tethered magnet and tested inside the Aeon Phocus to demonstrate the feasibility of magnetic field gradient control in a certified magnetic actuation system. This magnetic catheter has a distinct advantage over other types of flexible magnetic catheters that magnetic gradients are effective in the steering of the catheter. In fact, magnetic gradients are often less effective and typically ignored in other flexible catheters due to the relative stiffness of catheters.
Magnetically actuated catheters. a Schematic illustration of tethered magnet concept and actual tethered magnet inside a magnetic field. Reproduced with permission [83]. Copyright 2017, IEEE. b 3D mapping of magnetic catheter and epicardial evidence of ablation in vivo. Reproduced with permission [78]. Copyright 2011, Wolters Kluwer Health. c Schematic illustration of magnetic catheter deflection and force versus deflection angle graph. Reproduced with permission [93]. Copyright 2002, Wolters Kluwer Health. d Images of catheter tip with magnetic beads and deflection of the catheter in response to magnetic fields. Reproduced with permission. Copyright 2011, IEEE [98] and Copyright 2011 [97], John Wiley and Sons. e Schematic illustration of solenoid magnetic catheter and prototype with the solenoid covered by heat shrink tubing at the tip. Reproduced with permission [99]. Copyright 2014, Radiological Society of North America. f Images of three-magnet tip catheter and its dimensions. Reproduced with permission [100]. Copyright 2016, John Wiley and Sons. g Images of variable stiffness (VS) catheters with two and three variable stiffness segments (VSSs), and deflection of the VSS1 in the soft state. Reproduced with permission. Copyright 2017, IEEE [82] and under CC BY 4.0 (cropped) [101]
In 2011, Gang et al. [78] demonstrated the feasibility of a single-magnet catheter in an in vivo set of experiments with 10 pigs’ hearts (Fig. 8b). The magnetic actuation system used in this study was the Magnetecs’ CGCI system. The results showed that the catheter can reproducibly and effectively reach target ablation points within the pig’s left atrium. In 2002, another in vivo study was carried out by Faddis et al. (Fig. 8c) using a single-magnet catheter and the results showed that the ablation using the magnetic catheter was highly successful and precise in all four cardiac chambers, compared to conventional ablation procedure [93]. Though the performance of the single-magnet catheter was promising in the studies, there still remain more in vivo and clinical work to be done in the future as the limitations of experiments with the pig’s heart have been well reported and known [94]. Furthermore, in a couple of studies, the fluoroscopy time and procedure time were significantly longer [95] and the success rate of ablation was lower [96] with the magnetic catheters than with the conventional method. These poor outcomes were due to difficulty in the navigation of the magnetic catheter in certain regions of the atria and weaker contact forces exerted by the magnetic catheter. However, the physician’s level of competence in handling the magnetic actuation system is also important and should be taken into account when analyzing the results.
In 2011, a catheter microrobot containing ferromagnetic spheres in its tip was demonstrated and reported by researchers from Prof. Martel’s group (Fig. 8d) [97, 98]. They attached one to two ferromagnetic spheres at the distal tip of the catheter with different spacing between the spheres. The catheter microrobot was successfully steered and guided by applied magnetic gradients inside a magnetic resonance scanner. More ferromagnetic materials induced larger magnetic forces; however, the use of two ferromagnetic spheres caused undesired dipole–dipole interactions, which could be a problem when navigating into small branches.
In 2014, a study was carried out by Losey et al. [99] to compare in vitro navigation of a magnetically assisted remote controlled (MARC) catheter with manual navigation under real-time MRI system (Fig. 8e). The MARC catheter was equipped with a solenoid coil at its distal tip, through which the steering of the catheter was achieved when there was a 300 mA current input. The experimental results showed that the MARC catheter was clearly visible under MRI and was faster than manual catheterization.
A three-magnet-tip catheter has been reported and tested by different groups. Armacost et al. [75] investigated the accuracy, reproducibility and efficiency of the catheter in positioning its targeted locations, and Chun et al. [68] demonstrated its ability to perform tissue ablation with a higher success rate compared to a single-magnet-tip catheter. In 2015, Le et al. developed a mathematical model to predict the motions of a three-magnet tip catheter using an electromagnetic system (Fig. 8f) [100]. A three-magnet catheter tip provides advantages over a single-magnet catheter tip, which are the ability to create a greater deflection force and to change the orientation of the outer magnets more rapidly.
In 2017 and 2019, Chautems et al. [82, 101] presented prototypes of variable stiffness (VS) magnetic catheter robots for cardiac ablation and gastrointestinal endoscopy using two and three variable stiffness segments (VSSs) (Fig. 8g). In this work, a low melting point alloy (LMPA) was used due to its ability to change physical states from solid to liquid at relatively low temperatures. As for the fabrication process, a cylindrical filament of LMPA was obtained by injecting molten LMPA into a silicone tube placed on a heated plate. After LMPA curing, the silicone tube was removed and conductive copper wires were wound around the LMPA filament to create two independent heaters, and a new silicone tube was wrapped around the filament to encapsulate it. In the last fabrication step, a permanent magnet was glued to the distal end of the encapsulated filament and the other end of the filament was attached to a rigid shaft. When current is applied into the copper wires, the LMPA increases above the melting point and the VSS becomes soft, increasing the flexibility of the catheter. When the LMPA solidifies, the VSS becomes rigid; thus, the stiffness of the catheter increases. The Aeon Phocus was used for magnetic manipulation of the VS catheter robots. The ability to bend in multiple radii using a single permanent magnet seems to be one of the unique advantages that the VS robots can offer in catheter ablation and gastrointestinal endoscopy [101].
5 Challenges and future prospect of magnetic interventional technologies
There has been rapid progress in the fields of magnetic actuation, magnetically actuated interventional instruments and microrobotics in recent years to overcome the limitations of conventional interventional procedures, as was seen from this paper. It is plausible that the number of people suffering from vascular diseases is forecast to increase significantly over the next few decades due to the aging population, rapid population growth and growing prevalence of obesity worldwide [102,103,104,105], which could subsequently contribute to the increased demand for various types of vascular interventions. Therefore, we are of the opinion that the market and the research activities for the magnetically actuated interventional devices will grow in scale and popularity due to the advantages and benefits the devices can offer.
However, there exist technical issues and challenges that need to be addressed for successful implementation of the magnetic interventional technologies in the future. As it was observed in a couple of clinical studies, the X-ray exposure time [95] and interventional procedure time [96] are often significantly longer with the currently existing magnetic interventional techniques than with the conventional manual method. Patients undergoing vascular interventions would be in favor of the magnetic interventional technologies only if they can experience reduced fluoroscopy and procedure times with magnetic technologies. To realize this, the development and integration of closed-loop control, 3D imaging detection, master–slave units and a user-friendly control interface along with the magnetic interventional technologies that can help the physician perform vascular interventions with greater ease and speed are an important part of the future work.
6 Conclusions
In this paper, numerous approaches to the progress on the microrobotic techniques for steering and actuation of magnetic guidewires and catheters were reviewed. Several relevant research publications on commercial magnetic actuation systems and magnetically actuated interventional devices were summarized and discussed according to the pre-defined criteria, including their features, advantages and limitations. The findings of this review suggest that the magnetically actuated interventional devices and magnetic actuation systems still have a long way to go before they reach technical and medical specifications that can satisfy the regulatory requirements for the commercialization and deployment of the technologies for clinical use, despite the fact that there are some successful in vivo animal and clinical trials of the technologies.
Nevertheless, these technologies have shown promise as effective tools, improving the steerability of guidewires and catheters inside the complex vascular networks while reducing procedural time and X-ray radiation of the current interventional procedures. The microrobotic approaches to the actuation of guidewires and catheters using a magnetic field are not confined to basic research, as some of the magnetic catheters and actuation systems can already be found in the market today, and have been used to treat a number of cardiac arrhythmias worldwide. The current research outcomes and tested principle appear to suggest that the magnetic actuation techniques for vascular interventions are promising and the development of safer, more user-friendly and optimized technologies is worth considering.
References
Jager EW, Inganäs O, Lundström I (2000) Microrobots for micrometer-size objects in aqueous media: potential tools for single-cell manipulation. Science 288(5475):2335–2338
Charreyron SL, Zeydan B, Nelson BJ (2017) Shared control of a magnetic microcatheter for vitreoretinal targeted drug delivery. In: 2017 IEEE international conference on robotics and automation (ICRA), 2017. IEEE, pp 4843–4848
Park J, Jin C, Lee S, Kim JY, Choi H (2019) Magnetically actuated degradable microrobots for actively controlled drug release and hyperthermia therapy. Adv Healthc Mater 8(16):1900213
Vikram Singh A, Sitti M (2016) Targeted drug delivery and imaging using mobile milli/microrobots: a promising future towards theranostic pharmaceutical design. Curr Pharm Des 22(11):1418–1428
Lee S, Kim S, Kim S, Kim JY, Moon C, Nelson BJ, Choi H (2018) A capsule-type microrobot with pick-and-drop motion for targeted drug and cell delivery. Adv Healthc Mater 7(9):1700985
Jeon S, Kim S, Ha S, Lee S, Kim E, Kim SY, Park SH, Jeon JH, Kim SW, Moon C, Nelson BJ, Kim J-Y, Yu S-W, Choi H (2019) Magnetically actuated microrobots as a platform for stem cell transplantation. Sci Robot 4(30):eaav4317
Kim S, Qiu F, Kim S, Ghanbari A, Moon C, Zhang L, Nelson BJ, Choi H (2013) Fabrication and characterization of magnetic microrobots for three-dimensional cell culture and targeted transportation. Adv Mater 25(41):5863–5868
Ceylan H, Giltinan J, Kozielski K, Sitti M (2017) Mobile microrobots for bioengineering applications. Lab Chip 17(10):1705–1724
Lee S, Lee S, Kim S, Yoon C-H, Park H-J, Kim J-y, Choi H (2018) Fabrication and characterization of a magnetic drilling actuator for navigation in a three-dimensional phantom vascular network. Sci Rep 8(1):3691
Nelson BJ, Kaliakatsos IK, Abbott JJ (2010) Microrobots for minimally invasive medicine. Annu Rev Biomed Eng 12:55–85
Sitti M (2009) Miniature devices: voyage of the microrobots. Nature 458(7242):1121
Sitti M, Ceylan H, Hu W, Giltinan J, Turan M, Yim S, Diller ED (2015) Biomedical applications of untethered mobile milli/microrobots. Proc IEEE 103(2):205–224
Stone GW, Maehara A, Lansky AJ, De Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364(3):226–235
Kearney K, Hira RS, Riley RF, Kalyanasundaram A, Lombardi WL (2017) Update on the management of chronic total occlusions in coronary artery disease. Curr Atheroscler Rep 19(4):19
Touma G, Ramsay D, Weaver J (2015) Chronic total occlusions—current techniques and future directions. IJC Heart Vasc 7:28–39
Louvard Y, Lefèvre T, Morice M-C (2004) Percutaneous coronary intervention for bifurcation coronary disease. Heart 90(6):713–722
Roubin GS, Yadav S, Iyer SS, Vitek J (1996) Carotid stent-supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol 78(3):8–12
Levin DC, Rao VM, Parker L, Bonn J, Maitino AJ, Sunshine JH (2005) The changing roles of radiologists, cardiologists, and vascular surgeons in percutaneous peripheral arterial interventions during a recent five-year interval. J Am College Radiol 2(1):39–42
Willinsky R (2000) Use of a second microcatheter in the management of a perforation during endovascular treatment of a cerebral aneurysm. Am J Neuroradiol 21(8):1537–1539
Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, Curtis LH (2015) Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. J Am Coll Cardiol 65(9):920–927
Jaïs P, Haïssaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clémenty J (1997) A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 95(3):572–576
Verma A, Jiang C-y, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372(19):1812–1822
Schneider P (2019) Endovascular skills: guidewire and catheter skills for endovascular surgery. CRC Press, Boca Raton
Ammann P, Brunner-La Rocca HP, Angehrn W, Roelli H, Sagmeister M, Rickli MdH (2003) Procedural complications following diagnostic coronary angiography are related to the operator’s experience and the catheter size. Catheter Cardiovasc Interv 59(1):13–18
Suzuki S, Furui S, Kohtake H, Yokoyama N, Kozuma K, Yamamoto Y (2006) Radiation exposure to patient’s skin during percutaneous coronary intervention for various lesions, including chronic total occlusion. Circ J 70(1):44–48
Rosenthal LS, Mahesh M, Beck TJ, Saul JP, Miller JM, Kay N, Klein LS, Huang S, Gillette P, Prystowsky E (1998) Predictors of fluoroscopy time and estimated radiation exposure during radiofrequency catheter ablation procedures. Am J Cardiol 82(4):451–458
Miller DL, Balter S, Noonan PT, Georgia JD (2002) Minimizing radiation-induced skin injury in interventional radiology procedures. Radiology 225(2):329–336
Walsh SR, Cousins C, Tang TY, Gaunt ME, Boyle JR (2008) Ionizing radiation in endovascular interventions. J Endovasc Ther 15(6):680–687
Stratakis J, Damilakis J, Tsetis D, Gourtsoyiannis N (2007) Radiation dose and risk from fluoroscopically guided percutaneous transluminal angioplasty and stenting in the abdominal region. Eur Radiol 17(9):2359–2367
Hidajat N, Wust P, Felix R, Schröder RJ (2006) Radiation exposure to patient and staff in hepatic chemoembolization: risk estimation of cancer and deterministic effects. Cardiovasc Intervent Radiol 29(5):791–796
Lange HW, von Boetticher H (2006) Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheter Cardiovasc Interv 67(1):12–16
Karpelson M, Wei G-Y, Wood RJ (2012) Driving high voltage piezoelectric actuators in microrobotic applications. Sens Actuators, A 176:78–89
Flynn AM, Tavrow LS, Bart SF, Brooks RA, Ehrlich DJ, Udayakumar KR, Cross LE (1990) Piezoelectric micromotors for microrobots. In: IEEE symposium on ultrasonics, 1990. IEEE, pp 1163–1172
Kosa G, Shoham M, Zaaroor M (2007) Propulsion method for swimming microrobots. IEEE Trans Rob 23(1):137–150
Donald BR, Levey CG, McGray CD, Paprotny I, Rus D (2006) An untethered, electrostatic, globally controllable MEMS micro-robot. J Microelectromech Syst 15(1):1–15
Ebefors T, Mattsson JU, Kälvesten E, Stemme G (1999) A walking silicon micro-robot. In: Proceedings of transducers’ 99, 1999. pp 1202–1205
Bonvilain A, Chaillet N (2003) Microfabricated thermally actuated microrobot. In: 2003 IEEE international conference on robotics and automation (Cat. No. 03CH37422), 2003. IEEE, pp 2960–2965
Erdem EY, Chen Y-M, Mohebbi M, Suh JW, Kovacs GT, Darling RB, Bohringer KF (2010) Thermally actuated omnidirectional walking microrobot. J Microelectromech Syst 19(3):433–442
Glückstad J, Villangca MJ, Palima DZ, Bañas A (2017) Light-actuated microrobots for biomedical science. Spie Newsroom, Bellingham
Palima D, Glückstad J (2013) Gearing up for optical microrobotics: micromanipulation and actuation of synthetic microstructures by optical forces. Laser Photonics Rev 7(4):478–494
Singh DP, Uspal WE, Popescu MN, Wilson LG, Fischer P (2018) Photogravitactic microswimmers. Adv Func Mater 28(25):1706660
Hu W, Ishii KS, Fan Q, Ohta AT (2012) Hydrogel microrobots actuated by optically generated vapour bubbles. Lab Chip 12(19):3821–3826
Alapan Y, Yasa O, Schauer O, Giltinan J, Tabak AF, Sourjik V, Sitti M (2018) Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Robot 3(17):eaar4423
Taherkhani S, Mohammadi M, Daoud J, Martel S, Tabrizian M (2014) Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 8(5):5049–5060
Behkam B, Sitti M (2007) Bacterial flagella-based propulsion and on/off motion control of microscale objects. Appl Phys Lett 90(2):023902
Solovev AA, Mei Y, Bermúdez Ureña E, Huang G, Schmidt OG (2009) Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 5(14):1688–1692
Wu Z, Troll J, Jeong H-H, Wei Q, Stang M, Ziemssen F, Wang Z, Dong M, Schnichels S, Qiu T (2018) A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci Adv 4(11):eaat4388
Diller E, Sitti M (2014) Three-dimensional programmable assembly by untethered magnetic robotic micro-grippers. Adv Func Mater 24(28):4397–4404
Kim S, Lee S, Lee J, Nelson BJ, Zhang L, Choi H (2016) Fabrication and manipulation of ciliary microrobots with non-reciprocal magnetic actuation. Sci Rep 6:30713
Hu W, Lum GZ, Mastrangeli M, Sitti M (2018) Small-scale soft-bodied robot with multimodal locomotion. Nature 554(7690):81
Chautems C, Lyttle S, Boehler Q, Nelson BJ (2018) Design and evaluation of a steerable magnetic sheath for cardiac ablations. IEEE Robot Autom Lett 3(3):2123–2128
Jeon S, Hoshiar AK, Kim S, Lee S, Kim E, Lee S, Kim K, Lee J, Kim J-y, Choi H (2018) Improving guidewire-mediated steerability of a magnetically actuated flexible microrobot. Micro Nano Syst Lett 6(1):15
Ren Z, Hu W, Dong X, Sitti M (2019) Multi-functional soft-bodied jellyfish-like swimming. Nature. Communications 10(1):1–12
Silva AKA, Silva EL, Egito EST, Carriço AS (2006) Safety concerns related to magnetic field exposure. Radiat Environ Biophys 45(4):245–252
Nguyen BL, Merino JL, Gang ES (2010) Remote navigation for ablation procedures–a new step forward in the treatment of cardiac arrhythmias. Eur Cardiol 6(3):50–56
Petrů J, Škoda J (2012) Robot-assisted navigation in atrial fibrillation ablation—of any benefits? Cor et Vasa. 54(6):e408–e413
Chautems C, Zeydan B, Charreyron S, Chatzipirpiridis G, Pane S, Nelson BJ (2017) Magnetically powered microrobots: a medical revolution underway? Eur J Cardiothorac Surg 51(3):405–407
Jiles D (2015) Introduction to magnetism and magnetic materials. CRC Press, Boca Raton
Furlani EP (2001) Permanent magnet and electromechanical devices: materials, analysis, and applications. Academic Press, Cambridge
Liu C (2012) Foundations of MEMS. Pearson Education, London
Ryan P, Diller E (2016) Five-degree-of-freedom magnetic control of micro-robots using rotating permanent magnets. In: 2016 IEEE international conference on robotics and automation (ICRA), 2016. IEEE, pp 1731–1736
Boskma KJ, Scheggi S, Misra S (2016) Closed-loop control of a magnetically-actuated catheter using two-dimensional ultrasound images. In: 2016 6th IEEE international conference on biomedical robotics and biomechatronics (BioRob), 2016. IEEE, pp 61–66
Salmanipour S, Diller E (2018) Eight-degrees-of-freedom remote actuation of small magnetic mechanisms. In: 2018 IEEE international conference on robotics and automation (ICRA), 2018. IEEE, pp 1–6
Floyd S, Pawashe C, Sitti M (2008) An untethered magnetically actuated micro-robot capable of motion on arbitrary surfaces. In: 2008 IEEE international conference on robotics and automation, 2008. IEEE, pp 419–424
Ernst S, Ouyang F, Linder C, Hertting K, Stahl F, Chun J, Hachiya H, Bänsch D, Antz M, Kuck K-H (2004) Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation 109(12):1472–1475
Ernst S, Ouyang F, Linder C, Hertting K, Stahl F, Chun J, Hachiya H, Krumsdorf U, Antz M, Kuck K-H (2004) Modulation of the slow pathway in the presence of a persistent left superior caval vein using the novel magnetic navigation system Niobe. EP Eur 6(1):10–14
Pappone C, Vicedomini G, Manguso F, Gugliotta F, Mazzone P, Gulletta S, Sora N, Sala S, Marzi A, Augello G (2006) Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol 47(7):1390–1400
Chun JK-R, Ernst S, Matthews S, Schmidt B, Bansch D, Boczor S, Ujeyl A, Antz M, Ouyang F, Kuck K-H (2007) Remote-controlled catheter ablation of accessory pathways: results from the magnetic laboratory. Eur Heart J 28(2):190–195
Di Biase L, Fahmy TS, Patel D, Bai R, Civello K, Wazni OM, Kanj M, Elayi CS, Ching CK, Khan M (2007) Remote magnetic navigation: human experience in pulmonary vein ablation. J Am Coll Cardiol 50(9):868–874
Carpi F, Pappone C (2009) Stereotaxis Niobe® magnetic navigation system for endocardial catheter ablation and gastrointestinal capsule endoscopy. Expert Rev Med Dev 6(5):487–498
Atmakuri SR, Lev EI, Alviar C, Ibarra E, Raizner AE, Solomon SL, Kleiman NS (2006) Initial experience with a magnetic navigation system for percutaneous coronary intervention in complex coronary artery lesions. J Am Coll Cardiol 47(3):515–521
Thornton AS, Jordaens LJ (2006) Remote magnetic navigation for mapping and ablating right ventricular outflow tract tachycardia. Heart Rhythm 3(6):691–696
Choi MS, Oh Y-S, Jang SW, Kim JH, Shin WS, Youn H-J, Jung WS, Lee MY, Seong KB (2011) Comparison of magnetic navigation system and conventional method in catheter ablation of atrial fibrillation: is magnetic navigation system is more effective and safer than conventional method? Korean Circul J 41(5):248–252
Kiemeneij F, Patterson MS, Amoroso G, Laarman G, Slagboom T (2008) Use of the Stereotaxis Niobe® magnetic navigation system for percutaneous coronary intervention: results from 350 consecutive patients. Catheter Cardiovasc Interv 71(4):510–516
Armacost MP, Adair J, Munger T, Viswanathan RR, Creighton FM, Curd DT, Sehra R (2007) Accurate and reproducible target navigation with the stereotaxis Niobe® magnetic navigation system. J Cardiovasc Electrophysiol 18:S26–S31
Tsuchida K, García-García HM, van der Giessen WJ, McFadden EP, van der Ent M, Sianos G, Meulenbrug H, Ong AT, Serruys PW (2006) Guidewire navigation in coronary artery stenoses using a novel magnetic navigation system: first clinical experience. Catheter Cardiovasc Interv 67(3):356–363
Elrod J (2017) A new option for catheter guidance control and imaging. EPLab Digest, Devault
Gang ES, Nguyen BL, Shachar Y, Farkas L, Farkas L, Marx B, Johnson D, Fishbein MC, Gaudio C, Kim S (2011) Dynamically shaped magnetic fields: initial animal validation of a new remote electrophysiology catheter guidance and control system. Circul Arrhythm Electrophysiol 4(5):770–777
Nguyen BL, Merino JL, Shachar Y, Estrada A, Doiny D, Castrejon S, Marx B, Johnson D, Marfori W, Gang ES (2013) Non-fluoroscopic transseptal catheterization during electrophysiology procedures using a remote magnetic navigation system. J Atr Fibrillation 6(4):963
Filgueiras-Rama D, Estrada A, Shachar J, Castrejón S, Doiny D, Ortega M, Gang E, Merino JL (2013) Remote magnetic navigation for accurate, real-time catheter positioning and ablation in cardiac electrophysiology procedures. JoVE 74:e3658
Moya À, Sancho-Tello MJ, Arenal Á, Fidalgo ML, Brugada R, Ferrer JM, Merino JL, Mateas FR, Mont JLJREdC (2013) Innovations in heart rhythm disturbances: cardiac electrophysiology. Arrhythm Cardiac Pacing 66(2):116–123
Chautems C, Tonazzini A, Floreano D, Nelson BJ (2017) A variable stiffness catheter controlled with an external magnetic field. In: 2017 IEEE/RSJ international conference on intelligent robots and systems (IROS), 2017. IEEE, pp 181–186
Chautems C, Nelson BJ (2017) The tethered magnet: force and 5-DOF pose control for cardiac ablation. In: 2017 IEEE international conference on robotics and automation (ICRA), 2017. IEEE, pp 4837–4842
Li S, Zhao H, Shepherd RF (2017) Flexible and stretchable sensors for fluidic elastomer actuated soft robots. MRS Bull 42(2):138–142
Lin H-T, Leisk GG, Trimmer B (2011) GoQBot: a caterpillar-inspired soft-bodied rolling robot. Bioinspiration Biomim 6(2):026007
Ilievski F, Mazzeo AD, Shepherd RF, Chen X, Whitesides GM (2011) Soft robotics for chemists. Angew Chem Int Ed 50(8):1890–1895
Kratochvil BE, Kummer MP, Erni S, Borer R, Frutiger DR, Schürle S, Nelson BJ (2014) MiniMag: a hemispherical electromagnetic system for 5-DOF wireless micromanipulation. In: Experimental Robotics, 2014. Springer, Berlin, pp 317–329
Jeon S, Hoshiar AK, Kim K, Lee S, Kim E, Lee S, Kim J-y, Nelson BJ, Cha H-J, Yi B-J, Choi H (2019) A magnetically controlled soft microrobot steering a guidewire in a three-dimensional phantom vascular network. Soft Robot 6(1):54–68
Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ (2010) OctoMag: an electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans Rob 26(6):1006–1017
Kim Y, Parada GA, Liu S, Zhao X (2019) Ferromagnetic soft continuum robots. Sci Robot 4(33):eaax7329
Krings T, Finney J, Niggemann P, Reinacher P, Lück N, Drexler A, Lovell J, Meyer A, Sehra R, Schauerte P (2006) Magnetic versus manual guidewire manipulation in neuroradiology: in vitro results. Neuroradiology 48(6):394–401
Lalande V, Gosselin FP, Vonthron M, Conan B, Tremblay C, Beaudoin G, Soulez G, Martel S (2015) In vivo demonstration of magnetic guidewire steerability in a MRI system with additional gradient coils. Med Phys 42(2):969–976
Faddis MN, Blume W, Finney J, Hall A, Rauch J, Sell J, Bae KT, Talcott M, Lindsay B (2002) Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 106(23):2980–2985
Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH (1998) Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 193(1):105–119
Miyazaki S, Shah AJ, Xhaët O, Derval N, Matsuo S, Wright M, Nault I, Forclaz A, Jadidi AS, Knecht S (2010) Remote magnetic navigation with irrigated tip catheter for ablation of paroxysmal atrial fibrillation. Circul Arrhythm Electrophysiol 3(6):585–589
Vollmann D, Lüthje L, Seegers J, Hasenfuss G, Zabel M (2009) Remote magnetic catheter navigation for cavotricuspid isthmus ablation in patients with common-type atrial flutter. Circul Arrhythm Electrophysiol 2(6):603–610
Gosselin FP, Lalande V, Martel S (2011) Characterization of the deflections of a catheter steered using a magnetic resonance imaging system. Med Phys 38(9):4994–5002
Vonthron M, Lalande V, Bringout G, Tremblay C, Martel S (2011) A MRI-based integrated platform for the navigation of micro-devices and microrobots. In: 2011 IEEE/RSJ international conference on intelligent robots and systems, 2011. IEEE, pp 1285–1290
Losey AD, Lillaney P, Martin AJ, Cooke DL, Wilson MW, Thorne BR, Sincic RS, Arenson RL, Saeed M, Hetts SW (2014) Magnetically assisted remote-controlled endovascular catheter for interventional MR imaging: in vitro navigation at 1.5 T versus x-ray fluoroscopy. Radiology 271(3):862–869
Le VN, Nguyen NH, Alameh K, Weerasooriya R, Pratten P (2016) Accurate modeling and positioning of a magnetically controlled catheter tip. Med Phys 43(2):650–663
Chautems C, Tonazzini A, Boehler Q, Jeong SH, Floreano D, Nelson BJ (2019) Magnetic continuum device with variable stiffness for minimally invasive surgery. Advanced Intelligent Systems 1900086
Bonow RO, Eckel RH (2003) Diet, obesity, and cardiovascular risk. N Engl J Med 348(21):2057–2133
Smith SC, Collins A, Ferrari R, Holmes DR, Logstrup S, McGhie DV, Ralston J, Sacco RL, Stam H, Taubert K (2012) Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). J Am Coll Cardiol 60(22):2343–2348
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM (2010) Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 91(3):502–509
Mozaffarian D, Capewell S (2011) United Nations’ dietary policies to prevent cardiovascular disease. British Medical Journal Publishing Group, London
Acknowledgements
This work was supported in part by funding from the Ministry of Health and Welfare, Republic of Korea (Grant No. HI19C0655020019), the Ministry of Science and ICT (Grant No. NRF-2017K1A1A2013237), Republic of Korea, and DGIST (Grant No. 20-CoE-BT-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, J., Kim, Jy. & Choi, H. A review of magnetic actuation systems and magnetically actuated guidewire- and catheter-based microrobots for vascular interventions. Intel Serv Robotics 13, 1–14 (2020). https://doi.org/10.1007/s11370-020-00311-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11370-020-00311-0