Abstract
Introduction
Standard microguidewires used in interventional neuroradiology have a predefined shape of the tip that cannot be changed while the guidewire is in the vessel. We evaluated a novel magnetic navigation system (MNS) that generates a magnetic field to control the deflection of a microguidewire that can be used to reshape the guidewire tip in vivo without removing the wire from the body, thereby potentially facilitating navigation along tortuous paths or multiple acute curves.
Method
The MNS consists of two permanent magnets positioned on either side of the fluoroscopy table that create a constant precisely controlled magnetic field in the defined region of interest. This field enables omnidirectional rotation of a 0.014-inch magnetic microguidewire (MG). Speed of navigation, accuracy in a tortuous vessel anatomy and the potential for navigating into in vitro aneurysms were tested by four investigators with differing experience in neurointervention and compared to navigation with a standard, manually controlled microguidewire (SG).
Results
Navigation using MG was faster (P=0.0056) and more accurate (0.2 mistakes per trial vs. 2.6 mistakes per trial) only in less-experienced investigators. There were no statistically significant differences between the MG and the SG in the hands of experienced investigators. One aneurysm with an acute angulation from the carrier vessel could be navigated only with the MG while the SG failed, even after multiple reshaping manoeuvres.
Conclusion
Our findings suggest that magnetic navigation seems to be easier, more accurate and faster in the hands of less-experienced investigators. We consider that the features of the MNS may improve the efficacy and safety of challenging neurointerventional procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conventional microguidewires used in interventional neuroradiology utilize manual control mechanisms that demonstrate limitations that may affect the efficacy and safety of navigation, especially in tortuous vessels. Guidewire deflection mechanisms are inherently two dimensional with movement in the third dimension provided by torque transmitted by the operator handling the microguidewire proximally. However, torque transmission can be limited by frictional contact along tortuous paths. In addition, the radius of deflection is limited since the shape of the microguidewire is fixed at its tip before it is introduced into the microcatheter. Moreover, this predefined shape of the microguidewire tip might change within the vessel, especially during complicated and lengthy guiding manoeuvres.
Magnetic navigation is based on the interaction between an external magnetic field of specified direction and magnitude and the magnet that is located on the tip of the microguidewire. When the external magnetic field is applied, this magnet (and thereby the tip of the microguidewire) aligns itself with the field direction. The external magnets can be rotated and angled to change the field orientation. Therefore, the deflection and direction of the guidewire tip can be changed according to the specific needs of the physician while the guidewire is still in the patient’s vasculature [1–3].
To determine, whether this technique might prove useful for neuroradiological procedures, the following study was undertaken. Specifically, our aims were to determine: (a) whether the speed and accuracy of navigation was altered when using magnetic navigation in comparison to manual navigation in the hands of physicians with varying experience; (b) whether there were any differences in the learning curves for the two different methods in the hands of physicians with varying experience; (c) how much tortuosity of the vessel affected navigation employing the different methods; and (d) whether typically encountered aneurysms in the human cerebral vasculature could be reached with both types of microguidewires in a 3D vessel-phantom.
Methods
Magnetic navigation system
The Niobe II system (Stereotaxis, St. Louis, Mo.) is a magnetic navigation system (MNS) that comprises: (a) the navigation system, (b) a flat-detector fluoroscopic imaging suite (AXIOM Artis dFC, Siemens Medical Solutions, Forchheim, Germany), and (c) a digital control station. The navigation system consists of two permanent magnets, positioned on either side of the fluoroscopy table. The positions of the magnets relative to each other are computer controlled inside a fixed housing. While positioned in “navigate” position, they create a relatively uniform magnetic field (0.1 T) within a roughly spherical region approximately 15 cm in diameter inside the patient. The magnets create a constant precisely controlled magnetic field in the defined region of interest. This field enables 360° omnidirectional rotation of the device. The digital control station is sited in the control room. The magnetic field is steered via the user interface (touch screen monitor) both from within the control room and at the patient table. Using the Niobe system’s Navigant software (Stereotaxis), we were able to specify device direction and movement with point-and-click navigation tools via two- and three-dimensional interfaces. In addition, this module gives a range of preset controls including bulls-eye orientation, target-based navigation and anatomical overlays (Fig. 1). The control computer calculates the appropriate positions of the permanent magnets. The resultant composite magnetic field interacts with the tip of the microguidewire to align it parallel to the magnetic field. Each magnetic field manipulation requires approximately 5 s to activate.
Guidewires
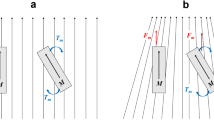
The magnetic microguidewire (MG, Cronus 0.014-inch guidewire, Stereotaxis) is equipped with a small (2 mm long) permanent magnet positioned at the tip that aligns itself with the direction of the externally controlled magnetic field to enable it to be steered effectively. By changing the orientation of the outer magnets relative to each other, the orientation of the magnetic field changes and thereby leads to deflection of the microguidewire (Fig. 2).
For manual navigation, a standard 0.014-inch Synchro microguidewire (SG, Boston Scientific, Fremont, Calif.) was chosen with the tip prebent according to the operator’s needs. For the microcatheter, an Excelsior 10-18 (Boston Scientific) was chosen and introduced via a 6F Envoy guiding catheter (Cordis, Fl.) into the tubing of the phantoms.
In vitro experiments
All runs were performed by four individuals with differing levels of training in microguidewire and catheter handling: one experienced neurointerventionalist, one neuroradiologist with moderate experience concerning neurointervention, one neurosurgeon without experience in microguidewire handling, and one graduate student. Before the testing began, training was provided with a training phantom for the use of the Navigant and Touch Screen software.
Three different models were used for the in vitro studies (see Figs. 3 and 4). Both two-dimensional phantoms were made from glass. Navigation could be performed without continuous fluoroscopy. A customized three-dimensional vessel phantom (Elastrat-Sarl, Geneva, Switzerland) included both carotid and vertebral arteries and a complete circle of Willis in realistic dimensions. A total of six aneurysms with different neck morphologies, sizes and angulations from the carrier vessel were present on this model (Fig. 4). The location of the aneurysms, their dimensions and other characteristics can be found in Table 1. In one aneurysm (left middle cerebral artery (MCA) bifurcation), there was an eccentric 80% stenosis present in the MCA proximal to the aneurysm. One aneurysm simulated a dissected vertebral artery aneurysm, in which the task was to navigate through the dissected area. Before navigation started in the 3D model, a 3D rotational angiogram was performed, uploaded into the Navigant system and registered. The navigation pathways were marked in advance. Magnetic vessel navigation was performed using the touch screen interface, with adjustments made using the field rotate mouse. Navigation in the aneurysm phantom was performed under continuous fluoroscopy, using a roadmap image from a contrast injection.
Three-dimensional aneurysm model with angiographic images of the six different aneurysms as described in Table 1
The phantoms were used wetted with a mixture of water and soap to simulate tissue lubricity. Additionally, in the three-dimensional phantom, flow was simulated by connecting both the inlet and outlet to a pump.
Three different tasks were performed to specifically evaluate speed of navigation in a two-dimensional model, accuracy of navigation in a two-dimensional model, and success of navigation in a complex three-dimensional model. For the first task, a predefined path employing 12 curves was navigated 15 times to evaluate the learning curves for the microcatheter navigation employing both the MG and the SG (Fig. 3a,b). The times needed to complete the manoeuvres were recorded. Timing was stopped when the microcatheter reached the predefined target point (end of the maze). To evaluate the accuracy of navigation of both wires in a tortuous vessel anatomy six acute turns had to be performed five times each in the second model (Fig. 3c,d) with the highest possible accuracy. The participants were informed that timing did not matter in this task. During the final experimental setup it was evaluated whether navigation into all aneurysms of the three-dimensional model (Fig. 4) was possible.
Results
Speed of navigation
Each of the four investigators performed 15 trials for this maze both with the MG and the SG. The mean time for completing this task with the MG was 53.2 s (SD 14.2 s), and for the SG 79.7 s (SD 83.7 s). The difference between the MG and the SG was highly statistically significant (t-test, P=0.0056). When evaluating the data in relation to the experience of the investigator, however, there were no statistically significant differences in speed for the experienced and the moderately experienced investigator (P=0.30 andP=0.13, respectively). However, for the less-experienced investigators the difference in speed of navigation was significant favouring the MG (P=0.06 for the neurosurgeon andP=0.01 for the graduate student). None of the four investigators demonstrated a clear learning curve with either MG or SG. However, in all but the experienced investigator, there was a high variability in the speed of navigation for the SG as indicated by a high standard deviation (Table 2).
Accuracy of navigation in tortuous vessels
Six acute turns had to be mastered in this maze with the highest possible accuracy in five consecutive trials. For the MG, each investigator made one wrong turn once, leading to an average mistake rate of 0.2 per trial for all investigators. With the SG, the experienced investigator made one wrong turn (average 0.2 per trial), whereas the less-experienced investigators made 17, 14, and 8 mistakes (average 3.4, 2.8, and 1.6 per trial), respectively.
Aneurysm navigation
For five aneurysms, entering the aneurysm with the microcatheter after careful navigation of the microguidewire into the aneurysm was attempted in five consecutive trials. In the aneurysm simulating a dissecting aneurysm the task was to navigate distal to the area of dissection. For all but one aneurysm this manoeuvre proved to be feasible with both the MG and the SG for all investigators. In particular, navigation into the left MCA aneurysm, which was located directly distal to an eccentric high-grade stenosis, and navigation through the “dissecting” aneurysm was readily feasible for all investigators employing both the MG and the SG. However, in a small right middle cerebral artery bifurcation aneurysm (Fig. 5) that demonstrated an acute angle from the carrier vessel, navigation with the SG was not possible, even after multiple reshaping attempts. Instead of entering the aneurysm, the SG kept on entering the lower M2 branch and navigation was finally abandoned. Navigation with the MG, on the other hand, was successful: the aneurysm was entered without any problems using an “overdriven” field direction (Fig. 5, frame d). There were no differences between the levels of experience in this experimental set-up.
Discussion
The first use of magnetism to direct an intravascular catheter was reported in 1951 [4]. A catheter with a steel tip could be navigated via an external magnet to areas of the vasculature that were previously inaccessible to standard guidewires; this design was subsequently revised by incorporating a central lumen for injection of contrast agent [5]. In 1991, the first human use of this technique was demonstrated in a neonate with complex congenital heart disease [6]. However, due to restrictions in size of the applied magnetic field, field strength and depth of the magnetic field, manipulations were only possible in a single plane and, therefore, this technique was not further pursued. The principle employed in the navigation system used in this investigation was first described in 1959 [7] and was further refined for intravascular use in 1968 [8]. This is based on the consideration that a magnetic object orients itself parallel to a prevailing uniform magnetic field. When using a multicoil arrangement, true three-dimensional control of a magnetic object is possible [8]. The basic principle of the current system is that individual magnetic fields generated from a complex arrangement and computer-guided orientation of solid magnets on each side of the patient superimpose upon each other to form a composite magnetic field for catheter manipulation. The first use of this system in animals was described in 2002 for cardiac interventions [2], and the first description of the human use of magnetic navigation was described in 2004 [3].
The principle of microguidewire manipulation with a magnetic navigation system is based on the following considerations. The external magnetic field, which is homogeneous in the local neighbourhood of the catheter tip, induces a torque on the permanent magnet located in the tip of the guidewire (just as the needle of a compass aligns itself to magnetic north). The wire tip magnet turns until its longitudinal axis is parallel to the surrounding magnetic field. The magnitude of the torque is equal to the product of the intrinsic magnetization (M) of the tip magnet, the length (L) of the tip magnet, the cross-sectional area (A) of the tip magnet, the magnetic field strength (B), and the sine of the angle of the permanent tip magnet relative to the magnetic field vector [1]. The torque can also be described by two identical forces acting in opposite directions on the two poles of the permanent magnet through the moment arm (L/2). Combining equations yields a description of the force exerted by the magnetic field on the catheter. The force exerted on the catheter is maximal when the guidewire orients perpendicular to the magnetic field and goes to zero as the catheter aligns parallel to the magnetic field [1].
Three experimental studies have thus far been carried out with a magnetic guidance system to derive phantom and animal data [9–11]. One study focused on a liver phantom comparing a magnetic and a conventional microguidewire [9], and another study used the same experimental set-up in an in vitro model of the female pelvis [10]. Both studies demonstrated a significantly shorter fluoroscopy time during magnetic navigation. The authors concluded that magnetic guidance allows precise navigation in a shorter time. In a pig model, the magnetic guidance system was used to direct a catheter on a nonlinear path through the brain to a predefined biopsy target. An endovascular route was not used in this study, but instead magnetic guidance was used to navigate to the biopsy site through the brain tissue. The authors concluded that magnetic guidance might be helpful for targeting the site of biopsy, especially when a nonlinear path was employed [11].
Clinical studies investigating the potential applications of magnetic navigation using this system have been reported. They have focused on cardiac interventions primarily employing magnetic ablation catheters instead of magnetic guidewires.
In one study, two patients with an accessory conduction pathway who presented with Wolff-Parkinson-White syndrome scheduled for catheter ablation were investigated [12]. In another study, a total of 42 patients underwent ablation of atrioventricular nodal reentry tachycardia with the use of the magnetic navigation system [3]. The authors concluded that magnetic navigation allowed remote-controlled navigation of an ablation catheter and proved to be helpful both for the patient and the physician. The ablation catheter held a stable position even with changing cardiac rhythms and complex atrial anatomy. Reapplication of a previously applied magnetic field vector allowed renavigation to a previously visited site and thereby shortened both mapping and fluoroscopy time. Moreover, after the diagnostic catheters were positioned, the electrophysiological study and the ablation process was performed remotely from within the control room, thereby reducing the fluoroscopic exposure time for the operator [3].
Although these descriptions are promising for cardiovascular applications, the potential use of this system in endovascular neuronavigation is limited by the size of the magnetic device (i.e. the ablation catheter). Only with the advent of a 0.014-inch microguidewire equipped with a magnetically steerable tip have the potential applications in neuroradiology become possible. This is the first description of the potential applications, problems and advantages of magnetic navigation for neurovascular interventions.
We have found that, using the magnetic guidewire, the speed of navigation was faster and the accuracy higher. Moreover, navigation through the phantoms with the magnetic guidewire demonstrated less variability (smaller standard deviation) of the time needed to complete the task.
These differences were most pronounced with the less-experienced investigators. These data suggest that magnetic navigation is easier, more accurate and faster in the hands of investigators not trained in interventional neuroradiology. The most pronounced differences were present in the accuracy of navigation between the magnetic and the standard guidewires. In the accuracy evaluation, the three less-experienced investigators made on average 2.6 wrong turns for six acute curves, whereas only 0.2 wrong turns were noted with the magnetic guidewire. Even the experienced investigator had the same results with little experience in magnetic navigation compared to his vast experience with manual navigation (0.2 wrong curves both for the magnetic and the standard guidewire). Unwanted or unpredictable translational movements were not encountered with magnetic navigation. In addition, we found that one aneurysm that demonstrated an acute angle from the carrier vessel (which is encountered exceedingly rarely in humans) could be navigated only with the magnetic guidewire. In the other investigated aneurysms, no statistically significant differences were noted between the two wire types.
Standard manually controlled guidewires have a predefined shape of the tip that cannot be changed while the guidewire is in the vessel thereby reducing the potential radius of deflection. Additionally, torque transmission can be limited by friction, especially in tortuous vessel anatomy. The predefined shape of the conventional microguidewire tip might also be altered with time within the vessel, especially during complicated and lengthy guiding manoeuvres. The magnetic navigation system might address some of these shortcomings. The use of magnetic force to control microguidewire position is accurate and facilitates control of subsequent catheter movement. The shape of the guidewire tip can be changed while in the vessel, thereby potentially facilitating navigation along tortuous paths or multiple acute curves. This feature might prove helpful, when navigating through acute branches to the distal vasculature such as during AVM embolization, or navigation across high-grade or exceedingly stenotic lesions that might be encountered during carotid stenting. Apart from telemedicine applications (that seem to us for the moment too speculative to discuss), potential future applications include the integration of three-dimensional maps into the guidance system for pretherapeutic planning of neurointerventional procedures. Regarding magnetic force, tip stiffness and the possibility of injuring the vessel wall, the wires have tip characteristics similar to “floppy” conventional guidewires and therefore demonstrate similar tissue forces. The magnetic field force itself has a clinically insignificant effect in increasing tip force on tissue. Regarding its stiffness, the guidewire tip prolapses within the same range of force as conventional wires whether the magnetic field is on or off. We would therefore anticipate no greater risk in aneurysmal tissue compared to a conventional manual wire and conventional techniques.
New devices such as microcatheters with a magnetic tip might prove helpful during aneurysm coiling to reach different parts of the aneurysm and to redirect the coils into different compartments of the aneurysm without having to replace the catheter. This technique could also be helpful to hold the catheter tip in position and to avoid luxation of the catheter tip out of the aneurysm during the coiling procedure. Prototypes of microcatheters have been tested with tip magnets and the Niobe system in vitro. Although they seem to be safe to use, the designs are still being modified by the company and are not yet effective for clinical use. In vivo animal experiments (such as in the aneurysm model of the rabbit) will be necessary to further determine the potential role of magnetic navigation in interventional neuroradiology [13].
From the present in vitro results we can foresee the following limitations and problems of the system. When the magnets are in the “navigate” position, biplanar fluoroscopy is not possible. This might be a disadvantage when navigating in difficult vessel anatomy or during coiling of an aneurysm. With the navigation system in place, the angle of the fluoroscopic system is limited to 28° for both left anterior oblique and right anterior oblique projections. Although this might not be of importance for some navigational manoeuvres, complex angulations, specific neck morphologies of aneurysms or acute angles of cerebral vessels might become superimposed and create challenges to guidewire handling. Three-dimensional road-mapping which was recently described, might, however, solve these two problems [14]. Clinical studies are needed to evaluate the benefits of magnetic navigation in a realistic environment in vivo.
Conclusions
Interventional procedures in the distal neurovasculature can be challenging and complex. Manually controlled guidewires have inherent functional limitations. Manual control of the distal tip becomes increasingly difficult as blood vessels become smaller, more tortuous and less accessible. With programmable omnidirectional distal tip control as offered by the magnetic navigation system, the physician is able to reshape the wire tip in vivo without removing or replacing the wire. This might prove helpful both to reduce procedure time and the number of wires used for complex cases. We were able to demonstrate that this could result in a higher accuracy of navigation and save fluoroscopy time. We therefore conclude that for selected complex cases, magnetic navigation might prove helpful for endovascular procedures.
References
Faddis MN, Lindsay B (2003) Magnetic catheter manipulation. Coron Artery Dis 14:25–27
Faddis MN, Blume W, Finney J, et al (2002) Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 106:2980–2985
Ernst S, Ouyang F, Linder C, et al (2004) Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation 109:1472–1475
Tillander H (1951) Magnetic guidance of a catheter with articulated steel tip. Acta Radiol 35:62–64
Tillander H (1956) Selective angiography of the abdominal aorta with a guided catheter. Acta Radiol 45:21–26
Ram W, Meyer H (1991) Heart catheterization in a neonate by interacting magnetic fields: a new and simple method of catheter guidance. Cathet Cardiovasc Diagn 22:317–319
Jenkins A, Parker H (1959) Electromagnetic support arrangement with three dimensional control. J Appl Phys 30:238–239
Yodh SB, Pierce NT, Weggel RJ, Montgomery DB (1968) A new magnet system for “intravascular navigation”. Med Biol Eng 6:143–147
Schiemann M, Killmann R, Kleen M, Abolmaali M, Finney J, Vogl TJ (2004) Vascular guide wire navigation with a magnetic guidance system: experimental results in a phantom. Radiology 232:475–481
Dietrich T, Kleen M, Killmann R, et al (2004) Evaluation of magnetic navigation in an in vitro model of uterine artery embolization. J Vasc Interv Radiol 15:1457–1462
Grady MS, Howard MA, Dacey RG, et al (2000) Experimental study of the magnetic stereotaxis system for catheter manipulation. J Neurosurg 93:282–288
Ernst S, Hachiya H, Chun JK, Ouyang F (2005) Remote catheter ablation of parahisian accessory pathways using a novel magnetic navigation system – a report of two cases. J Cardiovasc Electrophysiol 16:659–662
Krings T, Moller-Hartmann W, Hans FJ, et al (2003) A refined method for creating saccular aneurysms in the rabbit. Neuroradiology 45:423–429
Soderman M, Babic D, Homan R, Andersson T (2005) 3D roadmap in neuroangiography: technique and clinical interest. Neuroradiology 47:735–740
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krings, T., Finney, J., Niggemann, P. et al. Magnetic versus manual guidewire manipulation in neuroradiology: in vitro results. Neuroradiology 48, 394–401 (2006). https://doi.org/10.1007/s00234-006-0082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0082-3