Abstract
Purpose
Iron (Fe) oxides play an important role in regulating nitrification and N2O emissions, but there is very little study on the biological-chemical comprehensive effects of Fe oxides on nitrification and N2O emissions.
Materials and methods
A laboratory incubation experiment was performed to evaluate the effect of goethite addition on nitrification and N2O emissions from acidic and alkaline paddy soils.
Results and discussion
The cumulative N2O emissions from alkaline paddy soil were significantly higher than those from acidic paddy soil, no matter whether goethite had been added or not. Adding goethite decreased the average net rate of soil nitrification in acidic paddy soil by 33.2% in comparison with the treatment without adding goethite; however, adding goethite scarcely decreased the average net rate in alkaline paddy soil. Adding goethite increased the maximal N2O emissions by 85.6% in alkaline paddy soil, but had no obvious effect in acidic paddy soil. Adding goethite significantly increased the abundance of ammonia-oxidizing archaea (AOA) and bacteria (AOB) amoA genes in both alkaline and acidic paddy soils. High-throughput pyrosequencing of 16S rRNA gene showed that adding goethite significantly increased the relative abundance of Nitrosomonadaceae in alkaline paddy soil and that the dominant species of AOB and AOA were Nitrosomonadaceae and Nitrososphaeraceae, respectively.
Conclusions
N2O emissions in alkaline paddy soil were higher than those in acidic paddy soil. The enhancement of N2O emissions by goethite was more significant in alkaline paddy soil than in acidic paddy soil. Goethite stimulated the abundance of amoA gene (both of AOB and AOA) and participated in nitrification process via chemical reaction with intermediates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrous oxide (N2O) has about 298 times global warming potential higher than that of carbon dioxide over a 100-year time scale (IPCC, 2013; Zhu-Barker et al. 2015). Agricultural lands are one of the major N2O sources because of intensive nitrogen fertilization, especially in paddy soils with specific redoximorphic features (Hoben et al. 2011; Liu et al. 2011). The total annual N2O emissions from paddy soils in China were about 29.0 Gg N2O–N over the period of 1992–2002 (Zou et al. 2007). Deeper insight into the process and mechanism involved in N2O emissions is important for paddy soils (Wang et al. 2016). There are much high emissions of N2O during pre-flooding period (Zhao et al. 2011), and the dry-wet cycles can increase N2O emissions in paddy soils (Zou et al. 2007). Therefore, the fact that nitrification can produce N2O, especially under non-flooded conditions, should not be overlooked (He et al. 2016; Xin et al. 2016).
Nitrification is a biological process that spans all oxidation states of nitrogen from NH4+ to NO3−, including compounds with intermediary oxidation states such as hydroxylamine (NH2OH) and nitrite (NO2−) (Huang et al. 2016). In general, the factors that regulate the activity of nitrogen cycling microorganisms such as pH, soil water content, and iron and manganese oxides are assumed as the same factors that regulate nitrification and N2O emissions (Beaumont et al. 2002; Venkiteswaran et al. 2014; Jiang et al. 2015). It is widely recognized that nitrification is highly sensitive to pH (Curtin et al. 1998; Jiang et al. 2015). For example, it was reported that lime, rather than ammonium amendment, stimulated the growth of ammonia-oxidizing bacteria (AOB) and their nitrifying activity in acid forest soil. The net nitrification rate was increased by 3 times in the soil with lime application (pH 3.8) in comparison with the soil without lime application (pH 2.8) (Nugroho et al. 2007). In general, nitrification is an important pathway of N2O emissions in low-moisture soil (≤ 60% water-filled pore space) (Bateman and Baggs. 2005), whereas under the condition of deficiency oxygen, denitrification is the main way of N2O production (sequential enzymatic reduction of NO2− to NO and N2O). However, those dissimilatory processes can occur simultaneously in different microsites within the same soil (Hink et al. 2017). Fe and Mn oxides can act as electron acceptors or donors to play a critical role of influencing nitrification or denitrification under different soil moisture (Melton et al. 2014; Ding et al. 2014).
Fe oxides are abundant in many soils (Melton et al. 2014). The redox reaction of Fe is often related to the redox potential of the nitrogen, and affects nitrification and N2O emissions (Melton et al. 2014). Thus, knowing the relationship between Fe oxides and soil nitrification is especially significant for understanding their influence on N cycling process (Yang et al. 2012; Ding et al. 2014; Jiang et al. 2015; Huang et al. 2016). The direct participation of Fe in nitrification was first proposed by Li et al. (1988) as the Feammox reaction, which means anaerobic NH4+ oxidation coupled to Fe3+ reduction. Feammox usually occurs under anoxic condition in saturated soil, suggesting that Fe oxides can act as an electron acceptor and play a critical role in influencing N reactions (Park et al. 2009; Shrestha et al. 2009). In addition, Fe2+ oxidation coupled with denitrification via biotic and abiotic pathways is also observed in wetland soils, sediments, or anoxic microsites (Davidson et al. 2003; Straub et al. 2004; Smolders et al. 2010). Moreover, reactive nitrification intermediates such as NH2OH and NO2− may be coupled with biological process, in which one reactant can be produced enzymatically and then will undergo further biological reactions with Fe oxides to produce N2O or other products (Zhu et al. 2013; Zhu-Barker et al. 2015).
Fe oxides can affect microbial groups and their activities (Cheng et al. 2019). Meiklejohn (1953) found that Fe with low concentration stimulates the growth of nitrifying bacteria, whereas Fe with high concentration is toxic to nitrifying bacteria. Studies on the demand of AOB for Fe showed that when the Fe concentration in the medium of Nitrosomonas europaea increases from 0.2 to 10 μM, the activities of both amoA and hydroxylamine oxidoreductase decrease (Wei et al. 2006). However, Jiang (2015) demonstrated that 5% hematite can decrease both AOA and AOB abundance in Cambisols and Ferralsols. It is largely unknown whether the effect of Fe oxides on nitrifying microorganism is related to the types of Fe oxides or not. More and more evidences have showed that soil pH plays an essential role in shaping the distinct ecological niches of AOA and AOB (Erguder et al. 2009; Xi et al. 2017; Wang et al. 2019). Nitrification is driven by AOB rather than AOA in N-rich grassland soil and agricultural soil with pH about 7.0, whereas AOA controls nitrification in low-nutrient environment, for example, acidic agricultural soil (Di et al. 2009; Jia and Conrad, 2009). Moreover, ammonia as the substrate for both AOA and AOB, its concentration exponentially declines with decreasing pH due to the ionization of ammonia to ammonium (Jiang et al. 2015). Soil pH thus most likely determines the chemical form, concentration and availability of the substrate in association with nitrification (Kemmitt et al. 2006; Jiang et al. 2015). In addition, Fe oxides can affect the abundance and activities of AOA and AOB, thereby affecting the production rate of intermediates from biological process. For example, Huang (2016) found that 3% ferrihydrite stimulates net nitrification in low-pH soil (pH 5.1), while the opposite occurs in high-pH soil (pH 7.8). This implies that the response of nitrification to Fe oxides addition vary with different soil pH. Paddy soils are often characterized by Fe prone to redox reaction under short-term dry-wet alternation (Wang et al. 2016). Thus, the redox cycle of Fe affects nitrification and N2O emissions in paddy soils directly by involving in the chemical reaction (Huang et al. 2016) or indirectly by influencing microorganisms (Jiang et al. 2015). Therefore, understanding the relationship among Fe oxides, soil nitrification, and N2O emissions is significant for revealing the influence of Fe oxides on N cycling process in paddy soils.
N2O as byproduct can be produced biologically via hydroxylamine conversion in the process of ammonia oxidation mediated by AOA and AOB (Hink et al. 2017). In addition, N2O can directly come from the chemical reaction of Fe with intermediates such as NH2OH or NO2− (Zhu-Barker et al. 2015). Obviously, the reaction substrate required for the chemical process can be provided by the intermediates which are produced by biological process. In turn, Fe oxides affect the abundance and activity of AOA and AOB, thereby affecting the forming rate of intermediates produced by biological process. Thus, the effects of Fe on soil nitrification and N2O emissions are biological-chemical comprehensive effects. Some previous studies only focused on the effect of Fe oxides addition on soil nitrification, but not on the effect of Fe oxides on N2O emissions from nitrification (Jiang et al. 2015; Huang et al. 2016). Moreover, there are very few reports on the effect of Fe oxides on the diversity of nitrifying microorganisms. In order to comprehensively consider the chemical and biological effects of Fe oxides on soil nitrification and N2O emissions, we did not separate the chemical and biological effects of Fe oxides in present study. We carried out a laboratory experiment to study the effects of Fe oxides on nitrification, N2O emissions, and nitrifying microbial communities in two paddy soils. The guiding hypothesis of this study was that (1) the addition of Fe oxides might influence nitrification and N2O emissions under aerobic condition in paddy soils; (2) the effect of Fe oxides on N2O emissions might differ in the two paddy soils with different pH values; and (3) biological-chemical comprehensive effects might play a significant role in N2O formation during nitrification.
2 Materials and methods
2.1 Soils and sampling
Paddy soil samples were collected from the top layer (0–20 cm) of rice-rape rotation fields in Jingmen (30° 51′ N, 121° 6′ E) and Qianjiang (30° 41′ N, 121° 69′ E), Hubei Province, China. That region belongs to a subtropical monsoon climate and has a mean annual temperature of 16.1 °C and a mean annual rainfall of 949.4 mm. In order to study the response of nitrification to Fe oxides addition in soils with different pH, we selected acidic and alkaline paddy soils. The samples collected from Jingmen are acidic paddy soils (pH 5.5) developed on quaternary red earth, and the samples collected from Qianjiang are alkaline paddy soils (pH 7.9) developed on alluvial parent material.
Soil samples (0–20 cm) were taken with a 5-cm diameter soil core sampler from three field plots (5 m ×5 m). Five soil cores, about 2.0 kg soil, were collected from each plot. The soil samples were mixed thoroughly to reduce heterogeneity. Then, the soil samples were air-dried and ground to pass through a 20-mesh sieve and stored at 4 °Cfor the later incubation experiments. The physical and chemical properties of the soils are presented in Table 1. The pH value of the acidic paddy soil is 5.5 (H2O) and that of the alkaline paddy soil is 7.9. The organic matter and NO3−–N content in the acidic paddy soil is higher than that in the alkaline paddy soil. The concentration of active Fe (0.5 mol L−1 HCl extractable) in the acidic soil is significantly higher than that in the alkaline soil. However, the total Fe in the acidic soil is significantly lower than that in the alkaline soil, which may be mainly attributed to the different parent materials. In addition, the different leaching potential of the two soils with different pH may be another important reason (Huang et al. 2018).
2.2 Preparation of goethite, treatments, and replicates
Goethite was prepared using the procedure of Atkinson et al. (1968). Briefly, at first, an Fe solution (72.7 g Fe(NO3)3·9H2O in 400 mL distilled deionized water) was added to a base solution (23.5 g NaOH in 400 mL distilled deionized water) to form Fe hydroxide. Then, the pH value of the mixture was adjusted to 12 with 2.5 mol L−1 NaOH. With periodic shaking, the suspension was aged at 60 °C for 72 h, during which the suspension’s color changed from red to orange. The precipitate was centrifuged at 3900 rpm for 10 min, and then washed with ultrapure water until the conductivity of the supernatant was less than 20 mS m−1. After that, it was freeze-dried and ground again to pass 1-mm sieve to store at 4 °C for later use. The obtained goethite was identified by X-ray powder diffractometer (XRD), as shown in Fig. S1.
For each soil, we set up two treatments, one without goethite amendment (control) and the other with goethite amendment (Fe treatment). Goethite was added at 3% (by weight). The acidic paddy soil and alkaline paddy soil without or with goethite amendment was designated as pH 5.5 (control), pH 5.5 + 3% goethite, pH 7.9 (control), and pH 7.9 + 3% goethite, respectively. The four treatments were then stored at 4 °C for later incubation study.
2.3 Incubation experiment
The incubation experiment was conducted at 25 °C under laboratory-controlled conditions. The samples (all the 4 treatments) were pre-incubated at 20% water holding capacity (WHC) for 7 days for activation and stabilization of the microorganisms. After pre-incubation, aliquots (corresponding to 25 g dry soil) of the activated soils were placed into 250-mL culture bottles, which were covered with polyethylene film having needle-punctured holes to maintain aerobic conditions. Subsequently, (NH4)2SO4 was added into the bottles to make the N concentration reach 100 mg kg−1 dry soil (corresponding to 225 kg N ha−1 year−1), and the soil moisture was adjusted to 60% WHC. Then, each of the four treatments was made into 6 replicates, in which 3 were used for gas analysis and 3 for soil analysis. All the 24 samples were incubated in dark at 25 °C for 14 days, during which the loss of water through evaporation was compensated by adding distilled water every day.
In each treatment, 3 replicates were destructively sampled on day 0, 1, 3, 5, 7, and 14 for determining NH4+-N, NO3−-N, Fe(II), Fe(III), pH, and Eh. Right after the 7th day of incubation, their DNA was extracted for further analysis. The other 3 replicates were nondestructively sampled at the same sampling interval (day 0, 1, 3, 5, 7, and 14) for analyzing their N2O concentration in the headspace gas. N2O flux was determined as the change of N2O concentration in the headspace gas within 2 h.
2.4 Chemical analysis
N2O concentration of the gas samples was analyzed by GC (Agilent 7890A, Agilent Technologies Inc., Santa Clara, CA, USA) with an electron capture detector (ECD). Soil Eh was measured with an oxidation-reduction potentiometer (Nanjing Jaoyuan Analytical Instrument Company limited, China) using a platinum composite electrode. The pH was determined with a pH meter (Sartorius, Basic pH Meter PB-10, Germany). Ammonium was analyzed by the phenol hypochlorite method (Scheiner, 1976), and nitrate was analyzed by the ultraviolet spectrophotometry method (Cawse, 1967). Fe(II) and Fe(III) were extracted with 0.5 mol L−1 HCl from the soils. The extracted Fe(II) was analyzed by the ferrozine method (Viollier et al. 2000). The total extracted Fe was determined by the same procedure with the exception that hydroxylamine hydrochloride was added to the soil extracts to transform Fe(III) to Fe(II). The amount of Fe(III) was calculated as the difference between total extracted Fe and Fe(II).
2.5 Nucleic acid extraction and real-time quantitative PCR
The nucleic acid in soil was extracted from 0.5 g soil using a FastDNA spin kit (MPBIO Laboratories, Carlsbad, CA, USA). The quality and abundance of the extracted DNA was measured by gel electrophoresis (0.8% agarose) and NanoDropTM One Spectrophotometer (Thermo Fisher Scientific, MA, USA), and the extracted DNA was stored at −20 °C. The gene copy numbers of amoA genes of AOA and AOB were determined using real-time PCR (Bio-Rad Laboratory, CA, USA). The primers amoA-1 F (GGG GTT TCT ACT GGT GGT) and amoA-2 R (CCC CTC KGS AAA GCC TTC TTC) were used for ammonia-oxidizing bacteria for generating a 491 bp fragment (Francis et al. 2005); Arch-amoA F (STA ATG GTC TGG CTT AGA CG) and Arch amoA R (GCG GCC ATC CAT CTG TAT GT) were used for generating a 635 bp fragment (Rotthauwe. 1997), which are listed in Table S1 (see Supplementary information for details).
2.6 Pyrosequencing analysis and bioinformatics analysis
The 16S rRNA gene of the V4 regions was analyzed by pyrosequencing on an Illumina Hiseq2500 (Guangdong Magigene Biotechnology Co., Ltd. Guangzhou, China). Sequences were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) data analysis package. Based on specific sample barcodes, the reads were assigned to each sample, and then the barcodes and primer sequences were removed. Low-quality sequences were removed; only reads longer than 250 bp without ambiguous base pairs and with high average quality score were retained for further analysis. Sequences were clustered into operational taxonomic units (OTUs) with a 97% similarity threshold. The OUT richness, Shannon, Chao1, and PD whole_tree were analyzed using the QIIME software package. Principal component analysis (PCA) was conducted using the weighted UniFrac distance to evaluate the community similarity from the gene sequence data. Soil DNA quantitative PCR and pyrosequencing analysis were conducted by Magigene (Guangzhou, China). Genera and OTUs with more than 0.5% of the relative abundance and related to nitrogen cycle were selected.
2.7 Statistical analysis
The total N2O emissions were calculated by linear interpolation between measured values. The emission rate was expressed as the arithmetic mean of the three replicates. The changes in NO3−-N content with incubation period were modeled with a first-order reaction kinetic model, which is expressed as NNO3 = N0 + NP (1 − exp. (−K1∗t), or by a zero-order reaction kinetic model, which is expressed as NNO3 = N0 = K0∗t, where NNO3 is NO3−-N content at incubation time, t; N0 is NO3−-N content after pre-incubation (t = 0); Np is nitrification potential; and K1 and K0 are rate constants of the first- and zero-order reactions, respectively. The potential nitrification rate (Vp) was calculated from first-order kinetics as Vp = k1 * Np (Oorts et al. 2007).
Data (measured or calculated) were subjected to one-way ANOVA and mean values were analyzed using Duncan’s New Multiple Range Test at p < 0.05. All statistical analyses were conducted using SPSS statistical package. Principal component analysis (PCA) was conducted to analyze denitrifying community structure based on soil properties using the software CANOCO 5.0.
3 Results
3.1 Soil N2O emissions
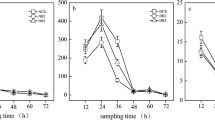
The N2O emission rate changed significantly during incubation, and it was significantly affected by the application of goethite in the alkaline paddy soil. The rate presented distinct difference in the acidic and alkaline paddy soils (Fig. 1). For instance, the N2O emission rate rapidly peaked on the first day after N fertilization, then declined gradually to a relatively low level within 5 days, and fluctuated at a lower level until the end of incubation in the alkaline paddy soil. Both the maximal rate and the cumulative amount of N2O emissions in the alkaline paddy soil were much higher than those in the acidic paddy soil. The N2O emission rate peaked at 8.5 and 4.6 μg N kg−1 h−1 for the alkaline paddy soil with and without adding 3% goethite, respectively, while the maximal N2O emission rate in the acidic paddy soil was very low for both treatments (0.19 and 0.17 μg N kg−1 h−1, respectively). The total N2O emissions from the alkaline paddy soil reached 446.7 μg N kg−1 during the 14 days of incubation after adding 3% goethite, which was significant higher than the total emissions (258.5 μg N kg−1) from the treatment without goethite addition. Similarly, the addition of goethite increased the cumulative N2O emissions in the acidic paddy soil (from 33.6 up to 59.5 μg N kg−1), but the increase was not significant.
3.2 Soil inorganic nitrogen
During the 14-day incubation, NO3−-N concentration increased significantly with the incubation time for both the acidic and alkaline soils (Fig. 2a). Under the treatment without goethite addition, the concentration of NO3−-N increased by 79.5 mg N kg−1 and 68.3 mg N kg−1 for the acidic soil and the alkaline soil at the end of incubation, respectively. In the alkaline soil, the concentration of NO3−-N under the treatment with goethite addition slightly decreased as compared with the treatment without goethite addition; however, in the acidic soil, the concentration of NO3−-N under the treatment with goethite addition showed no changes as compared with the control. Net nitrification kinetic was fitted best by a first-order model for both soils, and simulated parameters of nitrification are listed in Table 2. The potential rate of nitrification (Vp) in the acidic paddy soil with goethite addition was 16.1 mg N kg−1 day−1, which slightly decreased as compared with that of the control. Similarly, Vp decreased from 25.5 to 16.2 mg N kg−1 day−1 in the alkaline soil with and without goethite addition. Compared with the control, the addition of goethite decreased the average net rate of soil nitrification in the acidic soil by 33.2%, while the addition of goethite had little increase of the rate in the alkaline soil.
The concentration of NH4+-N decreased significantly with the increasing incubation time for both soils (Fig. 2b). For example, within the first 3 days of incubation, the concentration of NH4+-N decreased sharply from 176.4 to 75.1 mg N kg−1 in the acidic soil and from 96.1 to 22.1 mg N kg−1 in the alkaline soil without goethite addition. The average NH4+-N concentration was 18.58 mg N kg−1 for the acidic soil and 15.45 mg N kg−1 for the alkaline soil under the treatments without goethite addition at the end of the incubation. Similar trends were also found for the treatments with goethite addition, and the concentrations of NH4+-N decreased significantly with the increasing incubation time for both two soils.
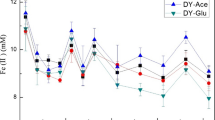
3.3 Dynamics of Fe production
The contents of Fe2+ and Fe3+ were measured during the 14-day incubation. The concentration of active Fe (0.5 mol L−1 HCl extractable) in the acidic soil was significantly higher than that in the alkaline soil (Fig. 3). Moreover, the average concentration of Fe3+ in the acidic soil was approximately 1.8 times higher than that in the alkaline soil during incubation, while the average concentration Fe2+ concentration in the acidic soil was less than half of that in the alkaline soil. The concentration of Fe2+ increased rapidly in the earlier incubation stage, and peaked on the third day of incubation in the alkaline soil and the fifth day in the acidic soil, and then fluctuated slightly in subsequent days. On the whole, the increase periods of Fe2+ concentration in both soils were in agreement with the periods of N2O emissions. The addition of goethite had no remarkable effect on Fe2+ concentration.
3.4 Abundances of AOA and AOB amoA genes
The addition of goethite strongly enhanced the AOA and AOB abundance (Fig. 4). In the acidic soil, the copy numbers of AOB amoA gene under the treatment with goethite addition was 19 × 105 copies g−1 soil, which was significantly higher than that under the control (9.9 × 105 copies g−1 soil) (p < 0.05). In the alkaline soil, the copy number of AOB amoA gene was 2.0 × 105 and 0.22 × 105 copies g−1 soil under the treatments with and without goethite addition, respectively. Similarly, the addition of goethite significantly increased the copy number of AOA amoA gene in both soils (p < 0.05). In the acidic soil, the addition of goethite increased the abundance of AOA and AOB amoA genes by 155.2% and 86.8% in comparison with that under the control; in the alkaline soil, the increase of the copy numbers was as high as 595.6% and 804.5%, respectively. In the acidic paddy soil, the AOA/AOB ratio was 0.60 and 0.83 under the treatments without and with goethite addition, respectively. Conversely, in the alkaline soil, the abundance of AOA amoA gene was higher than that of AOB amoA gene, and the AOA/AOB ratio was 2.53 and 1.94 under the treatments without and with goethite addition, respectively.
3.5 Variation in microbial diversity and community based on 16S rRNA gene
Based on 97% sequence similarity cutoff, the number of 16S rRNA gene OTUS ranged from 3130 to 3745 (Table S2). Compared with the control, the treatments with goethite addition increased the richness (Chao 1), observed species, phylogenetic diversity (PD_whole_tree), and Shannon (except for the pH 7.9 soil) indices calculated on the basis of 16S rRNA in both the soils (Fig. S2).
The principal component analysis (PCA) based on 16S rRNA gene was used to evaluate variations in communities. The analysis demonstrated that the bacterial community composition shifted between different treatments (Fig. S3). The first principal component axis (89.0% of contribution rate) showed variance in microbial community composition in the two soils, indicating different soil types can indeed affect microbial community composition. The second principal component axis (4.5% of contribution rate) showed variance of bacterial community composition between the treatment with goethite addition and the treatment without goethite addition.
Figure 5 shows the effect of goethite addition on the relative abundance of the dominant genera related to nitrogen cycle in the two soils. The relative abundance of Nitrososphaeraceae, Nitrosomonadaceae, Thermomonas, Gemmatimonadaceae, and Terrimonas in the alkaline soil was higher than that in the acidic paddy soil. In contrast, the relative abundance of Blastocatellaceae, Anaerolinea, Pseudolabrys, and Gemmatimonas in the alkaline soil was lower than that in the acidic soil. The addition of goethite significantly increased the relative abundance of Terrimonas and Nitrosomonadaceae in the alkaline soil and Gemmatimonas in the acidic soil.
Effects of 3% goethite addition on the relative abundance of dominant genera in two paddy soils. Error bars represent the standard deviation of three samples. Different letters above the columns denote significant differences at p < 0.05. Only genera with more than 0.5% of the relative abundance and related to nitrogen cycle were selected
4 Discussion
N2O is produced mainly from nitrification, nitrifier denitrification, and denitrification (Zhu-Barker et al. 2015; Chen et al., 2019), and nitrification is the principal pathway of N2O emissions under the oxic condition (Liu et al. 2019). The effects of Fe oxides on nitrification and N2O emissions, including stimulation, retardation, and inhibition, were reported in many ecosystems (Blaise et al. 1997; Jiang et al. 2015; Huang et al. 2016). Our results showed that N2O emissions rapidly peaked on the first day of incubation, and that was consistent with previous researches (Wang et al. 2016; Xin et al. 2016). It suggests that ammonium oxidation occurs immediately after NH4+ is added, and chemical ammonium oxidation contributes to the production of N2O in the initial stage (Wang et al. 2016). The addition of 3% goethite increases N2O emissions in both the soils, but the promoting effect is more significant in the alkaline paddy soil.
Firstly, Fe oxides can be utilized as terminal electron acceptors by many microorganisms, thus influencing microbial community structure and their activities (Meiklejohn 1953; Laufer et al. 2016; Zhang et al., 2019). Our results showed that both amoA genes of AOA and AOB were increased in the two soils with goethite addition (Fig. 4), and that was consistent with the promoting effect of goethite addition on the N2O emissions. That indicates goethite addition increases N2O emissions by promoting the abundance of AOA and AOB amoA genes. In the acidic soil, the addition of goethite increased the abundance of AOA and AOB amoA genes by 155.2% and 86.8% as compared with that under the control; in the alkaline soil, the increase was as high as 595.6% and 804.5%, respectively. The absolute increase in amoA genes was greater in the acidic soil, but the proportional increase was greater in the alkaline soil (Fig. 4). The results indicates that goethite addition has different promotion effects on the abundance of AOA and AOB amoA genes in the two soils, and has a stronger enhancement on N2O emissions from alkaline paddy soil than from acidic paddy soil. The copy number ratio of AOA/AOB amoA genes ranged from 0.60 to 0.83 in the acidic soil and from 1.94 to 2.53 in the alkaline soil, respectively. Some studies indicated that AOA may be the major member in aerobic ammonia oxidation both in acidic or near neutral soils (Li et al. 2015; Hink et al. 2018). However, AOB rather than AOA is a more active ammonia oxidizer in agricultural soil (Li et al. 2015). Those contrasting results imply that the relative importance of AOB and AOA may vary with environmental conditions (Huang et al. 2016). Hink et al. (2018) found that the amount of N2O emissions from ammonia oxidation dominated by AOA was only one-third of that by AOB. However, in our study, the AOA/AOB ratio is higher in the alkaline soil, implying that AOA is also dominant, but it is accompanied with higher N2O emissions. AOB generates N2O via enzymatic conversion of hydroxylamine to N2O, and also via the sequential reduction of NO2− to NO and N2O. In contrast, there is no genomic or physiological evidence for enzymatic production of N2O by AOA, and it is believed that NH3 oxidation associated N2O emissions results from an abiotic reaction between hydroxylamine and NO or NO2− (Kozlowski et al. 2014; Hink et al. 2017). This suggests abiotic process may also contribute to N2O emissions during ammonia oxidation dominated by AOA in alkaline paddy soil (Hink et al. 2017).
Furthermore, the addition of goethite altered the relative abundance of some genera in the two paddy soils (Fig. 5). Nitrosomonadaceae is ammonia-oxidizing bacteria (AOB) (Zeng et al., 2018), and the addition of goethite significantly increased the relative abundance of Nitrosomonadaceae in the alkaline paddy soil. Nitrososphaeraceae in the ammonia-oxidizing archaea (AOA) community was also increased under the treatments with goethite addition in both the soils. These results indicate that the dominant species of AOB and AOA are Nitrosomonadaceae and Nitrososphaeraceae, respectively. The addition of goethite tends to mainly increase Nitrosomonadaceae of AOB, thus promoting ammonia oxidation to produce N2O in the alkaline paddy soil. Moreover, Terrimonas is known as aerobic-denitrifying bacteria and considered being capable of performing aerobic-denitrifying process (Xie and Yokota, 2006), and it produces N2O at the optimal pH of neutral or alkalescency (7.0 ~ 8.0) (Ji et al., 2015). The abundance of Terrimonas was significantly increased in the alkaline paddy soil by goethite addition, indicating that goethite might also increase N2O production by stimulating the aerobic-denitrifying process. Gemmatimonas is obligate aerobic bacteria related to reduction of N2O (Park et al., 2017), and the addition of goethite significantly increased the relative abundance of Gemmatimonas in the acidic paddy soil. Therefore, N2O may undergo further reduction process in the acidic soil, and that may be the reason why the promotion effect of goethite addition on N2O emissions in acidic soil is not as significant as that in alkaline soil.
In addition, Fe oxides can also participate in nitrification process via chemical reaction with intermediates, thus affecting N2O emissions. In this study, we observed goethite addition stimulated N2O emissions, indicating that Fe affects some steps of the nitrification process (Yu et al. 2010; Wunderlin et al. 2012). Bremner et al. (1980) detected a correlation between the formation of N2O by the chemical decomposition of NH2OH and oxidized iron. The finding could be attributed to the following reaction (Eq. (1)) (Butler and Gordon, 1986):
In this study, we found a positive relation between Fe2+ and cumulative N2O emissions (r = 0.900, p < 0.01) and a negative correlation between Fe3+ and cumulative N2O emissions (r = −0.941, p < 0.01) (Table S3). The relationships may signify the occurrence of Fe3+ reduction to Fe2+ accompanying with nitrous oxide formation. The N2O emission rate increased significantly in the alkaline soil and slightly in the acidic soil (Fig. 1), indicating that N2O emissions are promoted by Fe oxides under oxic conditions. It was demonstrated from Eq. (1) that the oxidation of hydroxylamine coupled with Fe3+ reduction is accompanied with the formation of hydrogen ions, and that could be proved by the pH decrease in early stage of incubation (Fig. S4). Therefore, this reaction is more favorable to occur in the alkaline paddy soil than in the acidic paddy soil, resulting in the promotion effect of goethite addition on N2O emissions in the alkaline soil is stronger than in the acidic soil (Fig. 1). That indicates alkaline soil is more sensitive to the effect of goethite on N2O emissions. As forgotten drivers of N2O production (Zhu et al. 2013), Fe oxides play an important role in regulating N2O emissions through nitrification, especially in alkaline paddy soil. It is a feasible soil management measure to regulate the redox of iron through controlling the soil water condition to reduce N2O emissions. Zhao (2011) indicated that during pre-flooding period, there was much high emissions of N2O, which mainly came from nitrification. Therefore, it is possible to control the redox process of Fe by appropriately shortening the pre-flooding period and irrigating in advance to reduce the effect of Fe on N2O produced by nitrification, and that method is effective, especially in alkaline soils. However, in addition to feasibility and cost, that method requires consideration of potential effects on crop yield, and the potential effects will inevitably affect N2O emissions from other pathways. In any case, this study provides the basis for better understanding the important role of Fe in regulation of N2O emissions from nitrification of paddy soils.
Moreover, N2O emissions are also controlled in general by soil redox potential (Peng et al. 2011). The slight decrease and increase of Fe2+ and Fe3+ concentration (Fig. 3) as well as the fluctuation of Eh (Fig. S5) indicate that oxidation of Fe2+ and reduction of Fe3+ occurs during the experiment. It suggests that there are some anaerobic microsites in the incubation soils in which Fe2+ oxidation coupled with denitrification may occur. Cooper et al. (2003) found that goethite increases N2O production because of the reduction of NO2− and the simultaneous oxidation of Fe2+ to Fe3+. Similarly, we believe that iron-oxidation coupled denitrification also contributes to N2O emissions in our experiment. Thus, Fe oxides acting as electron acceptors through abiotic effect may affect the process that generates N2O.
It can be seen that goethite addition affects the abundance and activity of nitrifying microorganisms (e.g., AOA and AOB). At the same time, it can be demonstrated from the relation between Fe (including Fe2+ and Fe3+) and cumulative N2O emissions that Fe oxides, through reacting with nitrite or hydroxylamine, affect the nitrification process and N2O emissions. In turn, nitrifying microorganisms affect the production of nitrification intermediates, thus affecting the reaction process between Fe and nitrification intermediate. So, it can be inferred that Fe oxides affect the N2O emissions in the two paddy soils through the biological-chemical comprehensive effects. However, the deep mechanism may be more complicated and needs further exploring, especially to distinguish the contribution of the biotic and abiotic effects of Fe oxides.
5 Conclusions
The study indicates that addition of 3% goethite can increase N2O emissions in acidic and alkaline paddy soils. The enhancement of N2O emissions by goethite is more significant in alkaline paddy soil than in acidic paddy soil. The mechanisms possibly include the promotion effect of Fe oxides on microorganisms under oxic conditions and the effect of Fe acting as an alternate electron receptor to participate in the process of oxidizing ammonia to hydroxylamine. These results imply that Fe can be a potential regulator of N2O emissions from nitrification in paddy soils, especially in alkaline paddy soils.
References
Atkinson RJ, Posner AM, Quirk JP (1968) Crystal nucleation in Fe(III) solutions and hydroxide gels. Journal of Inorganic & Nuclear Chemistry 30:2371–2381. https://doi.org/10.1016/0022-1902(68)80247-7
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biology and Fertility of Soils 41:379–388. https://doi.org/10.1007/s00374-005-0858-3
Beaumont HJE, Hommes NG, Sayavedra SLA, Arp DJ, Aciero DM, Hooper AB, Westerhoff HV, Spanning RJM (2002) Nitrite reductase of nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J Bacteriol 184:2557–2560. https://doi.org/10.1128/jb.184.9.2557-2560.2002
Blaise D, Amberger A, Tucher SV (1997) Influence of iron pyrites and dicyandiamide on nitrification and ammonia volatilization from urea applied to loess brown earths (luvisols). Biol Fert Soils 24:179–182. https://doi.org/10.1007/s003740050228
Bremner JM, Blackmer AM, Waring SA (1980) Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biol Biochem 12:263–269. https://doi.org/10.1016/0038-0717(80)90072-3
Butler JH, Gordon LI (1986) Rates of nitrous oxide production in the oxidation of hydroxylamine by Iron(III). Inorg Chem 25:4573–4577. https://doi.org/10.1021/ic00245a024
Cawse PA (1967) The Determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst 92:311–315. https://doi.org/10.1039/an9679200311
Chen S, Di HJ, Cameron KC, Podolyan A, Shen JP, He JZ (2019) Effect of treated farm dairy effluents, with or without animal urine, on nitrous oxide emissions, ammonia oxidisers and denitrifiers in the soil. J Soil Sediment 19:2330–2345. https://doi.org/10.1007/s11368-018-02229-8
Cheng BY, Hua YM, Zhao JW, Liu GL, Wan XQ (2019) Nitrogen transformation mediated by nitrate-dependent iron oxidation in anoxic freshwater. J Soil Sediment. 20:1087–1096. https://doi.org/10.1007/s11368-019-02461-w
Cooper DC, Picardal FW, Schimmelmann A, Coby AJ (2003) Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appli Environ Microb 69:3517–3525. https://doi.org/10.1128/AEM.69.6.3517-3525.2003
Curtin D, Campbell C, Jalil A (1998) Effects of acidity on mineralization: pH dependence of organic matter mineralization in weakly acidic soils. Soil Biol Biochem 30:57–64. https://doi.org/10.1016/S0038-0717(97)00094-1
Davidson EA, Chorover J, Dail DB (2003) Amechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Change Biol 9:228–236. https://doi.org/10.1046/j.1365-2486.2003.00592.x
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2(9):621–624. https://doi.org/10.1038/ngeo613
Ding LJ, An XL, Li S, Zhang GL, Zhu YG (2014) Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence. Environ Sci Technol 48:10641–10647. https://doi.org/10.1021/es503113s
Erguder T, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869. https://doi.org/10.1111/j.1574-6976.2009.00179.x
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. P Natl Acad Sci USA 102:14683–14688. https://doi.org/10.1073/pnas.0506625102
He L, Zhao X, Wang S, Xing G (2016) The effects of rice-straw biochar addition on nitrification activity and nitrous oxide emissions in two Oxisols. Soil Till Res 164:52–62. https://doi.org/10.1016/j.still.2016.05.006
Hink L, Nicol GW, Prosser JI (2017) Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ Microbiol 00:00-00. https://doi.org/10.1111/1462-2920.13282
Hink L, Gubry RC, Nicol GW, Prosser JI (2018) The consequences of niche and physiological differentiation of acrchael and bacterial ammonia oxidisers for nitrous oxide emissions. Isme J 12:1084–1093. https://doi.org/10.1038/s41396-017-0025-5
Hoben JP, Gehl RJ, Millar N, Grace PR, Robertson GP (2011) Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Global Change Biol 17:1140–1152. https://doi.org/10.1111/j.1365-2486.2010.02349.x
Huang LM, Jia XX, Ming’an S, Chen LM, Han GZ, Zhang GL (2018) Phases and rates of iron and magnetism changes during paddy soil development on calcareous marine sediment and acid quaternary red-clay. Sci Rep 8(1):444. https://doi.org/10.1038/s41598-017-18963-x
Huang XR, Zhu BX, Horwath WR, Faeflen SJ, Luo HY, Xin XP, Jiang XJ (2016) Effect of iron oxide on nitrification in two agricultural soils with different pH. Biogeosciences 13:5609–5617. https://doi.org/10.5194/bg-13-5609-2016
IPCC (2013) Climate Change 2013: the physical science basis: working group I contribution to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press
Ji B, Yang K, Zhu L, Jiang Y, Wang HY, Zhou J, Zhang HN (2015) Aerobic denitrification: a review of important advances of the last 30 years. Biotechnol Bioproc E 20:643–651. https://doi.org/10.1007/s12257-015-0009-0
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11(7):1658–1671. https://doi.org/10.1111/j.1462-2920.2009.01891.x
Jiang XJ, Xin XP, Li SW, Zhou JC, Zhu TB, Müller C, Cai ZC, Wright AL (2015) Effects of Fe oxide on N transformations in subtropical acid soils. Sci Rep 5:8515. https://doi.org/10.1038/srep08615
Kemmitt SJ, Wright D, Goulding KW, Jones DL (2006) pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol Biochem 38:898–911. https://doi.org/10.1016/j.soilbio.2014.10.025
Kozlowski JA, Price J, Stein LY (2014) Revision of N2O-producing pathways in the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC 19718. Appl Environ Microbiol 80:4930–4935. https://doi.org/10.1128/aem.01061-14
Laufer K, Byrne JM, Glombitza C, Schmidt C, Jørgensen BB, Kappler A (2016) Anaerobic microbial Fe(II) oxidation and Fe(III) reduction in coastal marine sediments controlled by organic carbon content. Environ Microbiol 18:3159–3174. https://doi.org/10.1111/1462-2920.13387
Li H, Weng BS, Huang FY, Su JQ, Yang XR (2015) pH regulates ammonia-oxidizing bacteria and archaea in paddy soils in Southern China. Appl Microbiol Biot 99:6113–6123. https://doi.org/10.1007/s00253-015-6488-2
Li LM, Pan YH, Wu QT, Zhou XR, Li ZG (1988) Investigation on amorphous ferric oxide acting as an electron acceptor in the oxidation of NH4+ under anaerobic condition. Acta Pedologica Sinica 25:184–190
Liu C, Wang K, Meng SX, Zheng XH, Zhou ZX, Han SH, Chen DL, Yang ZP (2011) Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emission from a wheat-maize rotation field in northern China. Age Ecosyst Environ 140:226–233. https://doi.org/10.1016/j.agee.2010.12.009
Liu TL, Wang ZH, Wang SL, Zhao YP, Wright AL, Jiang XJ (2019) Responses of ammonia-oxidizers and comammox to different long-term fertilization regimes in a subtropical paddy soil. Eur J Soil Biol 93. https://doi.org/10.1016/j.ejsobi.2019.103087
Meiklejohn J (1953) Iron and the nitrifying bacteria. J Gen Microbiol 8:58–65. https://doi.org/10.1099/00221287-8-1-58
Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808. https://doi.org/10.1038/nrmicro3347
Nugroho R, Röling W, Laverman A, Verhoef H (2007) Low nitrification rates in acid scots pine forest soils are due to pH-related factors. Microb Ecol 53:89–97. https://doi.org/10.1007/s00248-006-9142-9
Oorts K, Merckx R, Gréhan E, Labreuche L, Nicolardot B (2007) Determinants of annual fluxes of CO2 and N2O in long-term no-tillage and conventional tillage systems in northern France. Soil and Tillage Research 95(1-2):133–148
Park D, Kim H, Yoon S (2017) Nitrous oxide reduction by an obligate aerobic bacterium, Gemmatimonas aurantiaca Strain T-27. Appl Environ Microb 83:e00502–e00517. https://doi.org/10.1128/AEM.00502-17
Park W, Nam YK, Lee MJ, Kim TH (2009) Anaerobic ammonia-oxidation coupled with Fe3+reduction by an anaerobic culture from a piggery wastewater acclimated to NH4+/Fe3+ medium. Biotechnol Bioproc E 14:680–685. https://doi.org/10.1007/s12257-009-0026-y
Peng SZ, Hou HJ, Xu JZ, Mao Z, Abudu S, Luo YF (2011) Nitrous oxide emissions from paddy fields under different water managements in southeast China. Paddy Water Environ 9:403–411. https://doi.org/10.1007/s10333-011-0275-1
Rotthauwe JH (1997) The ammonia monooxygenase structural gene amoA as a functional marker : molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microb 63:4704–4712. https://doi.org/10.1126/science.284.5411.63
Scheiner D (1976) Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res 10:31–36. https://doi.org/10.1016/0043-1354(76)90154-8
Shrestha J, Rich JJ, Ehrenfeld JG, Jaffe PR (2009) Oxidation of ammonium to nitrite under iron-reducing conditions in wetland soils. Soil Sci 174:156–164. https://doi.org/10.1097/ss.0b013e3181988fbf
Smolders AJP, Lucassen ECHET, Bobbink R, Roelofs JGM, Lamers LPM (2010) How nitrate leaching from agricultural lands provokes phosphate eutrophication in groundwater fed wetlands: the sulphur bridge. Biogeochemistry 98(1-3):1–7
Straub KL, Schönhuber WA, Buchholz-Cleven BEE, Schink B (2004) Diversity of ferrous Iron-Oxidizing, Nitrate-Reducing bacteria and their involvement in Oxygen-Independent Iron Cycling. Geomicrobiology Journal 21(6):371–378
Venkiteswaran JJ, Rosamond MS, Schiff SL (2014) Nonlinear response of riverine N2O fluxes to oxygen and temperature. Environ Sci Technol 48:1566–1573. https://doi.org/10.1021/es500069j
Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Van Cappellen P (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15:785–790. https://doi.org/10.1016/S0883-2927(99)00097-9
Wang JC, Wang JL, Rhodes G, He JZ, Ge Y (2019) Adaptive responses of comammox Nitrospira and canonical ammonia oxidizers to long-term fertilizations: implications for the relative contributions of different ammonia oxidizers to soil nitrogen cycling. Sci Total Environ 668:224–233. https://doi.org/10.1016/j.scitotenv.2019.02.427
Wang M, Hu R, Zhao J, Kuzyakov Y, Liu S (2016) Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils. Geoderma 271:173–180. https://doi.org/10.1016/j.geoderma.2016.02.022
Wei X, Vajrala N, Hauser L, Sayavedra-Soto LA, Arp DJ (2006) Iron nutrition and physiological responses to iron stress in Nitrosomonas europaea. Archives of Microbiology 186 (2):107–118
Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H (2012) Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res 46:1027–1037. https://doi.org/10.1016/j.watres.2011.11.080
Xi R, Long XE, Huang S, Yao H (2017) pH rather than nitrification and urease inhibitors determines the community of ammonia oxidizers in a vegetable soil. Amb Expr 7:129. https://doi.org/10.1186/s13568-017-0426-x
Xie CH, Yokota A (2006) Reclassification of [Flavobacterium] ferrugineum as Terrimonas ferruginea gen. nov., comb.nov., and description of Terrimonas lutea sp. Nov., isolated from soil. Int J Syst Evol Micr 56:1117–1121. https://doi.org/10.1099/ijs.0.64115-0
Xin XP, Jiang XJ, Su J, Yan XJ, Ni JP, Faeflen SJ, Huang XR, Wright AL (2016) Manganese oxide affects nitrification and ammonia oxidizers in subtropical and temperate acid forest soils. Catena 137:24–30. https://doi.org/10.1016/j.catena.2015.09.004
Yang WH, Weber KA, Silver WL (2012) Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction. Nat Geosci 5:538–541. https://doi.org/10.1038/Ngeo1530
Yu R, Kampschreur MJ, van Loosdrecht MCM, Chandran K (2010) Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ Sci Technol 44:1313–1319. https://doi.org/10.1021/es902794a
Zeng GM, Zhang LH, Dong HR, Chen YN, Zhang JC, Zhu Y, Yuan YJ, Xie YK, Fang W (2018) Pathway and mechanism of nitrogen transformation during composting: functional enzymes and genes under different concentrations of PVP-AgNPs. Bioresource Technol 253:112–120. https://doi.org/10.1016/j.biortech.2017.12.095
Zhang L, Jiang MH, Ding KR, Zhou SG (2019) Iron oxides affect denitrifying bacterial communities with the nirS and nirK genes and potential N2O emission rates from paddy soil. Eur J Soil Biol 93. https://doi.org/10.1016/j.ejsobi.2019.103093
Zhao X, Min J, Wang SQ, Shi WM (2011) Further understanding of nitrous oxide emission from paddy fields under rice/wheat rotation in South China. J Geophys Res-atmos 116(G2). https://doi.org/10.1029/2010JG001528
Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB (2015) The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126:251–267. https://doi.org/10.1007/s10533-015-0166-4
Zhu X, Silva LCR, Doane TA, Horwath WR (2013) Iron: the forgotten driver of nitrous oxide production in agricultural soil. Plos One 8:e60146. https://doi.org/10.1371/journal.pone.0060146
Zou JW, Huang Y, Zheng XH, Wang YS (2007) Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos Environ 41:8030–8042. https://doi.org/10.1016/j.atmosenv.2007.06.049
Funding
This work was supported financially by the National Key Research and Development Program of China (2018YFD0200500), the National Natural Science Foundation of China (41771270), and the National Natural Science Foundation of China (41761060 and 41601297).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Zucong Cai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
(1) Fe oxide–enhanced N2O emissions was more significant in alkaline paddy soil.
(2) Fe oxides increased the abundance of AOA and AOB amoA and thus promoted N2O emissions.
(3) Fe oxides mainly increased Nitrosomonadaceae-AOB in alkaline paddy soil.
(4) Fe oxides might participate in nitrification via chemical reaction to generate N2O.
Electronic supplementary material
ESM 1
(DOCX 215 kb)
Rights and permissions
About this article
Cite this article
Zuo, J., Hu, H., Fu, Q. et al. Biological-chemical comprehensive effects of goethite addition on nitrous oxide emissions in paddy soils. J Soils Sediments 20, 3580–3590 (2020). https://doi.org/10.1007/s11368-020-02685-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02685-1