Abstract
Purpose
NO3−-N reduction is the main way of soil nitrogen (N) loss, especially in saline-alkaline soil, and NO3−-N-reducing Fe(II) oxidation (NRFO) is an important but less known process for NO3−-N reduction. An anoxic cultivation experiment was carried out on coastal saline-alkaline paddy soil to determine the effect of the external environment on NRFO process and microbial community composition.
Methods
A laboratory cultivation experiment was studied to elucidate the effect of pH and acetate on NRFO in saline-alkaline soil. Nine different treatments were designed with two factors: (1) three pH levels: 7.5, 8.0, and 8.5; (2) three acetate concentrations: 15 (C1), 20 (C2), and 25 mM (C3). Concentration of Fe(II), Fe(III), NO3−-N, NO2−-N, and NH4+ and dynamic changes in microorganisms were assessed within 36 days.
Results
The reduction of NO3−-N showed a positive correlationship with Fe(II) oxidation and Fe(III) formation. In addition to Fe(II), acetate also participated in NO3−-N reduction as an electron donor. The reduction rate of NO3−-N was the highest in pH 8.5 + C3 treatment, in which the contribution of Fe(II) was 46.10%. The loss of NO3−-N was the least in pH 7.5 + C2 treatment, and the contribution of Fe(II) was 37.03%. Illumina high-throughput sequencing showed that the phyla of Proteobacteria and Firmicutes were enriched in all treatments. The classes of Alphaproteobacteria, Bacilli, and Gammaproteobacteria were found to be the dominant in all treatments. Pseudomonas, a NO3−-N reduction bacteria, was dominant in pH 8.5 + C3 treatment, in which the relative abundance was 24%. Meanwhile, a Fe(II) oxidizing bacteria, Acidovorax, was dominant in the pH 8.5 + C1 treatment, and the relative abundance was 15%.
Conclusion
In coastal saline-alkaline paddy field, high C input and high pH (pH 8.5 + C3 treatment) could increase NO3−-N reduction, which might be the important reasons for N loss. Therefore, proper application of organic fertilizer and N fertilizer should be paid attention to in actual production in the Yellow River Delta.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Yellow River Delta (YRD) has the advantages of flat terrain, sufficient illumination, and abundant water resources, but the soil salinization restricts the development of efficient ecological agriculture and regional economy in the YRD (Liu et al. 2012). Salinization resulted in low soil organic matter (SOM) content and poor soil fertilizer fixation in the YRD, and the local traditional planting habits aggravated the risk of nitrogen (N) loss from paddy soil. Therefore, how to improve the stability of N has become an issue of growing concern.
NO3−-N reduction is the main way of soil N loss. The metabolic pathway of microbial nitrate reduction is divided into denitrification (NO3−-N → NO2−-N → N2O → N2) and dissimilar nitrate reduction (NO3−-N → NO2−-N → NH4+), in which microbial denitrification is the main process controlling NO3−-N reduction (Weber et al. 2006). Fe(II) is ubiquitous in paddy soil. In the N and Fe biogeochemical cycling, nitrate-reducing Fe(II) oxidation (NRFO) plays an important role, in which the process is shown in the following equation (Liu et al. 2019; Zhao et al. 2017).
NRFO has been found to have chemical and biological mechanisms (Chen et al. 2020). Previous studies have been confirmed to present both Fe-rich and NO3−-N NRFO processes, such as in paddy soils (Wang et al. 2016) and sediment–water interface (Wang et al. 2015). Jamieson et al. (2018) found that 60–75% of Fe(II) oxidation in NRFO was a contribution from biological reactions. Organic carbon (OC) plays an important role in NO3−-N reduction, because NO3−-N-reducing microorganisms are mostly heterotrophic microorganisms that require organic carbon as a nutrient substrate to survive (Siemens et al. 2003). OC in soil can improve microbial activity and affect the composition of denitrifying microorganisms’ community (Miller et al. 2008, 2012). In the presence of OC, the NRFO process has been proven to be regulated by NO3−-reducing Fe(II) oxidation bacteria such as Acidovorax and Dechloromonas (Chakraborty and Picardal 2013). Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Archaea are NO3−-reducing Fe(II)-oxidizing microorganisms, which indicated their environmental importance (Kappler and Straub 2005).
Although the biological redox cycles of Fe and N under neutral pH conditions have been extensively studied (Zhao et al. 2015; Chen et al. 2018), few studies were concentrated on the reactions of Fe and N and microbial changes in alkaline environment, especially saline-alkaline soil. The saline-alkaline soil structure was poor, the pH was high, and the SOC content was pretty low (Wu, et al. 2019). The weaker N stabilization in this region was associated to the low C and high pH (7.5–8.5). It is necessary to explore the influence of different alkaline pH and OC on the process of the reactions of Fe and N. Therefore, a cultivation experiment was carried out with different pH and OC to explore (i) the carbon source and pH impact of the NRFO process in saline-alkaline paddy soil and (ii) the changes of abundance and diversity of microbial communities during NRFO in saline-alkaline paddy soil.
2 Materials and methods
2.1 Soil sampling
Soil sample (0–20 cm) was collected from a paddy field in October 2020 from Liwang Village, Kenli County, Shandong Province, China, where the soil type is mildly saline coastal tidal soil (Wu et al. 2020). Five samples were mixed into a composite sample, which were brought back to laboratory for cultivation experiment (within 48 h). The soil physicochemical properties were as follows: pH 8.64, SOM 12.78 g·kg−1, total nitrogen (TN) 0.97 g·kg−1, available potassium (AK) 104.24 mg·kg−1, available phosphorus (AP) 17.61 mg·kg−1, NO3−-N 1.33 mg·kg−1, NH4+-N 10.83 mg·kg−1, total Fe (Fet) 16.34 g·kg−1, citrate-bicarbonate-dithionite extractable Fe (Fed) 5.61 g·kg−1, and ammonium-oxalate extractable Fe (Feox) 1.65 g·kg−1.
2.2 NO3 −-N reduction incubation experiment
The NO3−-N reduction rate by Fe(II) under different acetate concentrations from 15 to 25 mM and different pH of 7.5 to 8.5 was determined by batch culture method. So the incubation experiment was conducted with two factors: (1) three pH levels: 7.5, 8.0, and 8.5; (2) three acetate concentrations: 15 (C1), 20 (C2), and 25 mM (C3). 2-Amino-2-(hydroxymethyl)-1, 3-propanediol (Trizma base, ≥ 99%) was purchased from Sigma-Aldrich (USA). NaNO3 (≥ 99%), FeCl2·4H2O (≥ 99%), and sodium acetate (≥ 99%) were purchased from the Tianjin Chemical Company.
Adjust the solution pH between 7.5 and 8.5 with 50 mM Trizma base buffer. All treatments were carried out in an anoxic Trizma base buffer; 40 mL of buffer was added to 100-mL serum bottles, and each serum bottle was added 0.4 g paddy soil, and sterilized in an autoclave at 121 °C for 60 min. To ensure anaerobic conditions, all solutions were injected with N2 (99.999%) for 30 min. The bottles were transferred to the anaerobic chamber and sealed with butyl stoppers. Fe(II) and other stock solutions were prepared with anoxic (99.999% N2 headspace) distilled deionized water in the anaerobic chamber (Li et al. 2015). The initial concentration of NO3−-N and Fe(II) was 10 and 5 mM, respectively. The detailed information of all treatments is presented in Table 1. And 24 replications were designed in each treatment. The time at which incubation bottles were sealed was recorded as 0 days. All experiments were repeated in triplicate; then, incubation and sampling were done in the anaerobic chamber at 25 °C in the dark for 36 days. Butyl rubber septa has been inserted by sterile needles for sampling of the solution. Samples were taken to determine the NO3−-N, NO2−-N, NH4+, Fe(II), and Fe(III) at 1, 3, 5, 7, 9, 12, 24, and 36 days, respectively. At 36 days, sediment samples were collected to determine the microbial community composition.
2.3 Chemical analysis
1,10-Phenanthroline method determined the dissolved Fe and extractable Fe concentration (Chen et al. 2018). The Fe(II) concentration and total Fe concentration in the extracted solution were determined by a UV–Vis spectrophotometer (UV-6000PC, Shanghai, China) at 510 nm with 0.1% o-phenanthroline reagent. Before adding the o-phenanthroline reagent, the sample was reduced with 10% hydroxylamine hydrochloride to determine the total Fe concentration in the extract. Subtraction of the total Fe concentration with the Fe(II) concentration could obtain Fe(III) concentration in a single sample.
NO3−-N, NO2−-N, and NH4+ concentrations were quantified by a continuous flow analyzer, in which Fe was removed by dialysis membrane and EDTA disodium salt solution (Chen et al. 2020).
2.4 DNA extraction and high-throughput sequencing
Depending on the manufacturer’s instructions of the PowerSoil™ DNA Isolation Kit (MO BIO Laboratories, USA), the soil genomic DNA was extracted from serum bottles and stored at − 45 °C for the following analytical steps. Universal primer set of F338 (50-ACTCCTACGGGAGGCAGCA-30) and R806 (50-GGACTACVSGGGTATCTAAT-30) was used. Three replicates were amplified for each DNA sample in a 30-mL reaction mixture using the following PCR procedure: initial denaturation at 95 °C for 3 min; 35 cycles at 94 °C denaturation for 30 s, primer annealing at 50 °C for 1 min, extension at 72 °C for 1 min, and a final extension of 10 min at 72 °C. The purified PCRs from each sample were combined in approximately equal quantities to produce a composite sample, which was then sequenced using a 250-bp pair sequencing kit on the Illumina HiSeq 2500 platform (Illumina, San Diego, CA, USA).
2.5 Analysis of microbial community composition and diversity
Bacterial 16S rRNA gene amplicon sequencing and MiSeq library construction were performed by Personalbio Biotechnologies Inc. (Shanghai, China). In this study, Illumina platform was used to perform double-ended sequencing on community DNA fragments. Then, Flash software (Magoč et al. 2011) was used to combine end reads to obtain the complete V3eV4 hypervariable region. The Quantitative Insights Into Microbial Ecology (QIIME) software pipeline was used to process the obtained sequences (Caporaso et al. 2010). Classification of OTUs was done using the Ribosomal Database Project classifier (Wang et al. 2007). Chao1, Shannon, and Simpson abundance-based indices were used to estimate the α-microbial biodiversity of the samples.
2.6 Statistical analysis
SPSS Statistics 20.0 was used for statistical analyses. Nine treatments were taken as experimental units to study the treatment effects. Two-way ANOVA was used to analyze the variance of the data with different treatments. In order to explore the relationship of NO3−-N, NO2−-N, NH4+, Fe(II), and Fe(III), Pearson correlation analysis was used. The plots in this work were generated using Origin Lab version 2018.
3 Results
3.1 Nitrate reduction
Within 36 days, NO3−-N concentration in all treatments showed a decreasing trend (Fig. 1). The reduction rate of NO3−-N in pH 8.0 was faster than that in pH 7.5 and pH 8.5. Under pH 8.5, the NO3−-N reduction rate in the C3 treatment was higher than that in C1 and C2 treatments, while in pH 8.0 it was highest in the C1 treatment; there was no significant difference among the three C treatments in pH 7.5. After 9 days of the reaction, NO3−-N concentration was about 6 mM, and it continued to decrease, while the Fe(II) was almost completely consumed. The consumption of NO3−-N was the fastest in the pH 8.5 + C3 treatment, which was changed from 6 to 1.18 mM in 36 days (Fig. 1c).
During the first 5 days of the reaction, almost no NO2−-N in all treatments was detected (Fig. 2). From day 6, few NO2−-N were detected, while on day 24, NO2−-N concentration in the pH 7.5 + C1 treatment was as high as 5.26 mM, and less than 2 mM of NO2−-N was also detected in the three acetate treatments in pH 8.5 (Fig. 2d, f). During the first 5 days, NH4+ concentration in all treatments was decreased (Fig. 2). After the 5th day, the increase and decrease of NH4+ fluctuated a little. At the end of the reaction, the concentration of NH4+ in all treatments was about 0.5 mM.
Since denitrification was the main process under flooding conditions, it was assumed that soil N loss was mainly emitted as N2O and N2. In this study, the gas products (such as N2O and N2) generated in denitrification process were not directly measured. The difference of NO3−-N, NO2−-N, and NH4+ concentrations before and after cultivation represents the generation of gas products. The change of soil NO3−-N, NO2−-N, and NH4+ concentration after cultivation is shown in Fig. 3. Compared with the initial N concentration, the N loss of all treatments was 59 ~ 83% at the end of cultivation. After 36 days, soil N loss in pH 8.5 level was higher than that in pH 7.5 and pH 8.0 levels. Under pH 8.0 and pH 8.5, soil N loss in the C3 treatment was higher than that in C1 and C2 treatments, while it was the highest in the C1 treatment under pH 7.5 (Fig. 3).
3.2 Fe(II) oxidation
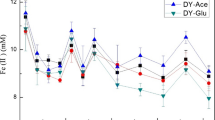
Under the three pH levels, Fe(II) concentration was sharply decreased from day 0 to day 3 in C1, C2, and C3 treatments, while from day 3 to day 5, it was increased, and then decreased again from day 5 to day 24 (Fig. 4a–c). In all treatments, more than 80% of the Fe(II) was completely oxidized within 9 days. On day 36, Fe(II) concentration in the C3 treatment was higher than that in C1 and C2 treatments; the latter two of which showed no significant difference (Fig. 4).
In the first 3 days of the reaction, Fe(III) concentration showed an increasing trend, and from the 3rd to 5th day, it was decreased rapidly, which might be due to the reduction of Fe(III) by acetate (Fig. 4d–f). In pH 8.0 and pH 8.5, Fe(III) was increased with the process of Fe(II) oxidation, while it was not equal to the decrease in Fe(II) (Fig. 4e, f).
Due to the simultaneity of NO3−-N reduction and Fe(II) oxidation, day 0 to day 9 of soil were selected as a specific period of Fe(II) oxidation, because of almost no Fe(II) was detected after day 9. We assumed that the NO3−-N reduction during day 0 to day 9 was due to Fe(II) oxidation; after day 9, it was due to other electron donors. Thus, the contribution of Fe(II) oxidation to NO3−-N reduction in all treatments was calculated to be 29.64%, 37.03%, 23.91%, 39.94%, 44.55%, 28.52%, 40.35%, 47.03%, and 46.10%, respectively.
3.3 Dynamic changes in microorganisms
During the reaction period, Illumina high-throughput sequencing generated 591,178 high-quality sequences from 9 analysis samples, and the sequence range of a single sample was 78,523 to 104,302. In the complete data set, 10,518 consensus OTUs were identified, with an average of 1169 OTUs per sample (Table S1). The average length of the analyzed sequences was 408 bases, and about 97% of the analyzed sequences were assigned at the phylum level, 95% at the order level, and 60–80% at the genus level.
In the original soil samples (0 days), the dominant phyla were Proteobacteria, Firmicutes, Chloroflexi, Acidobacteria, and Gemmatimonadetes. On day 36, Proteobacteria and Firmicutes dominated in all treatments (Fig. S1). Under pH 7.5, the relative abundance of Proteobacteria decreased from 78.71 to 34.50% and 46.78% in C1 and C2 treatments, respectively, while it increased from 78.71 to 82.04% in the C3 treatment (Fig. S1). Firmicutes was increased from 1.59 to 59.5%, 37.1%, and 10.1% in C1, C2, and C3 treatments, respectively. On day 36, the abundance of Actinobacteria and Bacteroidetes increased first and then decreased with the increase of C level (Fig. S1). Under pH 8.0, Proteobacteria showed no significant differences, which was all above 78% (Fig. S1). Firmicutes was increased from 1.59 to 8.84%, 2.37%, and 6.96% in C1, C2, and C3 treatments, respectively. Under pH 8.5, the relative abundance of Proteobacteria decreased from 78.71 to 55.3%, 44.7%, and 32.1% in C1, C2, and C3 treatments, respectively (Fig. S1). Meanwhile, Firmicutes showed an opposite trend, which was increased from 1.59 to 41.2%, 49.0%, and 64.0% in C1, C2, and C3 treatments, respectively.
At the class level, the dominant classes in the original soil samples were Alphaproteobacteria, Gammaproteobacteria, Clostridia, Bacteroidia, Deltaproteobacteria, Acidimicrobiia, and Gemmatimonadetes (Fig. S2). Under pH 7.5, Alphaproteobacteria dominated with a high relative abundance of 35%, 73%, and 59% in C1, C2, and C3 treatments at 36 days, respectively (Fig. S2). The relative abundance of Gammaproteobacteria increased from 19 to 52% and 26% in C1 and C3 treatments, respectively, while it decreased from 19 to 12% in the C2 treatment. Deltaproteobacteria was decreased from 13.8 to 0.19%, 0.68%, and 1.0% in C1, C2, and C3 treatments, respectively (Fig. S2). Under pH 8.0, Alphaproteobacteria was increased from 5.6 to 21.6%, 43.6%, and 49.4% in C1, C2, and C3 treatments, respectively (Fig. S2). Meanwhile, the relative abundance of Alphaproteobacteria showed the same trend under pH 8.5 (Fig. S2).
At the genus level, there were also significant differences in microbial composition between different treatments (Fig. 4). At pH 7.5, Paenisporosarcina, Microvirga, and Ensifer were enriched at the end of culture. The relative abundance of Paenisporosarcina in the C1 treatment was 41.45%, which was higher than that in C2 and C3 treatments, while Microvirga showed an opposite trend, which was increased from 16.52 to 41.09% (Fig. 4). When pH was 8.0, the dominant bacteria were Devosia, Lysobacter, Pseudomonas, Ensifer, and Allorhizobium—Neorhizobium—Pararhizobium—Rhizobium. Among them, the relative abundance of Devosia was 47.82% in the C2 treatment (Fig. 4). Under pH 8.5, the Paenisporosarcina relative abundance in C1, C2, and C3 treatment was 37.85%, 34.37%, and 61.23%, respectively (Fig. 4). The Pseudomonas relative abundance in the pH 8.0 + C3 treatment was 24.28%, while it was pretty low (1%) and showed no difference in the other treatments. Acidovorax was detected in pH 8.0 and pH 8.5, and the highest relative abundance was found in the pH 8.0 + C1 treatment, which was 14.97%. However, the relative abundance of anammox bacteria (e.g., Candidatus Scalindua and Candidatus Kuenenia Stuttgartiensis) was less than 1%, so anammox process could be excluded (Fig. 5).
4 Discussion
4.1 NO3 −-N reduction coupled with Fe(II) oxidation
This study simulated the process of NRFO in paddy soil. Denitrification results in the decrease of NO3−-N concentration during the incubation stage (Fig. 1). Denitrification was related to the reducing capacity of soil, which was mainly contributed by electron donors such as OC and Fe(II). Compared with C1, the higher concentration of acetate in C2 and C3 treatments accelerated the denitrification. In addition, the consumption of NH4+ also suggested the occurrence of nitrification (Fig. 2). This was consistent with previous studies that showed simultaneous nitrification and denitrification in paddy soils (Reddy and Patrick 1986). NH4+ concentration was positively correlated with Fe(III) concentration but negatively correlated with NO3−-N and Fe(II) in all treatments (Table 2), which proved that dissimilation into NH4+ process also occurred. The process of dissimilar NO3−-N reduction to NH4+ was also part of NRFO. NO2−-N was intermediate in both metabolic pathways of NO3−-N reduction. Each direction determines whether N was lost or becomes a nutrient that other plants could use. NH4+ could be absorbed and assimilated by rice and other crops, which has a certain promoting effect on agricultural production.
At 36 days, the lower Fe(II) concentration in all treatments indicated that NO3−-N drove the oxidation of Fe(II) (Fig. 3). This finding was consistent with previous research that demonstrated NO3−-N-driven Fe-redox cycle could solve the problem of excessive NO3−-N in groundwater (Roden 2012). These results indicated that NRFO exists in paddy soil (Ratering and Schnell 2001; Weber et al. 2006). In our study, Fe(III) concentration increase after 36 days of the reaction was not equivalent to the Fe(II) concentration decrease (Fig. 3). This phenomenon might be due to the oxidation of Fe(II) to produce insoluble Fe mineral products in flooded soil by NRFO microorganisms (Weber et al., 2006).
4.2 Effects of pH and acetate in NRFO
Three pH values (7.5, 8.0, and 8.5) were tested to examine the influence of solution pH on NRFO process and microbial change. The research on biological denitrification is mainly focused on circumneutral pH and previous studies have obtained contradictory results on the effect of pH on chemodenitrification. For example, Buchwald (2016) found that the rate of NO2−-N reaction was significantly increased when pH increased from 7 to 8, while Dhakal et al. (2013) reported that NO2−-N was reduced by Fe(II) and magnetite more rapidly at pH 5.5 than at pH 7.5. At higher pH, NO3−-N reduction rate was increased, but the accumulation of NO2−-N was decreased (Fig. 2d–f), which might be caused by the chemodenitrification of NO2−-N and Fe(II) (Klueglein and Kappler 2013). NO2−-N further reacted with Fe(II) to form N2O, N2 or NH4+, which reduced the accumulation of NO2−-N in the system (Fig. 2). The denitrification rate increased with the pH. In this study, soil N loss increased with the soil salinization. It was necessary to restore the degraded saline-alkaline soil.
Fe(II) oxidation strongly affected denitrification in anaerobic environment (Wang et al. 2016). Denitrifying bacteria could oxidize Fe(II) with the N2/N2O and other gas products (Wang et al. 2016). Most denitrifying bacteria require OC such as acetate, which is a carbon source to oxidize Fe(II) (Muehe et al. 2009). At the same pH level, the contribution of Fe(II) oxidation to NO3−-N reduction in the C2 treatment was higher than in the C1 treatment, which was proved that OC could serve as a carbon source to promote NRFO process (Figs. 1 and 3). But OC could not only promote the reaction as a carbon source, but also compete with Fe(II) as an organic electron donor. A study of microbial-driven NRFO reported that the reduction of NO3−-N was greater than the amount required for Fe(II) oxidation (Chen et al. 2018). In present research, almost no Fe(II) was detected in the system after day 9, while the NO3−-N concentration was still decreased during day 9 to day 36, which was due to other electron donors (Fig. 3). The amount of NO3−-N consumption implies that in addition to Fe (II), other electron donors reduce and consume NO3−-N, which may come from the OC stored in microbial cells during culture (Chen et al. 2018). Many NO3−-N-reducing bacteria could use organic electron donors to reduce NO3−-N (Muehe et al. 2009; Chakraborty and Picardal 2013). OC and Fe(II), as electron donors in paddy soil, competed with NO3−-N as electron acceptors, thus inhibiting their reaction with NO3−-N.
4.3 Microbial community diversity in NRFO
Denitrifying microorganisms were mainly heterotrophic anaerobic microorganisms. Under the long-term inundation and anaerobic environment of paddy soil, the increase of soil nutrients could greatly meet the demand for nutrients for the growth and reproduction of denitrifying microorganisms, thus stimulating the mass reproduction of denitrifying microorganisms. Consistent with the results of previous NRFO studies, Proteobacteria and Firmicutes dominated in all treatments after 36 days in this study (Fig. S1; Coby et al. 2011; Melton et al. 2014). Fe(II)-oxidizing bacteria Acidovorax and NO3−-N-reducing bacteria Pseudomonas (Fig. 4) were detected (Chakraborty and Picardal 2013), which participated in NRFO. Acidovorax is a Betaproteobacteria that could utilize NO3−-N and NO2−-N to oxidize Fe(II) in the presence of OC (Straub et al. 2004). Acidovorax has been reported as an anaerobic nitrate-dependent Fe(II) oxidant with complete genome sequence (Byrne-Bailey and Coates 2012). Pseudomonas is a Gammaproteobacteria, which belongs to autotrophic microorganisms (Su et al. 2015). The Acidovorax and Pseudomonas relative abundance was affected by pH and acetate concentration. Both Acidovorax and Pseudomonas existed at pH 8.0 and 8.5, and Acidovorax was higher in the C1 treatment and Pseudomonas was higher in the C3 treatment (Fig. 4). Besides, Microvirga, as a N-fixing bacteria (Malhotra et al. 2014), was detected out in this study, which might be one of the reasons for the increase in NH4+ concentration during the cultivation (Fig. 4). Anammox bacteria with a relative abundance of more than 1% were not detected in this study (e.g., Candidatus Scalindua and Candidatus Kuenenia Stuttgartiensis), so anammox could be excluded (Kuenen 2008).
4.4 Environmental implications
This study indicated that the reaction of NRFO was widespread in the natural environment. Most dominant genera in primitive paddy soil were enriched in all treatments, including Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and Chloroflexi (Fig. S1). This suggested that once the soil was changed from aerobic state to anoxic state, the functional microorganisms of NRFO in paddy soil could be readily developed while Fe(II) and NO3−-N were present (Chakraborty and Picardal 2013). OC could provide abundant material and energy source for the cell growth and metabolism of denitrifying bacteria, regulate the metabolic function and structure of soil denitrifying bacteria community, increase the diversity and abundance of denitrifying bacteria, and improve the denitrifying effect accordingly. On the other hand, OC could activate microbial respiration and accelerate the formation of soil anaerobic environment, thus increasing the rate of denitrification. In practice, excessive organic fertilizer and N fertilizer in saline-alkaline paddy soil could increase in the activity and abundance of denitrifying microorganisms, thus accelerating N loss. The reduction process of NO3−-N in saline-alkaline paddy soil was strongly affected by Fe(II) redox process and the input of OC, in which the increase of OC could promote NO3−-N reduction (Fig. 1). We believed that excessive carbon input in traditional fertilization is also one of the important reasons for N loss in the YRD (Kögel-Knabner et al. 2010). In actual production, the combination of organic fertilizer and N fertilizer should be paid attention to in consideration of the actual situation of the YRD.
5 Conclusions
Our experiments confirmed that under anaerobic conditions, different external conditions could influence response of NO3−-N and Fe(II) by affecting the microbial community in paddy soil. The reduction of NO3−-N was the fastest in the pH 8.5 + C3 treatment, and the slowest in the pH 7.5 + C1 treatment, because acetate, as an electron donor, participated in NO3−-N reduction. Proteobacteria and Firmicutes were the dominant phyla and the Alphaproteobacteria, Bacilli, and Gammaproteobacteria were the dominant classes. NO3−-N reduction bacteria Pseudomonas with the relative abundance of 24% was detected in the pH 8.5 + C3 treatment, and Fe(II)-oxidizing bacteria Acidovorax with the relative abundance of 15% was detected in the pH 8.5 + C1 treatment. In conclusion, 25 mM acetate input and high pH could increase the loss of NO3--N; hence, high pH and excessive C input in saline-alkaline soil might be the important reasons for N loss. Therefore, proper application of organic fertilizer and N fertilizer should be paid attention to in coastal saline paddy soil actual production.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Buchwald C, Grabb K, Hansel C, Wankel S (2016) Constraining the role of iron in environmental nitrogen transformations: dual stable isotope systematics of abiotic NO2- reduction by Fe(II) and its production of N2O. Geochim Cosmochim Acta 186. https://doi.org/10.1016/j.gca.2016.04.041
Byrne-Bailey KG, Coates JD (2012) Complete genome sequence of the anaerobic perchlorate-reducing bacterium azospira suillum strain PS. J Bacteriol 194:2767–2768. https://doi.org/10.1128/JB.00124-12
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Chakraborty A, Picardal F (2013) Induction of nitrate-dependent Fe(II) oxidation by Fe(II) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Appl Environ Microbiol 79:748–752. https://doi.org/10.1128/AEM.02709-12
Chen D, Liu T, Li X, Li F, Luo X, Wu Y, Wang Y (2018) Biological and chemical processes of microbially mediated nitrate-reducing Fe(II) oxidation by Pseudogulbenkiania sp. strain 2002. Chem Geol 476:59–69. https://doi.org/10.1016/j.chemgeo.2017.11.004
Chen D, Yuan X, Zhao W, Luo X, Li F, Liu T (2020) Chemodenitrification by Fe(II) and nitrite: pH effect, mineralization and kinetic modeling. Chem Geol 541:119586. https://doi.org/10.1016/j.chemgeo.2020.119586
Coby AJ, Picardal F, Shelobolina E, Xu H, Roden EE (2011) Repeated anaerobic microbial redox cycling of iron. Appl Environ Microbiol 77:6036–6042. https://doi.org/10.1128/AEM.00276-11
Dhakal P, Matocha CJ, Huggins FE, Vandiviere MM (2013) Nitrite Reactivity with Magnetite. Environ Sci Technol 47:6206–6213. https://doi.org/10.1021/es304011w
Jamieson J, Prommer H, Kaksonen AH, Sun J, Siade AJ, Yusov A, Bostick B (2018) Identifying and quantifying the intermediate processes during nitrate-dependent iron(II) oxidation. Environ Sci Technol 52:5771–5781. https://doi.org/10.1021/acs.est.8b01122
Kappler A, Straub K (2005) Geomicrobiological cycling of iron. Rev Mineral Geochem 59:85–108. https://doi.org/10.2138/rmg.2005.59.5
Klueglein N, Kappler A (2013) Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1 – questioning the existence of enzymatic Fe(II) oxidation. Geobiology 11:180–190. https://doi.org/10.1111/gbi.12019
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14. https://doi.org/10.1016/j.geoderma.2010.03.009
Kuenen JG (2008) Anammox bacteria: from discovery to application. Nat Rev Microbiol 6:320–326. https://doi.org/10.1038/nrmicro1857
Li B, Pan X, Zhang D, Lee DJ, Al-Misned FA, Mortuza MG (2015) Anaerobic nitrate reduction with oxidation of Fe(II) by Citrobacter freundii strain PXL1 – a potential candidate for simultaneous removal of As and nitrate from groundwater. Ecol Eng 77:196–201. https://doi.org/10.1016/j.ecoleng.2015.01.027
Liu P, Bai J, Ding Q, Shao H, Gao H, Xiao R (2012) Effects of water level and salinity on TN and TP contents in marsh soils of the Yellow River Delta, China. CLEAN - Soil Air Water 40:1118–1124. https://doi.org/10.1002/clen.201200029
Liu T, Chen D, Li X, Li F (2019)Microbially mediated coupling of nitrate reduction and Fe(II) oxidation under anoxic conditions. FEMS Microbiol Ecol 95.https://doi.org/10.1093/femsec/fiz030
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Malhotra J, Aparna K, Dua A, Sangwan N, Trimurtulu N (2014) Microbial and genetic ecology of tropical Vertisols under intensive chemical farming. Environ Monit Assess. https://doi.org/10.1007/s10661-014-4081-2
Melton ED, Rudolph A, Behrens S, Schmidt C, Kappler A (2014) Influence of nutrient concentrations on MPN quantification and enrichment of nitrate-reducing Fe(II)-oxidizing and Fe(III)-reducing bacteria from littoral freshwater lake sediments. Geomicrobiol J 31:788–801. https://doi.org/10.1080/01490451.2014.892765
Miller MN, Dandie CE, Zebarth BJ, Burton DL, Goyer C, Trevors JT (2012) Influence of carbon amendments on soil denitrifier abundance in soil microcosms. Geoderma 170:48–55. https://doi.org/10.1016/j.geoderma.2011.11.022
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyer C, Trevors JT (2008) Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol Biochem 40:2553–2562. https://doi.org/10.1016/j.soilbio.2008.06.024
Muehe EM, Gerhardt S, Schink B, Kappler A (2009) Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate reducing bacteria. FEMS Microbiol Ecol 70:335–343. https://doi.org/10.1111/j.1574-6941.2009.00755.x
Ratering S, Schnell S (2001) Nitrate-dependent iron(II) oxidation in paddy soil. Environ Microbiol 3:100–109. https://doi.org/10.1046/j.1462-2920.2001.00163.x
Reddy KR, Patrick WH (1986) Denitrification losses in flooded rice fields. In: De Datta S.K., Patrick W.H. (eds) Nitrogen economy of flooded rice soils. Developments in Plant and Soil Sciences, vol 26. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-4428-2_5.
Roden E, McBeth J, Blothe M, Percak-Dennett E, Fleming E, Holyoke R, Luther G, Emerson D (2012) The microbial ferrous wheel in a neutral pH groundwater seep. Front Microbiol 3.https://doi.org/10.3389/fmicb.2012.00172
Siemens J, Haas M, Kaupenjohann M (2003) Dissolved organic matter induced denitrification in subsoils and aquifers? Geoderma 113:253–271. https://doi.org/10.1016/S0016-7061(02)00364-6
Straub KL, Schönhuber WA, Buchholz-Cleven BEE, Schink B (2004) Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol J 21:371–378. https://doi.org/10.1080/01490450490485854
Su Jf, Shao Sc, Huang Tl, Ma F, Yang Sf Zhou Zm, Zheng Sc (2015) Anaerobic nitrate-dependent iron(II) oxidation by a novel autotrophic bacterium, Pseudomonas sp. SZF15. J Environ Chem Eng 3:2187–2193. https://doi.org/10.1016/j.jece.2015.07.030
Wang H, Hu C, Han L, Yang M (2015) Effects of microbial cycling of Fe(II)/Fe(III) and Fe/N on cast iron corrosion in simulated drinking water distribution systems. Corros Sci 100:599–606. https://doi.org/10.1016/j.corsci.2015.08.037
Wang M, Hu R, Zhao J, Kuzyakov Y, Liu S (2016) Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils. Geoderma 271:173–180. https://doi.org/10.1016/j.geoderma.2016.02.022
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/aem.00062-07
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764. https://doi.org/10.1038/nrmicro1490
Wu L, Wei C, Zhang S, Wang Y, Kuzyakov Y, Ding X (2019) MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J Clean Prod 235:901–909. https://doi.org/10.1016/j.jclepro.2019.07.043
Wu L, Zhang S, Wang J, Ding X (2020) Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: adsorption, column and field tests. Environ Pollut 261:114223. https://doi.org/10.1016/j.envpol.2020.114223
Zhao L, Dong H, Edelmann RE, Zeng Q, Agrawal A (2017) Coupling of Fe(II) oxidation in illite with nitrate reduction and its role in clay mineral transformation. Geochim Cosmochim Acta 200:353–366. https://doi.org/10.1016/j.gca.2017.01.004
Zhao L, Dong H, Kukkadapu RK, Zeng Q, Edelmann RE, Pentrak M, Agrawal A (2015) Biological redox cycling of iron in nontronite and its potential application in nitrate removal. Environ Sci Technol 49:5493–5501. https://doi.org/10.1021/acs.est.5b00131
Funding
This work was supported in part by the National Key Research and Development Projects, China (2017YFD0200200/2017YFD0200208-3), the Soil and Cultivation Position of Modern Agricultural Technology System Innovation Team in Shandong Province, China (SDAIT-17–05), and the Natural Science Fund of Shandong Province, China (ZR2020MC154).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xiaodong Ding, Yinghui Jiang. Data curation: Yinghui Jiang, Xiaodong Ding. Formal analysis: Yinghui Jiang, Xiaodong Ding. Funding acquisition: Xiaodong Ding, Shirong Zhang. Investigation: Yinghui Jiang, Shirong Zhang, Ruxue Wei. Methodology: Yinghui Jiang, Xiaodong Ding. Software: Yinghui Jiang, Ruxue Wei. Writing-original draft: Yinghui Jiang, Xiaodong Ding, Shirong Zhang. Writing-review and editing: Yinghui Jiang, Shirong Zhang, Xiaodong Ding, Ruxue Wei.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Jizheng He
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Y., Zhang, S., Wei, R. et al. Microbial community changes during anaerobic nitrate reduction and Fe(II) oxidation of a coastal saline paddy soil under alkaline pH. J Soils Sediments 22, 2720–2730 (2022). https://doi.org/10.1007/s11368-022-03247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03247-3