Abstract
Purpose
Stream sediment can control phosphorus (P) in the water column at baseflow. Two common laboratory analyses of sediment P are the equilibrium phosphate concentration at net zero sorption (EPC0) and P fractionation. Good sample handling ensures representative results, but oftentimes, studies rely on air-dried or freeze-dried samples, which alters sediment biogeochemistry. How and to what extent this influences EPC0 and P fractionation remains unclear. We therefore examine pretreatment effects on sediment EPC0 and P fractionation.
Materials and methods
We collected fine sediments (< 2 mm) from streams in the Tukituki River and Reporoa Basins in New Zealand (n = 31 sediments). Subsamples were then either kept fresh, frozen then lyophilized (freeze-dried), or dried at 40 °C for 2 weeks (air-dried). Measurements of EPC0 and P fractionation were made in triplicate. The sequential P fractionation scheme determined five different P pools: NH4Cl (labile P), NaOH reactive P (RP; metal oxide-bound P) and unreactive P (URP; organic P), HCl (Ca-mineral P), and residual P. Along with statistical comparisons between fresh results and the two pre-treatments, we explored correlations between pre-treatment effects and sediment physicochemical characteristics.
Results and discussion
The sediments had generally low EPC0 (majority < 0.020 mg P L−1), and uncertainty in EPC0 increased with concentration magnitude. While there were sediment-specific changes in EPC0 with pre-treatment, there was no consistent bias caused by pre-treatment. However, the differences between the fresh and air-dried sediment EPC0 were larger and more variable than between fresh and freeze-dried sediment. For P fractionation, the Tukituki sediments were enriched in HCl-P, while Reporoa sediments had more NaOH-RP and NaOH-URP. Despite large sediment-specific changes, the overall effects of freeze- and air-drying sediment were increased NH4Cl-P (estimated average effect, \( \widehat{\theta} \) = + 0.63 and + 3.7 mg P kg−1), no significant changes for NaOH-RP, contrasting changes in NaOH-URP (− 3.4 and + 3.3 mg P kg−1), and decreased HCl-P (− 40 and − 33 mg P kg−1).
Conclusions
We found that drying sediment significantly influenced EPC0 and P fractions (especially the NH4Cl-P fraction). Air-drying was particularly error-prone and should be avoided. The use of freeze-drying to preserve samples for later analyses and improve ease of handling may be used with appropriate consideration of the research objectives and the error introduced by freeze-drying. However, we recommend using fresh sediments for analyses whenever possible, as they best represent natural conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is a key limiting nutrient of primary production in aquatic ecosystems (Elser et al. 2007). Due to its numerous potential sources, variable chemical forms, and reactive transport in the environment, P pollution is difficult to target and mitigate (Sharpley et al. 2013; Powers et al. 2016). This transport is particularly complex in lotic systems, since numerous abiotic and biotic mechanisms control P fluxes (Reddy et al. 1999; House 2003; Withers and Jarvie 2008). Among abiotic factors, stream sediments are a major control of dissolved P in many streams (Jarvie et al. 2012; McDowell 2015). Since most fine sediments (< 2 mm) have a great capacity for P adsorption (Barrow 1983; Froelich 1988) and are themselves vectors for P derived from the original soil source (Condron and Newman 2011), studies of sediment P interactions are important for characterizing the transport of P in streams and to receiving water bodies.
Two common laboratory measurements for describing sediment P interactions in streams are the equilibrium phosphate concentration at net zero sorption (EPC0; Taylor and Kunishi 1971) and sediment P fractionation (Condron and Newman 2011; Wang et al. 2013). Equilibrium phosphate concentration at net zero sorption is the estimate of the equilibrium dissolved reactive P (DRP) concentration in the solution of batch experiments containing sediment where neither net desorption nor adsorption occurs (Froelich 1988). The EPC0 has been used to indicate the likely contribution of bed sediments in controlling water column DRP concentrations. For example, Jarvie et al. (2005) determined sediments to be an active sink for dissolved P at several stream sites subjected to wastewater treatment plant (WWTP) discharge since stream DRP concentrations were usually greater than the sediment EPC0. Similar studies of streams with high P loading find enriched EPC0 as an indication of previous P sequestration that might be released into solution again once in-stream DRP concentrations are reduced below the EPC0 (Ekka et al. 2006; Haggard and Stoner 2009).
Sediment P fractionation defines sequentially extracted pools of decreasingly bioavailable P. While some procedures may target compound-specific P (Golterman 1996), most produce operationally defined fractions (Wang et al. 2013). Phosphorus fractionation provides valuable information on the forms of P being transported by the sediment and their reactivity (and potential bioavailability). For example, in some lakes and reservoirs, Ca-P can be the largest pool in sediments (identified by acid extractions), where the release to solution is mostly mediated by bacteria (Tang et al. 2014; Li et al. 2016). Fractionation can also provide important information on P reactions with sediments; Lin et al. (2009) found that P adsorbed to river sediments in isotherm experiments was primarily associated with Fe oxide minerals and (to a lesser extent) Al oxide minerals extracted by NaOH and NH4F, respectively.
Data generated from either sediment EPC0 or P fractionation relies on robust laboratory methods to be representative of the study system and comparable between studies. For EPC0, previous work has highlighted that solution ionic strength and Ca2+ concentrations need to be similar to the study stream, since low ionic strength and low Ca2+ (e.g., deionized water) can reduce the sediment’s affinity for P (Klotz 1988; Rietra et al. 2001; Lucci et al. 2010). Additionally, pH is pivotal to the sorption process (Barrow 1983), where lower pH generally increases adsorption affinity (Meyer 1979; Klotz 1988; Huang et al. 2016). A less significant factor is the temperature during incubation, where greater temperatures can increase reaction rates and EPC0 (Barrow 1983; Klotz 1988; McDowell et al. 2017). For P fractionation, the main variables lie with the choice of the fractionation scheme, where the investigator must consider: compound-specific versus operationally defined P pools (Golterman 2002), the number of pools to measure, and the appropriate analytical methods to accurately measure concentrations (He and Honeycutt 2005; Nascimento et al. 2015). Condron and Newman (2011) and Wang et al. (2013) provide helpful reviews on available P fractionation methods and guidance for choosing the appropriate method.
One laboratory variable that has not been formally addressed is the pre-treatment of sediment for storage and handling purposes. Often, fresh (wet) sediment is preferred for sediment P analyses, as changes that occur through drying are avoided (Haggard et al. 2007; Lottig and Stanley 2007; Condron and Newman 2011). However, logistical constraints often prevent the timely analysis or handling (e.g., weighing) of fresh sediment, so drying may be needed for long-term storage. For example, the intensive study design of stream P by Stutter et al. (2010) did not allow for all sediments to be analyzed fresh in the same timeframe; therefore, air-dried (30 °C) sediments were used with the acceptance of the error introduced by drying. It is usually, but not always, acknowledged that air-drying sediment can alter redox properties (Phillips and Lovley 1987; Baldwin 1996), organic matter structure (Turner et al. 2007), and microbial content (Qiu and McComb 1995; Worsfold et al. 2005) of the sediment, and therefore is likely to alter P sorption and fractionation results (Klotz 1988; Condron and Newman 2011).
An alternative pre-treatment for sediments that is utilized by some protocols (e.g., Ruttenberg 1992) is freeze-drying. Although recommended by some workers for sediment P analyses (Pettersson et al. 1988), freeze-drying is known to affect redox conditions (Phillips and Lovley 1987), some pools of nitrogen (Worsfold et al. 2008), and disrupt soil organic matter (Bartlett and James 1980). Some studies of P fractionation in lake sediments and similar systems suggest systematic differences between fresh and freeze-dried sediments (Barbanti et al. 1994; Goedkoop and Pettersson 2000), but little work has focused on the implications for stream sediments. Freeze-drying may provide a reasonable alternative for sediment storage when analysis of fresh sediments is not feasible.
This study discusses the effect of sediment pre-treatment on EPC0 and P fractionation results. Using multiple stream sediments from two contrasting catchments, we compare results when analyzing sediments fresh, freeze-dried, and air-dried. We hypothesized that air-drying would produce the largest differences in EPC0 and P fractions compared to fresh sediment data, but freeze-dried sediments would be more comparable to fresh sediments. Additionally, we hypothesized that freeze-drying and air-drying would produce results with less variation among replicate analyses than fresh samples.
2 Materials and methods
2.1 Study sites
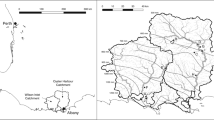
Sediment sampling sites were located in two catchments on the North Island of New Zealand: the Tukituki and the Reporoa Basin. The Tukituki Basin is dominated (in New Zealand Soil Classification) by Brown and Pallic soils which equate to Dystrochrepts and Aquepts or Fragiochrepts, respectively, in US Soil Taxonomy (Hewitt 2010). Land use is dominated by high production exotic grasslands (77% as of 2012). The Tukituki Basin receives approximately 800 mm of rainfall annually, and mean annual temperature is 14.5 °C. The Reporoa Basin is dominated by pumice soils (Vitrands in USDA soil taxonomy; Hewitt 2010); land use (as of 2012) is predominantly dairy farming (44%) and exotic forestry (39%). Mean annual rainfall in the Reporoa Basin varies from 1100 to 1550 mm, and mean annual temperature is 12.6 °C (Piper 2005).
Sampling took place during baseflow conditions in austral summer 2016/2017. Sediments were sampled from the submerged streambed at a variety of stream locations within each catchment (n = 28) and from some floodplain sites in the Tukituki catchment (n = 3). The Tukituki stream substrates were mostly gravel, sand, and some silt, while Reporoa stream substrates were sandy with a few cases of high silt (~ 80%); floodplain samples were predominantly sand. Further details about the study sites and their water quality can be found for the Tukituki catchment in Quinn et al. (2018) and for the Reporoa catchment in McDowell et al. (2018).
2.2 Sampling and preparation
Surficial sediments (uppermost 1 to 3 cm) were collected with a shovel during baseflow conditions. These sediments were located within the stream, near the centroid of flow, so as to target sediments under active flow. The stream sites, while at baseflow, were shallow and slow enough so as to prevent excessive winnowing of fine sediments during removal. We consider the streambed sediments to be oxic at time of collection as judged by the dissolved oxygen in the water column (saturation was generally ≥ 100%) and sufficient streamflow (and thus hyporheic exchange). However, it is possible that anoxic micro-zones may be present, even at these shallow depths, depending on the biogeochemical context (De Falco et al. 2016; Reeder et al. 2018). Samples were wet-sieved in the field to < 2 mm with minimal exposure to air, kept cool (4 °C), and in the dark during transit to the laboratory.
Approximately 5 g dry weight (dw) from each sediment sample was dried at 104 °C to determine moisture content. Approximately 20 g dw of sediment was used for each pre-treatment. For the freeze-dried sediments, the subsample was first frozen (− 20 °C) for at least 24 h before being quickly transported to a freeze-drier for desiccation. The air-dried sediments were prepared by drying at 40 °C (with ventilation) for 2 weeks. Air- and freeze-dried samples were stored at 4 °C until analysis.
For each pre-treatment, pH was measured in a 1:5 sediment to solution ratio (e.g., 1 g dw in 5 mL of solution) in D.I. water. For other physicochemical characteristics of the sediment, subsamples of the freeze-dried sediment were as follows: microwave digested with nitric acid plus hydrogen peroxide; then analyzed via inductively coupled plasma optical emission spectrometry for total Al, Ca, Fe, Mg, K, Mn, Na, and Zn content; analyzed for total C content via a CN elemental analyzer; and analyzed for anion storage capacity (ASC; Blakemore et al. 1987).
All sediments (n = 31) were analyzed for P fractions, but only the Tukituki sediments (n = 20) were used for sorption experiments.
2.3 Sorption experiments
Batch experiments for EPC0 were carried out in triplicate for each sediment following the methodology of Lucci et al. (2010). Solutions of KH2PO4 at 0, 0.01, 0.1, 1, 5, and 25 mg P L−1 were prepared with a background of 0.003 M CaCl2 to simulate in-stream ionic strength and Ca2+ concentrations (Klotz 1988; Lucci et al. 2010). A pH buffer was not used for these solutions; however, all solutions were in the range 4.5–5.6. A higher pH (closer to the stream pH) would possibly alter the measured EPC0 depending on the specific sediment surface chemistry regulating P uptake (Bolan and Barrow 1984; Klotz 1988; Huang et al. 2016). Using a sediment to solution ratio of 1:20, sediment samples were incubated with each solution for approximately 24 h via an end-over-end shaker near room temperature (~ 20 °C). After centrifugation (2400g for 20 min), the supernatant was analyzed colorimetrically either via the molybdenum blue method (Murphy and Riley 1962) or the malachite green method (Ohno and Zibilske 1991; D’Angelo et al. 2001). We preferred the malachite green method with a micro-plate reader for low concentrations (e.g., < 0.1 mg P L−1), since its detection limit (0.006 mg P L−1; D’Angelo et al. 2001) is more sensitive than what we could achieve with the molybdenum blue method on our spectrophotometer (~ 0.02 mg P L−1 for a 1-cm light-path). Standards prepared with the same background matrix were used for each batch of analyses. Adsorption or desorption of P was calculated as the mass of P either released into solution or removed from solution divided by the mass of sediment.
2.4 Phosphorus fractionation
We determined P fractions in each sediment using the Hieltjes and Lijklema (1980) method with some modifications. The sediments were sequentially extracted (at 1:100 sediment to solution ratio) with NH4Cl (loosely sorbed P), NaOH (inorganic P associated with metal oxides; Danen-Louwerse et al. 1993), and HCl (inorganic P associated with calcium minerals). A wash step with NH4Cl was included between NaOH and HCl extractions to prevent any significant carryover (Condron and Newman 2011). The NaOH extract was also analyzed for total P (NaOH-TP; Pettersson et al. 1988) after an acid-persulfate autoclave digestion (USEPA 1978). After the HCl extraction, the residual pellet was dried, ground via a mortar and pestle, and digested for remaining P (residual P) by block digestion with H2SO4 and H2O2 (Olsen and Sommers 1982). The NaOH extracts were analyzed for reactive P (NaOH-RP) with a modified molybdenum blue method suitable for alkaline extracts (Dick and Tabatabai 1977; He and Honeycutt 2005), which prevents the hydrolysis of organic P known to occur with the analysis of reactive P via the single-solution molybdenum blue method of Murphy and Riley (1962). Here, we denote the difference between NaOH-TP and NaOH-RP as unreactive P (NaOH-URP), which we consider to be primarily comprised of organic P (He and Honeycutt 2005). All other extracts and digested extracts were neutralized and then analyzed with the molybdenum blue method of Murphy and Riley (1962).
Phosphorus fractionations were done in triplicate. Two separate observations of HCl-P (from two different sediment samples, one from the fresh and one from the freeze-dried pre-treatments) were censored due to extreme values (~ 800–1200 mg P kg−1 greater than other replicates and samples). Laboratory replicates were summarized with a geometric mean (to account for skewness) and used for statistical comparisons (see below).
2.5 Determination of EPC0
Sediment EPC0 was calculated as the x-intercept of the linear sorption model of P sorption on initial (rather than equilibrium) solution DRP concentration using only the solution concentrations from the 0 mg P L−1 treatment up to the lowest treatment with all triplicate points indicating positive sorption (in this study, either 0.01 or 0.1 mg P L−1). Further explanation of the rationale behind this approach can be found in the Electronic Supplementary Material 1.
In addition to calculating the EPC0, the uncertainty about the EPC0 was calculated in order to compare the variations induced by sediment pre-treatment. A 95% confidence interval about the x-intercept was estimated with a likelihood-based approach (see example 4 in Harding 1986). An example of this calculation is shown in Fig. S1 (Electronic Supplementary Material 1).
2.6 Statistical comparisons
To test our hypothesis that sediment pre-treatment would affect P fractions and EPC0, comparisons were made using the Wilcoxon signed-rank test (Hollander et al. 2013). The null hypothesis of the test is that there is zero shift in location due to the pre-treatment (i.e., the paired differences have a distribution symmetric about a common median (θ) that is equal to zero); the data were treated as repeated measures where the T+ statistic and p value were computed for each comparison (fresh vs freeze-dried, fresh vs air-dried, and freeze-dried vs air-dried). Additionally, an estimate of θ (\( \widehat{\theta} \)) and its 95% confidence interval were computed for evaluating the alternative case where θ ≠ 0 (Hollander et al. 2013; Ugarte et al. 2015). The statistic \( \widehat{\theta} \) (which carries the same units as the observations) estimates, on average, both the magnitude and direction of pre-treatment effect when the null hypothesis is rejected.
Further, we explored the differences in P fractions and EPC0 between pre-treatments with the sediment physicochemical data (given in Table S1, Electronic Supplementary Material 2). We used Spearman’s ρ to measure the correlations between variables.
All analyses were performed in R ( R Core Team 2017). The data and code used in this manuscript are provided online at https://doi.org/10.6084/m9.figshare.6157772.v1.
3 Results
3.1 EPC0 and its uncertainty
The EPC0 of the Tukituki sediments varied from 0.011 to 0.055 mg P L−1 for fresh sediments, 0.012 to 0.052 mg P L−1 for freeze-dried sediments, and 0.003 to 0.130 mg P L−1 for air-dried sediments (Fig. 1). Most of these sediments had relatively low EPC0 (< 0.020 mg P L−1) regardless of the pre-treatment used. The uncertainty about EPC0 (here, the width of the 95% confidence interval for EPC0) was correlated with the magnitude of EPC0 (Spearman ρ = 0.439; Fig. 2) where uncertainty was as low as 0.002 mg P L−1 and as high as 0.053 mg P L−1.
It can be seen from Fig. 1 that the disparity between pre-treatment methods varied for each sediment. For example, sediment T11 had practically identical results for each pre-treatment, while air-drying reduced EPC0 in other sediments such as T9 and T1. Additionally, the air-dried pre-treatment had the greatest uncertainty for sediment T9, while the fresh pre-treatment was the most variable for T1. Because effects of pre-treatment were different among each sediment, a Wilcoxon signed-rank test showed no uniform effect of pre-treatment for any of the three comparisons (p > 0.5; Table 1). This result is further illustrated in Fig. 3. While differences in measured EPC0 with magnitudes of approximately 0.010 mg P L−1 or more are ecologically relevant for sediment P studies, the distribution of these differences is largely centered near zero (\( \widehat{\theta} \) of 0.0004 and − 0.0032 mg P L−1 for the freeze-dried and air-dried comparisons, respectively; Table 1). Thus, no consistent bias can be attributed to pre-treatment based on our data.
However, we note that the variation in pre-treatment differences is considerably greater for air-drying in comparison to freeze-drying. In Fig. 3, the standard deviation of comparisons and the inter-quartile range (IQR; difference between the 25th and 75th percentiles) are 0.010 and 0.004 mg P L−1 for the freeze-dried comparison; for the air-dried comparison, the standard deviation and IQR were 0.034 and 0.016 mg P L−1. This result suggests that air-drying induces more deviation in EPC0 from the fresh sediment EPC0 than freeze-drying.
Tables S1 and S2 (Electronic Supplementary Material 2) give the physicochemical characteristics of the sediments and their Spearman rank correlations with the EPC0 values, respectively. Greater EPC0 in the fresh sediments coincided with greater sediment Al, K, Mg, and Zn (at α = 0.05). Changes in EPC0 due to freeze-drying (positive values being an increase in EPC0 relative to the fresh sediment EPC0) were negatively correlated with Al but positively correlated with anion storage capacity (ASC). For the air-dried pre-treatment, changes in EPC0 were negatively correlated with sediment Al, Fe, Mg, and Mn content. There were no significant correlations of the EPC0 measures with pH nor with changes in pH caused by drying.
3.2 Phosphorus fractionation
Sediment P fractions for each pre-treatment are shown for the Tukituki and Reporoa samples (Fig. 4). The fresh Tukituki sediments were relatively low in the labile fractions, NH4Cl and NaOH-RP (medians of ~ 0.4 mg P kg−1 and 19 mg P kg−1, respectively). Unreactive P in the NaOH fraction was also low for Tukituki sediments (maximum of 19 mg P kg−1 but median of 4.4 mg P kg−1). However, the Tukituki sediments were enriched in the HCl fraction (range of 250 to 410 mg P kg−1). The fresh Reporoa sediments were also low in NH4Cl-P (majority < 0.5 mg P kg−1) but had a relatively large amount of NaOH-RP (median of 280 mg P kg−1). Additionally, the Reporoa sediments had medians of 9 mg P kg−1 in NaOH-URP and 140 mg P kg−1 in HCl-P.
Phosphorus fractionations in stream sediments from the Tukituki Basin (top row) and Reporoa Basin (bottom row) for each pre-treatment (fresh, freeze-dried, and air-dried). Note that within each sediment, the pre-treatments are ordered as fresh, freeze-dried, and then air-dried, and note the difference in scales for each row
Sediment pre-treatment effects on P fractionation are summarized in Table 2. Both drying methods increased the NH4Cl P fraction as compared to the fresh sediment, with an average increase of 0.63 mg P kg−1 and 3.7 mg P kg−1 for freeze-drying and air-drying, respectively. Further, the increase in NH4Cl-P due to pre-treatment was 3.1 mg P kg−1 greater for air-drying than freeze-drying. Pre-treatment effects on the NaOH-RP fraction were mixed: Changes due to either freeze-drying or air-drying were not significant at α = 0.05 although Fig. 4 suggests some decreases with drying—particularly for the Reporoa sediments (median values of differences with fresh Reporoa data were − 22 and − 76 mg P kg−1 for freeze-dried and air-dried, respectively). Results of NaOH-URP indicated a decrease with freeze-drying (\( \widehat{\theta} \) = − 3.4 mg P kg−1), but an increase with air-drying (\( \widehat{\theta} \) = 3.3 mg P kg−1). Both drying methods caused significant decreases in HCl-P in the sediments (\( \widehat{\theta} \) = − 40.4 and − 33.3 mg P kg−1 for freeze-drying and air-drying, respectively). The residual P fraction was unaffected by drying methods.
Spearman rank correlations of fresh sediment P and differences in sediment P due to pre-treatment with sediment characteristics for each P fraction are given in Table S3 (Electronic Supplementary Material 2) and are only summarized here. NH4Cl-P content was positively correlated with metals (Al, Fe, Mg, Mn), but negatively correlated with ASC. While no correlations were apparent for freeze-drying, changes in NH4Cl-P due to air-drying were negatively correlated with metals (e.g., greater amounts of P in the air-dried pre-treatment relative to fresh sediment corresponded with less amounts of Fe), but positively correlated with total C and ASC. NaOH-RP was negatively correlated with Ca, Mg, and pH, but positively correlated with Na, total C, and ASC; changes due to either drying method were negatively correlated with Na, ASC, and—just for the freeze-dried pre-treatment—total C. While some correlations were evident for the NaOH-URP, HCl-P, and residual P fractions in the fresh sediment, no noteworthy correlations were evident for the pre-treatment effects.
4 Discussion
4.1 Effects of pre-treatment on EPC0
In this study, we report considerable variability in sediment EPC0 dependent on the pre-treatment used (Figs. 1 and 3), yet no consistent bias due to pre-treatment (Table 1). To our knowledge, few studies have examined changes in EPC0 with drying. Klotz (1988) measured an increase in one sediment EPC0 from 0.011 to 0.021 mg P L−1 with air-drying at 80 °C—well within the differences calculated here (Fig. 3). With a focus on lake sediments, Twinch (1987) compared fresh sediments and air-dried sediments (at room temperature) and measured significant increases in EPC0 with drying (means of 0.095 and 0.284 mg P L−1, respectively). These results cast doubt on the use of drying, particularly air-drying, for sediment P studies; however, the mechanisms for these changes in EPC0 remain unclear.
For the case of an increased EPC0 with drying (i.e., decrease of P affinity), at least two complementary biotic mechanisms are possible: lysing of microbial P (to be flushed when in contact with solution) and the removal of any potential biotic uptake by killing microbes. The flushing of microbial P upon drying has been demonstrated in lake sediments (Qiu and McComb 1995; Baldwin 1996) as well as after microbial death via less sediment-perturbing methods (e.g., autoclaving and irradiation; Meyer 1979; Klotz 1988; Haggard et al. 1999). McDowell (2003) showed an inverse relationship between microbial biomass P and desorbable P (as CaCl2-extracted P) in stream sediments except where high organic carbon was present. Thus, the effects of both air-drying and freeze-drying could alter microbial P interactions and shift EPC0 upward, particularly in sediments where biotic P uptake is relatively high (Lottig and Stanley 2007).
A possible abiotic mechanism for increasing EPC0 with drying is the shift of sediment Fe from amorphous Fe (oxyhydr)oxides to more crystalline Fe oxides. Phillips and Lovley (1987) demonstrated that both air-drying and freeze-drying sediments oxidizes poorly crystalline Fe (as measured by oxalate extracts). This oxidation could shift the Fe species from more amorphous, highly P-reactive Fe (oxyhydr)oxides to more crystalline, less P-reactive Fe oxides (Golterman 2004; Jan et al. 2015), i.e., the metal oxides are aged (see also discussion below). More crystalline metal oxides may also explain the positive correlation observed between fresh sediment ASC (higher for more sorptive sediments) and increased EPC0 after freeze-drying, although the same effect was insignificant for the air-dried pre-treatment (Table S2; Electronic Supplementary Material 2). Using lake sediments, Baldwin (1996) tested the effects of desiccation and oxidation (via air-drying) compared to oxidation alone on P adsorption and concluded that oxidation is the primary factor in reducing P affinity. The speciation of the sediment Fe in the current study was not measured, but average total Fe for the sediments in the EPC0 experiments was 17.5 g kg−1 (standard deviation of 2.24 g kg−1; n = 20). Therefore, oxidizing and aging of sediment Fe may have increased EPC0 in some of the dried pre-treatments, particularly the air-dried treatment (Fig. 3).
Mechanisms for decreasing EPC0 (i.e., an increase in P affinity) with drying are less clear. Sorption processes are typically stronger at lower pH (Barrow 1983; Huang et al. 2016), which could be important for the cases of decreasing sediment pH with drying, but we found no relation between these variables for our data (Table S2, Electronic Supplementary Material 2). As drying may affect organic matter content (Barbanti et al. 1994; Turner et al. 2007), an indirect effect may be tied to the competition between organic matter and phosphate for sorption sites (Guan et al. 2006).
Considering fresh sediment EPC0 as the “ideal” EPC0, one objective was to identify a drying technique that induces the least variation in EPC0 measurements. We have shown that freeze-drying introduces a modest amount of variation in EPC0 (standard deviation of comparisons 0.010 mg P L−1; IQR of 0.004 mg P L−1), but air-drying produces considerably more variation (standard deviation of comparisons 0.034 mg P L−1; IQR of 0.016 mg P L−1; Fig. 1). These figures are also reflected in the 95% confidence intervals for \( \widehat{\theta} \) in Table 1 (− 0.0022 to 0.0021 and − 0.0086 to 0.025 mg P L−1 for the freeze-dried and air-dried comparisons, respectively). Therefore, we recommend that, in cases where fresh sediment analyses are not practical, freeze-drying is the better alternative for sediment preservation before sorption analyses. We further recommend caution when using dried sediments for individual sorption studies where the goal is to characterize reactive transport processes (e.g., House and Denison 2002); the variability induced by drying may alter the interpretation of how the natural sediments behave in situ.
Additionally, as we have shown that uncertainty in EPC0 increases with magnitude (Fig. 2), care should be exercised in EPC0 measurements not only for very low concentrations but also for relatively high concentrations (e.g., > 0.030 mg P L−1). Several studies examining impacted streams in a variety of settings have measured EPC0 values ranging from near detection limits (i.e., < 0.010 mg P L−1) to more than 1 mg P L−1 (Ekka et al. 2006; McDowell 2015; Weigelhofer 2017). Often, EPC0 is used as an indicator variable, where changes in EPC0 are examined over multiple sampling periods or sites (Jarvie et al. 2005; Ekka et al. 2006), or used to correlate with baseflow DRP concentrations (McDowell 2015). These general uses of EPC0 may not be significantly affected by the uncertainty we describe here, but other specific calculations may be more tenuous (e.g., percentage EPC saturation; Jarvie et al. 2005).
4.2 Effects of pre-treatment on P fractionation
Drying pre-treatments of stream sediments can alter P fractions, thus confounding important P biogeochemical processes. We have demonstrated that, generally, the largest P fraction in a sediment is the most susceptible to error caused by drying (e.g., HCl-P in the Tukituki sediments and NaOH-RP in the Reporoa sediments; Fig. 4). However, error in smaller P fractions (e.g., NH4Cl-P) should not be ignored as these pools can represent highly bioavailable pools (Pettersson et al. 1988; Condron and Newman 2011; Wang et al. 2013). Indeed, the labile pools such as NH4Cl-P are critical as they also correlate with P available for exchange with the water column (McDowell 2015). In this study, we showed consistent increases in NH4Cl-P (0.63 to 3.7 mg P kg−1; Table 2) with either drying pre-treatment, with air-drying being the most severe. This is likely due to either lysing of microbial P (Qiu and McComb 1995; McDowell 2003) or reduction in sediment P affinity (Baldwin 1996), but NH4Cl is also capable of dissolving some small amounts of CaCO3, Al, or Fe oxide-bound P (Hieltjes and Lijklema 1980; Pettersson et al. 1988)—there is not enough information to discern if any interactions between drying and labile calcite or metal P compounds took place.
Sediments with larger amounts of NaOH-RP (i.e., the Reporoa sediments; mean of 330 mg P kg−1) had the largest changes in the NaOH-RP fraction; however, there was no consistent pre-treatment effect for all sediments (Table 2). The fact that more of the sediments studied here were relatively poor in NaOH-RP (i.e., the Tukituki sediments; mean of 22 mg P kg−1) may have weakened any possible effects of pre-treatment on this fraction. Decreases in this somewhat labile fraction with either freeze-drying or air-drying have been reported, where the P may instead appear in more labile fractions (Dieter et al. 2015) or potentially become occluded by either particle aggregation (Twinch 1987) or aging metal oxides (Schlichting and Leinweber 2002; Hjorth 2004), thus appearing as more recalcitrant P. While the fractionation scheme we employed here does not partition between various metal oxide-bound P fractions (e.g., amorphous Fe oxy(hydr)oxides extracted first with bicarbonate-dithionite (BD) followed by extraction of more crystalline Fe and Al oxides with NaOH; Jan et al. 2015), it is conceivable that oxidation and aging of metal oxides incurred by either drying method would alter the speciation and lability of metal oxide-bound P (Phillips and Lovley 1987). In fact, when studying air-drying effects on sediment P, Dieter et al. (2015) used a similar P fractionation scheme to that of the current study but included a BD step: Both NH4Cl-P and BD-P increased after the studied lake sediments were air-dried, concomitant with a decrease in NaOH-RP (reduced or redox-insensitive Fe and Al oxide-bound P in this context) and NaOH-URP. Therefore, when drying sediments from a strongly reduced environment (many lakes, wetlands, and some streams; Reddy et al. 1999) or when using a more detailed P fractionation scheme, pre-treatment effects may be magnified.
The sediments studied here were relatively low in alkaline-extracted URP (overall mean of 12 mg P kg−1), as compared to other New Zealand stream sediments (average NaOH-URP ranging from 35 to 57 mg P kg−1; McDowell and Hill 2015). Yet, there was a moderate decrease in NaOH-URP with freeze-drying (\( \widehat{\theta} \) = − 3.4 mg P kg−1) and increase with air-drying (\( \widehat{\theta} \) = 3.3 mg P kg−1; Table 2). Turner et al. (2007) studied the effects of air-drying and freeze-drying on NaOH-EDTA extracts for wetland soils and also had conflicting results: Between pre-treatments, there were sample-specific changes in both total P recoveries and speciation (via 31P nuclear magnetic resonance spectroscopy; e.g., changes in proportions of phosphate and various organic P compounds). It remains unclear what mechanisms are most important, as potential drying effects include enhancing organic P recoveries in alkaline extracts by increasing organic P lability or by disrupting organic matter as well as the potential to solubilize some organic P (particularly during air-drying) so that it is instead detected as part of the labile P fractions (Barbanti et al. 1994; Cade-Menun et al. 2005; Turner et al. 2007; Dieter et al. 2015).
The HCl-P fraction largely contains various Ca-P minerals (e.g., apatite) and is typically stable (Wang et al. 2006), but small amounts may be mobilized for lower pH at the microscale (Golterman 2004). Both pre-treatments resulted in large decreases in this fraction (− 40 and − 33 mg P kg−1 for freeze-drying and air-drying, respectively; Table 2). Schlichting and Leinweber (2002) reported similar findings for P fractionation in a peat soil: Acid-extracted P was significantly reduced for freeze-drying and air-drying pre-treatments, which the authors attributed to decreased solubility of Ca-phosphates.
5 Conclusions
If EPC0 is to be a useful parameter for describing sediment P interactions in streams, then methods should be as robust and replicable as possible. While previous work has recommended using solutions that match the stream chemistry, we further recommend using fresh sediments for EPC0 measurements whenever possible. However, in cases of logistical and handling constraints, freeze-drying should be a preferred storage method (with tacit acknowledgement of potential errors of approximately 0.01 mg P L−1). In agreement with our hypothesis, air-drying increased variability and uncertainty compared to fresh (and freeze-dried) sediments and should be avoided in the measurement of EPC0.
For P fractionation, we recommend that the pre-treatment of samples should be uniform within a given study and that, when comparing results to studies employing differing pre-treatments, caution should be used. In particular, we have shown that labile P fractions (e.g., NH4Cl-P) are the most susceptible to changes with drying, where air-drying had the most dramatic effect. Given the changes in P fractions that can occur with any drying method, we also share the past recommendations that fresh sediment data should be the most representative of in situ conditions and the favored pre-treatment.
References
Baldwin DS (1996) Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir. Limnol Oceanogr 41:1725–1732
Barbanti A, Bergamini MC, Frascari F, Miserocchi S, Rosso G (1994) Critical aspects of sedimentary phosphorus chemical fractionation. J Environ Qual 23:1093–1102
Barrow NJ (1983) A mechanistic model for describing the sorption and desorption of phosphate by soil. Eur J Soil Sci 34:733–750
Bartlett R, James B (1980) Studying dried, stored soil samples—some pitfalls. Soil Sci Soc Am J 44:721
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils. NZ soil bureau scientific report 80, Lower Hutt, New Zealand
Bolan NS, Barrow NJ (1984) Modelling the effect of adsorption of phosphate and other anions on the surface charge of variable charge oxides. J Soil Sci 35:273–281
Cade-Menun BJ, Benitez-Nelson CR, Pellechia P, Paytan A (2005) Refining 31P nuclear magnetic resonance spectroscopy for marine particulate samples: storage conditions and extraction recovery. Mar Chem 97:293–306. https://doi.org/10.1016/j.marchem.2005.05.005
Condron LM, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 11:830–840
D’Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Qual 30:2206–2209
Danen-Louwerse H, Lijklema L, Coenraats M (1993) Iron content of sediment and phosphate adsorption properties. Hydrobiologia 253:311–317
De Falco N, Boano F, Arnon S (2016) Biodegradation of labile dissolved organic carbon under losing and gaining streamflow conditions simulated in a laboratory flume. Limnol Oceanogr 61:1839–1852
Dick WA, Tabatabai MA (1977) Determination of orthophosphate in aqueous solutions containing labile organic and inorganic. J Environ Qual 6:82–85
Dieter D, Herzog C, Hupfer M (2015) Effects of drying on phosphorus uptake in re-flooded lake sediments. Environ Sci Pollut Res 22:17065–17081
Ekka SA, Haggard BE, Matlock MD, Chaubey I (2006) Dissolved phosphorus concentrations and sediment interactions in effluent-dominated Ozark streams. Ecol Eng 26:375–391
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Froelich PN (1988) Kinetic control of dissolved phosphate in natural rivers and estuaries: a primer on the phosphate buffer mechanism. Limnol Oceanogr 33:649–668
Goedkoop W, Pettersson K (2000) Seasonal changes in sediment phosphorus forms in relation to sedimentation and benthic bacterial biomass in Lake Erken. Hydrobiologia 431:41–50
Golterman HL (1996) Fractionation of sediment phosphate with chelating compounds: a simplification, and comparison with other methods. Hydrobiologia 335:87–95
Golterman HL (2002) With special attention to phosphate fractionation in sediments. Hydrobiologia 471:3–4
Golterman HL (2004) The chemistry of phosphate and nitrogen compounds in sediments, 1st edn. Kluwer Academic Publishers, Dordrecht
Guan XH, Shang C, Chen GH (2006) Competitive adsorption of organic matter with phosphate on aluminum hydroxide. J Colloid Interface Sci 296:51–58
Haggard BE, Smith DR, Brye KR (2007) Variations in stream water and sediment phosphorus among select Ozark catchments. J Environ Qual 36:1725–1734
Haggard BE, Stanley EH, Hyler R (1999) Sediment-phosphorus relationships in three northcentral Oklahoma streams. Trans ASAE 42:1709–1714
Haggard BE, Stoner RJ (2009) Long-term changes in sediment phosphorus below a rural effluent discharge. Hydrol Earth Syst Sci Discuss 6:767–789
Harding EF (1986) Modelling: the classical approach. J R Stat Soc 35:115–134
He Z, Honeycutt CW (2005) A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun Soil Sci Plant Anal 36:1373–1383
Hewitt AE (2010) New Zealand soil classification (NZSC), 3rd edn. Manaaki Whenua Press, Landcare Research, Lincoln
Hieltjes AHM, Lijklema L (1980) Fractionation of inorganic phosphates in calcareous sediments. J Environ Qual 9:405–407
Hjorth T (2004) Effects of freeze-drying on partitioning patterns of major elements and trace metals in lake sediments. Anal Chim Acta 526:95–102
Hollander M, Wolfe DA, Chicken E (2013) The one-sample location problem. In: nonparametric statistical methods, 3rd edn. Wiley, Hoboken, pp 39–114
House WA (2003) Geochemical cycling of phosphorus in rivers. Appl Geochem 18:739–748
House WA, Denison FH (2002) Exchange of inorganic phosphate between fresh waters and sediments. Environ Sci Technol 36:4295–4301
Huang L, Fang H, He G, Chen M (2016) Phosphorus adsorption on natural sediments with different pH incorporating surface morphology characterization. Environ Sci Pollut Res 23:18883–18891
Jan J, Borovec J, Kopáček J, Hejzlar J (2015) Assessment of phosphorus associated with Fe and Al (hydr)oxides in sediments and soils. J Soils Sediments 15:1620–1629
Jarvie HP, Jürgens MD, Williams RJ, Neal C, Davies JJL, Barrett C, White J (2005) Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye. J Hydrol 304:51–74
Jarvie HP, Sharpley AN, Scott JT, Haggard BE, Bowes MJ, Massey LB (2012) Within-river phosphorus retention: accounting for a missing piece in the watershed phosphorus puzzle. Environ Sci Technol 46:13284–13292
Klotz RL (1988) Sediment control of soluble reactive phosphorus in Hoxie Gorge Creek, New York. Can J Fish Aquat Sci 45:2026–2034
Li H, Liu Y, Cao X, Song C, Zhou Y (2016) Functions of calcium-bound phosphorus in relation to characteristics of phosphorus releasing bacteria in sediment of a Chinese shallow lake (Lake Wabu). Geomicrobiol J 33:751–757
Lin C, Wang Z, He M, Li Y, Liu R, Yang Z (2009) Phosphorus sorption and fraction characteristics in the upper, middle and low reach sediments of the Daliao river systems, China. J Hazard Mater 170:278–285
Lottig NR, Stanley EH (2007) Benthic sediment influence on dissolved phosphorus concentrations in a headwater stream. Biogeochemistry 84:297–309
Lucci GM, McDowell RW, Condron LM (2010) Evaluation of base solutions to determine equilibrium phosphorus concentrations (EPC0) in stream sediments. Int Agrophysics 24:157–163
McDowell RW (2015) Relationships between sediment chemistry, equilibrium phosphorus concentrations, and phosphorus concentrations at baseflow in rivers of the New Zealand National River Water Quality Network. J Environ Qual 44:921–929
McDowell RW (2003) Sediment phosphorus chemistry and microbial biomass along a lowland New Zealand stream. Aquat Geochem 9:19–40
McDowell RW, Elkin KR, Kleinman PJA (2017) Temperature and nitrogen effects on phosphorus uptake by agricultural stream-bed sediments. J Environ Qual 46:295–301
McDowell RW, Hill SJ (2015) Speciation and distribution of organic phosphorus in river sediments: a national survey. J Soils Sediments 15:2369–2379
McDowell RW, Simpson ZP, Stenger R, Depree C (2018) The influence of a flood event on the potential sediment control of baseflow phosphorus concentrations in an intensive agricultural catchment. J Soils Sediments. https://doi.org/10.1007/s11368-018-2063-7
Meyer JL (1979) The role of sediments and bryophytes in phosphorus dynamics in a head water stream ecosystem. Limnol Oceanogr 24:365–375
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nascimento CAC, Pagliari PH, Schmitt D et al (2015) Phosphorus concentrations in sequentially fractionated soil samples as affected by digestion methods. Sci Rep 5:1–13
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895
Olsen SR, Sommers LE (1982) Phosphorus. In: Klute A, Page AL (eds) Methods of soil analysis part 2, 2nd edn. American Society of Agronomy, Madison, pp 403–430
Pettersson K, Boström B, Jacobsen OS (1988) Phosphorus in sediments—speciation and analysis. Hydrobiologia 170:91–101
Phillips EJP, Lovley DR (1987) Determination of Fe(III) and Fe(II) in oxalate extracts of sediment. Soil Sci Soc Am J 51:938–941
Piper J (2005) Water resources of the Reporoa Basin. Environment Waikato Regional Council, Hamilton
Powers SM, Bruulsema TW, Burt TP, Chan NI, Elser JJ, Haygarth PM, Howden NJK, Jarvie HP, Lyu Y, Peterson HM, Sharpley AN, Shen J, Worrall F, Zhang F (2016) Long-term accumulation and transport of anthropogenic phosphorus in three river basins. Nat Geosci 9:353–356
Qiu S, McComb AJ (1995) Planktonic and microbial contributions to phosphorus release from fresh and air-dried sediments. Mar Freshw Res 46:1039–1045
Quinn JM, Rutherford K, Depree C et al (2018) Nutrient-periphyton interactions along a temperate river and response to phosphorus input reduction. In: Proceedings of the 4th international conference water resources and wetlands. Romanian Limnogeographical Association, Tulcea, pp 10–18
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing, Vienna
Reddy KR, Kadlec RH, Flaig E, Gale PM (1999) Phosphorus retention in streams and wetlands: a review. Crit Rev Environ Sci Technol 29:83–146
Reeder WJ, Quick AM, Farrell TB, Benner SG, Feris KP, Tonina D (2018) Spatial and temporal dynamics of dissolved oxygen concentrations and bioactivity in the hyporheic zone. Water Resour Res 54:2112–2128
Rietra RPJJ, Hiemstra T, Van Riemsdijk WH (2001) Interaction between calcium and phosphate adsorption on goethite. Environ Sci Technol 35:3369–3374
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37:1460–1482
Schlichting A, Leinweber P (2002) Effects of pretreatment on sequentially-extracted phosphorus fractions from peat soils. Commun Soil Sci Plant Anal 33:1617–1627
Sharpley AN, Jarvie HP, Buda A, May L, Spears B, Kleinman P (2013) Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J Environ Qual 42:1308–1326
Stutter MI, Demars BOLL, Langan SJ (2010) River phosphorus cycling: separating biotic and abiotic uptake during short-term changes in sewage effluent loading. Water Res 44:4425–4436
Tang X, Wu M, Dai X, Chai P (2014) Phosphorus storage dynamics and adsorption characteristics for sediment from a drinking water source reservoir and its relation with sediment compositions. Ecol Eng 64:276–284
Taylor AW, Kunishi HM (1971) Phosphate equilibria on stream sediment and soil in a watershed draining an agricultural region. J Agric Food Chem 19:827–831
Turner BL, Newman S, Cheesman AW, Reddy KR (2007) Sample pretreatment and phosphorus speciation in wetland soils. Soil Water Manag Conserv 71:1538–1546
Twinch AJ (1987) Phosphate exchange characteristics of wet and dried sediment samples from a hypertrophic reservoir: implications for the measurements of sediment phosphorus status. Water Res 21:1225–1230
Ugarte MD, Militino AF, Arnholt AT (2015) Probability and statistics with R, 2nd edn. CRC Press, New York
USEPA (1978) Method 365.3. All forms of phosphorus. In: Methods of chemical analysis of water and wastes. U.S. Environmental Protection Agency, Cincinatti
Wang C, Zhang Y, Li H, Morrison RJ (2013) Sequential extraction procedures for the determination of phosphorus forms in sediment. Limnology 14:147–157
Wang S, Jin X, Zhao H, Wu F (2006) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids Surf A Physicochem Eng Asp 273:109–116
Weigelhofer G (2017) The potential of agricultural headwater streams to retain soluble reactive phosphorus. Hydrobiologia 793:149–160
Withers PJA, Jarvie HP (2008) Delivery and cycling of phosphorus in rivers: a review. Sci Total Environ 400:379–395
Worsfold PJ, Gimbert LJ, Mankasingh U et al (2005) Sampling, sample treatment and quality assurance issues for the determination of phosphorus species in natural waters and soils. Talanta 66:273–293
Worsfold PJ, Monbet P, Tappin AD, Fitzsimons MF, Stiles DA, McKelvie ID (2008) Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: a review. Anal Chim Acta 624:37–58
Acknowledgements
We thank Craig Depree and Kerry Costley (NIWA) for the help in sampling; Roger Creswell, Vicky Zhang, Lynne Clucas, and Leanne Hassall for providing some analyses and general aid in laboratory work; Gustavo Boitt for assisting with P fractionation and providing interesting discussions; and the editor-in-chief and an anonymous reviewer for providing keen comments on an earlier version of this manuscript.
Funding
Financial support was from the Our Land and Water National Science Challenge and AgResearch.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Brian Kronvang
Rights and permissions
About this article
Cite this article
Simpson, Z.P., McDowell, R.W. & Condron, L.M. The error in stream sediment phosphorus fractionation and sorption properties effected by drying pretreatments. J Soils Sediments 19, 1587–1597 (2019). https://doi.org/10.1007/s11368-018-2180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2180-3