Abstract
Sediment drying associated with large water level fluctuations is an increasingly common feature of temporary streams and lakes worldwide. Drying-induced sediment aeration and re-flooding periodically alter redox conditions, and therefore stimulate redox-sensitive processes influencing phosphorus (P) binding forms. We experimentally tested the effects of drying on P binding forms, and the P sorption potential, by drying and re-flooding lake sediments in the laboratory. Wet and dried fine sediments were re-flooded in columns, and the overlying water was continuously re-stocked to a constant P concentration. We measured changes in P forms, P uptake rates, and the pore water dynamics in each column over 36 weeks. Drying decreased the fraction of stable P, stimulated the mineralization of organic P, and increased the proportion of labile and reductant-soluble forms. Drying of sediment furthermore reduced its P sorption affinity and capacity by up to 32 % in batch equilibrium experiments, and led to a fourfold increase in sediment compaction which increased P uptake rates by a factor of 1.7 in sediment column experiments. Compaction due to drying also induced the development of a sharp gradient below which P was mobilized. These results indicate that in fine sediments, a single drying event can result in the transformation of P components into more labile forms which accumulate in the uppermost sediment layer, therefore raising the potential for a pulsed P release under reducing conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most aquatic systems experience water level fluctuations, which temporarily expose the previously flooded sediments not only to air but also to desiccation. Such water level fluctuations occur seasonally or as single events in lakes and reservoirs (Wantzen et al. 2008b), and temporary streams are also a common phenomenon (Tockner et al. 2009). Sediment drying can range spatially from minor water level fluctuations affecting only the littoral zone, to large drawdown events which also affect the profundal zone of a lake. Understanding the processes associated with sediment drying is important because the frequency and spatial extent of sediment drying will increase globally, due to changes in climate and land use, as well as increased water withdrawal (Coops et al. 2003; Havens and Steinman 2015).
Drying and re-flooding of sediments can have marked effects on biological, chemical, and physical processes (Lake 2003), especially redox-sensitive processes such as phosphorus (P) dynamics and carbon (C) turnover (Fabre 1988; de Vicente et al. 2010). A significant portion of the sediment P may be bound to iron (Fe) oxides and hydroxides. The binding of Fe to P depends on the following: (i) whether Fe occurs in amorphous or more crystalline structures (the latter have less binding places than the former) (Qiu and McComb 2002), and (ii) whether Fe occurs as ferric Fe(III) or ferrous Fe(II) (the latter has a lower sorption potential than the former); thus, P dynamics is redox-sensitive (Roden and Edmonds 1997). P also occurs in organic P forms, the mineralization of which depends on redox conditions, too (Aller 1994).
Drying and re-flooding of sediments can also affect biologically mediated P dynamics. A significant amount of P can accumulate in microbial cells, and this P may be released when these cells rupture upon extreme drying or rapid re-flooding (Turner and Haygarth 2001), or under anoxic conditions (Reddy et al. 1999).
The effects of droughts on P dynamics are of particular relevance to the trophic state of freshwater ecosystems, which is often controlled by the availability of P to autotrophs in the water column. However, those effects are not yet fully understood, particularly in rivers and lakes (Dahm et al. 2003; Withers and Jarvie 2008). Changes in P availability during drying and re-flooding have been widely studied in forested soils, agricultural sites, floodplains, and wetlands (for example Grierson et al. 1998; Baldwin and Mitchell 2000; Turner and Haygarth 2001; Nguyen and Marschner 2005; Zak and Gelbrecht 2007; Gilbert et al. 2014). However, detailed information on changes in P availability in sediments during desiccation and re-flooding of sediments in rivers and lakes is rare, and mostly focused on short-term P release (Steinman et al. 2014; Tang et al. 2014).
Most studies from rivers and lakes report increased P release following drying and re-flooding, due to bacterial mineralization of organic material (Qiu and McComb 1994; Watts 2000a; Xiao et al. 2012). In contrast, microbially driven P release after re-flooding may be restricted, as especially sulfate-reducing bacteria experience an osmotic and oxic shock (Mitchell and Baldwin 1998). A shortage of sulfide in the oxidized sediments decreases Fe reduction and Fe fixation to sulfide, and can therefore prevent the immediate release of P (Caraco et al. 1989).
Detailed data are either contradictory or absent concerning the underlying processes and changes in the sediment sorption capacities, the sorption affinity for P, and the chemical P binding forms in the sediments. For example, although Baldwin (1996) and Kerr et al. (2010) both reported reduced P sorption affinity in dried sediments due to crystallization of amorphous Fe oxides (mineral aging), their reports were inconsistent regarding the changes in P binding forms. In contrast, Brand-Klibanski et al. (2007) reported increased soil P sorption capacity after soil flooding and subsequent soil drying, due to an increase in the soil content of amorphous Fe oxides.
Re-flooding is often associated with increased concentrations of organic and inorganic nutrients in the water (first flush) that are released from accumulated organic material, such as leaf litter and manure, and from microbial organic matter turnover in soils and sediments (Birch 1960; Watts 2000a, b; Corstanje and Reddy 2004; Qiu et al. 2004; McComb et al. 2007; Brothers et al. 2014). Additionally, P concentrations are often greater from point sources, such as effluents from sewage treatment plants, during periods of water shortage and diffuse sources, such as outputs from intensively managed agricultural fields, increase P concentrations during following first flush events (Withers and Jarvie 2008). In addition to these external P inputs, P is also supplied to the sediments by seston sedimentation, especially in lakes (Hupfer et al. 1995; Kleeberg 2002).
Information is lacking on the potential of dried and re-flooded sediments to retain these P inputs, and conclusions are unreliable regarding the trophic conditions of affected ecosystems. More detailed information would therefore be advantageous to evaluate possible effects on a perennial aquatic system when surface water disappears. In addition, the P uptake capacity of sediments, which represents the potential to remove P from the water column, has often been estimated by the microcosm batch equilibrium technique (Nair et al. 1984). In this technique, the sediment particles move loosely in the water. However, this method disregards sediment bulk density and diffusion pathways within the sediment profile, thus probably overestimating the sorption potential. Furthermore, the stratification of geochemical gradients such as P, Fe, and sulfate concentrations in the pore water cannot be simulated. Comparison of P uptake in experimental sediment columns (mesocosms) would be more precise (Reddy et al. 1999; Young and Ross 2001).
The objective of our study was to investigate the dynamics of P mobilization and uptake in Fe-rich sediments: those exposed to drying and re-flooding with P-enriched water, and undried re-flooded sediments. We compare and evaluate the results of the two approaches: the batch equilibrium technique and sediment column study. We hypothesized that: (i) oxidative processes during drying would significantly increase the fraction of labile P, due to the mineralization of organic P, and (ii) drying would significantly reduce the P uptake potential of the sediments, due to a loss of potential sorption places by mineral aging and shifts in the P binding forms.
Methods and material
Sediment sampling and drying
A total of 17 undisturbed sediment cores (ø 6 cm) were collected in February 2011 with PVC tubes from one single location of the profundal zone of Lake Müggelsee, a shallow polymictic and eutrophic lake southeast of Berlin, Germany (52°26′N, 13°39′E). The lake has an approximate area of 7.3 km2, with a mean depth of 4.9 m. The limnological and sediment properties of the lake were previously described in detail by Driescher et al. (1993) and Kleeberg and Kozerski (1997). Profundal sediment cores were chosen because these sediments had never been exposed to previous drying events.
The diagram in Fig. 1 gives an overview about the processing of the 17 sediment cores and the applied study techniques.
Each of the 17 cores was sliced into three separate layers: upper layer (0–2.5 cm), lower layer (>2.5–12.5 cm), and base layer (>12.5 cm). Every layer of each core was sieved (<2 mm), and then for each of the three layers, all 17 samples were pooled into three plastic containers. Subsamples of the upper and lower layers were air-dried on trays under laboratory conditions (20 °C) for 4 weeks (termed “dried” hereafter) to reach 3 % residual gravimetric moisture, a moisture level observed in situ in sediments of temporary waters (Kerr et al. 2010). Meanwhile, water saturated un-dried sediments, (termed “original wet” hereafter) of each of the three layers were kept in closed dark containers at 4 °C.
Batch equilibrium experiment
To determine changes in sediment P sorption affinity and sorption capacity due to sediment drying (Reddy et al. 1999), three sorption models were fitted to the sorption curves. We applied the commonly used linear, Langmuir, and Freundlich sorption isotherms to ensure comparability with other studies. Sorption curves (i.e., sorbed P vs. equilibrium P concentrations) were determined in a standardized method under aerobic conditions (batch equilibrium experiment—BEE) at 20 °C, following Nair et al. (1984). Twelve 0.5 g dry mass (DM) aliquots of dried and original wet sediments, from both upper and lower layers, were shaken in centrifuge plastic tubes for 3 h in 20 mL of a KH2PO4 solution, with 1 of 12 P concentrations: 0, 0.5, 1.0, 2.5, 5.0, 10, 25, 50, 100, 200, 250, and 500 mg L−1 (Kleeberg et al. 2010). The slurry was centrifuged (10,000 × g), and the supernatant was passed through 0.45 μm cellulose acetate syringe filters (Whatman) prior to the determination of equilibrium soluble reactive P (SRP). The SRP concentrations were determined with a spectrophotometer (San++ CFA, Skalar, Breda, Netherlands) following the molybdate blue method (Murphy and Riley 1962).
The SRP concentrations in the pore water of the wet sediment, prior to the addition of a P solution, were determined by the same procedure of sediment centrifugation and photometric detection. For P sorption calculations, these values were included in the initial P concentration in the solution (Kleeberg et al. 2010). However, the results were not different whether pore water P was included or excluded, but were routinely included.
The concentration of sorbed P (q) was calculated from the difference between the initial and equilibrium P concentrations (c) in the solution: q = V solution × (c initial − c equilibrium) / m sediment. The P sorption capacity was determined as q max from the Langmuir model, and the P sorption affinity was defined by the sorption energy (model coefficient k in all models) and EPC0 (concentration at zero net sorption, q = 0 in the linear and Langmuir models). To properly calculate q max with the Langmuir isotherm, it is typical to choose a wide range of P concentrations, including unnaturally high concentrations. However, such high P concentrations may lead to P precipitation in the batch tube, which artificially increases P uptake potential, and therefore overestimates the calculated q max. The P sorption potential is therefore more accurately estimated using a range of low concentrations, and fitting a linear isotherm, with linear coefficient k and EPC0 being the important estimates (Brand-Klibanski et al. 2007). The range of concentrations was therefore curtailed for proper fitting of isotherms.
Sediment column experiment
To investigate P uptake under stratified conditions over long time spans, we created eight artificially stratified sediment cores by re-packing the three pooled sediment layers into PVC tubes (ø 6 cm). These tubes allowed for undisturbed P diffusion in the pore water and long-term transformation and exchange processes (Reddy et al. 1999).
A total of eight PVC tubes were first loaded with a 15-cm depth of the wet base layer, to allow upward microbial re-colonization into the dried sediments above. The tubes were then stocked with the upper and lower layers, maintaining the original sequence with the upper layer on top: four tubes were stocked with dried sediment layers, and four tubes were stocked with original wet sediment layers. The sediment dry mass of the dried upper and lower layers was equal to the dry mass of the wet sediments of the upper and lower layers (determined at 105 °C for 8 h). The upper layer was 2.5 cm thick in the wet sediments and 0.5 cm thick in the dried sediments. The lower layer was 10 cm thick in the wet sediments and 2.5 cm thick in the dried sediments.
Each of the eight artificial sediment cores in the PVC tubes was slowly flooded from above with tap water (<50 μg SRP L−1), avoiding sediment surface disturbance and allowing the air enclosed in the dried sediments to escape. A water column of 25 cm above the sediments was finally obtained.
The PVC tubes were put into large racks and placed in a climate chamber at 10 °C (annual mean temperature of the surface water in Lake Müggelsee). The overlying water was constantly aerated using flexible tubes, to maintain a constant oxygen level (saturation) throughout the water column. After 5 days of adaption, KH2PO4 stock solution was added to the water for the first time to obtain a P concentration of 2 mg L−1, exceeding the P concentration of the original pore water (0.9 mg L−1 in the upper layer and 1.4 mg L−1 in the lower layer, determined as total dissolved P after centrifugation).
Over a 36-week period, sediment P uptake rates were determined by measuring the SRP concentrations in samples from the overlying water. After each sampling, abstracted water volume was replaced (again with tap water which had in contrast to lake water constant conditions during the period of 36 weeks) and P concentrations were returned to 2 mg L−1 using the P stock solution. Measurement frequency ranged from twice a week (weeks 0–5) to biweekly (weeks 30–36) (Fig. 3). Water pH and specific conductivity were measured in the overlying water every 3 weeks with dipping probes (WTW, Weilheim, Germany).
To determine the dynamics of P, Fe2+, and sulfate (SO4 2−) concentrations in the pore water, two tubes with wet sediments, and two tubes with dried sediments, had been equipped with rhizon pore water samplers (Eijkelkamp, Giesbeek, Netherlands). The pore water was sampled on five dates, using 1 mL syringes and transferred into safe lock microtubes (Eppendorf, Germany), and analyzed for the following: (i) SRP and Fe2+ with a spectrophotometer (Sunrise, Tecan, Männedorf, Switzerland) using microtiter plates (Laskov et al. 2007), and (ii) SO4 2− with ion chromatography (LC-10A, Shimadzu, Kyoto, Japan). The samples determined for Fe2+ analysis were in a separate set of vials, to which diluted (1:50) sulfuric acid was immediately added (1:12), and vials were immediately closed in order to prevent Fe precipitation and oxidation.
The SO4 2− reduction rates and Fe2+ mobilization/consumption rates were calculated by integrating concentrations across pore water depth, and so the differences of concentrations between pore water sampling dates.
At the end of the 36-week experiment, sediments from the four columns without rhizon pore water samplers were sliced into layers at 1.0-cm increments, using a sediment core cutter, by pushing wet sediment cores through the PVC tube. For each increment, the samples of two columns were combined with either previously dried sediments or original wet sediments.
Sequential phosphorus fractionation
To determine changes in P binding fractions due to drying and P uptake, we performed P fractionation by a sequential extraction procedure, following Psenner et al. (1984), with slight modifications as described in Hupfer et al. (1995). This P fractionation was performed on (i) original wet and laboratory dried sediments of the upper and lower layers, and (ii) the samples from the sliced sediment columns at the completion of the sediment column experiment.
The five extractants, used in sequence, were solutions of ammonium chloride (NH4Cl), bicarbonate/dithionite (BD), sodium hydroxide (NaOH), hydrochloric acid (HCl), and persulfate digestion (K2S2O8). In the extracts, SRP and total P (TP) (after 5 % K2S2O8 digestion at 120 °C) were measured with a spectrophotometer (San++ CFA, Skalar, Breda, Netherlands). The main fraction of organic P was determined as non-reactive P (NRP) in the NaOH extracts and calculated as the difference between TP and SRP. The dissolved Fe2+ and Mn2+ concentrations in the BD extract were measured with atom absorption spectrophotometry.
For each sampled sediment column increment, the changes of P, Fe2+, and Mn2+ concentrations were calculated using the reference data from the corresponding layer of the original wet or laboratory dried sample. The P concentrations of extracted fractions given on volumetric basis were calculated using the bulk density of the corresponding wet or dried layer.
The TP concentrations in the sediments were determined by (i) direct photometric measurements after H2SO4/H2O2 digestion at 160 °C (Kleeberg et al. 2010), and (ii) direct measurements after microwave-assisted aqua regia dissolution and detection with an ICP-OE spectrometer (iCAP 6000, Thermo Scientific, USA). The TP concentrations, which were calculated as the sum of P fractions and by H2SO4/H2O2 digestion, accounted for ≥83 and ≥94 % of TP by ICP-OES detection, respectively.
In addition to P, five other elements (Al, Fe, Ca, Mn, and Mg) were also simultaneously extracted from the original sediment by aqua regia dissolution and measured by ICP-OES detection.
Further subsamples of sediment were used for determination of loss on ignition (450 °C) and detection of total C and N concentrations with a C/N elemental analyzer (Elementar vario EL, Hanau, Germany).
Statistical analyses
The statistical analyses were performed using R software (version 2.14.2, Ihaka and Gentleman 1996) and Office Excel software (Microsoft, 2010) with the significance level set to α = 0.05. For reasons of clarity and comprehensibility, applied tests are given together with the results.
Results
Sediment properties
The sediment was rich in C, Ca, and Fe. It contained about 16.5 % total C and 1.5 % total N, and was characterized by the atomic ratios of Fe/S/P = 10:4:1 and H/C/N/S = 43:36:3:1 (Table 1). The sediment contained, per gram DM, the following: 130.2 mg Ca, 2.3 mg Mg, 64.0 mg Fe, 1.5 mg Mn, and 11.1 mg Al. The loss on ignition accounted for 25 % of the total dry mass.
During the sediment column experiment, a small proportion of organic C and N compounds were mineralized in both layers of the dried sediments, and in the upper layer of the wet sediments, as indicated by the slight decrease in %C and %N. Proportionally more N than C was mineralized, which led to a slight increase in the C/N ratio in these zones (Table 1).
Sediment drying
During drying in the laboratory, the sediments cracked and compacted. For the upper layer, this compaction was to 20 % of the former thickness (from 0.08 to 0.38 g DM cm−3), and for the lower layer, this was to 25 % of the former thickness (from 0.10 to 0.40 g DM cm−3). The compaction was irreversible over the course of the whole sediment column experiment (36 weeks) because the sediments did not re-expand during re-flooding.
Drying also led to a shift in the proportion of P fractions in both layers (Table 2). The amounts of directly available P (NH4Cl-P) and reductant-soluble P (BD-P) increased, while more stable Fe and Al oxide-bound P (NaOH-SRP) and organic P (NaOH-NRP) decreased. The increase in NH4Cl-P was an average of four times greater than was the amount of P taken up from the evaporating pore water (~11 μg P g−1 DM) during experimental sediment drying.
Sediment drying also increased the concentrations of reductant-soluble Fe (BD-Fe) (upper layer: from 8.1 ± 0.2 to 10.3 ± 0.1 mg Fe g−1 DM; lower layer: from 3.7 ± 0.1 to 7.9 ± 0.2 mg Fe g−1 DM) and reductant-soluble Mn (BD-Mn) (upper layer: from 0.33 ± 0.11 to 0.68 ± <0.01 mg Mn g−1 DM; lower layer: from 0.26 ± <0.01 to 0.64 ± 0.03 mg Mn g−1 DM). The molar ratio of Fe/P in the BD extract thereby increased from 2.5 to 2.8 in the upper layer, and from 1.8 to 3.2 in the lower layer.
The two more stable P fractions (HCl-P and residual P) remained unaffected by drying, and the TP yield (sum of all P fractions) in dried sediments was not significantly different from that in wet sediments.
Batch equilibrium experiment
The range of low P concentrations in the present study (c <10 mg L−1 or q <400 μg g−1) could be described by a linear model, but could not be properly included in Freundlich and Langmuir models (Fig. 2). Models were therefore curtailed to ensure prediction accuracy. The linear sorption equation was applied only for P sorption values q <400 μg g−1 DM, for the Freundlich model q >500 μg g−1 DM were selected, and the Langmuir equation was defined only for q ≥0 μg g−1 DM (Table 3) (Barrow 1978, 2008; Reddy et al. 1999).
Measured and modeled isotherms of equilibrium P concentration vs. change in sorbed P concentration of two layers of sediments, 0–2.5 and 2.5–12.5 cm, from Lake Müggelsee. Isotherms for original wet and laboratory dried samples determined with a batch equilibrium experiments. a, b Entire range of P concentrations; c, d low concentrations. Diagrams include equations for the Freundlich isotherms (for q >0 μg P g−1 DM). Details of linear, Langmuir, and curtailed Freundlich equations are given in Table 3
Our BEE demonstrated that drying decreased the P sorption potential. Equilibrium P concentrations at zero net sorption in the upper and lower layers were six times higher in dried than in wet sediments for the upper layer and three times higher in dried than in wet sediments for the lower layer (Table 3, EPC0). Dried sediments also exhibited a slower increase in sorbed P with rising P concentrations than did wet sediments (Table 3, k in all models). Both observations, higher EPC0 and lower k values, indicated a reduced sorption affinity of dried sediments compared to wet sediments. The maximum P sorption capacity, predicted by the Langmuir model, showed sorption capacities reduced by 15 and 32 % for dried sediments in the upper and lower layers, respectively (Table 3, q max).
Sediment column experiment
During the 5-day adaption time prior to P addition to the water columns, the four wet sediments already took up 0.2 ± 1.5 mg P m−2 on average, while the four dried sediment columns showed a first flush P release of 6.7 ± 5.6 mg m−2. The re-flooding of sediment columns, and P-enrichment of the water, induced initial P uptake rates which were 1.7 times faster in previously dried sediments (55 ± 8 mg m−2 day−1) than in wet sediments (31 ± 6 mg m−2 day−1) (Fig. 3). The P uptake rates differed significantly between approaches (ANCOVA on log10 transformed data, with date as covariate: F = 38.34, P < 0.001) and decreased more strongly and steadily in dried sediment columns than in wet sediment columns. In the wet sediment columns, the P uptake rates initially decreased, and then increased again after 16 weeks, and exceeded the rates in the dried sediment after 20 weeks (17 ± 6 vs. 7 ± 1 mg m−2 day−1 by the end of the experiment at 36 weeks). Replicates of previously dried sediments were very similar to each other, whereas variation of the wet sediments was greater than found in the dried treatment (deviation bars, Fig. 3).
Temporal changes in rate of phosphorus (P) uptake in sediment columns that were re-flooded with P-enriched water (constantly re-stocked to 2 mg L−1) over 36 weeks after having been dried or not dried (wet). Data are means ± sd, n = 4; four columns contained original wet sediments, and four columns contained previously dried sediments
The total quantity of P uptake during 36 weeks did not significantly differ between previously dried sediments (4.1 ± 0.2 g m−2) and wet sediments (3.4 ± 0.8 g m−2). The pH of the overlying water during P addition was higher for the previously dried sediments (8.6 ± 0.1, n = 48) than for the wet sediments (8.3 ± 0.2, n = 48) (t = 8.54, df = 88, P < 0.001). In addition, the specific conductivity was higher for the previously dried sediments (1096 ± 211 μS cm−1, n = 40) than for the wet sediments (665 ± 102 μS cm−1, n = 40) (t = 11.67, df = 56, P < 0.001).
Sequential phosphorus fractionation
Considering that the sediment compacted during drying, the absolute amounts of all six P fractions by cubic centimeter volume (i.e., P concentrations in μg cm−2 per 0.5 cm depth) at different depths in wet and dried sediments are shown in Fig. 4. There was a high accumulation of reductant-soluble P (BD-P) within 0–1 cm in previously dried sediments due to compaction and a high uptake of P (dried, 1.97 mg cm−3 vs. wet, 0.33 mg cm−3 within 0–1 cm).
Phosphorous (P) fractions determined by sequential extraction of lake sediments that were re-flooded with P-enriched water (constantly re-stocked to 2 mg L−1) over 36 weeks after having been dried or not dried (wet). BD, bicarbonate/dithionite; NRP, non-reactive P; SRP, soluble reactive P; TP, total P. Dashed lines indicate the boundaries between the former upper and lower layers (0–2.5, >2.5–12.5 cm) and the wet base material (>12.5 cm) that were originally sampled from the lake before homogenization, drying, and refilling of sediment columns. Only the dried sediment columns were sampled down to the base layer. Values are given on a volumetric base calculated as mass of P per surface area within 0.5 cm increments of sediment depth. Values are means of laboratory duplicates obtained from pooled sediment samples
To focus on the changes in P forms during 36 weeks, we compared the concentration of P fractions in sliced sediment cores after the addition of P to previously dried or wet sediments to the original material from the upper and lower layers (Fig. 5a–c). To isolate the effects of P addition only, from the combined effects of P addition and sediment drying, on the various P fractions, we calculated changes in the P fractions of dried sediments in two ways: (i) as the difference from the original wet sediments (combined effects) (Fig. 5b), and (ii) as the difference from the original dried sediments that were not yet re-flooded (P addition effect) (Fig. 5c). A comparison of these calculations revealed that in the upper 2 cm, the changes of combined effects were additive, meaning that P was moved from NaOH fractions to more labile fractions during sediment drying (see also Table 2) and also during the subsequent column incubation with P addition to the columns (stronger decrease of NaOH-P in Fig. 5b than in Fig. 5c). In contrast, in the layers below 2 cm, shifts in P binding fractions were partly compensatory, which means that P in the NaOH-SRP fraction was lost during drying, and regained during P addition (stronger increase in Fig. 5c than in Fig. 5b). In contrast, the BD fraction increased during drying and decreased during P addition (stronger decrease in Fig. 5c than in Fig. 5b). Both methods of calculation revealed the same implications: above 2 cm depth, the NH4Cl-P and DB-P fraction increased, whereas below 2 cm the NaOH-SRP fraction increased, and each of the other fractions decreased. Therefore, the following results from dried sediment columns refer only to the difference from the original dried sediments (Fig. 5c).
Changes in phosphorus (P) fractions with depth determined by sequential extraction of lake sediments that were re-flooded with P-enriched water (constantly restocked to 2 mg L−1) for 36 weeks after having been dried or not dried (wet): a, b changes referring to the original wet sediment material; c changes referring to dry sediment, isolating the effect of P addition after re-flooding. Negative values indicate a loss of P, while positive values indicate an increase of P in a specific fraction. BD, bicarbonate/dithionite; DM, dry mass of sediment; NRP, non-reactive P; SRP, soluble reactive P; TP, total P. The dashed lines indicate the boundaries between the former upper and lower layers (0–2.5, 2.5–12.5 cm) and the wet base material (>12.5 cm) that were originally sampled from the lake before homogenization, drying, and refilling of sediment columns. Values are means of laboratory duplicates obtained from pooled sediment samples. Figure excludes fractions with negligible changes (HCl-P, residual-P)
For both wet and dried sediments approaches, the added P was mainly taken up in the BD fraction (mainly from amorphous and redox-sensitive Fe and Mn oxides, P uptake up to 1798 and 2480 μg g−1 in wet and dried sediments, respectively (Fig. 5a, c)), while P was lost from NaOH-SRP (up to −354 and −315 μg g−1 in wet and dried sediments, respectively (Fig. 5a, c). The concentrations of NH4Cl-P increased slightly in both approaches (up to 68 μg g−1 in previously dried sediments).
In wet sediments, the net P uptake increased with depth down to 4.5 cm due to changes in P fractions; below this depth, the changes in P fractions were negligible. In contrast, in previously dried sediments, net P uptake was observed to a depth of only 2 cm, with a maximum uptake zone between 0.5 and 1 cm, where P uptake was greater than in the wet sediments. Below 2 cm, the previously dried sediments experienced a net P loss: while NaOH-SRP increased (up to 550 μg g−1), P was lost from BD fraction (up to −423 μg g−1) and from organic compounds (NaOH-NRP) (up to −244 μg g−1). Previously dried sediments lost P from organic compounds (NaOH-NRP) across all depths, whereas wet sediments gained organic P across all depths (up to 159 μg g−1).
In both approaches, the total increase in P content in the layers analyzed by fractionation was greater than the total amount of P removed from the water column (wet, 4.8 vs. 3.4 g m−2; dried, 6.9 vs. 4.1 g m−2).
Sediment drying increased the concentrations of BD-Fe (upper layer, 8.1 ± 0.2 to 10.3 ± 0.1 mg Fe g−1 DM; lower layer, 3.7 ± 0.1 to 7.9 ± 0.2 mg Fe g−1 DM) and BD-Mn (upper layer, 0.33 ± 0.11 to 0.68 ± <0.01 mg Mn g−1 DM; lower layer, 0.26 ± <0.01 to 0.64 ± 0.03 mg Mn g−1 DM). After re-flooding and P uptake, the wet sediments showed a greater increase in BD-Fe and BD-Mn than did the dried sediments (Fig. 6). In the wet sediment, the BD-Fe concentrations increased down to a depth of 6 cm and also expressed a relatively high Fe/P ratio (a mean of 2.6) in the BD fraction. The BD-Fe accounted for 9–18 % of the total Fe content of the wet sediment. In dried sediments, the BD-Fe concentrations decreased rapidly below 1 cm depth (the former upper layer), and therefore had relatively low Fe/P ratios (a mean of 1.6), approaching saturation of Fe oxides with P. The BD-Fe therefore rapidly declined from 16 % down to 4 % of total Fe.
Changes in the concentrations of Fe2+ and Mn2+, determined from a bicarbonate/dithionite extract during sequential fractionation, of lake sediments that were re-flooded in sediment columns with P-enriched water (constantly restocked to 2 mg L−1) for 36 weeks, after having been dried or not dried (wet). Gray shaded areas indicate changes in concentrations of Fe2+ (left) and Mn2+ (right) in wet (dark gray) and dried (light gray) sediments. The dashed line indicates the molar Fe/P ratio in the BD fraction at the end of the experiment
In the wet sediments, the concentration of HCl-extractable Fe decreased below 1 cm depth by an average of 3.3 ± 1.7 mg Fe g−1 DM. In contrast, in dried sediments, the concentration of HCl-Fe increased by an average of 5.4 ± 1.1 mg Fe g−1 DM. In both approaches, changes in BD-Fe were negatively correlated with changes in Fe extracted with HCl (r = −0.90, t = −4.56, df = 5, P < 0.01), especially for layers below a depth of 1 cm, indicating a shift between these Fe fractions.
In both approaches, reductant-soluble Mn (BD-Mn) declined abruptly below 1 cm (Fig. 6), a pattern consistent with total Fe and Mn concentrations (Table 1). In wet sediments, losses of HCl-extractable Mn were <0.2 mg Mn g−1 DM, contrasting with the situation in dry sediments in which there was an increase of 0.2 ± 0.1 mg Mn g−1 DM.
Pore water dynamics
The pore water P concentrations increased up to a maximum of 7 mg L−1 in the deepest sampled layers of the wet sediments (about 10 cm) by the end of P addition at week 36 (Fig. 7). In the upper layers of previously dried sediments, the concentration of pore water P reached a maximum concentration at a depth of 2 cm and was greater than that in the corresponding layers of wet sediments. In contrast, in wet sediments, concentrations of pore water P steadily increased with depth, reaching maximum concentration at approximately 10 cm.
Vertical profiles for concentrations of soluble reactive phosphate (SRP), ferrous iron, and sulfate in the pore water of sediment columns over the course of 36 weeks of P addition to the overlying water column of previously dried (open circles, two replicate columns) and wet (full circles, two replicates) lake sediments
In wet sediments, the Fe2+ concentrations were initially negligible down to a depth of 3 cm, then increased temporarily, but finally declined again towards the end of the experiment. In contrast, in previously dried sediments, the Fe2+ concentrations rapidly increased at depths of 0–4 cm, peaking at 2 cm depth with a maximum concentration of 50 mg L−1 at week 3. Concentrations decreased thereafter, approaching concentrations in the wet sediments.
In wet sediments, the SO4 2− concentrations in the pore water decreased steadily during the experiment, providing no relevant differences between different depths. In contrast, in previously dried sediments, there was an initial increase in SO4 2− concentrations of up to 1423 mg L−1 at a depth of 2 cm (this accounted for about 16.5 % of the total S being in oxidized form). After a lag time of approximately 3 weeks, these concentrations declined rapidly being similar to concentrations in wet sediments by the end of the experiment. In the same 2 cm, SO4 2− concentrations decreased to a minimum at week 8.
Maximum rates of SO4 2− reduction and Fe2+ mobilization/consumption were observed for the previously dried sediments, and for the interval between weeks 3 and 8, reaching 798 mg m−2 day−1 for SO4 2− and 15 mg m−2 day−1 for Fe2+. The development of these rates from one sampling to the next sampling was positively correlated (r = 0.88, t = 5.74, df = 10, P < 0.001), suggesting that the redox potential was low enough to cause: (i) Fe(III) oxides to become reduced, leading to Fe2+ production, and (ii) SO4 2− to become reduced, leading to S2− production, which precipitated the dissolved Fe2+, thus removing it from the pore water.
Discussion
Sediment drying
The drying of previously non-dried lake sediments caused an irreversible compaction and a shift in the proportions of the P binding fractions (Table 2). The amounts of reductant-soluble P (BD-P) and directly available P (NH4Cl-P) increased due to losses from Fe and Al oxide-bound P (NaOH-SRP), and from the mineralization of organic P (NaOH-NRP). This shift in composition of P forms in the sediments from Lake Müeggelsee was in accordance with the results from previous studies performed on dried river sediments (Kerr et al. 2010), reservoir sediments (Baldwin 1996), and marshland soils (de Groot and Fabre 1993).
The increase in reductant-soluble P was likely due to the oxidation of Fe(II) and the formation of Fe(III) oxyhydroxides, which preferentially bind P (Fox 1989). The Fe(III) would be reduced to Fe(II) again during BD extraction (Psenner et al. 1984) releasing the bound P. The increase in directly available P could not be explained by the uptake of P from evaporating pore water alone. The increase was most likely of microbial origin, possibly released from lysed and disrupted cells under the stress of desiccation and the osmotic shock of rapid re-flooding (Birch 1960; Baldwin and Mitchell 2000; Turner et al. 2003). Mineralization of organic P was likely to be facilitated by the aeration of previously anoxic sediments (Qiu and McComb 1994) and was associated with a decrease in the C and N content of the dried sediments (Table 1).
Phosphorus sorption affinity and capacity
Drying caused a decrease in the P sorption affinity and capacity of sediments (Table 3), as has been observed in earlier studies (Baldwin 1996; Watts 2000a; de Vicente et al. 2010; Xiao et al. 2012), with sorption parameters very similar to the results of Kerr et al. (2010). We, too, observed a sixfold increase in EPC0 for laboratory dried sediments, exceeding the results of Twinch (1987), where only a threefold increase of EPC0 was observed. A threefold increase of EPC0 was however reported by Kerr et al. (2010) for in situ dried sediments.
The decrease in P affinity and capacity may have been due to numerous causes.
-
(i)
The formation of sediment aggregates during compaction may have occluded potential sorption places in dried sediments.
-
(ii)
The sediments contained a large fraction of C, which might have formed coatings on the mineral grains. Drying can change the molecular structure of these C compounds, affecting the surface associations of C on the mineral grains and complexations, which may have influenced P sorption capacity or affinity. Significant amounts of P may have been released from C metal-bridged P complexations due to drying and re-flooding increasing the EPC0.
-
(iii)
The mineralization of organic material during drying, and cell lysis and disruption of microbes during extreme drying, could also have released P upon re-flooding, increasing the EPC0 (Turner and Haygarth 2001; Xiao et al. 2012).
-
(iv)
Death of microbial cells during drying and disruption might also have restricted microbial P uptake compared to that in the wet sediments. Kamp-Nielsen (1974) observed reduced P uptake from sterilized sediments and Watts (2000a) observed a reduced P release from un-sterilized sediments. Our BEE was performed without fumigation or sediment sterilization, so that biological P uptake was enabled in addition to chemical P sorption. This may therefore explain the greater P sorption potential in our wet samples compared to our dried samples.
-
(v)
The strong drying may have facilitated the formation of a crystalline structure of amorphous Fe hydroxides, in a process known as mineral aging. This process involves the loss of potential sorption places for P, thereby reducing sorption capacity (Lijklema 1980; Baldwin et al. 2000; Qiu and McComb 2002; de Vicente et al. 2010).
-
(vi)
Finally, the sorption affinity in wet sediments might have been overestimated, due to aeration of anoxic wet sediments during shaking in the batch solution that was not deoxygenized. Traces of oxygen in the batch tube may have led to the oxidation of Fe(II) to amorphous Fe(III) oxides, increasing the amount of P sorption places and enhancing P binding. Brand-Klibanski et al. (2007) described this as an artifact that causes decreased EPC0 values for reduced soils, when the batch solution is not under anaerobic (N2) conditions. We did not use a N2 environment in the batch experiment to ensure identical conditions for both approaches, and we therefore might have created an artifact that led to underestimation of the EPC0 for wet samples that were originally anoxic. However, the fully oxidized dried sediments in our study still had a much higher EPC0 than the wet sediments that potentially oxidized during shaking. Brand-Klibanski et al. (2007) also reported that P capacity and k values were unaffected by the artifact.
The EPC0 value for the dried upper sediment layer even exceeded the long-term mean TP concentrations in the water of Lake Müggelsee during summer (but not TP concentration in the pore water). This implies that the dried sediments of Lake Müggelsee may act as a major P source if drying and re-flooding occurred.
Phosphorus uptake in sediment columns
The sediment column experiment partly confirmed, and partly contradicted, the results of the BEE. The results of the BEE were confirmed only during the final third of the sediment column experiment, when wet sediments had higher uptake rates than previously dried sediments (Fig. 2). During the first two thirds of the sediment column experiment, dried sediments had higher uptake rates.
Initially, as with previously dried sediments, the uptake rates in wet sediments decreased due to the rising saturation of P, but then the uptake rates in wet sediments eventually increased. An active population of microbiota may have contributed to biological P uptake processes in the sediments, as explained for the BEE. This suggestion is supported by the observed increase in organic P fractions in wet sediments, whereas organic P was lost in dried sediments. To corroborate this, phospho-lipid fatty acid analysis was performed on residual sediment samples, revealing that the bacterial biomass was about 40 % lower in previously dried sediments than in wet sediments, even after 8 months of re-flooding (suppl. Fig. ESM 1). This was somewhat surprising because the non-dried base layer should have facilitated the microbial re-colonization of dried sediments. The structure of bacterial groups, however, did not differ between the two approaches, suggesting that the single drying treatment did not select for specialized groups of bacteria within the time span of our experiment, but was reported by Baldwin and Mitchell (2000).
At the beginning of the sediment column experiment, P uptake rates in columns with previously dried sediments were almost double those in wet sediment columns, contrasting with the results of the BEE. These higher rates may be an effect of sediment compaction during drying. During the BEE, wet and dried sediment samples moved loosely through the liquid in shaken centrifuge tubes; thus, the original bulk density was irrelevant to the P uptake potential. In contrast, the higher density of dried sediments in the columns (compared to wet sediments) provided a higher density of potential sorption places within shorter distances (Reddy et al. 1999), which superimposed the effect of reduced sorption affinity as determined by BEE. This proposed mechanism was supported by the findings that P uptake in previously dried sediments was restricted to the upper 2 cm, and was much greater there than in the wet sediments, where P also diffused into deeper layers. This may have led to higher uptake rates in previously dried sediments compared to wet sediments, despite lower sorption affinity and lower sorption capacity in dried sediments.
These findings imply that results from nutrient sorption experiments involving the shaking of fine sediment must be interpreted carefully, taking into consideration in situ bulk density when accounting for the effects of drying and re-flooding. In other words, uptake rates cannot always be directly deduced from sorption affinity and capacity, as determined by a BEE.
Below the primary P uptake zone at 2 cm, shifts in P fractions appeared to be reversed, indicating a border between oxidative and reductant processes and products. We therefore suggest the development of a sharp redox gradient in previously dried sediments, while in wet sediments the conditions changed more gradually. In previously dried sediments, reductant-soluble Fe and Mn decreased abruptly below 1 cm (Fig. 6). Within the top 1 cm, more Fe and Mn were in oxidized forms than in reduced forms due to drying and water column aeration, and could therefore be reduced and extracted with BD. Below 1 cm, anoxic conditions facilitated the reduction of Fe and Mn; thus, there were fewer oxidized and reductant-soluble Fe and Mn forms. The reductant-soluble Mn increased in the uppermost sediment layer and then abruptly declined. A comparable decrease in total Mn concentrations was not observed from the surface to 6.5 cm (Table 1), but it is possible that Mn was mobilized in even deeper layers, diffused upwards, and was finally precipitated at the oxygenated surface layer. In contrast, not only reductant-soluble Mn but also reductant-soluble Fe increased in wet sediments, even after re-flooding, to a maximum depth of 6 cm, and both may have been sequestered from the HCl fraction.
The results from pore water analysis in wet sediments also suggested that redox conditions did not foster Fe reduction, and a sharp gradient may not have developed. The aeration of water columns in the laboratory, and higher diffusion rates due to lower bulk densities, may have supported higher redox levels in the wet sediment columns compared to the original sediments in the lake or dried sediment columns. The supposed higher redox levels are supported by the fact that almost no P was mobilized from the BD fraction in wet sediments, while it was lost in the anoxic layers of previously dried sediments (Fig. 5a, c). There, this P fraction was fixed in more stable metal oxide compounds that were less sensitive to anoxic conditions, as indicated by the increase in NaOH-SRP (Fig. 5c). In addition, much more P was mobilized from previously dried sediments than from wet sediments, as indicated by the increasing P concentrations in the pore water (Fig. 7). Remarkably, the pore water P concentrations below 2 cm exceeded the water column P concentration of 2 mg L−1 after only 3 weeks of re-flooding in both approaches. These high P concentrations would have restricted diffusive P transport to deeper layers (>2 cm).
Despite the high pore water P concentrations, in the wet sediments, P uptake was at its maximum between 2.5 and 4.5 cm. The P was unlikely to be taken up prior to the increase in pore water P because the amount of P taken up in this depth far exceeded the amount of P removed from the water column within the first 3 weeks. A high P uptake in this layer was more likely to be due to the corresponding increase in reductant-soluble Fe within the same layer (Fig. 6). The additional P must have been mobilized in deeper layers, then diffused upwards, and was sorbed to Fe compounds, as indicated by the increase in reductant-soluble P (Fig. 5) and the slope of the pore water P profile (Fig. 7). This is in accordance with the surplus of P uptake detected by sequential fractionation compared to the total amount of P removed from the water column. Future studies might test this by not including a wet base layer.
The temporal and spatial development of P, Fe2+, and SO4 2− concentrations in the pore water also demonstrated the change between aerobic and anaerobic processes (Fig. 7). During sediment preparation (sieving and mixing), and especially during sediment drying, oxidative processes resulted in the formation of Fe(III) and SO4 2−. Hence, high amounts of SO4 2− were released into the pore water and overlying water after re-flooding, which explained the increase in conductivity in columns with dried sediments (Baldwin et al. 2000). With the onset of oxygen depletion after re-flooding, Fe(III) was reduced again, as indicated by the increase of Fe2+ in the pore water.
With the onset of SO4 2− respiration at further decreasing redox levels, sulfate and Fe2+ concentrations decreased, likely due to the formation of S2−, precipitating Fe2+ (FeS x ) (Baldwin et al. 2000). The SO4 2− respiration indicated one of two unexpected events: (i) obligate anaerobic bacteria were not killed or suppressed by the desiccation (Mitchell and Baldwin 1998; Schönbrunner et al. 2012) or (ii) they quickly re-colonized from the non-dried base layer.
Pore water concentrations were highly dynamic at a depth of 2 cm where Fe2+ concentrations temporarily peaked, and SO4 2− was rapidly depleted (Fig. 7). However, the SO4 2− reduction rates for the sediments from Lake Müggelsee were within the range of other lakes (Holmer and Storkholm 2001). The high SO4 2− consumption may be due to previous sediment drying stimulating aerobic metabolism, resulting in a high oxygen demand upon re-flooding (Schönbrunner et al. 2012). This led to a rapid oxygen depletion at depth of 1–2 cm, while the layers above received oxygen from the aerated supernatant water. The Fe(III) was rapidly reduced at the same depth as which a lot of P was taken up in the BD fraction. This resulted in low Fe/P ratios in the BD extracts approximating saturation of Fe with P.
In anoxic layers below 2 cm of the previously dried sediments, we observed a net loss of TP from the sediments, raising pore water P concentrations. The suspected fixation of Fe in sulfides mobilized P and decoupled the Fe-P-cycle in dried sediments. The mobilized P most likely diffused upwards, and was retained at the lower edge of the assumed redox gradient, as indicated by high TP concentrations 1–2 cm immediately below the assumed redox gradient, and below the peak depth of TP increase at 0.5–1 cm (Fig. 5). The increase in TP concentrations in upper layers, with simultaneous TP decrease in deeper layers, was also described by de Groot and van Wijck (1993), who also attributed this to an upward flux of P.
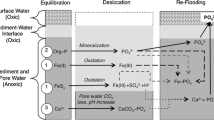
Conceptual model
To summarize the results from our experiments and their interpretation, Fig. 8 shows a simplified conceptual model of the processes of P mobilization and uptake in sediments that were re-flooded with P enriched water. The conceptual model compares our main findings between sediments that had been dried or not dried (wet) prior to the re-flooding. It highlights the relative composition and changes of the most important P binding forms (stacked bars), and the most pronounced processes in the Fe-S-P cycling. This includes the irreversible sediment compaction and shift of organic P and metal oxide-bound P to reductant soluble iron-bound P due to sediment drying. After re-flooding, the added P was taken up in reductant soluble P in both treatments. It was also taken up in organic forms in the wet sediments, while the organic P content decreased in the dried sediments. Wet sediments had a lower concentration of (reductant soluble) P per sediment mass than the dried sediments. This was compensated by the fact that the P uptake layer in wet sediments was thicker than in dried sediments. Wet sediments developed a smooth gradient of redox relevant processes while dried sediments supposedly developed a sharp redox gradient. The aeration during drying followed by reducing conditions induced an internal cycling of Fe, S, and consequently P. This included the oxidation of iron and sulfide during drying, which produced sulfate and increased reductant soluble Fe-bound P. After re-flooding, reducing conditions facilitated the reduction of sulfate and iron, and the precipitation of FeS x induced the mobilization of P in deeper layers of previously dried sediments.
Simplified conceptual model of phosphorus (P) uptake and mobilization in lake sediments flooded with P-enriched water (a), compared to sediments that were dried prior to re-flooding with P-enriched water (b). Diagram shows the most pronounced processes and changes in P pools with respect to the effect of drying. The four stacked bars indicate the relative concentration of sequentially extractable P pools in the sediment at a particular layer: diagonal hatch, FeOOH ~ P, P bound to iron oxyhydroxides (reductant soluble P); light gray, MeO ~ P, metal oxide bound (NaOH-extractable SRP), dark gray, Porg, organic P (NaOH extractable NRP); black, other P fractions. Other abbreviations: Eh, redox potential; P diss , dissolved P
We detected no drying-related changes in HCl-extractable P (Table 2), whereas an increase in HCl-P in dried sediments was reported by Baldwin (1996). Kerr et al. (2010) detected inconsistent changes in this fraction, depending on sediment characteristics. This variability suggests that the form and amplitude of sediment reactions to drying and re-flooding are dependent on the native composition of the sediment. We used sediments which had a high total Fe/P ratio and a high total S content. Most P was therefore bound to Fe and was highly sensitive to changes in redox conditions with a high potential for SO4 2−-mediated P mobilization. In Fe-poor or more calcareous sediments, the Ca-bound P may be differently affected. Studies comparing different sediment types are necessary to elucidate the particular effects associated with each type. This would be important to deduce possible management implications for various aquatic ecosystems.
Considering the mean P uptake rates at the end of our experiment and presuming that the rates remain stable, another 10 weeks would be needed for the wet sediments to reach the same total amount of retained P as the dried sediments. In other words, within 46 weeks (almost one year), both systems would be able to retain the same amount of P. However, the dynamics and structure of the sediments would be different, with undisturbed wet sediments apparently being more efficient at long-term P uptake than are dried and re-flooded sediments. De Groot and van Wijck (1993) proposed repeated drying and re-flooding as a management tool to remove P from an aquatic system by taking advantage of greater P release from dried soils, and subsequent flush out of sediment P. Our results show that inundation after drying may indeed lead to a mobilization of P under anaerobic conditions in deeper layers of sediment.
However, P release might be restricted in the surface layer due to the oxidation of the Fe as a P binding partner (Wilson and Baldwin 2008). Drawdown does not seem to be a short-term option for the removal of P from the water body, as anoxic conditions have to establish first. However, compaction of sediment during drying will concentrate P in the surface layer, from where it can be released easily under anoxic conditions and flushed out, or contribute to higher P concentration in the water column.
Another proposition for management of reservoirs, lakes, and re-flooded fens is to remove the drying-degraded upper layer, as critically discussed by Kleeberg and Kohl (1999), Watts (2000a), Van Wichelen et al. (2007), Zak and Gelbrecht (2007), and Smit and Steinman (2015). This layer releases P due to enhanced mineralization of the high organic matter content. If this layer is removed, the release of P and other substances upon re-flooding would be restricted, the function of retaining P would be preserved in the remaining sediment, and the removed material could be transferred to agricultural sites to serve as a natural fertilizer.
Total lake drawdown is an extreme case of sediment exposition to aeration and drying. Many lakes have regular water level fluctuations that affect only part of the ecosystem; thus, it is difficult to predict the effects of littoral drying on the whole ecosystem. The extent to which a system is affected strongly depends on the morphology, with shallow lakes such as Lake Müggelsee, or lakes with broad and shallow littoral zones, being the most sensitive (Wantzen et al. 2008a). In addition, the consequences of sediment drying for rivers are distinct from those of lakes because they are more affected by longitudinal flow and are therefore characterized by shorter residence times, higher turnover rates, and transportation, resulting in a more intense aquatic-terrestrial link (Wantzen et al. 2008a), favoring for instance the input of nutrients such as P.
The present study emphasizes that one single drying event can markedly influence processes involving the transformation of P forms, and functions of uptake and mobilization within the sediment, which are primarily driven by oxidation and sediment compaction. When perennial systems, with sediment characteristics similar to those in this study, dry out, P will accumulate in the near surface layer, representing an important P source after re-flooding. However, as the sediment compaction was irreversible, compaction in repeated drying/re-flooding cycles is limited to the layer of freshly settled material. In intermittent systems, the effect of compaction will be more important for single or first occurring drying events, than for repeated drying events. In addition, the effect of compaction will be strongly dependent on sediment composition. Very fine sediments will be more affected by compaction than will sediments consisting of larger solid grains, as found in upstream rivers, where fine particles rarely settle. However, in intermittent systems, pools of standing water with settling fine particles can develop during drying events in rivers. Nonetheless, the effects related to sediment compaction will be most important in lakes experiencing the first drying event and least important in intermittent streams.
Conclusion
The drying of formerly permanently inundated sediments stimulated mineralization of organic P compounds, resulted in a shift towards labile P, and led to an irreversible compaction of the sediments (Fig. 8).
The sediment drying reduced the P uptake potential of loose sediment particles in a solution, but not of stratified sediments. The comparison of two experimental setups showed that P uptake characteristics of fine, carbon-rich sediments should not be deduced solely from short-term, small-scale experiments involving sediment shaking, but from experiments considering sediment stratification.
Processes upon re-flooding with P-enriched water facilitated the formation of a sharp redox gradient, promoting the mobilization of P in the anoxic layer due to organic P mineralization and decoupling of the Fe-P-cycle. A single drying and re-flooding event can result in the accumulation of reductant-soluble P in the near surface sediment layers, thus raising the potential for P release into the water body under reducing conditions.
Abbreviations
- BD:
-
Bicarbonate/dithionite
- BEE:
-
Batch equilibrium experiment
- DM:
-
Dry mass
- EPC0 :
-
Equilibrium phosphorus concentration at net zero sorption
- NRP:
-
Non-reactive (with molybdate blue reagent) phosphorus
- q max :
-
Maximum sorbed phosphorus
- SRP:
-
Soluble reactive (with molybdate blue reagent) phosphorus
- TP:
-
Total phosphorus
References
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114:331–345
Baldwin D (1996) Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir. Limnol Oceanogr 41:1725–1732
Baldwin D, Mitchell A (2000) The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: a synthesis. Regul River 16:457–467
Baldwin D, Mitchell A, Rees G (2000) The effects of in situ drying on sediment-phosphate interactions in sediments from an old wetland. Hydrobiologia 431:3–12
Barrow NJ (1978) Description of phosphate adsorption curves. J Soil Sci 29:447–462
Barrow NJ (2008) The description of sorption curves. Eur J Soil Sci 59:900–910
Birch H (1960) Nitrification in soils after different periods of dryness. Plant Soil 12:81–96
Brand-Klibanski S, Litaor MI, Shenker M (2007) Overestimation of phosphorus adsorption capacity in reduced soils: an artifact of typical batch adsorption experiments. Soil Sci Soc Am J 71:1128–1136
Brothers S, Köhler J, Meyer N, Attermeyer K, Grossart HP, Mehner T, Scharnweber K, Hilt S (2014) A feedback loop links brownification and anoxia in a temperate, shallow lake. Limnol Oceanogr 59:1388–1398
Caraco N, Cole JJ, Likens GE (1989) Evidence for sulfate-controlled phosphorus release from sediments of aquatic systems. Nature 341:316–318
Coops H, Beklioglu M, Crisman T (2003) The role of water-level fluctuations in shallow lake ecosystems—workshop conclusions. Hydrobiologia 506–509:23–27
Corstanje R, Reddy K (2004) Response of biogeochemical indicators to a drawdown and subsequent reflood. J Environ Qual 33:2357–2366
Dahm C, Baker M, Moore D, Thibault J (2003) Coupled biogeochemical and hydrological responses of streams and rivers to drought. Freshwater Biol 48:1219–1231
de Groot CJ, Fabre A (1993) The impact of desiccation of a freshwater marsh (Garcines Nord, Camargue, France) on sediment-water-vegetation interactions. Part 3: the fractional composition and the phosphate adsorption characteristics of the sediment. Hydrobiologia 252:105–116
de Groot CJ, van Wijck C (1993) The impact of desiccation of a freshwater marsh (Garcines Nord, Camargue, France) on sediment-water-vegetation interactions. Part 1: the sediment chemistry. Hydrobiologia 252:83–94
de Vicente I, Andersen FO, Hansen HCB, Cruz-Pizarro L, Jensen HS (2010) Water level fluctuations may decrease phosphate adsorption capacity of the sediment in oligotrophic high mountain lakes. Hydrobiologia 651:253–264
Driescher E, Behrendt H, Schellenberger G, Stellmacher R (1993) Lake Müggelsee and its environment—natural conditions and anthropogenic impacts. Int Rev Hydrobiol 78:327–343
Fabre A (1988) Experimental studies on some factors influencing phosphorus solubilization in connection with the drawdown of a reservoir. Hydrobiologia 159:153–158
Fox LE (1989) A model for inorganic control of phosphate concentrations in river waters. Geochim Cosmochim Acta 53:417–428
Gilbert JD, Guerrero F, de Vicente I (2014) Sediment desiccation as a driver of phosphate availability in the water column of Mediterranean wetlands. Sci Total Environ 466–467:965–975
Grierson P, Comerford N, Jokela E (1998) Phosphorus mineralization kinetics and response of microbial phosphorus to drying and rewetting in a Florida Spodosol. Soil Biol Biochem 30:1323–1331
Havens KE, Steinman AD (2015) Ecological responses of a large shallow lake (Okeechobee, Florida) to climate change and potential future hydrologic regimes. Environ Manag 55:763–775
Holmer M, Storkholm P (2001) Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw Biol 46:431–451
Hupfer M, Gächter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57:305–324
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Kamp-Nielsen L (1974) Mud-water exchange of phosphate and other ions in undisturbed sediment cores and factors affecting the exchange rates. Arch Hydrobiol 73:218–237
Kerr J, Burford M, Olley J, Udy J (2010) The effects of drying on phosphorus sorption and speciation in subtropical river sediments. Mar Freshw Res 61:928–935
Kleeberg A (2002) Phosphorus sedimentation in seasonal anoxic Lake Scharmützel, NE Germany. Hydrobiologia 472:53–65
Kleeberg A, Kohl JG (1999) Assessment of the long-term effectiveness of sediment dredging to reduce benthic phosphorus release in shallow Lake Müggelsee (Germany). Hydrobiologia 394:153–161
Kleeberg A, Kozerski H (1997) Phosphorus release in Lake Großer Müggelsee and its implications for lake restoration. Hydrobiologia 342:9–26
Kleeberg A, Herzog C, Jordan S, Hupfer M (2010) What drives the evolution of the sedimentary phosphorus cycle? Limnologica 40:102–113
Lake P (2003) Ecological effects of perturbation by drought in flowing waters. Freshw Biol 48:1161–1172
Laskov C, Herzog C, Lewandowski J, Hupfer M (2007) Miniaturized photometrical methods for the rapid analysis of phosphate, ammonium, ferrous iron, and sulfate in pore water of freshwater sediments. Limnol Oceanogr 4:63–71
Lijklema L (1980) Interaction of orthophosphate with iron(III) and aluminum hydroxides. Environ Sci Technol 14:537–541
McComb AJ, Qiu S, Bell RW, Davis AJ (2007) Catchment litter: a phosphorus source mobilized during seasonal rainfall. Nutr Cycl Agroecosyst 77:179–186
Mitchell A, Baldwin D (1998) Effects of desiccation/oxidation on the potential for bacterially mediated P release from sediments. Limnol Oceanogr 43:481–487
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nair P, Logan T, Sharpley A, Sommers L, Tabatabai M, Yuan T (1984) Interlaboratory comparison of a standardized phosphorus adsorption procedure. J Environ Qual 13:591–595
Nguyen B, Marschner P (2005) Effect of drying and rewetting on phosphorus transformations in red brown soils with different soil organic matter content. Soil Biol Biochem 37:1573–1576
Psenner R, Pucsko R, Sager M (1984) Fractionation of organic and inorganic phosphorous compounds in lake sediments, an attempt to characterize ecologically important fractions. Arch Hydrobiol 70:111–155
Qiu S, McComb A (1994) Effects of oxygen concentration on phosphorus release from reflooded air-dried wetland sediments. Aust J Mar Freshwat Res 45:1319–1328
Qiu S, McComb A (2002) Interrelations between iron extractability and phosphate sorption in reflooded air-dried sediments. Hydrobiologia 472:39–44
Qiu S, McComb A, Bell R, Davis J (2004) Phosphorus dynamics from vegetated catchment to lakebed during seasonal refilling. Wetlands 24:828–836
Reddy K, Kadlec R, Flaig E, Gale P (1999) Phosphorus retention in streams and wetlands: a review. Crit Rev Environ Sci Technol 29:83–146
Roden EE, Edmonds JW (1997) Phosphate mobilization in iron-rich anaerobic sediments: microbial Fe(III) oxide reduction versus iron-sulfide formation. Arch Hydrobiol 139:347–378
Schönbrunner IM, Preiner S, Hein T (2012) Impact of drying and re-flooding of sediment on phosphorus dynamics of river-floodplain systems. Sci Total Environ 432:329–337
Smit JT, Steinman AD (2015) Wetland sediment phosphorus flux in response to proposed hydrologic reconnection and warming. Wetlands. doi:10.1007/s13157-015-0655-1
Steinman AD, Ogdahl ME, Weinert M, Uzarski DG (2014) Influence of water-level fluctuation duration and magnitude on sediment-water nutrient exchange in coastal wetlands. Aquat Ecol 48:143–159
Tang X, Wua M, Li Q, Lin L, Zhao W (2014) Impacts of water level regulation on sediment physic-chemical properties and phosphorus adsorption-desorption behaviors. Ecol Eng 70:450–458
Tockner K, Uehlinger U, Robinson CT, Tonolla D, Siber R, Peter FD (2009) European rivers. In: Likens GE (ed) Encyclopedia of inland waters, 1st edn. Elsevier, Academic Press, Oxford, pp 366–377
Turner B, Haygarth P (2001) Biogeochemistry—phosphorus solubilization in rewetted soils. Nature 411:258
Turner B, Driessen J, Haygarth P, McKelvie I (2003) Potential contribution of lysed bacterial cells to phosphorus solubilisation in two rewetted Australian pasture soils. Soil Biol Biochem 35:187–189
Twinch A (1987) Phosphate exchange characteristics of wet and dried sediment samples from a hypertrophic reservoir: implications for the measurements of sediment phosphorus status. Water Res 21:1225–1230
Van Wichelen J, Declerck S, Muylaert K, Hoste I, Geenens V, Vandekerkhove J, Michels E, De Pauw N, Hoffmann M, De Meester L, Vyverman W (2007) The importance of drawdown and sediment removal for the restoration of the eutrophied shallow Lake Kraenepoel (Belgium). Hydrobiologia 584:291–303
Wantzen KM, Junk WJ, Rothhaupt KO (2008a) An extension of the floodpulse concept (FPC) for lakes. Hydrobiologia 613:151–170
Wantzen KM, Rothhaupt KO, Moertl M, Cantonati M, Toth L, Fischer P (2008b) Ecological effects of water-level fluctuations in lakes: an urgent issue. Hydrobiologia 613:1–4
Watts C (2000a) Seasonal phosphorus release from exposed, re-inundated littoral sediments of two Australian reservoirs. Hydrobiologia 431:27–39
Watts C (2000b) The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 431:13–25
Wilson JS, Baldwin DS (2008) Exploring the ‘Birch effect’ in reservoir sediments: influence of inundation history on aerobic nutrient release. Chem Ecol 24:379–386
Withers PJA, Jarvie HP (2008) Delivery and cycling of phosphorus in rivers: a review. Sci Total Environ 400:379–395
Xiao WJ, Song CL, Cao XY, Zhou YY (2012) Effects of air-drying on phosphorus sorption in shallow lake sediment, China. Fresenius Environ Bull 21:672–678
Young EO, Ross DS (2001) Phosphate release from seasonally flooded soils: a laboratory microcosm study. J Environ Qual 30:91–101
Zak D, Gelbrecht J (2007) The mobilization of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 85:141–151
Acknowledgments
The authors acknowledge contributions from other IGB colleagues. We like to thank S. Schiller, S. Jordan, and B. Schütze for their help with sampling and experimental setup. Sincere thanks are also given to S. Schell, G. Siegert, H.-J. Exner, K. Premke, and A. Krüger for analytical support. Critical comments by A. Kleeberg and J. Lewandowski improved the manuscript. We thank Søren Brothers and Sarah Poynton for language corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 107 kb)
Rights and permissions
About this article
Cite this article
Dieter, D., Herzog, C. & Hupfer, M. Effects of drying on phosphorus uptake in re-flooded lake sediments. Environ Sci Pollut Res 22, 17065–17081 (2015). https://doi.org/10.1007/s11356-015-4904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4904-x