Abstract
Purpose
Contamination of sediments with heavy metals (HMs) is a worldwide environmental issue, due to the negative ecological effects of HMs. Sediments are an important component of aquatic ecosystems, impacting the transformation and transfer of HMs in the environment. Thus, remediating sediments polluted by HMs is a crucial activity within the full aquatic ecosystem remediation process, and economical, effective, and environmentally friendly remediation techniques are urgently needed.
Materials and methods
We reviewed the existing literature on sediment remediation techniques and developments in the fields of environmental science and engineering, attempting to provide a better understanding of the advances of remediation techniques and new research directions for sediments contaminated by HMs.
Results and discussion
This review summarized remediation methods (e.g., physical–chemical strategies, biological strategies, and combined techniques) used to treat sediments contaminated with HMs. This included analyzing the mechanisms associated with biological remediation technologies and their combination with other methods. Then, the review summarized the factors influencing the selection of remediation methods and evaluated the prospects of new emerging remediation methods.
Conclusions
Bioimmobilization techniques (e.g., phytostabilization and microorganism immobilization) have received increased attention because of their low remediation cost and environmental compatibility. Furthermore, particular attention has been paid to explore the role of sulfate-reducing bacteria in decreasing heavy metal mobility. The review provides a useful theoretical foundation and technology reference for the remediation of sediment polluted by HMs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sediment pollution has emerged as a global environmental problem since the 1980s, and has received great attention (Peng et al. 2009; Burton 2010; Perelo 2010). Contaminants of particular concern are both organic and inorganic (Perelo 2010; Vandenbossche et al. 2014). Among different pollutants, heavy metals and metalloids (HMs, e.g., As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn) have received significant attention in the multidisciplinary geosciences and environmental sciences, due to their negative ecological effects, including degradation resistance, bioaccumulation, and biomagnification (Ali et al. 2013; Niu et al. 2013; Vandenbossche et al. 2014; Dixit et al. 2015).
Worldwide, many countries and regions are facing the issue of HMs pollution in sediment (Table 1). Qian et al. (2015) summarized the HM content in sediment, reporting data from 20 countries across six continents. They found that HM pollution in sediment is widespread. Zhu and Wang (2012) also summarized the HM content, focusing on the sediments sampled from the main river systems of China. They found serious sediment pollution, particularly in the Haihe River and Pearl River, where Cd and Hg have created high potential ecological risk. In addition to natural sources (e.g., mineral weathering and volcanic activity), anthropogenic activities, primarily associated with industrial processes (e.g., mining and metallurgy), are the major sources of metal enrichment in sediments (Peng et al. 2009; Chiang et al. 2012; Ali et al. 2013; Vandenbossche et al. 2014; Akcil et al. 2015).
Districts with significant industrial development generally have high concentrations of HMs in sediment. For example, maximum Cd levels in Keratsini Harbor in Greece, East London harbor, and Port Elizabeth harbor in South Africa have reached 1000 mg kg−1 (Fatoki and Mathabatha 2001; Galanopoulou et al. 2009). These levels exceed the probable effect concentration (PEC) value for Cd (Table 1), indicating that levels are likely to have adverse effects on aquatic organisms (MacDonald et al. 2000). The sediments of limnetic, estuarine, and coastal ecosystems in southeastern China have also been impacted by industrial pollution and other factors, and are also heavily contaminated by HMs. This is particularly the case for coastal sediments (Pan and Wang 2012; Tang et al. 2014), where the maximum concentrations of many HMs have exceeded the PEC (MacDonald et al. 2000) and the grade III of marine sediment quality in China (GAQSIQ 2002) (Table 1). The elevated contamination of HMs along China’s coastal environment (include both seawater and sediment) may increase the risk of human exposure to HMs (Pan and Wang 2012).
Sediments are a critical compartment of aquatic ecosystems; these ecosystems are the main sink and source of HMs (Burton 2010; Chiang et al. 2012; Zhang et al. 2014). Dissolved metals can enter water bodies in different ways and can accumulate in sediments through adsorption, precipitation/coprecipitation, and biological effects. This results in HM concentrations that are far greater in sediments (by orders of magnitude) than in the overlying water. It has been reported that the anthropogenic inputs of HMs to aquatic systems have reached 0.3–1 × 106 t year−1 (Schwarzenbach et al. 2006). In some conditions, > 99% of HMs can be stored in sediments (Peng et al. 2009). However, once the environmental or physicochemical conditions change (e.g., pH, Eh, and dissolved oxygen, etc.), HMs accumulated in sediments can be released into the overlying water, possibly enter the food web, and create adverse environmental effects (Horowitz 1991; Peng et al. 2009; Akcil et al. 2015). Thus, sediments significantly affect the transformation and transfer of HMs in the environment; moreover, as part of sediment management processes, treating sediments contaminated by HMs is crucial to the full aquatic ecosystem remediation process.
This review summarizes the remediation methods used to treat sediments polluted by HMs, from the perspectives of physical–chemical strategies, biological strategies, and techniques that combine these strategies.
2 Physical-chemical strategies
2.1 Capping
Capping is an attractive, non-intrusive, and cost-effective method for remediating contaminated sediments (Mohan et al. 2000; Vandenbossche et al. 2014). Sandy materials (e.g., clean sediment, sand, and gravel) or amendments (e.g., apatite, rock phosphate, lime, and zeolite) are usually mixed or tiered in specific proportions, and then placed on contaminated sediments. The purpose of capping is to decrease the solubility, mobility, and transfer rate of HMs in sediment, through physical–chemical isolation, or sediment stabilization. The cap is typically composed of one or more of the following elements (Mohan et al. 2000; Vandenbossche et al. 2014): (i) stabilizing layer (e.g., geotextiles), providing local stability to the native sediments to support the added weight of the cap; (ii) isolation layer (e.g., sand), isolating the contaminants from the environment; (iii) filter layer (e.g., gravel), providing hydraulic protection to the base isolation layer; and (iv) armor layer (e.g., stone), protecting the filter and base isolation layers from erosion (Fig. 1).
The low cost and environmentally friendly nature of the process are some of the main advantages of capping. However, capping also has some disadvantages. These include high workloads, difficulty in maintaining cap homogeneity, particularly in complex riverbeds, and increasing sediment volume while decreasing water capacity. As such, capping is generally not appropriate for shallow water bodies or for water bodies with large water flows, as the capping material can be easily washed away (Mohan et al. 2000; Vandenbossche et al. 2014).
Capping can be classified as passive (or inactive) or reactive (or active) (Vandenbossche et al. 2014). Passive capping uses clean and neutral material to cover contaminated sediments. This capping creates a physical barrier to isolate polluted sediment from the surrounding environment. Active capping decreases the mobility, toxicity, and bioavailability of contaminants using chemical reaction between capping materials (e.g., apatite, clays, zeolite, and activated carbon) and contaminants. Passive capping usually has higher thickness than active capping. Thus, passive capping is unsuitable in shallow areas, under existing marine structures, or in sensitive habitats (Vandenbossche et al. 2014).
According to the different adding manner of capping materials, capping can also be classified as overwater or underwater (Zhang et al. 2016b). Overwater capping adds capping material through the overlying material, whereas underwater capping adds capping material directly onto the water bottom (Fig. 1). Overwater capping is easy to operate, but can result in large water disturbances and an uneven capping surface. Underwater capping usually requires less capping material and disturbs water less; it also results in a more even capping effect. Capping can be applied both in situ and ex situ to remediate contaminated sediment.
2.2 Washing
Sediment washing is a relatively simple and useful ex situ remediation technology, where a solution is added to the polluted sediment to transfer contaminants from sediment to aqueous solution (Mulligan et al. 2001; Peng et al. 2009). Washing includes two steps: the solubilization of metals and the removal of solubilized metals (Akcil et al. 2015). To enhance the sediment washing performance, different additives are used to facilitate the solubilization, dispersal, and desorption of metal contaminants from polluted sediments (Peng et al. 2009; Akcil et al. 2015). Ideal additives meet two important criteria: high treatment efficiency and environmental compatibility (e.g., low toxicity and biodegradability) (Akcil et al. 2015). Common additives include inorganic acids (e.g., hydrochloric acid, sulfuric acid, and nitric acid), organic acids (e.g., oxalic, citric, gluconic and ascorbic acids), chelators (e.g., EDDS, EDTA, and NTA), and surfactants (e.g., rhamnolipids and sophorolipids) (Peng et al. 2009; Akcil et al. 2015).
Some additives, however, may adversely affect the ecological environment. For example, although EDTA may be a widely used and more efficient extraction agent to remove some HMs (e.g., Cd; Polettini et al. 2006), its high environmental persistence may adversely affect the ecological environment (Gorby et al. 1998; Bohuslavek et al. 2001; Meers et al. 2005b; Peng et al. 2009). Beolchini et al. (2013) evaluated the efficiency of different chemical leaching agents (including sulfuric, oxalic and citric acids) and bioleaching processes (using different acidophilic bacterial strains) on HM mobilization (e.g., As, Cr, Ni, and Zn) in contaminated harbor sediments. Considering both resource requirements and emissions, the researchers found that diluted sulfuric acid is better than other treatments to decrease the environment impact. Akcil et al. (2015) reviewed the main washing additives in detail.
Washing is suitable for HMs weakly associated with sediment particles (e.g., exchangeable, hydroxides, carbonates, and reducible oxides phases), and for coarse-grained sediments (e.g., sands and gravels) (Mulligan et al. 2001; Peng et al. 2009). However, dredged sediment is usually fine-grained, and the finest particles are more polluted and more difficult to wash because of the high surface area available for adsorption (Akcil et al. 2015). This is a critical limitation in applying the washing technique. This means that extraction tests should be conducted to determine optimal criteria (e.g., chemical type and dosage, contact time, agitation, temperature, and extraction steps) required to meet regulatory requirements (Mulligan et al. 2001; Akcil et al. 2015). Furthermore, inorganic acids may not work well with calcareous sediments, because protons can be neutralized by calcite and carbonate dissolution (high acid-neutralizing capacity) (Fonti et al. 2013); chelating agents can effectively treat dredged sediment contaminated with both organic pollutants and HMs (Peng et al. 2009).
2.3 Immobilization
Immobilization (also called stabilization) strategies have also been proposed as an in situ/ex situ remediation solution for sediments contaminated with metals, particularly for dredged sediment (Akcil et al. 2015). This method reduces the solubility, mobility, and bioavailability of HMs using different amendments, by adsorption, oxidation, reduction, and precipitation. The approach is an alternative to extracting HMs, and while it cannot remove metals from sediment, it is still common because of its low cost and rapid remediation effect (Peng et al. 2009). Common amendments include inorganic, organic, and complex formulation stabilizing agents (Fan et al. 2016). Widely used inorganic amendments include silico-calcium materials (e.g., CaO, CaO2, MgO and fly ash, etc); phosphates (e.g., rock phosphate, calcium hydrophosphate, and hydroxyapatite); iron-bearing materials (e.g., Fe(OH)3, FeCl3, FeSO4, Fe2(SO4)3, and Fe0); aluminum salts (e.g., aluminum sulfate, aluminum chloride, and aluminum polychloride); and mineral-based amendments (e.g., zeolite, diatomite, and bentonite). Organic amendments generally include turf, farmyard manure, and green manure. A complex formulation amendment is a mix of inorganic amendments and/or organic amendments.
Xu (2017) found a combined stabilizing agent (potassium dipropyl dithiophosphate and humic acid) achieved > 90% stabilization efficiency in treating Cd, Cu, Pb, and Zn in sediment. Jośko et al. (2013) found that carbonaceous materials (e.g., activated carbon, biochars, and multi-walled carbon nanotubes) reduced the negative effect of contaminated sediment on Lepidium sativum. Huang et al. (2017) also found that the extractable fraction of Cd and Zn declined when sediment was treated with biochar, however, adding a high concentration of biochar (> 50 mg kg−1) decreased enzymes activity and microbial abundance, and altered the microbial community structure.
Amendments usually have high cation exchange capacity, are environmentally friendly, and are economically reasonable (Peng et al. 2009; Akcil et al. 2015; Heyden and Roychoudhury 2015). The stabilizing effect of amendments on HMs is influenced by sediment characteristics, amendment type, HM type and concentrations, remediation method, remediation time, and evaluation methods. This complicates the comparing of immobilization efficiencies of different sediments contaminated with different HMs. For example, the changing of HM speciation is usually used to assess the stable efficiency of HMs in sediment, and to reveal the remediation mechanism; however, it is difficult to compare immobilization efficiencies due to different sequential extraction methods (Table 2). These difficulties make it challenging to choose the appropriate amendments and application dosage. Chiang et al. (2012) proposed a strategic framework to systematically address the development of an in situ sediment remediation solution (e.g., finding effective sorbent mixtures) through assessment, feasibility, and performance studies. These strategies provide guidance to researchers in the field of HM remediation, and bridge the gap between laboratory tests and field applications.
Some nanometer materials (e.g., nano-zero-valent iron (nZVI), nanohydroxyapatite, nanosized metal oxides) have higher reactivity and sorption abilities than the same materials at normal sizes (Akcil et al. 2015). As such, these technologies have been applied as an amendment with metal-contaminated sediments, initially for soil or solid waste remediation. Many scientific questions remain because of the complexity and specificity of the sediment. For instance, Chen et al. (2016b) found that nano-zero-valent iron/activated carbon composite (nZVI/AC) could effectively immobilize HMs (e.g., Cd, Cr, Cu, and Pb) in sediment from Huangpu River, China, by converting relatively weakly bound HMs into more strongly bound species (Table 2).
However, Kumar et al. (2014, 2015) found that nZVI had an inhibitive effect on sulfate-reducing bacteria (SRB) in aquifer sediment; this decreased biostabilization. Fajardo et al. (2012) found that applying nZVI reduced the availability and mobility of Zn and Pb in contaminated soil. The nZVI also significantly changed the structure and composition of the bacteria population. Pawlett et al. (2013) found that nZVI not only changed the structure and composition of soil bacteria population, but also significantly reduced microbial biomass. These results show that nZVI toxicity can be highly dose- and species-dependent. Thus, as a new environmental restoration material, there is significant uncertainty associated with nanometer materials, and more research is needed on their environmental behavior, toxicity mechanisms, and bioavailability (Fajardo et al. 2012). Other important sediment remediation and management research topics include the reduction, reuse, and recycling of immobilized sediments (Wang et al. 2015a, b; Couvidat et al. 2016).
2.4 Electrochemical remediation
Electrochemical remediation (also called electrokinetic treatments) involves passing a low-intensity electric current (e.g., AC or DC fields) between a cathode and an anode embedded in polluted sediment in wet condition (Mulligan et al. 2001; Peng et al. 2009; Akcil et al. 2015; Pedersen et al. 2015). The electric field causes the transport of ions, small charged particles, and water between the electrodes. Positive ions move to the negatively charged cathode, while negative ions move to the positively charged anode (Peng et al. 2009) (Fig. 2). When the remediation process has been completed, the contaminants concentrated around the electrode can be treated with various physical–chemical methods, including electroplating, precipitation/coprecipitation, pumping water near the electrodes, complexing with ion-exchange resins, or other methods (Mulligan et al. 2001; Peng et al. 2009; Akcil et al. 2015; Pedersen et al. 2015).
Electrodialytic cell set-up for sediment treatment (source: Pedersen et al. 2015)
Electrochemical remediation is appropriate for fine-grained sediment, because fine particles (e.g., clay) can adsorb most metals, thus having high electric conductivity and a strong electric field (Mulligan et al. 2001; Peng et al. 2009). Metals that are present as soluble ions and that are bound to soils as oxides, hydroxides, and carbonates can be removed by this method, as well as other ions, such as cyanide and nitrate, and radionuclides (e.g., Sr and U) (Mulligan et al. 2001). This method has the advantages of having no or few by-products, and is easy to control. Further, HM recovery can help recover costs (Mulligan et al. 2001; Akcil et al. 2015).
The main mechanisms of electrochemical remediation include electromigration (charged chemical movement), electro-osmosis (fluid movement), electrophoresis (charged particle movement), and electrolysis (chemical reactions due to the electric field) (Mulligan et al. 2001; Peng et al. 2009). Electromigration is considered to be the main transfer mechanism of HMs, because the transfer rate using this method is higher than achieved using other methods (Mulligan et al. 2001; Peng et al. 2009). Current density, time, cell set-up, stirring rate, dry/wet material ratio, and sediment properties can influence remediation efficiency. Of these factors, remediation time and current density usually have the greatest effects (Pedersen et al. 2015).
The electrode reactions can produce OH− and H+ at the cathode and anode, respectively (Peng et al. 2009). If the pH is not controlled, the H+ in the anode will migrate through the sediment towards the cathode, while the OH− will migrate towards the anode (Peng et al. 2009; Pedersen et al. 2015) (Fig. 2). The pH in sediment depends on the migration level of OH− and H+. For example, increasing the OH− content increases the pH value around the cathode. When HMs encounter this type of basic condition (i.e., high pH), they are likely to be adsorbed onto soil particles or form precipitates such as hydroxides and oxyhydroxides; on the contrary, in acidic condition, those ions desorb, solubilize, and migrate (Peng et al. 2009; Pedersen et al. 2015). As a result, the increasing pH around the cathode impedes the removal of HMs. Desorbing agents (e.g., acidification and surfactants) have been effectively used to increase contaminant removal efficiency, by solubilizing the metal hydroxides, carbonates, or other species adsorbed onto sediment particles, and protonate the organic functional groups (Peng et al. 2009). Future research is needed to better select desorbing agents and optimize the soil or sediment electrochemical remediation process (Kaya and Yukselen 2005; Nystroem et al. 2006).
2.5 Thermal treatments
Thermal treatments include thermal extraction and vitrification. After a dewatering pretreatment, heat is used to treat the contaminated sediment. The high temperature destroys most organic pollutants in the sediment through oxidation. Meanwhile, many HMs can be immobilized in the sediment matrix. However, some metals, such as As, Cd, and Hg, can be volatilized, and others, such as As, Mo, and V, can become more leachable, due to oxyanions formation (Mulligan et al. 2001; Akcil et al. 2015). Thermal treatments are mainly used to treat organic contaminants. Temperature and retention time are the two dominant factors driving decontamination level (Mulligan et al. 2001; Akcil et al. 2015). At 100–500 °C, some organic contaminants, such as low-molecular hydrocarbons and polycyclic aromatic hydrocarbons (PAHs), can be removed through thermal desorption and vaporization. However, when temperature is > 800 °C, organic contaminants can be completely destroyed, and some inorganic contaminants in the sediment can be evaporated (e.g., As, Cd, and Hg) or immobilized by melting (i.e., vitrification) (Mulligan et al. 2001; Zoubeir et al. 2007; Akcil et al. 2015).
There are several commercially available thermal chemical treatment processes, including Cement Lock, X-Trax™ process, Novosol® process, and Mercury Recovery Services (Mulligan et al. 2001; Zoubeir et al. 2007; Akcil et al. 2015). Cement Lock is a typical thermal treatment technique and has been used to remediate sediment, especially dredged sediment. During the process (Fig. 3), contaminated sediment and lime are firstly mixed and added into a rotary kiln reactor smelter. After being melted (1200–1600 °C), quenched, and pulverized, the mixture can be used for blend cement products. In order to remove the acid gas, volatilized HMs, and other combustion products in the off-gases, gas processing equipment (e.g., particulate filter and activated carbon filter) is required. Pilot tests using this technique result in estimated costs of US$20–30/m3 (Mulligan et al. 2001).
Process flow diagram of Cement Lock (adapted from Mulligan et al. 2001)
3 Biological strategies
3.1 Phytoremediation
Phytoremediation involves using plants and associated microorganisms to partially or completely remediate selected contaminants from soil, sludge, sediments, wastewater, and groundwater (Ali et al. 2013; Dixit et al. 2015). There are three reasons to use phytoremediation to treat polluted land: (1) for risk containment (phytostabilization); (2) for phytoextraction of HMs with market value (e.g., Au, Ni, and Tl); and (3) for durable land management, using phytoextraction to improve soil quality to facilitate subsequent crop cultivation with a high market value (Ali et al. 2013). As a green technology with a positive public perception, phytoremediation is a novel, efficient, cost-effective, environmentally and eco-friendly, in situ applicable, and solar-driven remediation strategy (Ali et al. 2013). The method has been widely used to remove organic (e.g., polychlorinated biphenyls, PAHs, nitroaromatic and halohydrocarbon, etc.) and inorganic (e.g., radionuclide and HMs) pollutants from various environmental media, including wastewater, soil, and sediment (e.g., shallow rivers, lakes, and wetlands) (Bert et al. 2009; Peng et al. 2009; Perelo 2010; Ali et al. 2013; Mani and Kumar 2014; Dixit et al. 2015).
Phytoremediation techniques include phytovolatilization, phytodegradation, phytofiltration, phytoextraction, phytostabilization, and rhizo(sphere) degradation (Fig. 4). Of these, phytoextraction (known as phytosequestration, phytoabsorption, or phytoaccumulation) is a critical biochemical process to remove HMs from contaminated environmental media (Ali et al. 2013; Dixit et al. 2015). Metal phytoextraction includes three steps (Vassilev et al. 2004; Bert et al. 2009): (1) cultivation of suitable plant species at the polluted site; (2) harvest metal-enriched biomass from the site; and (3) postharvest treatment to produce market value (e.g., energy recovery from thermal treatment).
Different processes involved in the phytoremediation of HMs (source: Dixit et al. 2015)
Phytoextraction efficiency depends on many factors, including the bioavailability of HMs, soil properties, HM speciation, and the plant species (Ali et al. 2013). Ideal plants for phytoextraction need a high growth rate, significant above-ground biomass, a widely distributed and highly branched root system, ability to accumulate the target HMs from soil, ability to translocate the accumulated HMs from roots to shoots, ability to tolerate the toxicity of target HMs, ability to adapt to prevailing environmental and climatic conditions, resistance to pathogens and pests, easy cultivation and harvest, and herbivore repulsion to avoid food chain contamination (Vassilev et al. 2004; Bert et al. 2009; Ali et al. 2013; Dixit et al. 2015).
Two main factors drive the phytoextraction capacity of a plant: shoot metal content and shoot biomass. However, hyperaccumulation and hypertolerance are more important for phytoremediation than high biomass (Ali et al. 2013). There has been significant research about HM removal using phytoremediation, with particular focus on using hyperaccumulators to degrade and detoxify contaminants; this is because of the efficacy and cost efficiency of the approach (Dixit et al. 2015). The criteria used for hyperaccumulation varies by metal; however, hyperaccumulators are considered to be plant species that usually accumulate > 100 mg kg−1 dry weight of Cd; > 1000 mg kg−1 dry weight of Cu, Ni, and Pb; or > 10,000 mg kg−1 dry weight Mn and Zn in plant shoots when grown in HM-rich soils (Ali et al. 2013; Dixit et al. 2015). Many researches have reviewed hyperaccumulators and their application in remediation (e.g., Ali et al. 2013; Mani and Kumar 2014; Dixit et al. 2015). Significant research is focusing on screening and exploiting hyperaccumulators with high remediation potential (e.g., transgenic plants) (Kotrba et al. 2009); this research is critical to the effective application of phytoremediation. However, the ecological influence of phytoremediation using transgenics must also be carefully evaluated (Kotrba et al. 2009).
Rhizosphere microorganisms are of great importance to plant growth and their ability to tolerate HMs (Dixit et al. 2015). The rhizosphere is an important component and main mechanism of phytoremediation. Phytoremediation costs are expected to be < 25% of some other remediation techniques, including in situ soil mixing/solidification/stabilization, water flooding/soil flushing/soil washing, electrokinetics, and chemical reduction/oxidation (Mani and Kumar 2014). Phytoremediation is a green and promising technique to remediate HM-contaminated soils; however, there are also limitations, such as low biomass and slow growth rate lead to long remediation timeframes; there is difficulty in mobilizing the more tightly-bound fractions of HMs; the approach only applies at low to moderate pollution level of HMs (not heavily polluted environmental media); and mismanagement and improper care may lead to risks of food chain contamination (Ali et al. 2013).
Phytoremediation is mostly used in dredged sediments, using ex situ approaches (Akcil et al. 2015; Doni et al. 2015; Choudhury et al. 2016). Research shows that some hydrophytes can also decrease HM toxicity through plant uptake and rhizosphere microorganism activity (Peng et al. 2009). As such, phytoremediation can also be used for in situ sediment remediation. For example, Xie et al. (2016) found that Hydrilla verticillata had a better comprehensive restoration effect than Vallisneria natans and Ceratophyllum demersum in Cu and Pb co-polluted sediments. Qiao et al. (2016) concluded that Vallisneria natans can serve as a pioneer plant to ecologically restore Cd and Zn co-polluted sediments. However, direct uptake by hydrophytes is usually small, while the indirect reactions (e.g., stimulation of microbial activity, redox reactions/formation, and precipitation of insoluble metal compounds in the rhizosphere) may play a relatively important role (Clemente et al. 2005; Peng et al. 2009; Ahemad 2014). Therefore, more researchers have focused on remediating HM-contaminated sediments using microorganisms.
3.2 Microbial remediation
3.2.1 Microbial resistance to metals
Microorganisms are widespread in contaminated media. Microorganisms have developed many strategies to evade the stress and toxicities associated with different HMs (Ahemad 2014; Fls et al. 2017). Mechanisms used by microorganisms to resist metals include exclusion using a permeability barrier, intracellular and extracellular sequestration, active transport efflux pumps, enzymatic detoxification, and reductions in cellular sensitivity to metal ions (Nies 1999; Bruins et al. 2000; Ahemad 2014). Microorganisms can mineralize organic pollutants to generate end products (e.g., CO2 and H2O), or to generate metabolic intermediates that serve as main substances for cell growth (Dixit et al. 2015). Inorganic contaminants (e.g., HMs) cannot be directly degraded into harmless compounds. However, microorganisms can change the chemical form, mobility, toxicity, and bioavailability of HMs through growth metabolism and metabolic products. Interactions between microbial cells and HMs mainly occur through biosorption, bioaccumulation, bioassimilation, bioprecipitation, bioleaching, biodegradation/biosynthesis, and biotransformation (Fig. 5).
Biosorption describes the association of soluble HMs with the cell surface through complexation (e.g., electrostatic, covalent, exopolysaccharides), chelation/coordination, reduction, precipitation, cation/anion-exchange (Tabak et al. 2005; Ahemad 2014; Fls et al. 2017). For example, many HMs can bind onto anionic groups (e.g., amine, amide, carboxyl, hydroxyl, sulfhydryl, and sulfonate) and extracellular polymers (e.g., polysaccharides, proteins, and humic substances). This reduces HM toxicity by forming complexes or by creating a useful barrier around the cell (Tsezos 2009; Ahemad 2014).
Bioaccumulation is the retention and concentration of a substance within an organism. In this process, solutes are transported from the outside of the microbial cell through the cellular membrane into the cell cytoplasm, where the metal is sequestered (Tabak et al. 2005).
Bioassimilation of HMs involves the active transport of a microbial cell’s siderophores. In aerobic conditions, iron is mainly present as Fe(III). Due to low solubility in water (i.e., 10−18), Fe(III) cannot be obtained by microbes as a free ion (Tabak et al. 2005). In order to solve this problem, microbes produce siderophores, which are low-molecular-weight chelating agents that bind with iron and transport it into the cell using an energy-dependent process (John et al. 2001). Meanwhile, some metals (e.g., Pu) can form complexes with siderophores, and many of these complexes are recognized by cell uptake proteins (John et al. 2001; Tabak et al. 2005).
Bioprecipitation involves using microbial metabolism to transform soluble species to insoluble hydroxides, carbonates, phosphates, and sulfides (Tsezos 2009). For example, bioprecipitation of HMs using microbiologically produced sulfides (e.g., SRB) is an efficient method to immobilize HMs (Tabak et al. 2005).
Bioleaching is the dissolution of metallic minerals and the release of associated metals through microorganism activity. The best-known strains—Fe/S-oxidizing bacteria (e.g., Thiobacillus and Leptospirillum ferrooxidans)—can oxidize iron and sulfide, producing sulfuric acid and releasing associated HMs into an aqueous solution (Tabak et al. 2005; Akcil et al. 2015). This approach has been used in large-scale operations to recover metals from ores.
Biodegradation usually refers to the oxidation of organic contaminants. However, the biodegradation of some organic complexing agents (e.g., EDTA and NTA) significantly affects HM mobility, toxicity, and bioavailability in subsurface environments (Tabak et al. 2005).
Biotransformation (e.g., methylation/reduction, dealkylation/oxidation) can change the chemical form of HMs, altering HM mobility, toxicity, and bioavailability. For example, direct enzymatic reduction through metal-reducing microorganism can reduce soluble and mobile Cr(VI), Tc(VII), and U(VI) into insoluble and immobile Cr(III), Tc(IV), and U(IV), respectively; the reduced products (e.g., Fe(II) and H2S) of metal-reducing microorganisms and SRB can also indirectly reduce Cr(VI), Tc(VII), and U(VI) (Tabak et al. 2005; Tsezos 2009; Ahemad 2014).
Interactions between microbial cells with HMs provide a bioremediation strategy for microorganisms. Biotransformation, biosorption, and bioaccumulation are the main three kinds of microbial interaction processes that affect HM toxicity and transport, playing a critical role in microbial remediation (Tabak et al. 2005). In a narrow sense, microbial remediation refers to bioremediation (Mulligan et al. 2001; Akcil et al. 2015; Dixit et al. 2015). Microbial remediation uses microorganisms to remove or fix HMs. The approach has low costs and is non-invasive; it can be done on-site and coupled with physical or chemical treatment technologies (Mani and Kumar 2014). Microbial remediation has been considered as a safe, easy, and effective technology (Dixit et al. 2015; Fls et al. 2017). However, microbial remediation also has some disadvantages (Tsezos 2009; Mani and Kumar 2014; Akcil et al. 2015). For example, it is time-consuming and has limited real applications; it can be difficult to predict the bioremediation effect; and the related mechanisms are complicated and not always fully understood. As a new and most promising bioremediation technique, however, microorganisms have been applied to restore wastewater, soil, and solid waste. It has also been used more recently to restore sediments. For HM-contaminated sediments, there are two different strategies: biomobilization and bioimmobilization.
3.2.2 Biomobilization
Unlike organic contaminations, HMs cannot be biodegraded. Instead, the speciation of HMs can be changed (e.g., mobilized or immobilized) through a biogeochemical process, changing the HM mobility, toxicity, and bioavailability. Biomobilization has been widely used to remediate HM-contaminated sediment. This process usually involves two steps. First, HMs are mobilized into a solution using biological methods (e.g., adding microorganism directly, microbial preparation, and biostimulation). Second, dissolved HMs are separated into solid and liquid phases and then treated. Bioleaching is one of the most common approaches to biomobilization, as it uses the effects of biological oxidation and acid production to translate insoluble metallic compounds into soluble ion states. This method has been widely used to leach ore (Oliveira et al. 2014); treat mine tailings (Park et al. 2014; Nguyen and Lee 2015); and bioremediate environmental media (soil, sludge, and sediment) contaminated by HMs (Seidel et al. 2004, 2006; Gan et al. 2015, 2016; Zeng et al. 2015a, b).
Microorganisms used in bioleaching mainly include chemoautotrophic bacteria and fungi (single and composite strain), such as Leptospirillum ferrooxidans, Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, and Aspergillus niger. Nguyen and Lee (2015) found that 42.4, 45.0, 47.7, 92.0, and 67.2% of As, Cu, Fe, Mn, and Zn, respectively, in mine tailings can be removed using a mixture culture of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans, after 500 h at 0.5% elemental sulfur concentration. Zeng et al. (2015a, b) found that Aspergillus niger strain SY1 can remove Cd, Cu, Pb, and Zn from dredged sediments contaminated with multiple metals, particularly Cd, with a removal rate > 90%.
Gan et al. (2016) suggested that the acid-tolerant microorganisms Aspergillus niger and Rhodotorula can degrade dissolved organic matter. This effect can help Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum, and Acidithiobacillus thiooxidans accelerate sulfur and pyrite use; the bioleaching efficiency of Cd, Cu, Mn, and Zn reached 84.2, 90.9, 94, and 94.7%, respectively. Subsequent research from Gan et al. (2015) found that when bioleaching multiple HMs from polluted sediment, a moderately thermophilic consortium (Sulfobacillus thermosulfidooxidans and Acidithiobacillus caldus) achieved higher acidification and metal solubilization efficiencies than did pure strains. The solubilization efficiency for Cd, Cu, Mn, and Zn reached 89, 94, 95, and 98%, respectively, while the efficiency for As, Hg, and Pb was only 45, 34, and 22%, respectively.
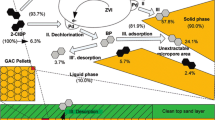
Direct and indirect mechanisms are both involved in solubilizing metals (Mulligan et al. 2001; Chen and Lin 2004; Akcil et al. 2015; Gan et al. 2015). Direct leaching solubilizes metal sulfides to metal sulfates through enzymatic oxidation (Eq. 1, see below); in the indirect mechanism, bacteria oxidized elemental sulfur or reduced sulfur compound to sulfuric acid. It lowers the pH and subsequently enhances metal solubilization (Eqs. 2 and 3). However, it is widely accepted that there is no direct mechanism of biological metal sulfide oxidation. On the contrary, the true factors that solubilize metals from ores are the indirect mechanisms (Vera et al. 2013; Akcil et al. 2015). The dissolution of metal-bearing minerals can follow two different reaction pathways. The pathways depend on the acid-solubility of the sulfides involved: acid-insoluble metal sulfides (e.g., pyrite, molybdenite, tungstenite) are exclusively oxidized through electron extraction (Eqs. 4 and 5, the thiosulfate pathway, Fig. 6a); and acid-soluble metal sulfides (e.g., sphalerite, galena, arsenopyrite, chalcopyrite, hauerite) are dissolved by the combined actions of Fe(III) oxidative and proton attacks (Eqs. 6–8, the polysulfide pathway, Fig. 6b).
Schematic comparison of the thiosulfate (a) and polysulfide (b) mechanisms in bioleaching metal sulfides (MS metal sulfides, M2+ metal cations, Af Acidithiobacillus ferrooxidans, At Acidithiobacillus thiooxidans and Lf Leptospirillum ferrooxidans) (from Schippers and Sand 1999; Vera et al. 2013; Akcil et al. 2015)
Bioleaching involves combining proton attacks and oxidation processes, as such, sediments having high levels of metal sulfides and other reduced metal forms should be especially appropriate for bacterial leaching techniques (Akcil et al. 2015). The sulfur oxidation rate is the critical factor impacting bioleaching. It is influenced by pH (Park et al. 2014; Fonti et al. 2015b), ORP, temperature, DOM (Gan et al. 2016), the ratio of sulfur added to total sediment solids (Tsai et al. 2003), types of elemental sulfur (Seidel et al. 2006), dosage (Oliveira et al. 2014; Nguyen and Lee 2015; Porzionato et al. 2017), and mode of addition (Porzionato et al. 2017). Tichy et al. (1998) and Seidel et al. (2006) suggested that during suspension leaching, biological sulfur yields better results than technical sulfur. Porzionato et al. (2017) found that heap leach systems with superficial scattered sulfur perform better than systems with sulfur integrated into the mixture. Compared to other approaches, bioleaching has lower costs, lower energy requirements, higher environmental safety, and more operational flexibility (Zeng et al. 2015b). However, it also has some disadvantages: metal solubilization experiences slow kinetics; the process is time-consuming; there are fewer applications; and there is a risk of groundwater contamination (Mani and Kumar 2014; Zeng et al. 2015a).
When only a simple microbiological approach is used to remediate sediment, the removal effect for some HMs is insufficient. This inefficiency is particularly true for sediments contaminated by multiple HMs. Nguyen and Lee (2015) found that a mixed culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans experienced a low As, Cu, and Fe removal rate (range 42.4–47.7%). Gan et al. (2015) also found that a moderately thermophilic consortium (Sulfobacillus thermosulfidooxidans and Acidithiobacillus caldus) obtained a low As, Hg, and Pb removal rate (range 22–45%). Combining microbiological approaches with other methods has become a focus for biomobilization research. For example, Zeng et al. (2015a) found that the process combined with bioleaching and a Fenton-like reaction was an effective approach to remove HMs and to enhance the dewaterability of polluted dredged sediments. After adding H2O2, the Fenton-like reaction was activated, and the Cd, Cu, Pb, and Zn removal rates were increased from 90 to 99.5%, 60 to 70%, 20 to 39%, and 60 to 70%, respectively.
3.2.3 Bioimmobilization
Bioimmobilization refers to using biological methods (e.g., adding microorganism directly, microbial preparation, and biostimulation) to transform toxic metallic compounds into low- or non-toxic states through biosorption, bioaccumulation, bioprecipitation, and biotransformation. The goal of bioimmobilization is to reduce HM solubility, mobility, bioavailability, and toxicity without completely removing them from sediments. Bioimmobilization is significantly different from biomobilization and other physical–chemical immobilization approaches. Compost and SRB have served as bioimmobilization agents to stabilize HMs and restore soil, sludge, and sediment.
Compost can break down organic contaminants. Mattei et al. (2016) found that co-composting dredged sediment along with green waste (mainly consisting of mixed tree branches) significantly degraded PAHs. Compost is considered an appropriate method in reclaiming dredged sediments, opening opportunities for their use as technosol or as a plant-growing substrate. Macía et al. (2014) also proved the viability of the ecological management of marine dredged sediments through the elaboration of technosols. Although co-composting is an inefficient technique in reducing HMs, it can transform exchangeable HMs into more stable organic-bound forms (Mattei et al. 2016). Considerable research suggests that HM bioavailability could be reduced through complexation, adsorption, reduction, and volatilization during composting (Park et al. 2011; Mattei et al. 2016; Hazarika et al. 2017).
However, some research has reached the opposite conclusions. For example, it had been found that compost amendments have significantly reduced the risk of human exposure to toxic As concentrations (Kumpiene et al. 2008). However, Fang et al. (2017) found that repeatedly applying composted sewage sludge to soils contributed to the formation of reducing conditions. This enhanced the leached As concentrations by approximately one order of magnitude. Beesley et al. (2014) found that when olive mill waste compost was used as an amendment with heavily contaminated mine soil, it solubilized a considerable amount of As into the pore water. Walker et al. (2004) also found that compost (prepared from a mixture of olive leaves and the solid fraction of olive mill wastewater in a pilot plant) increased plant-available Cu, Mn, and Zn in soil; cow manure prevented soil acidification and decreased HMs bioavailability in amended soils. Hazarika et al. (2017) found that rotary drum composting of paper mill sludge efficiently reduced bioavailable and leachable fractions of HMs. In general, using compost to stabilize HMs is common for restoring soil and sludge, but not sediment (Akcil et al. 2015).
With respect to HM bioimmobilization, researchers have examined the role of SRB in decreasing HM mobility by generating sulfides. Sulfides have a very low solubility product constant and support HM precipitation, recycling, and reuse (Muyzer and Stams 2008). Previous research has shown that SRB effectively immobilizes HMs. Thus, SRB have been successfully used in wastewater treatment (e.g., acid mine drainage) (Vitor et al. 2015; Zhang and Wang 2016); this provides a reference for its application in other environmental matrices, such as soil and sediment. However, few studies have been conducted using SRB to remediate soil or sediment. Mamouni et al. (2002) studied the influences of electron donor and acceptor on SRB bioremediation of soil polluted by trichloroethene and Ni. Groudev et al. (2014) studied the effect of SRB biostimulation on radionuclides (e.g., Ra and U) and non-ferrous metals (e.g., Cd, Cu, and Zn) for in situ remediation. Fonti et al. (2015a) studied changes in bacterial diversity during the SRB biostimulation of contaminated marine sediments.
4 Combined methods
Physical–chemical methods, also known as traditional techniques, are usually highly efficient in remediation, but often have high costs. Biological methods are considered promising strategies; they are usually environmentally friendly, but require significant remediation time and are unstable in their remediation efficiency. As such, both methods have advantages and disadvantages. Due to the heterogeneous nature and compositional complexity of sediment, a single physical–chemical method or biological method cannot usually achieve an ideal remediation effect. This is particularly true when numerous HMs contaminate the sediment. Combining the methods helps maximize their advantages, enhancing remediation efficiency. Combined methods can be classified as (1) physical–chemical methods with phytoremediation, (2) physical–chemical methods with microorganisms, (3) phytoremediation with microorganisms, and (4) group technology.
4.1 Physical–chemical methods with phytoremediation
Low hyperaccumulator biomass limits the application of phytoremediation. During HM phytoextraction in soil or sediment remediation, metal complexing agents (e.g., EDTA and EDDS) are usually used first to activate HMs. Then, plants (e.g., sunflower and willow) with high growth rates and large biomass are used to extract HMs, increasing remediation efficiency (Meers et al. 2005a, 2005b, 2007; Bert et al. 2009; Shahid et al. 2014). Combined physical–chemical methods and phytoremediation are usually used to remediate dredged sediment. Meers et al. (2005a, 2005b) examined HM mobilization (e.g., Cd, Cu, Zn, and Ni) into a solution of dredged sediment-derived surface soil, applying EDTA or EDDS. They found that both EDTA and EDDS enhanced the phytoextraction of Brassic rapa, Helianthus annuus, Cannabis sativa, and Zea mays.
Based on this, Meers et al. (2007) utilized EDDS and five willow species (Salix spp.) to remediate sediment-derived surface soils polluted at different levels and sandy soils in pot experiments. They found that Salix schwerinii, Salix dasyclados, and Salix fragilis had better phytoextraction for Cd and Zn, and the promoting effect of EDTA was influenced by the type of environmental media, and HM type and level. Based on existing studies, although EDTA has poor biodegradability, it remains the most efficient organic ligand in increasing metal solubilization, uptake, and translocation. This is because EDTA can form highly soluble and stable metal-EDTA complexes (Shahid et al. 2014). EDTA-enhanced metal phytoremediation is affected by different biogeochemical processes in the plants, soil, metal, and the EDTA itself (Meers et al. 2005a, 2005b, 2007; Shahid et al. 2014). There are two concerns when applying EDTA (Shahid et al. 2014). First, the metal-EDTA complexes have a relatively low biodegradability and can modify the bio-physico-chemical properties of the soil or sediment. Second, adding EDTA may increase HM field leaching, generating groundwater pollution and environmental risk.
4.2 Physical–chemical methods with microorganisms
The combined method of applying physical–chemical methods and microorganisms is mainly used in bioleaching and biostabilization. Tan (2011) used Filamentous Bacteria to enhance the bioleaching of Acidithiobacillus ferrooxidans to treat sediments. The study found that applying Filamentous bacteria can reduce treatment time, and significantly increase Pb removal efficiency. Bioleaching is influenced by pH, ORP, HM speciation, and sediment properties. Liu (2016) investigated the effect of sodium dodecyl sulphate (SDS) on bioleaching Cd, Cu, and Zn from Xiangjiang sediment using sulfur-oxidizing bacteria (Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans). Results showed that SDS could increase the surface hydrophilia of sulfur powder, the solubility of elemental sulfur, the interaction between sulfur and sulfur-oxidizing bacteria, and the sulfur oxidation rate. When the SDS dose ranged from 0 to 0.5 g L−1, the removal rate of Cd, Cu, and Zn increased to 87.74, 84.48, and 83.08%, respectively.
Zeng et al. (2015a) studied the effect of a Fenton-like reaction in promoting the bioleaching of Aspergillus niger strain SY1 to remediate dredged sediment. The study found that adding H2O2 initiated a Fenton-like reaction, leading to further metal removal. The removal efficiencies of Cd, Cu, Pb, and Zn increased from 90 to 99.5%, 60 to 70%, 20 to 39%, and 60 to 70%, respectively. The combined process efficiently improved the dewaterability of contaminated dredged sediments.
Microorganism immobilization studies have focused on using SRB to decrease HM mobility by generating sulfides (Kumar et al. 2014, 2015; Li et al. 2016). However, three technical problems limit SRB bioremediation. First, ideal environmental conditions (e.g., pH, Eh, SO42−, T, and electron donors) for SRB growth cannot usually be met simultaneously, leading to poor field test performance (Mamouni et al. 2002; Muyzer and Stams 2008; Kumar et al. 2014). Second, SRB are sensitive to acidity and high HM concentrations, inhibiting SRB activity, particularly when SRB cells are directly suspended in the medium (Hsu et al. 2010; Zhang et al. 2016a). Finally, an excess carbon source supply can cause secondary pollution by organic matter (Min et al. 2008; Chai et al. 2009).
To address these problems, studies have tried to enhance bioremediation efficiency and stability by creating a suitable environment, using an immobilization technique and an inner cohesive nutrient source. Karri et al. (2005) found that a zero-valent iron (Fe0) can provide a low oxidation reduction environment for SRB; the hydrogen produced through the chemical corrosion of Fe0 can be used as the electron donor for SRB. Recent studies by Kumar et al. (2014, 2015) also found that adding Fe0 can strengthen the antijamming capability of SRB to pH and Eh, and enhance the biostabilization efficiency of HMs (e.g., Zn). The integrated Fe0 and SRB system was used by Li et al. (2016) to treat sediment that was heavily polluted with HMs in the Xiangjiang River in Hunan Province, China. The results showed that the remediation efficiency of Fe0 integrated with SRB was better than the single system of Fe0 or SRB on their own. This is because more stable fractions of HMs were produced in the integrated system than in the single system. The amount of HM leaching was also significantly lower in the integrated system than in the single system.
Chai et al. (2009) and Min et al. (2008) previously studied immobilized SRB and inner cohesive nutrient source techniques. These methods provided good SRB growth conditions, ensuring low effluent COD concentrations and a high HM removal rate. However, these techniques have been mainly used to treat wastewater, not soil or sediment (Hsu et al. 2010; Zhang et al. 2016a). New research conducted by Li et al. (2017) demonstrated that immobilized SRB beads with inner cohesive nutrients also effectively stabilized HMs to treat sediment. For example, the stabilization efficiencies of HMs (e.g., Cd, Cu, Pb, and Zn) were higher than using free SRB. Further, the beads can be reused several times, and secondary pollution is avoided. These improvements have enhanced the biostabilization efficiency of SRB. However, sediment composition is complex, and identifying the immobilized product is difficult. As such, the biostabilization mechanism associated with SRB in treating sediment is unclear, especially with respect to the changing bacterial community composition and the relationships between microorganisms. These are critical for biostabilization regulation and control.
4.3 Phytoremediation and microorganisms
It is common to combine phytoremediation and microorganism use; this combined approach has been widely used in soil remediation and in the ex situ remediation of dredged sediment. Microorganisms that survive in contaminated environmental media have developed many strategies to change the chemical forms of HMs in the rhizosphere (Fig. 7), and further change HM bioavailability of HMs and phytoextraction of plants (Ahemad 2014; Sarwar et al. 2017). Besides the bioaccumulation and biotransformation mechanisms of the microorganism itself, the microorganisms use the following mechanisms to promote plant growth (Ahemad 2014): antibiotic production, N2 fixation, insoluble phosphorus solubilization, siderophores production, phytohormones production, the lowering of ethylene concentrations, antifungal metabolite production, and induced systemic resistance. Microorganisms used to promote plant growth to remediate HMs mainly include saprophytic and symbiotic plant growth promoting bacteria (PGPB) and mycorrhizal fungi (saprophytic and symbiotic) (Philippot et al. 2013; Sarwar et al. 2017).
The rhizosphere (AMF arbuscular mycorrhizal fungi) (source: Philippot et al. 2013)
Considerable research shows that metal-resistant PGPB can be used as a bioinoculant or biofertilizer, significantly improving plant growth in HM-contaminated/stressed soils and enhancing phytoremediation efficiency (Ahemad 2014). Ahemad (2014) reviewed the prospects of using PGPB for bacteria-assisted phytoremediation. The research found that using bacteria with metal detoxifying traits, combining with plant-beneficial properties, is a promising, cost-effective, and environmentally friendly metal bioremediation method. Farwell et al. (2007) found that, after being treated with Pseudomonas putida UW4, shoot biomass and Ni accumulation of Brassica napus increased. When studying HM-contaminated sediments, Wan (2012) found that endophytic bacteria S. nematodiphila LRE07 became resistant to Cd, Cu, and Cr. Endophyte inoculation of S. nematodiphila LRE07 enhanced the photosynthetic pigment and growth of Solanum nigrum L, and S. nematodiphila LRE07 improved the antioxidative capability of its host plant and reduced ROS injury caused by Cd exposure. This was primarily due to increased mineral element uptake and antioxidant enzyme activities. Moreover, endophytic bacteria could survive in plants and continued its activity in the plant’s offspring seeds.
These studies were conducted using nutrient solution cultures, without pot experiment and field tests. However, the studies show that endophytic bacteria may have a significant potential in remediating soil and sediment contaminated by HMs. Liu (2015) found that the Cd-resistant microbes Empedobacter brevis and Delftia tsumhatensis could enhance the phytoremediation effect (e.g., plant growth and Cd phytoextraction) of Brassica juncea, Solanum nigrum L., Lolium perenne L., and Elymus dahuricus Turcz.
Mycorrhizal fungi are a major component of living organisms in the root zone and live in association with most higher plants in different forms (Sarwar et al. 2017). The associations between fungi and plant roots can benefit plants in various ways, including enhancing the availability of plant nutrients through an extensive hyphal network (Sarwar et al. 2017). These fungal associations can also modify the chemical composition of root exudates, soil pH, and HM bioavailability in the soil (Sarwar et al. 2017). Arbuscular mycorrhizal fungi (AMF) are the most common mycorrhizal fungi (Fig. 7), influencing HM uptake and accumulation in plants, and increasing plant tolerance to HMs. These processes are also influenced by the plant and fungi types, and soil characteristics (Merlos et al. 2016; Wu et al. 2016).
There are two main viewpoints on the effect of fungi. Some researchers have found that fungi can increase HM phytoextraction. Other researchers have found that fungi can reduce HM phytoextraction, increasing plant tolerance to HMs. Chen et al. (2003) found that inoculating Trifolium pratense L. with arbuscular mycorrhiza Glomus mosseae increased plant yields and Zn phytoextraction. Li (2011) found that Glomus mosseae can also increase the exchangeable and carbonate-bounded forms of HMs in dredged sediment. This enhanced the phytoextraction of HMs (e.g., Cd, Cr, Pb, and Zn) by maize, Lolium multiflorum Lam, and Medicogo sativa L.
However, Wu et al. (2016) found that, after being inoculated with Rhizophagus irregularis, the extraradical mycelium of arbuscular mycorrhiza could uptake and transmit Cr to the mycorrhizal roots of Taraxacum platypecidum Diels. However, Cr migration from roots to shoots was restrained, immobilizing the Cr in roots and relieving the plant of Cr phytotoxicity. Merlos et al. (2016) found that two maize cultivars with different copper tolerances (the Cu-sensitive cv. Orense and the Cu-tolerant cv. Oropesa.) experienced increased Cu concentrations after being inoculated with Rhizophagus irregularis. The mycorrhizal plant cv. Orense may have experienced an increase in Cu tolerance due to an increased induction of shoot phytochelatin biosynthesis through symbiosis. Hou et al. (2016) found that Rhizophagus intraradices can also improve Glycyrrhiza uralensis Fisch growth, including plant biomass, phosphorus, and chlorophyll levels. When applying biogas residue, Rhizophagus intraradices significantly reduced Cu and Pb concentrations in Glycyrrhiza uralensis Fisch.
4.4 Group technology
Group technology refers to the combination of three or more remediation methods. This approach is emerging as a trend in sediment remediation. During real-world, practical remediation processes, a single technique generally does not obtain an ideal result, and single techniques cannot generally be universally applied. Thus, selection methods must depend on local conditions. Combining different methods should be done using an approach that fully uses each method’s advantages, enhancing remediation results as much as possible.
However, this type of remediation method has not been widely applied in field experiments. Seidel et al. (2004) used group technology to treat HM-contaminated dredged sediments on a pilot scale. This involved conditioning the dredged sludge with plants, applying solid-bed leaching of HMs using microbially produced sulfuric acid, and revitalizing the leached sediment by adding CaCO3 and compost. The study found that Phalaris arundinacea was the most suitable for conditioning sediment; after 21 days of subsequent bioleaching, most of the metal contaminants were leached (Cd, Co, Mn, Ni, and Zn were removed at a rate of 61–81%, Cu was reduced by 21%, and Cr and Pb were almost immobilized). After alkalization of the bioleaching process water and solid-liquid separation, the leached sediment was treated with 5% pulverized limestone and 3% compost. The revitalized sediments were then exposed to the weather. After revitalization, growth rates were compared using pot experiments, with Phaseolus vulgaris, Brassica rapa, and oats Avena sativa. These tests demonstrated that the plants grew as well on revitalized sediment as on agricultural reference soil.
Yu (2007) and Yu et al. (2009) used in situ stabilization, oxidizing sulphides by aerating sediment, stabilizing HMs using phosphates, and improving the sediment dewatering capacity by injecting lime and flocculants. Ex situ composting followed, treating HM-contaminated river sediments in a practical engineering application. Results showed that, after in situ stabilization, the extracted content of HMs (e.g., Cu, Pb, and Zn) significantly decreased by 65–90%. After high-temperature aerobic composting, the exchangeable and carbonate-bounded forms of HMs (e.g., Cu and Zn) were both reduced. After the full treatment process, the sediment was a candidate for use as a fertilizer for riparian plants.
5 Choice of remediation methods
Heavy metals cannot be degraded by biochemical processes; they can only be transformed between soluble and insoluble forms (Peng et al. 2009; Akcil et al. 2015). This process changes the chemical forms and toxicity of HMs. Only determining the total content of HMs cannot provide sufficient information for their mobility and bioavailability in aquatic benthic ecosystems. Instead, HM toxicity and bioavailability depend heavily on their speciation (Peng et al. 2009; Burton 2010; Fonti et al. 2015a). The remediation methods (physical–chemical strategies, biological strategies, and combined methods) reviewed above essentially increase either metal solubility (mobilization) or stability (immobilization), to reduce HM toxicity and bioavailability (Akcil et al. 2015).
These two remediation strategies are usually used in in situ and ex situ remediation technologies, respectively (Peng et al. 2009). In in situ remediation, HM is not thoroughly removed from sediment; instead, the method enhances stability between HMs and sediments using methods such as adsorption, precipitation, and complexation. Ex situ remediation is designed to move HMs away from the sediment, and is usually followed by additional treatment of the HM water solution using physico–chemical and biological methods.
Table 3 summarizes the advantages and disadvantages of in situ and ex situ remediation. In situ remediation approaches usually include amendments, sand capping, and phytoremediation; ex situ remediation approaches usually include washing, electrochemical remediation, flotation, and ultrasonic-assisted extraction (Peng et al. 2009; Akcil et al. 2015). Adopting a remediation technology usually depends on specific sediment characteristics, such as metal loads, particle size distributions, and metal species distribution (Peng et al. 2009; Akcil et al. 2015). In addition, when choosing a remediation method, the function of water body should also be considered. This includes assessing the need to dredge the port, wharf, or river (especially urban) systems, and considering other factors, such as financial and human costs.
6 Prospects
There are many remediation technologies available to treat contaminated sediments. Immobilization methods cannot fully remove HMs from sediment; however, compared to other methods, they are less expensive, require less time, are easily operated, create less environmental disturbance, and result in low contaminant releases. Immobilization methods have significant potential as an in situ remediation technique. Compared with traditional physical–chemical immobilization methods (e.g., adding amendments), bioimmobilization techniques (e.g., phytostabilization and microorganism immobilization) have received increased attention, because of their low remediation cost and environmental compatibility. Microorganism immobilization, and its combination with other methods to restore sediments, has become a research hotspot in the environmental science and engineering fields.
Sulfur-reducing bacteria (SRB) have a better immobilization effect on HMs compared to other approaches, making it a more promising approach for sediment remediation. However, the majority of research on SRB has been in wastewater treatment settings. Few studies had been conducted using SRB for soil or sediment remediation. Complications remain with the use of SRB, including poor remediation efficiency in field tests, sensitivity to acidity and high contaminant concentrations, and excess carbon source production that causes secondary pollution of organic matter. Using SRB has exhibited low and unstable remediation efficiency with certain HMs, particularly when there are multiple contaminants. This results in less than ideal outcomes.
More positively, the successful application of immobilized SRB in wastewater treatment provides a reference for its application in other environmental matrices, such as soil and sediment. Using immobilized SRB to treat sediment contaminated by HMs may solve the problems associated with SRB, enhancing its remediation efficiency. However, for sediment remediation, research should focus on the technologies used to prepare immobilized SRB, optimizing experimental scales, and further revealing biostabilization mechanisms.
References
Ahemad M (2014) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.11.020
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36. https://doi.org/10.1016/j.jclepro.2014.08.009
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7):869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJ (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202. https://doi.org/10.1016/j.envpol.2013.11.026
Beolchini F, Fonti V, Rocchetti L, Saraceni G, Pietrangeli B, Dell’Anno A (2013) Chemical and biological strategies for the mobilisation of metals/semi-metals in contaminated dredged sediments: experimental analysis and environmental impact assessment. Chem Ecol 29(5):415–426. https://doi.org/10.1080/02757540.2013.776547
Bert V, Seuntjens P, Dejonghe W, Lacherez S, Thuy HT, Vandecasteele B (2009) Phytoremediation as a management option for contaminated sediments in tidal marshes, flood control areas and dredged sediment landfill sites. Environ Sci Pollut Res 16(7):745–764. https://doi.org/10.1007/s11356-009-0205-6
Bohuslavek J, Payne JW, Liu Y, Jr BH, Xun L (2001) Cloning, sequencing, and characterization of a gene cluster involved in EDTA degradation from the bacterium BNC1. Appl Environ Microb 67(2):688–695. https://doi.org/10.1128/AEM.67.2.688-695.2001
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45(3):198–207. https://doi.org/10.1006/eesa.1999.1860
Burton GA (2010) Metal bioavailability and toxicity in sediments. Crit Rev Environ Sci Technol 40(9-10):852–907. https://doi.org/10.1080/10643380802501567
Chai LY, Min XB, Ning T, Wang YY (2009) Mechanism and kinetics of Zn(II) removal from wastewater by immobilised beads of SRB sludge. Int J Environ Pollut 37(1):20–33. https://doi.org/10.1504/IJEP.2009.024468
Chen SY, Lin JG (2004) Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: effects of sulfur concentration. Water Res 38(14-15):3205–3214. https://doi.org/10.1016/j.watres.2004.04.050
Chen BD, Li XL, Tao HQ, Christie P, Wong MH (2003) The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 50(6):839–846. https://doi.org/10.1016/S0045-6535(02)00228-X
Chen WF, Wang W, Zhang X, Zhang J (2016a) Stabilization of heavy metals in contaminated river sediment by nanozero-valent iron/activated carbon composite. J Environ Eng 142(12):04016068. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001147
Chen WF, Zhang J, Zhang X, Wang W, Li Y (2016b) Investigation of heavy metal (Cu, Pb, Cd, and Cr) stabilization in river sediment by nano-zero-valent iron/activated carbon composite. Environ Sci Pollut Res 23(2):1460–1470. https://doi.org/10.1007/s11356-015-5387-5
Chiang YW, Santos RM, Ghyselbrecht K, Cappuyns V, Martens JA, Swennen R, Van Gerven T, Meesschaert B (2012) Strategic selection of an optimal sorbent mixture for in-situ remediation of heavy metal contaminated sediments: framework and case study. J Environ Manag 105:1–11. https://doi.org/10.1016/j.jenvman.2012.03.037
Choudhury MR, Islam MS, Ahmed ZU, Nayar F (2016) Phytoremediation of heavy metal contaminated buriganga riverbed sediment by Indian mustard and marigold plants. Environ Prog Sustain 35(1):117–124. https://doi.org/10.1002/ep.12213
Clemente R, Walker DJ, Bernal MP (2005) Uptake of heavy metals and As by Brassica juncea grown in a contaminated soil in Aznalcollar (Spain) the effect of soil amendments. Environ Pollut 138(1):46–58. https://doi.org/10.1016/j.envpol.2005.02.019
Couvidat J, Benzaazoua M, Chatain V, Bouzahzah H (2016) Environmental evaluation of dredged sediment submitted to a solidification stabilization process using hydraulic binders. Environ Sci Pollut Res 23(17):17142–17157. https://doi.org/10.1007/s11356-016-6869-9
Dixit R, Wasiullah EY, Malaviya D, Pandiyan K, Singh U, Sahu A, Shukla R, Singh B, Rai J, Sharma P, Lade H, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212. https://doi.org/10.3390/su7022189
Doni S, Macci C, Peruzzi E, Iannelli R, Masciandaro G (2015) Heavy metal distribution in a sediment phytoremediation system at pilot scale. Ecol Eng 81:146–157. https://doi.org/10.1016/j.ecoleng.2015.04.049
Fajardo C, Ortiz LT, Rodriguez-Membibre ML, Nande M, Lobo MC, Martin M (2012) Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: a molecular approach. Chemosphere 86(8):802–808. https://doi.org/10.1016/j.chemosphere.2011.11.041
Fan YC, Wang M, Tan LL, Wu QG, Ge YQ, Zhou WN, Zhang X (2016) Heavy metal-contaminated sediments in China: a review of current situation and solidification remediation. Anhui Agric Sci Bull 22:97–101 (in Chinese)
Fang W, Delapp RC, Kosson DS, van der Sloot HA, Liu J (2017) Release of heavy metals during long-term land application of sewage sludge compost: percolation leaching tests with repeated additions of compost. Chemosphere 169:271–280. https://doi.org/10.1016/j.chemosphere.2016.11.086
Farwell AJ, Vesely S, Nero V, Rodriguez H, McCormack K, Shah S, Dixon DG, Glick BR (2007) Tolerance of transgenic canola plants (Brassica napus) amended with plant growth-promoting bacteria to flooding stress at a metal-contaminated field site. Environ Pollut 147(3):540–545. https://doi.org/10.1016/j.envpol.2006.10.014
Fatoki OS, Mathabatha S (2001) An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water SA 27:233–240
Fls DA, Navoni JA, do Amaral VS (2017) The use of bacterial bioremediation of metals in aquatic environments in the twenty-first century: a systematic review. Environ Sci Pollut Res 24:16545–16559
Fonti V, Dell'Anno A, Beolchini F (2013) Influence of biogeochemical interactions on metal bioleaching performance in contaminated marine sediment. Water Res 47(14):5139–5152. https://doi.org/10.1016/j.watres.2013.05.052
Fonti V, Beolchini F, Rocchetti L, Dell’Anno A (2015a) Bioremediation of contaminated marine sediments can enhance metal mobility due to changes of bacterial diversity. Water Res 68:637–650. https://doi.org/10.1016/j.watres.2014.10.035
Fonti V, Dell’Anno A, Beolchini F (2015b) Biogeochemical interactions in the application of biotechnological strategies to marine sediments contaminated with metals. Nova Biotechnol Chim 14:12–31
Galanopoulou S, Vgenopoulos A, Conispoliatis N (2009) Anthropogenic heavy metal pollution in the surficial sediments of the Keratsini Harbor, Saronikos Gulf, Greece. Water Air Soil Pollut 202(1-4):121–130. https://doi.org/10.1007/s11270-008-9962-y
Gan M, Jie S, Li M, Zhu J, Liu X (2015) Bioleaching of multiple metals from contaminated sediment by moderate thermophiles. Mar Pollut Bull 97(1-2):47–55. https://doi.org/10.1016/j.marpolbul.2015.06.040
Gan M, Song Z, Zhu J, Liu X (2016) Efficient bioleaching of heavy metals from contaminated sediment in batch method coupled with the assistance of heterotrophic microorganisms. Environ Earth Sci 75(6):457. https://doi.org/10.1007/s12665-016-5307-0
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (GAQSIQ) (2002) Marine sediment quality (GB 18668–2002)
Gorby YA, Frank Caccavo J, Harvey Bolton J (1998) Microbial reduction of cobaltIIIEDTA- in the presence and absence of manganese (IV) oxide. Environ Sci Technol 32(2):244–250. https://doi.org/10.1021/es970516r
Groudev S, Georgiev P, Spasova I, Nicolova M (2014) Decreasing the contamination and toxicity of a heavily contaminated soil by in situ bioremediation. J Geochem Explor 144:374–379. https://doi.org/10.1016/j.gexplo.2014.01.017
Hazarika J, Ghosh U, Kalamdhad AS, Khwairakpam M, Singh J (2017) Transformation of elemental toxic metals into immobile fractions in paper mill sludge through rotary drum composting. Ecol Eng 101:185–192. https://doi.org/10.1016/j.ecoleng.2017.02.005
Heyden BPVD, Roychoudhury AN (2015) Application, chemical interaction and fate of iron minerals in polluted sediment and soils. Curr Pollut Rep 1(4):265–279. https://doi.org/10.1007/s40726-015-0020-2
Horowitz AJ (1991) A primer on sediment-trace element chemistry, 2nd edn. Lewis Publishers, Michigan
Hou SJ, Li T, Lin G, Wu SL, Chen BD (2016) The influences of biogas residue and arbuscular mycorrhizal fungi on growth and mineral nutrition of Glycyrrhiza uralensis. Acta Sci Circumst 36:4453–4460 (in Chinese)
Hsu HF, Jhuo YS, Kumar M, Ma YS, Lin JG (2010) Simultaneous sulfate reduction and copper removal by a PVA-immobilized sulfate reducing bacterial culture. Bioresour Technol 101(12):4354–4361. https://doi.org/10.1016/j.biortech.2010.01.094
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553. https://doi.org/10.1016/j.chemosphere.2017.01.130
John SG, Ruggiero CE, Hersman LE, Tung C, Neu MP (2001) Siderophore mediated plutonium accumulation by Microbacterium flavescens (JG-9). Environ Sci Technol 35(14):2942–2948. https://doi.org/10.1021/es010590g
Jośko I, Oleszczuk P, Pranagal J, Lehmann J, Xing B, Cornelissen G (2013) Effect of biochars, activated carbon and multiwalled carbon nanotubes on phytotoxicity of sediment contaminated by inorganic and organic pollutants. Ecol Eng 60:50–59. https://doi.org/10.1016/j.ecoleng.2013.07.064
Karri S, Sierra-Alvarez R, Field JA (2005) Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnol Bioeng 92(7):810–819. https://doi.org/10.1002/bit.20623
Kaya A, Yukselen Y (2005) Zeta potential of soils with surfactants and its relevance to electrokinetic remediation. J Hazard Mater 120(1-3):119–126. https://doi.org/10.1016/j.jhazmat.2004.12.023
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol Adv 27(6):799–810. https://doi.org/10.1016/j.biotechadv.2009.06.003
Kumar N, Omoregie EO, Rose J, Masion A, Lloyd JR, Diels L, Bastiaens L (2014) Inhibition of sulfate reducing bacteria in aquifer sediment by iron nanoparticles. Water Res 51:64–72. https://doi.org/10.1016/j.watres.2013.09.042
Kumar N, Chaurand P, Rose J, Diels L, Bastiaens L (2015) Synergistic effects of sulfate reducing bacteria and zero valent iron on zinc removal and stability in aquifer sediment. Chem Eng J 260:83–89. https://doi.org/10.1016/j.cej.2014.08.091
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28(1):215–225. https://doi.org/10.1016/j.wasman.2006.12.012
Li YN (2011) Contamination and bioremediation of heavy metal-organic complex in urban sewage river sediment. Ph.D. thesis, Tianjin University, Tianjin, China (in Chinese)
Li X, Wu Y, Zhang C, Liu Y, Zeng G, Tang X, Dai L, Lan S (2016) Immobilizing of heavy metals in sediments contaminated by nonferrous metals smelting plant sewage with sulfate reducing bacteria and micro zero valent iron. Chem Eng J 306:393–400. https://doi.org/10.1016/j.cej.2016.07.079
Li X, Dai L, Zhang C, Zeng G, Liu Y, Zhou C, Xu W, Wu Y, Tang X, Liu W, Lan S (2017) Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient. J Hazard Mater 324(Pt B):340–347. https://doi.org/10.1016/j.jhazmat.2016.10.067
Liu XL (2015) Micro-phyto combined remediation on heavy metal polluted channel dredged sediment. MSc thesis, Tianjin University of Science and Technology, Tianjin, China (in Chinese)
Liu W (2016) Effect of sodium dodecyl sulphate on bioleaching of Cd, Cu and Zn from Xiangjiang sediment by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. MSc thesis, Hunan University, Changsha, China (in Chinese)
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Con Tox 39(1):20–31. https://doi.org/10.1007/s002440010075
Macía P, Fernández-Costas C, Rodríguez E, Sieiro P, Pazos M, Sanromán MA (2014) Technosols as a novel valorization strategy for an ecological management of dredged marine sediments. Ecol Eng 67:182–189. https://doi.org/10.1016/j.ecoleng.2014.03.020
Mamouni RE, Jacquet R, Gerin P, Agathos SN (2002) Influence of electron donors and acceptors on the bioremediation of soil contaminated with trichloroethene and nickel: laboratory- and pilot-scale study. Water Sci Technol 45(10):49–54
Mani D, Kumar C (2014) Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol 11(3):843–872. https://doi.org/10.1007/s13762-013-0299-8
Mattei P, Cincinelli A, Martellini T, Natalini R, Pascale E, Renella G (2016) Reclamation of river dredged sediments polluted by PAHs by co-composting with green waste. Sci Total Environ 566-567:567–574. https://doi.org/10.1016/j.scitotenv.2016.05.140
Meers E, Ruttens A, Hopgood M, Lesage E, Tack FM (2005a) Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 61(4):561–572. https://doi.org/10.1016/j.chemosphere.2005.02.026
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack FM (2005b) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58(8):1011–1022. https://doi.org/10.1016/j.chemosphere.2004.09.047
Meers E, Vandecasteele B, Ruttens A, Vangronsveld J, Tack FMG (2007) Potential of five willow species (Salix spp.) for phytoextraction of heavy metals. Environ Exp Bot 60(1):57–68. https://doi.org/10.1016/j.envexpbot.2006.06.008
Merlos MA, Zitka O, Vojtech A, Azcon-Aguilar C, Ferrol N (2016) The arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci 253:68–76. https://doi.org/10.1016/j.plantsci.2016.09.010
Min X, Chai L, Zhang C, Takasaki Y, Okura T (2008) Control of metal toxicity, effluent COD and regeneration of gel beads by immobilized sulfate-reducing bacteria. Chemosphere 72(7):1086–1091. https://doi.org/10.1016/j.chemosphere.2008.04.001
Mohan RK, Brown MP, Barnes CR (2000) Design criteria and theoretical basis for capping contaminated marine sediments. Appl Ocean Res 22(2):85–93. https://doi.org/10.1016/S0141-1187(00)00003-1
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85(1-2):145–163. https://doi.org/10.1016/S0304-3894(01)00226-6
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6(6):441–454. https://doi.org/10.1038/nrmicro1892
Nguyen VK, Lee JU (2015) Effect of sulfur concentration on microbial removal of arsenic and heavy metals from mine tailings using mixed culture of Acidithiobacillus spp. J Geochem Explor 148:241–248. https://doi.org/10.1016/j.gexplo.2014.10.008
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biot 51(6):730–750. https://doi.org/10.1007/s002530051457
Niu B, Hong S, Yuan J, Peng S, Wang Z, Zhang X (2013) Global trends in sediment-related research in earth science during 1992–2011: a bibliometric analysis. Scientometrics 98:511–529
Nystroem GM, Pedersen AJ, Ottosen LM, Villumsen A (2006) The use of desorbing agents in electrodialytic remediation of harbour sediment. Sci Total Environ 357(1-3):25–37. https://doi.org/10.1016/j.scitotenv.2005.04.040
Oliveira DMD, Sobral LGS, Olson GJ, Olson SB (2014) Acid leaching of a copper ore by sulphur-oxidizing microorganisms. Hydrometallurgy 147-148:223–227. https://doi.org/10.1016/j.hydromet.2014.05.019
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421-422:3–16. https://doi.org/10.1016/j.scitotenv.2011.03.013
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J Hazard Mater 185(2-3):549–574. https://doi.org/10.1016/j.jhazmat.2010.09.082
Park J, Han Y, Lee E, Choi U, Yoo K, Song Y, Kim H (2014) Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep Purif Technol 133:291–296. https://doi.org/10.1016/j.seppur.2014.06.054
Pawlett M, Ritz K, Dorey RA, Rocks S, Ramsden J, Harris JA (2013) The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ Sci Pollut Res 20(2):1041–1049. https://doi.org/10.1007/s11356-012-1196-2
Pedersen KB, Kirkelund GM, Ottosen LM, Jensen PE, Lejon T (2015) Multivariate methods for evaluating the efficiency of electrodialytic removal of heavy metals from polluted harbour sediments. J Hazard Mater 283:712–720. https://doi.org/10.1016/j.jhazmat.2014.10.016
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2-3):633–640. https://doi.org/10.1016/j.jhazmat.2008.04.061
Perelo LW (2010) Review: in situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater 177(1-3):81–89. https://doi.org/10.1016/j.jhazmat.2009.12.090
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11(11):789–799. https://doi.org/10.1038/nrmicro3109
Polettini A, Pomi R, Rolle E, Ceremigna D, De Propris L, Gabellini M, Tornato A (2006) A kinetic study of chelant-assisted remediation of contaminated dredged sediment. J Hazard Mater 137(3):1458–1465. https://doi.org/10.1016/j.jhazmat.2006.04.022
Porzionato N, Tufo A, Candal R, Curutchet G (2017) Metal bioleaching from anaerobic sediments from Reconquista River basin (Argentina) as a potential remediation strategy. Environ Sci Pollut Res 24(33):25561–25570. https://doi.org/10.1007/s11356-016-6717-y
Qian G, Chen W, Lim TT, Chui P (2009) In-situ stabilization of Pb, Zn, Cu, Cd and Ni in the multi-contaminated sediments with ferrihydrite and apatite composite additives. J Hazard Mater 170(2-3):1093–1100. https://doi.org/10.1016/j.jhazmat.2009.05.093
Qian Y, Zhang W, Yu L, Feng H (2015) Metal pollution in coastal sediments. Curr Pollut Rep 1(4):203–219. https://doi.org/10.1007/s40726-015-0018-9
Qiao YL, Li MH, Xie PJ, Yan LR, Zhu JF (2016) A study on the absorption of cadmium and zinc in the water sediments with submerged plants. J Zhejiang Univ 43:601–609 (in Chinese)
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721. https://doi.org/10.1016/j.chemosphere.2016.12.116
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microb 65:319–321
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313(5790):1072–1077. https://doi.org/10.1126/science.1127291
Seidel H, Löser C, Zehnsdorf A, Hoffmann P, Schmerold R (2004) Bioremediation process for sediments contaminated by heavy metals: feasibility study on a pilot scale. Environ Sci Technol 38(5):1582–1588. https://doi.org/10.1021/es030075d
Seidel H, Wennrich R, Hoffmann P, Loser C (2006) Effect of different types of elemental sulfur on bioleaching of heavy metals from contaminated sediments. Chemosphere 62(9):1444–1453. https://doi.org/10.1016/j.chemosphere.2005.06.003
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, Nadeem M, Nasim W, Dumat C (2014) EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam 23(4):389–416. https://doi.org/10.1080/15320383.2014.831029
Shin W, Kim YK (2015) Stabilization of heavy metal contaminated marine sediments with red mud and apatite composite. J Soils Sediments 16:726–735
Tabak HH, Lens P, van Hullebusch ED, Dejonghe W (2005) Developments in bioremediation of soils and sediments polluted with metals and radionuclides—1. Microbial processes and mechanisms affecting bioremediation of metal contamination and influencing metal toxicity and transport. Rev Environ Sci Bio 4(3):115–156. https://doi.org/10.1007/s11157-005-2169-4
Tan XY (2011) Promotion effect of filamentous bacteria on bioleaching of heavy metals from contaminated sediment. MSc thesis, Hunan University, Changsha, China (in Chinese)
Tang W, Shan B, Zhang H, Zhang W, Zhao Y, Ding Y, Rong N, Zhu X (2014) Heavy metal contamination in the surface sediments of representative limnetic ecosystems in eastern China. Sci Rep 4:7152
Tichy R, Rulkens WH, Grotenhuis JTC, Nydl V, Cuypers C, Fajtl J (1998) Bioleaching of metals from soils or sediments. Water Sci Technol 37:119–127
Tsai LJ, Yu KC, Chen SF, Kung PY, Chang CY, Lin CH (2003) Partitioning variation of heavy metals in contaminated river sediment via bioleaching: effect of sulfur added to total solids ratio. Water Res 37(19):4623–4630. https://doi.org/10.1016/j.watres.2003.07.003
Tsezos M (2009) Metal-microbes interactions: beyond environmental protection. Adv Mater Res 71-73:527–532. https://doi.org/10.4028/www.scientific.net/AMR.71-73.527
Vandenbossche M, Jimenez M, Casetta M, Traisnel M (2014) Remediation of heavy metals by biomolecules: a review. Crit Rev Env Sci Tec 45:1644–1704
Vassilev A, Schwitzguebel JP, Thewys T, Van Der Lelie D, Vangronsveld J (2004) The use of plants for remediation of metal-contaminated soils. Sci World J 4:9–34. https://doi.org/10.1100/tsw.2004.2
Vera M, Schippers A, Sand W (2013) Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—part a. Appl Microbiol Biot 97(17):7529–7541. https://doi.org/10.1007/s00253-013-4954-2
Vitor G, Palma TC, Vieira B, Lourenço JP, Barros RJ, Costa MC (2015) Start-up, adjustment and long-term performance of a two-stage bioremediation process, treating real acid mine drainage, coupled with biosynthesis of ZnS nanoparticles and ZnS/TiO2 nanocomposites. Miner Eng 75:85–93. https://doi.org/10.1016/j.mineng.2014.12.003
Walker DJ, Clemente R, Bernal MP (2004) Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 57(3):215–224. https://doi.org/10.1016/j.chemosphere.2004.05.020
Wan Y (2012) A study on mechanism and application of endohytic bacteria in heavy metal phytoremediation. PhD thesis, Hunan University, Changsha, China (in Chinese)
Wang L, Kwok JS, Tsang DC, Poon CS (2015a) Mixture design and treatment methods for recycling contaminated sediment. J Hazard Mater 283:623–632. https://doi.org/10.1016/j.jhazmat.2014.09.056
Wang L, Tsang DC, Poon CS (2015b) Green remediation and recycling of contaminated sediment by waste-incorporated stabilization/solidification. Chemosphere 122:257–264. https://doi.org/10.1016/j.chemosphere.2014.11.071
Wen J, Yi Y, Zeng G (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69. https://doi.org/10.1016/j.jenvman.2016.04.046
Wu S, Zhang X, Chen B, Wu Z, Li T, Hu Y, Sun Y, Wang Y (2016) Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ Exp Bot 122:10–18. https://doi.org/10.1016/j.envexpbot.2015.08.006
Xie PJ, Li MH, Yan LR, Qiao YL (2016) Remediation of Cu and Pb co-polluted sediments by three submerged plants. J Agro-Environ Sci 35:757–763 (in Chinese)
Xu Y (2017) Stabilization of heavy metal-contaminated sediment with a chelator and humic acid mixture. Water Air Soil Poll 228(1):20. https://doi.org/10.1007/s11270-016-3198-z
Yan M, Zeng G, Li X, He J, Chen G, Huang D, Tang L, Lai C, Zhang C, Li X, Wang L, Guo Z, Tao W (2017) Incentive effect of bentonite and concrete admixtures on stabilization/solidification for heavy metal-polluted sediments of Xiangjiang River. Environ Sci Pollut Res 24(1):892–901. https://doi.org/10.1007/s11356-016-7527-y
Yu GW (2007) In situ sediment remediation of heavily polluted Tidal River: technologies research and application. PhD thesis, Sun Yat-Sen University, Guangzhou, China (in Chinese)
Yu G, Lei H, Bai T, Li Z, Yu Q, Song X (2009) In-situ stabilisation followed by ex-situ composting for treatment and disposal of heavy metals polluted sediments. J Environ Sci 21(7):877–883. https://doi.org/10.1016/S1001-0742(08)62357-8
Zeng X, Twardowska I, Wei S, Sun L, Wang J, Zhu J, Cai J (2015a) Removal of trace metals and improvement of dredged sediment dewaterability by bioleaching combined with Fenton-like reaction. J Hazard Mater 288:51–59. https://doi.org/10.1016/j.jhazmat.2015.02.017
Zeng X, Wei S, Sun L, Jacques DA, Tang J, Lian M, Ji Z, Wang J, Zhu J, Xu Z (2015b) Bioleaching of heavy metals from contaminated sediments by the Aspergillus niger strain SY1. J Soils Sediments 15(4):1029–1038. https://doi.org/10.1007/s11368-015-1076-8
Zhang M, Wang H (2016) Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner Eng 92:63–71. https://doi.org/10.1016/j.mineng.2016.02.008
Zhang C, Yu ZG, Zeng GM, Jiang M, Yang ZZ, Cui F, Zhu MY, Shen LQ, Hu L (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Int 73:270–281. https://doi.org/10.1016/j.envint.2014.08.010
Zhang M, Wang H, Han X (2016a) Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 154:215–223. https://doi.org/10.1016/j.chemosphere.2016.03.103
Zhang YH, Huang LL, Yang LK, Liu CY, Wang CJ, Zhang ZB, Sun CZ (2016b) In-situ remediation technology for river sediments contaminated by heavy metals. Water Purif Technol 35:26–32 (in Chinese)
Zhu QQ, Wang ZL (2012) Distribution characteristics and source analysis of heavy metals in sediments of the main river systems in China. Earth Environ 40:305–313 (in Chinese)
Zoubeir L, Adeline S, Laurent CS, Yoann C, Truc HT, le Benoit G, Federico A (2007) The use of the Novosol process for the treatment of polluted marine sediment. J Hazard Mater 148(3):606–612. https://doi.org/10.1016/j.jhazmat.2007.03.029
Funding
This work was supported by the National Natural Science Foundation of China (51778031, 21707006 and 51708012), Beijing Natural Science Foundation (8142027), Excellence Foundation of BUAA for Ph.D. Students (2017068), and the open projects of collaborative innovation center of Suzhou Regional Development (2015SZXTZXKF04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gijs D. Breedveld
Rights and permissions
About this article
Cite this article
Peng, W., Li, X., Xiao, S. et al. Review of remediation technologies for sediments contaminated by heavy metals. J Soils Sediments 18, 1701–1719 (2018). https://doi.org/10.1007/s11368-018-1921-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-1921-7