Abstract
Purpose
Grasslands are an important ecosystem covering about a quarter of the earth’s surface. Different management practices of grassland ecosystems can have a major impact on the sustainability of these ecosystems. The objective of this study was to determine the impact of different pasture management practices on soil properties and microbial communities in the semi-arid grassland ecosystem in Inner Mongolia.
Materials and methods
Long-term experimental plots were established in the semi-arid grasslands of Inner Mongolia to study the effect of different grazing practices on soil properties and microbial communities. The treatments included (1) enclosure from grazing since 1983 (E83), (2) enclosure from grazing since 1996 (E96), and (3) continuous free grazing (FG). We collected the soil samples from these treatments to study soil properties and microbial biomass abundance and diversity. An incubation study was also conducted using soils from E96 and FG treatments to determine the growth responses of ammonia oxidizers to urea addition.
Results and discussion
Soil organic matter and total N increased when the grassland was enclosed from grazing, but soil fertility did not increase further with continued enclosure extending from 1996 to 1983. Enclosure also increased microbial biomass but did not significantly affect the microbial diversity. Both ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) grew when supplied with urea-N, but the growth rate was higher in the soil from FG than in the soils from enclosed areas. The phospholipid fatty acids (PLFAs) of bacteria i15:0, 16:1 ω7c, 16:1 ω5c, 16:0, 18:1 ω7c, and actinomycetes 10-Me-16:0 used the most of the 13C-urea in both the E83 and FG soils. There was higher incorporation of 13C in PLFA 16:0 in the E83 soil after 3 and 7 days incubation, compared with the FG soil, suggesting higher metabolic activity in the E83 soil than the soil from the FG treatment. Most of the effects by the different pasture management practices were confined to the surface soil (0–20 cm), and there was minimal effect in the subsoils (below 20 cm).
Conclusions
These results suggest that enclosure of grassland from grazing not only affects soil fertility but also microbial biomass and ammonia-oxidizing populations. Microbial communities are sensitive to pasture management changes, and these have implications to nutrient cycling and management in these grassland ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Grasslands cover approximately 25.4 % of the total land area of the earth (Watson et al. 2000). At present, China’s total grassland area is approximately 400 million hectares, accounting for 11.7 % of the earth’s total grassland area and 41.7 % of China’s land area (Liao and Jia 1996). Grassland is an important terrestrial ecosystem and one of the most widely distributed ecosystems in the world. In terms of climate change, grassland has a significant impact on global carbon source/sink dynamics and carbon cycling (Cheng et al. 2012).
Overgrazing has not only decreased the productivity of grasslands but also resulted in a dramatic decline in the quality of forage and plant diversity, thus having a negative impact on the sustainability of grassland (Li et al. 1994). This is manifested not only by a loss of biomass but also by the invasion of undesirable plant species (Karnieli et al. 2013). The original dominant species in Inner Mongolia grassland were Leymus chinensis and Stipa grandis, but were gradually dominated by Artemisia frigida, due to overgrazing (Li and Wang 1999). Overgrazing also has adverse impacts on soil physical, chemical, and biological properties (Li and Zhao 2005).
Large areas of grasslands in China have been severely degraded due to overgrazing (Chen 1990). In order to prevent further degradation and restore degraded grasslands, areas of degraded land are fenced off from grazing for periods of time. During this recovery phase of degraded grasslands, pasture composition and microbial communities undergo significant changes. For example, Shan et al. (2008) found that the productivity and species diversity of severely degraded grassland increased after 14 years of enclosure but decreased after 14 years of enclosure. The soil nutrient contents also increased significantly during the restoration phase (Shan et al. 2009).
Our knowledge and understanding of microbial community composition and abundance in relation to the different phases of grasslands management is limited. This limits our ability to predict and manage grasslands under a changing climate. It also limits our understanding on how grasslands will impact on and respond to climate change. Therefore, further studies are required to determine changes in microbial communities in grasslands as affected by animal grazing and different restoration phases of grassland management.
The objective of this study was to determine the effect of different pasture management on soil nutrition availability and microbial communities in the semi-arid grasslands of Inner Mongolia.
2 Materials and methods
2.1 Study site
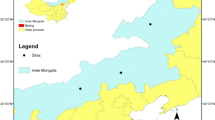
The research site, Baiyinxile grassland, is situated in Eastern Inner Mongolia (N 43° 33′ 12″–43° 33′ 35″, E 116° 42′ 26″–116° 42′ 31″; 1000–1500 m above sea level). The region has a temperate semi-arid continental climate with an annual average temperature of −0.1 °C, annual mean precipitation of 280–350 mm, and annual evaporation 4–5 times that of the precipitation. From March to May, the weather is windy and the monthly average wind speed is 4.9 m s−1. Frost-free period is about 90 days. The soil is chestnut soil (Mollisols), on gently sloping terrain. L. chinensis and S. grandis are typical original pasture species in the region, but because of overgrazing, other invading species, including Agropyron michnoi, A. frigida, Potentilla acaulis, and Cleistogenes squarrosa have become dominant species.
2.2 Field experimental design
Three pasture management practices (two different periods of enclosure and one without enclosure with continuous grazing) were studied in terms of their impact on soil properties and microbial communities. One treatment of non-grazed enclosure plots had been enclosed free from grazing since 1983 (E83), and the plot size was 600 × 400 m; another non-grazed enclosure treatment had been enclosed since 1996 (E96), and the plot size was 80 × 400 m. The non-enclosed plots with continuous grazing was used as a control treatment where nomads were allowed to graze throughout the year since 1983 (FG), and the plot size was 600 × 50 m. The plots were arranged in a random design in the field.
Five soil cores at three different depths (0–20, 20–40, and 40–60 cm) were collected randomly from three plots for each treatment in October 2013, and they were mixed for each depth to form one composite sample for each plot. The soil samples were placed in sterile plastic bags, sealed and transported to the laboratory on ice. One part was sieved through 2.0 mm for analysis of chemical properties according to the methods described by Sparks et al. (1996), subsamples were stored at −80 °C for DNA extraction and molecular analysis, and another part was incubated by stable isotope probing to determine differences of soil microbial structure community immediately after arrival to the laboratory.
2.3 Soil microbial biomass analysis
The fumigation extraction method was used to determine soil microbial biomass C (MBC) and soil microbial biomass N (MBN) (Brookes et al. 1985; Vance et al. 1987). Briefly, 10 g (oven-dry equivalent) was weighed into 100 mL bottles. Three unfumigated samples from each treatment were immediately extracted with 50 mL 1.0 M K2SO4 on a rotary shaker at 220 rpm for 30 min and then filtered through medium-speed qualitative filter paper (Chantigny et al. 2008). The remaining samples (triplicates) were fumigated with alcohol-free chloroform for 24 h at 25 °C. Excess chloroform was removed by repeated evacuation, and then the samples were extracted and filtered as described above. The filtrates were stored at −20 °C prior to analysis for MBC and MBN.
2.4 Stable isotope probing microcosms
Soil microcosms were constructed by adding approximately 50 g (dry weight basis) of soil from the E83 and FG (0–20 cm) treatments to 100 mL plastic cans. The plastic cans were covered with lids which had a hole (the diameter is 1 cm) pierced on the top. The soils were maintained at 60 % water holding capacity in the soil microcosms for 1 week prior to use. Following this pre-incubation period, soils were subjected to one of three treatments: CK (0), 13C (200 mg kg−1 13C- urea-N), 12C (200 mg kg−1 12C- urea-N). Either 2.72 mL of sterile deionized water (control) or 2.72 mL of a solution containing 20 mg mL−1 urea-N labeled or unlabeled (equivalent to additions of 200 mg kg−1 urea-N) was added to the soils. The labeled urea applied had a 13C enrichment at 99 atom % (Sigma-Aldrich, USA). This gave a total of six treatments (CK-E83, 13C-E83, 12C-E83 and CK-FG, 13C-FG, 12C-FG) with three replicates. The microcosms were randomly arranged and incubated at 4 °C in the dark. Deionized water was added every day to maintain constant water holding capacity. A sample of soil was taken from each microcosm after 1, 3, and 7 days following urea addition. Samples were freeze-dried within 3 h and stored at −80 °C for lipid extraction and DNA extraction. Phospholipid fatty acids (PLFAs) were extracted from soils 1 and 3 days after urea addition, and DNA were extracted from the soil samples taken after 7 days to analyze ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB).
2.4.1 Lipid extraction and PLFA analyses
Lipid extraction and PLFA analyses were performed using the modified Bligh and Dyer method (1993). Briefly, approximately 3.0000 g freeze-dried soil was extracted twice using 22.8 mL single-phase mixture (1:2:0.8 v/v/v) of chloroform: methanol and citrate buffer (0.15 M, pH 4.0). The phospholipids were separated from neutral and glycolipids on a silicic acid column and were methylated using a mild alkaline methanolysis to derivatize them to their respective fatty acid methyl esters (FAMES). The FAMES were separated on a gas chromatograph equipped with a flame ionization detector (MIDI Inc., Newark, DE, USA). Methyl nona decanoate fatty acid (19:0) was added, prior to the derivatization, as an internal concentration standard to quantify the phospholipids. The 13C/12C ratios of individual PLFAs were analyzed by GC-C-IRMS using a Trace GC Ultra gas chromatograph with combustion column attached via a GC Combustion III to a Delta V Advantage isotope ratio mass spectrometer (Thermo, Germany). The total 13C enrichment rate (pt) of PLFAs in each soil were calculated according to the formulas described by Wang et al. (2014). PLFAs i15:0, a15:0, and i16:0 were used as biomarkers for Gram-positive bacteria (16:1ω7c, 18:1ω7c, and cy 19:0ω7c) and for Gram-negative bacteria (Wilkinson 1988) (18:2ω6c and 18:1ω9c) for saprophytic fungi, and 16:1ω5c for arbuscular mycorrhizal fungi (AMF) (Olsson 1999).
2.4.2 Quantification of AOA and AOB genes by real-time PCR
LightCycler®480 Real-Time PCR System (Roche Applied Science, CA) was used for real-time PCR. PCR primers used to amplify regions of functional genes from ammonia-oxidizing bacteria are Arch-amoAF, Arch-amoAR, amoA1F, and amoA2R. A typical 20 μL reaction contained 10 μL of SYBR® Premix Ex Taq™ II (TaKaRa), 0.4 μL each primer for final concentrations of each primer pair combination used. Details of the method have been given in Di et al. (2014).
2.5 Statistical analysis
Means and least significant difference at the 5 % significance level were calculated by one-way ANOVA. The molar percent of the individual PLFAs and percentage distribution of 13C among total PLFA-C were standardized to unit variance (scaling) after generating a correction matrix and were then subjected to principal component analysis (PCA) to explain the variation of data. All statistical analyses were made using Excel 2010 and SPSS 20.0.
3 Results
3.1 The influence of different management practices on soil nutrient status
Basic chemical properties of soil samples are given in Table 1. There was substantial field variability of the soil properties among the replicate plots. Soil pH increased with soil depth, whereas soil available K, organic matter, and total N generally decreased with depth. In the 0–20 cm layer, the organic matter content in the E96 treatment was 52.3 % higher than in the FG treatment, and 36.0 % higher in the E83 than in the FG treatment. Total nitrogen in the E96 and E83 treatments was 53.2 and 55.7 % higher than in the FG treatment, at 0–20 cm, respectively, and was 17.3 and 26.9 % higher than in the FG treatment at 20–40 cm depth. The CEC of soil samples followed the order: E83 > E96 > FG at 0–20 cm. However, the management practices did not lead to consistent soil fertility changes in the subsoil layers.
3.2 Impact of management on microbial communities
The MBC followed the order E83 > E96 > FG in the 0–20 cm depth (Table 2). The MBN was similar in the E83 and E96 treatments and both were higher than in the FG treatment. The MBC in the 20–40 cm layer followed the order E96 > E83 > FG. There was no difference in MBN between the treatments at the 20–40 cm layer. However, at the 40–60 cm depths, the MBC and MBN were higher in the FG treatment than in the two enclosure treatments.
3.3 Stable isotope probing
3.3.1 The diversity response of soil microbial community to urea addition
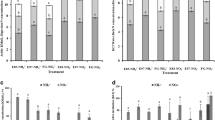
Soils under the E83 and FG treatments (only these two treatments were chosen) contained a variety of PLFAs comprising of saturated, unsaturated, methyl-branched, and cyclopropane fatty acids (Fig. 1). Twenty-four PLFAs with chain lengths from C14 to C20 were identified. The PLFA patterns varied in response to FG and E83 as revealed by their relative abundance (Fig. 1). Most 13C in both the free-grazing and enclosed treatments was incorporated into i15:0, 16:1 ω7c, 16:1 ω5c, 16:0, 10-Me-16:0, and 18:1 ω7c; a lesser amount was also incorporated into a15:0, i16:0, i17:0, 18:1ω9c, and 18:0. The urea-derived C in 16:1ω5c from E83 was almost twice that from FG at the third day. These above lipids together accounted for 95 % of the total 13C that was incorporated (Fig. 1c, d). Most 13C incorporation into PLFAs at the third day decreased compared with day 1 in FG, and the 13C incorporated into i16:0 in the E83 treatment declined sharply while increased in the FG treatment.
Moles C of individual and labeled 13C of the total PLFAs in E83 and FG. a Moles C in E83 after 1 and 3 days incubation, b moles C in FG after 1 and 3 days incubation, c relative abundance (mol %) of individual 13C-PLFA from urea-derived 13C in E83 after 1 and 3 days incubation, and d relative abundance (mol %) of individual 13C-PLFA from urea-derived 13C in FG after 1 and 3 days incubation
To investigate the effect of urea addition on soil microbial community composition of E83 and FG, PCA was performed on the molar percentage of individual PLFAs to the total PLFA pool (Fig. 2). The first principal component (PC1) accounted for 50.0 % of the variation and the second (PC2) showed a further 21.7 % variation as 12C-PLFA (Fig. 2b). The PCA showed that the first principal component (PC1) accounted for 42.8 % of the variation and the second (PC2) accounted for 32.3 % variation as 13C-PLFA (Fig. 2c). In contrast to percentage molar abundance of total (12C + 13C) PLFAs, the 13C incorporation showed no significant correlation between either PC1 or PC2 and soil management (Figs. 1a, b and 2c).
a A loading plot of two components from the PCA on the PLFA composition. Percentage of variance explained by each component is indicated within parenthesis on each axis; b principal analysis of 12C-PLFA composition at 1 and 3 days; c principal component analysis of 13C-PLFA composition of soil samples at 1 and 3 das
3.3.2 The growth response of ammonia oxidizers to urea addition
Ammonium-N concentration rapidly reached to a maximum due to urea hydrolysis and then decreased gradually. The nitrate-N concentration increased after the application of urea, following the nitrification of ammonium. The nitrate-N concentration in the FG treatment was much higher than that in the E83 in the third and seventh day (Fig. 3).
Following urea application, both AOB and AOA communities grew, as indicated by the increased abundance of amoA gene copy numbers after 3 and 7 days incubation (Fig. 4). However, the growth rate was higher in the soil from the FG treatment than in the soil from the E83 treatment. The growth rate is also higher for AOB than for AOA following urea application.
4 Discussion
4.1 Basic chemical properties
Results from this study clearly showed that pasture management had a major impact on soil properties and soil nutrient availability. Overall, soil nutrient fertility status increased when the grassland was enclosed; however, the fertility did not always continue to increase when the enclosure period increased from 1996 to 1983. This would indicate that as the enclosure period increased, the soil nutrient status would probably have reached a stable level and any further enclosure from grazing would probably not substantially change the soil nutrient status further (Ao et al. 2011). Therefore, there might be an optimum period of enclosure after which the grassland can be reverted back to grazing again. The challenge therefore is to determine the optimum enclosure that is required to restore the pasture and soil conditions. The decrease in soil pH in the surface layer of this alkaline soil was attributed to atmospheric acid deposition and plant root excretions in this alkaline soil (Grennfelt and Hultberg 1986). However, the impact of pasture management on the soil properties was mainly confined to the surface layer and the impact on subsurface soils was minimal.
4.2 Soil microbial diversity and biomass
Results from this study showed that pasture management also had an impact on microbial biomass. Microbial biomass was kept at a higher level under enclosure than under grazing, demonstrating that enclosure helped to restore the soil microbial biomass. Similar results have also been reported by other researchers (Holt 1997; Ma et al. 2005; Shrestha and Stahl 2008). For example, Holt (1997) reported that after 6–8 years of heavy beast grazing of two semi-arid grasslands in northeast Australia, soil microbial biomass decreased by about 24–51 %. Ma et al. (2005) also reported that after 22 years of sheep grazing of the L. chinensis grassland in Inner Mongolia, the surface soils’ microbial biomass carbon decreased by about 27.9–38.2 %.
However, the species richness indices and diversity indices did not change significantly in the different treatments. Therefore, grazing decreased the microbial biomass, but did not result in major changes in diversity of the microbial communities.
4.3 Stable isotope probing microcosms
In the current study, the effect of urea on microbial community composition by 13C-PLFA, through the addition of C and N, was much smaller than the effects of pasture management (Fig. 2), indicating that pasture management plays an important role in determining microbial composition of soils (Chaparro et al. 2012). However, the PCA charts of total PLFA molar abundance and 13C-PLFA molar abundance (Figs. 1 and 2) indicated that urea addition did affect the allocation of urea-derived 13C to microbes more than overall microbial community composition, and this agrees with findings reported by Hamer et al. (2009). Over 75 % of the urea-derived C was found in fungi 18:1ω9c, actinomycetes 10-Me-16:0, Gram-negative bacteria 16:1ω7c, 16:1ω5c, and Gram-positive bacteria i15:0, 16:0 (Fig. 1). PLFA 16:0 is widespread in different microbial populations, and is often used as a tool for the evaluation of total microbial biomass (Salomonová et al. 2003). The much higher incorporation of 13C in PLFA 16:0 in the E83 soil after 3 and 7 days incubation, compared with the FG soil, would suggest higher metabolic activity in the E83 soil than the soil from the FG treatment.
It is interesting that the copy numbers of ammonia-oxidizing bacteria in the soil from the FG treatment was higher than that in the soil from the E83 treatment, indicating that ammonia-oxidizing bacteria communities might have been conditioned by the continued N cycling in the forms of urine and dung from the grazing animal. Therefore, the ammonia-oxidizing bacteria communities responded more rapidly following the addition of the N substrate in the form of urea. It is also interesting that both AOA and AOB grew following urea application. This was in contrast to earlier findings where AOB rather than AOA grew following the additions of animal urine on a fertile dairy pasture soil (Di et al. 2009, 2014). The AOB and AOA growth following urea application in this study may also reflect the low fertility nature of this grassland soil compared with the high fertility dairy pasture soils used in the study by Di et al. (2009, 2014). Therefore, in these semi-arid low fertility grassland soils, AOA may play a significant role in N cycling, and this aspect requires further investigation.
5 Conclusions
This study clearly showed that pasture management of semi-arid grasslands has a major impact on the soil fertility, microbial biomass, and ammonia-oxidizing populations. Although soil nutrient status was higher in the soil under enclosure since 1997 but the soil nutrient status did not continue to increase with increased length of enclosure extending back to 1983. It is therefore important to define the optimum period for enclosure for different ecosystems. This study also showed that enclosure from animal grazing also had an impact on the microbial biomass but not on the microbial diversity. Enclosure also resulted in slower growth of ammonia-oxidizing bacteria in response to urine application. The bacteria i15:0, 16:1ω7c, 16:1ω5c, 16:0, 18:1ω7c, and actinomycetes 10-Me-16:0 used the most of 13C-labeled urea. The implications of this on nutrient cycling and grassland ecosystem function requires further investigation.
References
Ao YM, Jiao Y, Xu Z (2011) The changes of carbon and nitrogen storage of plant-soil system of enclosure years in typical steppe. Ecol Envir Sci 20:1403–1410
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Chantigny MH, Angers DA, Kaiser K, Kalbitz K (2008) Extraction and characterization of dissolved organic matter. Soil sampling and methods of analysis. CRC Press, pp 617–635
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fert Soil 48:489–499
Chen ZZ (1990) Natural degradation of grassland ecosystem and its regulation in our country. China’s prevention and control of land degradation research. Science and Technology of China Press, pp 86–89
Cheng JM, Cheng J, Yang XM, Liu W, Chen FR (2012) Spatial distribution of carbon density in grassland vegetation of the Loess Plateau of China. Acta Ecol Sin 32:226–237
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68
Grennfelt P, Hultberg H (1986) Effects of nitrogen desposition on the acidification of terrestrial and aquatic ecosystems. Water Air Soil Pollut 30:945–963
Hamer U, Potthast K, Makeschin F (2009) Urea fertilisation affected soil organic matter dynamics and microbial community structure in pasture soils of Southern Ecuador. Appl Soil Ecol 43:226–233
Holt JA (1997) Grazing pressure and soil carbon, microbial biomass and enzyme activities in semi-arid northeastern Australia. Appl Soil Ecol 5:143–149
Karnieli A, Bayarjargal Y, Bayasgalan M, Mandakh B, Dugarjav C, Burgheimer J (2013) Do vegetation indices provide a reliable indication of vegetation degradation? A case study in the Mongolian pastures. Int J Remote Sens 34:6243–6262
Li YH, Wang S (1999) Response of plant and plant community to different stocking rates. Grassland of China 3:11–19
Li YQ, Zhao JB (2005) Effects of overgrazing on ecological and environmental construction and measurement. Journal of Desert Research 25:404–408
Li ZH, Hao P, Liu ZL (1994) Chinese grassland degraded community recovery succession research. Journal of Inner Mongolia University 1:88–98
Liao GF, Jia YL (1996) Rangeland resources of China. Chinese Science and Technology Press, Beijing
Ma XZ, Wang YF, Wang SP, Wang JZ, Li CS (2005) Impacts of grazing on soil carbonfractions in the grasslands of XiLin river basin, Inner Mongolia. Acta Phytoecologica Sinica 29:569–576
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Salomonová S, Lamačová J, Rulík M, Rolčík J, Čáp L, Bednář P, Barták P (2003) Determination of phospholipid fatty acids in sediments. Acta Univ Palacki Olomuc Facultas Rerum Nat Chem 42:39–49
Shan GL, Xu Z, Ning F, Ma YB, Li LH (2008) Iinfluence of exclouse year on community structure and species diversity on a typical steppe. Acta Prataculturae Sinica 17:1–8
Shan GL, Xu Z, Ning F, Jiao Y (2009) Influence of seasonal exclosure on plant and soil characteristics in typical steppe. Acta Prataculture Sinica 18:3–10
Shrestha G, Stahl PD (2008) Carbon accumulation and storage in semi-arid sagebrush steppe: effects of long-term grazing exclusion. Agr Ecosyst Environ 125:173–181
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner, ME (1996) Methods of soil analysis. Part 3-Chemical methods. Soil Science Society of America Inc.
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang J, Thornton B, Yao HY (2014) Incorporation of urea-derived 13C into microbial communities in four different agriculture soils. Biol Fert Soil 50:603–612
Watson RT, Noble IR, Bolin B, Ravindranath NH, Verardo DJ, Dokken DJ (2000) Land use, land-use change and forestry: a special report of the Intergovernmental Panel on Climate Change. Cambridge University Press
Wilkinson SG (1988) Gram-negative bacteria. Microbial Lipids 1:299–488
Acknowledgments
This work was funded by the National Key Basic Research Program of China (2014CB138801) and by the Natural Science Foundation of China (41271272 and 41401266).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Rights and permissions
About this article
Cite this article
Liu, J., Zhang, Q., Li, Y. et al. Effects of pasture management on soil fertility and microbial communities in the semi-arid grasslands of Inner Mongolia. J Soils Sediments 16, 235–242 (2016). https://doi.org/10.1007/s11368-015-1210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1210-7