Abstract
Purpose
The aims of this study were to isolate an alkaliphilic humus-reducing bacterium, investigate the fastest microbial reduction of humus analog as affected by different cultivation, and examine its ability for iron(III) oxide reduction and organochlorine pollutants (OCPs) degradation.
Materials and methods
A strain of pure culture, designated as HN01, was isolated from cassava dreg compost using anaerobic enrichment procedure with glucose as the electron donor and anthraquinone-2,6-disulphonate (AQDS) as the sole terminal electron acceptor. The isolate strain was identified using phenotypic and phylogenetic analysis. Iron(III) oxides and OCPs were chosen as potential electron acceptors. Strict anaerobic techniques and sterile conditions were applied throughout the incubation experiments, purged with O2-free N2 for 15 min. The concentration of reduced AQDS and Fe(II) was then quantified using a UV–vis spectrophotometer. The concentration of OCPs was analyzed by gas chromatography with a micro-electron capture detector. Cell number was determined by direct plate counting on aerobic Luria–Bertani medium agar medium at pH 9.
Results and discussion
(1) Strain HN01 was identified as Kocuria rosea, and the AQDS reduction by HN01 was observed in NaCl concentrations below 12 % (w/v) (optimum, 10 %) and pH ranges of 6.0–10.0 (optimum, 9.0) with sucrose as electron donor at 30 °C; (2) glucose, sucrose, methanol, ethanol, glycerol, and acetate were the favorable electron donors for AQDS reduction by strain HN01; (3) the strain had the ability of reducing iron(III) oxides in the presence of sucrose at pH 9.0 and its Fe(III)-reducing capacity ranked as goethite (α-FeOOH) > lepidocrocite (γ-FeOOH) > haematite(α-Fe2O3); and (4) the strain could effectively dechlorinate p,p′-DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane), and the dechlorination rate reached 71.3 %.
Conclusions
This is the first report of a strain of K. rosea capable of reducing AQDS, iron (III) oxides, and p,p′-DDT, which extends the diversity of the alkaliphilic and halotolerant humus/Fe(III)-reducing bacterium associated with dechlorination. The strain may have the potential to be used for bioremediation of an anoxic alkaline wastewater or site contaminated with OCPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The decay of soil and sedimentary organic matter yields organic compounds with a high molecular weight, termed humic substances (humus) (Stevenson 1994). Humus can serve as the terminal electron acceptor for microbial respiration coupling with organic compound oxidation, and this biochemical process driven by microbes has been recognized as the main pathway for humus reduction in soils and sedimentary environments (Gralnick and Newman 2007; Lovley et al. 1996; Roden et al. 2010; Scott et al. 1998). The microbe-reduced humus can transfer electrons to insoluble minerals and refractory organics, such as iron(III) oxides (Liu et al. 2007; Wolf et al. 2009), azo dyes (Hong et al. 2007; Rodrigues da Silva et al. 2012), nitroaromatic compounds (Bhushan et al. 2006), and chlorinated organic contaminants (Cao et al. 2012; Wang et al. 2009). Therefore, there is a growing interest in humic-reducing microorganisms (HRMs) and humus-mediated reduction of extracellular substrates in recent years (Lovley et al. 1996; Gralnick and Newman 2007; Roden et al. 2010; Wu et al. 2012).
It is well recognized that HRMs are widespread in nature; more than a hundred HRMs have been isolated from a broad diversity of environments, mainly with circumneutral pH (Lovley et al. 2004; Wu et al. 2009). However, there are few reports on alkaliphilic HRM and the process that it can mediate substrate transformation under alkaline conditions. Only relatively recently have researchers turned their attention to alkaliphilic HRM, and several alkaliphilic humus-reducing bacteria were isolated, including Alkaliphilus peptidofermentans, Bacillus pseudofirmus, Corynebacterium humireducens, Natronincola ferrireducens, and Natronincola peptidovoranshave (Ma et al. 2012; Wu et al. 2011; Zhilina et al. 2009a, b).
The solubility of humus is strongly pH dependent, and a high pH would increase the fraction of dissolved humus that is accessible to bacteria. However, in alkaline environments, iron species are generally insoluble, solid-phase minerals; humus-mediated reduction of iron(III) oxides will become significantly higher compared with circumneutral pH levels. The mineralogy and geochemistry of alkaline systems are complex and cannot be simply deduced from those studies conducted under near or below neutral pH conditions. For instance, Fe(III) reduction had not been recognized to occur beyond a pH of 9.0 before alkaliphilic bacteria capable of Fe(III) reduction was isolated from alkaline environments (Gorlenko et al. 2004; Ma et al. 2012; Ye et al. 2004). Therefore, the finding of alkaliphilic HRM is helpful in thoroughly understanding the mechanisms of electron transferring between cells and extracellular electron acceptors. Moreover, these strains might play an important role in organic matter oxidation, element biogeochemical cycles, and pollutant detoxification in alkaline environments (Hobbiea et al. 2012; Wu et al. 2012).
We conducted a study on an isolate from a cassava dreg composting reactor that was continuously reacting for 35 days and in mesophilic and maturation phase of pH 8.5–9.0 to determine the isolate's possible humus-reducing activity, optimum humus reduction conditions, and alternative electron acceptors for its anaerobic metabolism. The humus analog anthraquinone-2,6-disulfonate (AQDS) was used in the enrichment and reduction experiments. The specific objectives of this study were to: (1) characterize and identify the new isolate, alkaliphilic strain HN01; (2) determine its humus-reducing activity and optimum reducing conditions; (3) explore its potential capacity of reducing iron(III) oxides (goethite (α-FeOOH), lepidocrocite (γ-FeOOH) or hematite(α-Fe2O3)) and degrading organochlorine pollutants (OCPs) under alkaline conditions. To our knowledge, this is a new research on describing the capacity of Kocuria rosea to reduce humus, Fe(III), and OCPs.
2 Materials and methods
2.1 Chemicals
α-FeOOH was synthesized according to the procedures of Li et al. (2008). Preparation of γ-FeOOH and α-Fe2O3 followed the method described by Li et al. (2007a). The hexane of high-performance liquid chromatography grade and the anhydrous sodium sulfate of analytical grade were purchased from Guangzhou Chemical Reagent Co. (China). AQDS of 98.6 % purity was purchased from Sigma-Aldrich (Tokyo, Japan). γ-Hexachlorohexanes (γ-HCH; 98.2 %), 1,1-(2,2,2-trichloroethylidene)bis[4-chloro-benzene] (p,p′-DDT; 98.6 %), and 1-chloro-4-[2,2,2-trichloro-1-(2-chlorophenyl)ethyl]benzene (o,p′-DDT; 98.6 %) of analytical grade were obtained from Accu Stangard (New Haven, USA). A mixture of standard solution containing p,p′-DDT, p,p′-DDE, p,p′-DDD, o,p′-DDT, o,p′-DDE, and o,p′-DDD, with 10.0 mg l−1 per compound was purchased from Labor Dr. Ehrenstorfer, Germany. All of the chemicals were used as received, without further purification.
2.2 Enrichment and isolation
According to previous studies, quinones serve as electron-accepting moieties when microorganisms transfer electrons to humus, and AQDS has been used extensively as a humus analog in studies on humus for microbial respiration (Lovley et al. 1996; Scott et al. 1998). Thus, we selected AQDS to replace humus in the enrichment and reduction experiments. The inoculum source for enrichment was the cassava dreg compost in mesophilic and maturation phase of pH 8.5–9.0. Primary enrichments were initiated in anaerobic culture serum bottles (purged with O2-free N2 for 15 min, sealed with a butyl-rubber stopper and aluminum cap) containing mineral salts medium (MSM) added with 5 mmol l−1 sucrose (electron donor) and 0.5 mmol l−1 AQDS (electron acceptor). The preparation of MSM was performed according to Wu et al. (2011). The bottles were cultured at 30 °C in the dark. Positive enrichments for AQDS reduction were qualitatively determined by the color change of the aqueous solution from transparent to red. The primary enrichments were then successively transferred to fresh medium three times. Finally, the mixed solutions were serially diluted and plated onto aerobic Luria–Bertani medium (LB medium: peptone, 10 g l−1; NaCl, 10 g l−1; yeast extract, 5 g l−1; pH 9.0). Afterwards, distinct colonies were picked and streaked three times on agar plates, and each colony was tested for AQDS reduction in sterilized anaerobic medium. The single colony of interest was stored in a glycerol tube at −20 °C.
2.3 Phenotypic and 16S rRNA analysis
The motility of cells was tested by the hanging drop method. Physiological–biochemical characteristics were determined by the standard methods (Buchanan and Gibbons 1974). The salt tolerance of strain HN01 was determined on LB medium (pH 9.0) by varying NaCl concentrations from 0 to 15 % (w/v). The 16S rRNA gene of the isolate was amplified by PCR as described in Li et al. (2007b). The obtained sequences were then aligned with related 16S rRNA sequences from GenBank data libraries (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the Ribosomal Database Project (http://rdp.cme.msu.edu/seqmatch). Corresponding nucleotide sequences of representatives of the genus Kocuria were aligned using the program CLUSTAL X (Thompson et al. 1997). The phylogenetic tree was constructed with the software package MEGA version 4.0 using the maximum-parsimony and neighbor-joining method according to Kimura 2-parameter model and bootstrap analyses based on 1,000 replicates.
2.4 Batch anaerobic incubation experiments
All reduction and degradation experiments were conducted in 25.2-ml serum bottles under anaerobic and sterile conditions (Wu et al. 2011), performed in three sets, and all the bottles were incubated in the dark. Resting cell suspension of HN01 was used throughout the experiments. The suspension was aerobically prepared in LB medium at 30 °C and then harvested at late log phase by centrifugation (8,000×g at 4 °C for 10 min). Pellets were then washed twice and resuspended in sterile fresh MSM to an optical density of about 1.5 (λ = 600 nm).
To test the pH range for AQDS reduction, each bottle containing 20 ml MSM with 1 ml cell suspension, 0.5 mmol l−1 AQDS, and 5 mmol l−1 sucrose was adjusted to pH 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, or 11.0. The pH was maintained using 20-mmol l−1 phosphate buffer (for pH 5.0, 6.0, 7.0, 8.0) or carbonate buffer (for pH 9.0, 10.0, 11.0) (Wu et al. 2011). The medium without cells served as the control set.
For testing the effect of NaCl% (m/v) on AQDS reduction by HN01, mixed cultures containing AQDS (1 mmol l−1), cell suspension (1 ml), sucrose (5 mmol l−1), and carbonate buffer (20 mmol l−1; pH 9.0) were supplied with NaCl% (m/v) of 0.5, 1, 2, 3, 5, 7, 10, 12, 15, or 20 %.
To investigate the effect of alternative electron donors on AQDS reduction, seven possible organic substrates were tested, including acetate, pyruvate, glycerol, methanol, ethanol, glucose, and sucrose, with pH 9.0 and without NaCl%. The medium without cells served as the control set.
Under favorable conditions (i.e., pH, NaCl%, and electron donor) for AQDS (1 mmol l−1) microbial reduction by strain HN01, we investigated the cell growth coupling with AQDS reduction by the variation of active cell numbers.
The ability of strain HN01 to use different electron acceptors was studied in the serum bottles containing 1 ml cell suspension, 20 ml MSM, 5 mmol l−1 of sucrose (electron donor), carbonate buffer (20 mmol l−1; pH 9.0), and one of the following substrates as an electron acceptor: 5 mmol l−1 of Fe(III) oxides (α-FeOOH, γ-FeOOH, or α-Fe2O3) and 1 mg l−1 of organochlorine pollutants (γ-HCH, o,p′-DDT, or p,p′-DDT). Two control assays were performed under the same conditions: an abiotic set without bacterial cells and a biotic set without the addition of sucrose.
2.5 Chemical analysis
Triplicate bottles were used for chemical analysis at each sampling interval. The concentration of the reduced AQDS (AH2QDS) was quantified with a UV–vis spectrophotometer (UV-3600, Japan) at a wavelength of 386 nm (pH 5.0–6.0 sets) or 408 nm (pH 7.0–11.0 sets), which was due to the different AH2QDS UV–vis spectra at different pH levels (Liu et al. 2007; Wu et al. 2012). The total Fe(II), including dissolved and sorbed Fe(II), was quantified photometrically at 510 nm after being extracted using 0.5 mol l−1 HCl for 1.5 h and reaction with 1,10-phenanthroline. Dissolved Fe(II) was determined by removing the mineral and sorbed Fe(II) from the aqueous phase using a 0.22-mm syringe filter and then assaying the filtrate by colorimetric method (Li et al. 2010).
For analysis of organochlorine compounds (γ-HCH, o,p′-DDT, p,p′-DDT, and their intermediates), the sample bottles were immediately added with 20 ml hexane, and the mixtures were extracted using the ultrasonic bath as described in Cao et al. (2010). The quantification of the organochlorine compounds was carried out with an Agilent 6890 GC/mECD gas chromatograph and AT7693 auto sampler. The separation was performed on a fused silica capillary column (Aligent 1909/J-413; 30 m × 320 μm × 0.25 μm). The carrier gas was the high pure N2 (99.999 %) with a flow of 1.0 ml min−1 and the make-up gas at 60 ml min−1. The injector and detector temperatures were 250 and 300 °C, respectively. The GC oven temperature was programmed as follows: initial temperature of 100 °C held for 1 min, increased to 160 °C at a rate of 100 °C min−1, then increased to 270 °C at a rate of 10 °C min−1. Samples (1 μl) were injected in splitless mode. GC peaks were identified by accurate assignment of retention times for each standard; quantitative calculation was conducted with the external standard method. The organic chlorine compounds were quantitatively determined by comparing the area under each peak with the area under the standard peak. The resulting correlation coefficients for the calibration curves of the compounds were all greater than 0.995. The detection limits of quantification of target compound ranged from 0.01 to 0.04 μg l−1.
3 Results
3.1 Identification of the strain
The vegetative cells of isolated strain HN01 were coccus, 0.5–0.7 μm in diameter. The cells occurred singly or in pairs, were nonmotile, and stained Gram positive in both the exponential and stationary growth phases. After 48-h incubation on an LB agar plate, the colonies were uniformly round, 0.5–1.0 mm in diameter, red, wet, and with a distinct smooth morphology.
The optimum growth temperature for the strain ranged from 15 to 30 °C, and no growth was observed at 45 and below 5 °C. Microbial growth was observed between pH 6.0 and 10.0, and the optimum pH was 8.5–9.0. The strain can survive at NaCl concentrations of 0–12 % (optimum, 0–7 %), but not in 13 % NaCl. Strain HN01 had more similar characteristics with K. rosea ATCC 186T compared with other closely related species, was oxidase-positive and gelatin negative, and produced acid from glucose and fructose, but not from glycerol and lactose. No activity was detected for nitrate reduction, lysine decarboxylase, and arginine dihydrolase (Table 1). In terms of overall phenotype characteristics, the isolate should resemble Kocuria. Furthermore, chemotaxonomic characteristics of the strain and the topology of the 16S-rRNA-based phylogenetic tree (Fig. 1) clearly indicated that the nearest phylogenetic neighbors (sequence similarity values ranged from 96.0 to 99.7 %) of strain HN01 were members of the genus Kocuria. The strain had the highest similarity of 99.6 % with K. rosea D40 (JN192402). Consequently, the strain was identified as K. rosea strain HN01, which was deposited in the China General Microbiological Culture Collection Center (CGMCC no. 5810).
3.2 AQDS reduction
Here, we investigated the optimal cultivating conditions for AQDS reduction by the isolate HN01 and if it can grow during anaerobic metabolism. The color change of the culture liquid from transparent to yellow or red was a good indicator for AQDS reduction.
The AQDS reduction by HN01 using sucrose as the electron donor varied at different pH levels (Fig. 2). After 5 days of incubation, as the color of the solution changed, the AH2QDS concentrations in the treatments of AQDS + HN01 + sucrose at pH 7.0, 8.0, 9.0, and 10.0 increased to 0.10, 0.15, 0.18, and 0.13 mmol l−1, respectively. In contrast, only less than 0.038 mmol l−1 AH2QDS was formed in the treatments at pH 5.0, 6.0, and 11.0, respectively. The reduction in the abiotic control without cells was negligible (data not shown). The results indicated that the pH ranges for AQDS reduction by strain HN01 were within 7.0–10.0, and the optimum pH was 9.0. To the best of our knowledge, only few alkaliphilic bacteria capable of reducing humus and quinone have been reported. The identification of strain HN01 expands the species that can rapidly reduce AQDS at high pH levels.
Additionally, the reduction of AQDS was studied at various increasing NaCl% (v/w) of the cultivation medium (i.e., from 0.5, 1, 2, 3, 5, 7, 10, 12, 15, and 20 %). We found that the concentration of AH2QDS was increased with the increasing of NaCl% content from 0 to 12 %. However, the reduction content was decreased while the NaCl level greater than 12 %, and no reduction was measured at the 20 % NaCl level (Fig. 3). These results showed that: NaCl% was also a main factor influencing the reduction of AQDS by strain HN01, and the optimum NaCl concentrations for AQDS reduction were between 7 and 12 %.
The production of AH2QDS over a 5-day incubation in the AQDS + sucrose + HN01 treatment with different NaCl% (v/w). The experiments used 5 mmol l−1 sucrose and 1.0 mmol l−1 AQDS, and were performed under anaerobic conditions at 30 °C in the dark. Error bars represent standard deviation of the mean (n = 3)
Seven types of organic substrates were tested as the alternative electron donors for AQDS reduction. As shown in Fig. 4, the production of AH2QDS increased with time in the incubations with acetate, glycerol, methanol, ethanol, glucose, and sucrose, while no AH2QDS was detected in the incubations with pyruvate. The concentration of produced AH2QDS ranked as an order of glucose > sucrose > ethanol > methanol > glycerol > acetate. In the biotic (HN01 + AQDS) and abiotic (AQDS + organic substrates) controls, nearly no AH2QDS was detected (data not shown). These results indicated that glucose, sucrose, ethanol, methanol, glycerol, and acetate could serve as favorable electron donors for AQDS reduction by strain HN01, and the reduction rate depended on the type of organic substrates. Organic substrate such as pyruvate was not available as an electron donor with respect to AQDS reduction.
The effect of electron donors (5 mmol l−1) on AQDS (0.5 mmol l−1) reduction by strain HN01 at 2- and 5-day intervals. Electron donors: acetate (Ace), glucose (Glu), sucrose (Suc), pyruvate (Pyr), methanol (Meth), ethanol (Eth), and glycerol (Gly). The experiments were conducted in the medium not added with NaCl and at pH 9.0, and were performed under anaerobic conditions at 30 °C in the dark. Error bars represent standard deviation of the mean (n = 3)
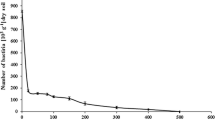
In the sucrose-fed incubation (pH 9.0; NaCl, 10 %), significant microbial growth and AQDS reduction were observed over 5 days. From Table 2, there was no obvious increase in active cell number of HN01 in both AQDS + HN01 + sucrose and HN01 + sucrose treatments, which indicated that HN01 cannot ferment sucrose and conserve energy to support microbial growth coupling AQDS reduction.
3.3 Iron(III) oxides reduction
The Fe(III)-reducing activity of strain HN01 was explored with three types of iron(III) oxides: α-FeOOH, γ-FeOOH, and α-Fe2O3. After 25 days (Fig. 5), no Fe(II) was formed in the abiotic control without active cells, and less than 0.06 mmol l−1 of Fe(II) was observed in the two biotic controls (without sucrose and with dead cells). In contrast, the total Fe(II) concentration in the active tests using α-FeOOH, γ-FeOOH, or α-Fe2O3 as the electron acceptor reached 0.16, 0.087, and 0.066 mmol l−1, respectively. The results suggested that (1) Fe(III) reduction by HN01 was a biological process because it required both active cells and sucrose, (2) all the three types of iron(III) oxides can be reduced by the strain with sucrose as the electron donor, and (3) the reduction rate of α-FeOOH was the highest, followed by γ-FeOOH and α-Fe2O3.
3.4 p,p′-DDT biodegradation
Figure 6 shows the anaerobic degradation of three types of organochlorine pollutants by strain HN01 with sucrose as the electron donor at pH 9.0. After 25-day incubation, the concentration of p,p′-DDT in the controls lacking sucrose (biotic control) or active cells (abiotic control) remained almost unchanged, demonstrating that p,p′-DDT was persistent in the absence of microbial activity of HN01, and the chemical reduction of p,p′-DDT by sucrose was negligible. In contrast, p,p′-DDT was significantly degraded in the active treatments, and the degradation rate reached 71.3 %. These results indicated the overall success of the biodegradation process of p,p'-DDT by HN01. However, as there was no decrease of γ-HCH and o,p'-DDT in all related treatments, it was suggested that strain HN01 cannot perform γ-HCH/o,p′-DDT transformation involving anaerobic metabolism.
Anaerobic degradation of OCPs (γ-HCH, o,p′-DDT, and p,p′-DDT) by strain HN01 over 25-day incubation. The experiments used 5 mmol l−1 sucrose and 1.0 mmol l−1 γ-HCH, o,p′-DDT, or p,p′-DDT, and were performed under anaerobic conditions at 30 °C in the dark. Error bars represent standard deviation of the mean (n = 3)
It was reported that DDT firstly degrades to DDD (anaerobic biotransformation product) or DDE (Cao et al. 2012). The GC analysis during the incubation period showed no p,p′-DDE was detected, while a variable amount of p,p′-DDD was quantified as 0.34 mg l−1 at 10 days, and the concentration decreased to 0.21 mg l−1 at the end of incubation (Fig. 7). This indicated that p,p′-DDT was firstly dechlorinated to p,p′-DDD but not p,p′-DDE in the treatments of p,p′-DDT + HN01 + sucrose. The total priority pollutants (p,p′-DDTs; i.e., p,p′-DDT, p,p′-DDE, and p,p′-DDD) decreased more than 60 % after 25 days of incubation, suggesting the overall success of the bio-dechlorination process of p,p′-DDT by HN01.
The major biotransformation products of p,p′-DDT by strain HN01 at 10- and 25-day incubation periods in the p,p′-DDT + HN01 + sucrose treatments. The experiments used 5 mmol l−1 sucrose and 1.0 mmol l−1 p,p′-DDT, and were performed under anaerobic conditions at 30 °C in the dark. Error bars represent SD of the mean (n = 3)
4 Discussion
Microorganism-mediated electron transfer between electron donors inside the cells and extracellular substances has been recognized as a significant biochemical process in anoxic soils or sediments (Lovley et al. 1996, 2004; Hernandez and Newman 2001; Jiang and Kappler 2008). Various substances, including minerals, electrodes, humus, soluble metal ions, and organic pollutants, can serve as the terminal electron acceptor for microbial anaerobic metabolism (Gralnick and Newman 2007). To date, the subject of extracellular reduction in neutral environments has been most thoroughly explored through studies concerning the reduction of humus and iron minerals. Only relatively recently, however, have researchers turned their attention to alkaliphilic humus/Fe(III)-reducing microorganisms and related process under alkaline conditions, and only a few strains have been isolated from natural or artificial alkaline environments (Hobbiea et al. 2012; Zhilina et al. 2009ab).
According to Ulukanli and Diğrak (2002), alkaline-adapted microorganisms can be classified into two groups, alkaliphiles and alkalitolerants, the former requiring actually alkaline media for growth and the optimum growth above pH 9; the latter can grow at pH values more than 9 or 10 but the optimum pH at around 7 or less. Strain HN01 could get growth at pH of 6–10 and obtain optimum growth at pH 9; thus, it was classified as alkaliphiles. Less than five alkaliphilic strains (alkaliphiles) capable of reducing extracellular substances have been reported, and the strains include A. peptidofermentans (Zhilina et al. 2009a), Anaerobranca californiensis (Gorlenko et al. 2004), B. pseudofirmus (Ma et al. 2012), Bacillus sp (Pollock et al. 2007), Corynebacterium humicreducens (Wu et al. 2011).
Among the factors influencing the microbial reduction of humus or Fe(III), types of electron donors are considered to be one of the most important factors (Lovley et al. 2004; Ma et al. 2011). Organic acids and sugars are usually used to study the microbial reduction of humus. Among them, acetate is the most important electron donor in many sedimentary environments (Lovley et al. 2004), but only partial humus-reducing bacteria can utilize it, while sugars might be the most effective electron donors according to our recent studies (Ma et al. 2012; Wu et al. 2011). For example, Ma et al. (2012) tested the effect of electron donors on AQDS reduction by B. pseudofirmus MC02, and the experimental results showed that the extent to which the AQDS reduction varied as a sequence of glucose > sucrose > lactate > glycerol > citrate, and acetate could not be utilized for AQDS reduction by the strain. The concentration of produced AH2QDS by C. humireducens ranked as an order of sucrose > acetate > lactate > ethanol > formate, and citrate, propionate, and glycerol were not available electron donors with respect to AQDS reduction (Wu et al. 2011). Seven possible organic substrates could serve as alternative electron donors for AQDS reduction by K. rosea HN01 in this study with a sequent effect in the order of glucose > sucrose > ethanol > methanol > glycerol > acetate, and pyruvate was not associated with AQDS reduction. The physical and chemical characteristics of Fe(III) are the main factors which influence Fe(III) reduction rate by Fe(III)-reducing microbes (Liu et al. 2001; Roden and Zachara 1996; Wu et al. 2010). Consistent with the previous reports, the reduction of iron(III) oxides by K. rosea HN01 was consistent with their surface area and crystalline property, ranking as α-FeOOH > γ-FeOOH > α-Fe2O3; Among these Fe(III), α-Fe2O3 reduction proceeded with the most difficulty, probably due to its smallest surface area (29.4 m2 g−1); α-FeOOH and γ-FeOOH are with similar surface areas (120.93 and 115.44 m2 g−1), whereas α-FeOOH's crystal size (41.9 nm) is extremely larger than γ-FeOOH (13.7 nm), and this is likely the reason for the different Fe(III) microbial reduction rates.
The newly isolated strain HN01 belonged to the genus Kocuria, which is taxonomically dissected from the genus Micrococcus to accommodate phylogenetically distinct actinobacteria (Stackebrandt et al. 1995) and composed of 18 species: K. rosea (the type species), Kocuria aegyptia, Kocuria atrinae, Kocuria carniphila, Kocuria flava, Kocuria gwangalliensis, Kocuria halotolerans, Kocuria himachalensis, Kocuria koreensis, Kocuria kristinae, Kocuria marina, Kocuria palustris, Kocuria polaris, Kocuria rhizophila, Kocuria salsiccia, Kocuria sediminis, Kocuria turfanensis, and Kocuria varians (Bala et al. 2012; Li et al. 2006; Liu 2011). Among them, there are more than five strains capable of growing under alkaline and saline conditions, including K. aegyptia, K. atrinae, K. flava, K. turfanensis (Zhou et al. 2008), K. halotolerans (Tang et al. 2009), K. koreensis (Park et al. 2010), K. marina, K. polaris, and K. sediminis (Bala et al. 2012). Compared with these alkaline-adapted microorganisms, K. rosea has much more potential applications in the field of environment protection. As a strain of environmental origin, K. rosea has been reported to be capable of decolorization of malachite green, azo, triphenylmethane, and industrial dyes (Parshetti et al. 2006, 2010) and also active in feather degradation (Coello and Vidal 2002). In our study, we found the new anaerobic metabolism of K. rosea, which was capable of effectively reducing extracellular soluble substrates (AQDS; p,p′-DDT) and insoluble iron(III) oxides under extreme alkaline conditions. The reducing pathway was proposed as: electrons transferred from organic substrates to extracellular substrates by a series of electron carriers inside the cells; in this biochemical process, extracellular substrates (AQDS, iron(III) oxides, p,p′-DDT), as the terminal electron acceptors, were reductive transformed or degraded into reducible form, coupling with the oxidation of organic substrates, which served as the electron donor.
5 Conclusions
A novel halotolerant, alkaliphilic humus-reducing bacterium, designated as HN01, was isolated from a cassava dreg composting reactor in mesophilic and maturation phase of pH 8.5–9.0. The strain was a Gram-positive, facultatively anaerobic, nonfermentative, nonmotile, coccus-shaped bacterium and was identified as K. rosea HN01 according to phylogenetic analysis based on 16S rRNA gene sequences. The AQDS reduction by strain HN01 was observed at NaCl concentrations below 12 % (w/v) (optimum, 10 %) and pH levels of 6.0–10.0 (optimum, 9.0) with sucrose as electron donor at 30 °C. Glucose, sucrose, methanol, ethanol, glycerol, or acetate could also serve as favorable electron donors for AQDS reduction. Coupling with sucrose as the electron donor, the strain could effectively transfer electrons from active cells to extracellular iron(III) oxides and p,p′-DDT. This was the first report that a strain of the Kocuria genus was involved in AQDS/Fe(III) reduction and p,p′-DDT dechlorination under anaerobic alkaline conditions. Such findings explore the new environmental functions of the Kocuria genus and also provide opportunity for the development of bioremediation of alkaline wastewater and other organic-contaminated environments containing humus.
References
Bala M, Kaur C, Kaur I, Khan F, Mayilraj S (2012) Kocuria sediminis sp. nov., isolated from a marine sediment sample. Antonie van Leeuwenhoek 101:469–478
Bhushan B, Halasz A, Hawari J (2006) Effect of iron(III), humic acids and anthraquinone-2,6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp. EDB2. J Appl Microbiol 100:555–563
Buchanan RE, Gibbons NE (eds) (1974) Bergey's manual of determinative bacteriology. Eighth edition. Williams and Wilkins, Baltimore
Cao F, Li FB, Liu TX, Huang DY, Wu CY, Feng CH, Li XM (2010) Effect of Aeromonas hydrophila on reductive dechlorination of DDTs by zero-valent Iron. J Agric Food Chem 58:12366–12372
Cao F, Liu TX, Wu CY, Li FB, Li XM, Yu HY, Tong H, Chen MJ (2012) Enhanced biotransformation of DDTs by an iron- and humic-reducing bacteria Aeromonas hydrophila HS01 upon addition of goethite and AQDS. J Agric Food Chem 60:11238–44
Coello N, Vidal L (2002) Kocuria rosea as a new feather degrading degrading bacteria. Appl Microbiol 2:165–175
Gorlenko V, Tsapin A, Namsaraev Z, Teal T, Tourova T, Engler D, Mielke R, Nealson K (2004) Anaerobranca californiensis sp. nov., an anaerobic, alkalithermophilic, fermentative bacterium isolated from a hot spring on Mono Lake. Int J Syst Evol Microbiol 54:739–743
Gralnick JA, Newman DK (2007) Extracellular respiration. Mol Microbiol 65:1–11
Hernandez ME, Newman DK (2001) Extracellular electron transfer. Cell Mol Life Sci 58:1562–1571
Hobbiea SN, Li XZ, Basena M, Stingl U, Brune A (2012) Humic substance-mediated Fe(III) reduction by a fermenting Bacillus strain from the alkaline gut of a humus-feeding scarab beetle larva. Syst Appl Microbiol 35:226–232
Hong YG, Gou J, Xu ZC, Xu MY, Sun GP (2007) Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. J Microbiol Biotechnol 17:428–437
Jiang J, Kappler A (2008) Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ Sci Technol 42:3563–3569
Li WJ, Zhang YQ, Schumann P, Chen HH, Hozzein WN, Tian XP, Xu LH, Jiang CL (2006) Kocuria aegyptia sp. nov., a novel actinobacterium isolated from a saline, alkaline desert soil in Egypt. Int J Syst Evol Microbiol 56:733–737
Li FB, Li XZ, Li XM, Liu TX, Dong J (2007a) Heterogeneous photodegradation of bisphenol A with iron oxides and oxalate in aqueous solution. J Colloid Interf Sci 311:481–490
Li WJ, Xu P, Schumann P, Zhang YQ, Pukall R, Xu LH, Stackebrandt E, Jiang CL (2007b) Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China) and emended description of the genus Georgenia. Int J Syst Evol Microbiol 57:1424–1428
Li FB, Wang XG, Li YT, Liu CS, Zeng F, Zhang LJ, Hao MD, Ruan HD (2008) Enhancement of the reductive transformation of pentachlorophenol by polycarboxylic acids at the oxide–water interface. J Colloid Interf Sci 321:332–341
Li FB, Li XM, Zhou SG, Zhuang L, Cao F, Huang DY, Xu W, Liu TX, Feng CH (2010) Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide. Environ Pollut 158:1733–1740
Liu DY (2011) Micrococcus and Kocuria. In: Liu DY (ed) Molecular detection of human bacterial pathogens. CRC Press, Boca Raton, pp 111–115
Liu C, Kota S, Zachara JM, Fredrickson JK, Brinkman CK (2001) Kinetic analysis of the bacterial reduction of goethite. Environ Sci Technol 35:2482–2490
Liu CX, Zachara JM, Foster NS, Strickland J (2007) Kinetics of reductive dissolution of hematite by bioreduced anthraquinone-2,6-disulfonate. Environ Sci Technol 41:7730–7735
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Ma C, Wang YQ, Zhuang L, Huang DY, Zhou SG, Li FB (2011) Anaerobic degradation of phenanthrene by a newly isolated humus-reducing bacterium, Pseudomonas aeruginosa strain PAH-1. J Soils Sediments 11:923–929
Ma C, Zhuang L, Zhou SG, Yang GQ (2012) Alkaline extracellular reduction: isolation and characterization of an alkaliphilic and halotolent bacterium, Bacillus pseudofirmus MC02. J Appl Microbial 112:883–891
Park EJ, Roh SW, Kim MS, Jung MJ, Shin KS, Bae JW (2010) Kocuria koreensis sp. nov., isolated from fermented seafood. Int J Syst Evol Microbiol 60:140–143
Parshetti G, Kalme S, Saratale G, Govindwar S (2006) Biodegradation of malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov 53:492–498
Parshetti GK, Telke AA, Kalyanib DC, Govindwarb SP (2010) Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J Hazard Mater 176:503–509
Pollock J, Weber KA, Lack J, Achenbach LA, Mormile MR, Coates JD (2007) Alkaline iron(III) reduction by a novel alkaliphilic, halotolerant, Bacillus sp. isolated from salt flat sediments of soap lake. Appl Microbiol Biotechnol 77:927–934
Reddy GSN, Prakash JSS, Prabahar V, Matsumoto GI, Stackebrandt E, Sisinthy S (2003) Kocuria polaris sp. nov., an orange-pigmented psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int J Syst Evol Microbiol 53:183–187
Roden EE, Zachara JM (1996) Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Roden EE, Kappler A, Bauer I, Jiang J, Paul A, Stoesser R, Konishi H, Xu HF (2010) Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nature Geosci 3:417–421
Rodrigues da Silva ME, Firmino PI, Dos Santos AB (2012) Impact of the redox mediator sodium anthraquinone-2,6-disulphonate (AQDS) on the reductive decolourisation of the azo dye Reactive Red 2 (RR2) in one- and two-stage anaerobic systems. Bioresour Technol 121:1–7
Scott DT, Mckinght DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32:2984–2989
Stackebrandt E, Koch C, Gvozdiak O, Schumann P (1995) Taxonomic dissection of the genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int J Syst Bacteriol 45:682–692
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley, New York
Tang SK, Wang Y, Lou K, Mao PH, Xu LH, Jiang CL, Kim CJ, Li WJ (2009) Kocuria halotolerans sp. nov., an actinobacterium isolated from a saline soil in China. Int J Syst Evol Microbiol 59:1316–1320
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Ulukanli Z, Diğrak M (2002) Alkaliphilic micro-organisms and habitat. Turk J Biol 26:181–191
Wang YB, Wu CY, Wang XJ, Zhou SG (2009) The role of humic substances in the anaerobic reductive dechlorination of 2,4-dichlorophenoxyacetic acid by Comamonas koreensis strain CY01. J Hazard Mater 164:941–947
Wolf M, Kappler A, Jiang J, Meckenstock RU (2009) Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ Sci Technol 43:5679–5685
Wu CY, Li FB, Zhou SG (2009) Humus respiration and its ecological significance. Acta Ecol Sin 29:1535–1542
Wu CY, Zhuang L, Zhou SG, Li FB, Li XM (2010) Fe(III)-enhanced anaerobic degradation of 2,4-dichlorophenoxyacetic acid by a dissimilatory Fe(III)-reducing bacterium Comamonas koreensis CY01. FEMS Microb Ecol 71:106–113
Wu CY, Zhuang L, Zhou SG, Li FB (2011) Corneybacterium humicreducens sp. nov., a alkaliphilic humic-reducing bacterium isolated from a microbial fuel cell. Inter J Syst Evol Microbiol 61:882–887
Wu CY, Zhuang L, Zhou SG, Yuan Y, Yuan T, Li FB (2012) Humic substance-mediated reduction of iron(III) oxides and degradation of 2,4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5. Microb Biotech 6:141–149
Ye Q, Roh Y, Carroll SL, Blair B, Zhou J, Zhang CL, Fields MW (2004) Alkaline anaerobic respiration: isolation and characterization of a novel alkaliphilic and meal-reducing bacterium. Appl Environ Microbiol 70:5595–5602
Zhilina TN, Zavarzina DG, Kolganova TV, Lysenko AM, Tourova TP (2009a) Alkaliphilus peptidofermentans sp. nov., a now alkaliphilic bacterial soda lake isolate capable of peptide fermentation and Fe(III) reduction. Microbiology 78:445–454
Zhilina TN, Zavarzina DG, Osipov GA, Kostrikina NA, Tourova TP (2009b) Natronincola ferrireducens sp. nov., and Natronincola peptidovorans sp. nov., new anaerobic alkaliphilic peptolytic iron-reducing bacteria isolated from soda lakes. Microbiology 78:455–467
Zhou GL, Luo XS, Tang YL, Zhang L, Yang Q, Qiu YJ, Fang CX (2008) Kocuria flava sp. nov. and Kocuria turfanensis sp. nov., airborne actinobacteria isolated from Xinjiang, China. Int J Syst Evol Microbiol 58:1304–1307
Acknowledgments
The authors thank the National Natural Science Foundations of China (no. 41101477, 21007091) and the Fundamental Research Funds for Environment and Plant Protection Institute, CATAS (no. 2011Hzs1J006, 2010hzsZDZX001) for financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Chun-Yuan Wu and Nan Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, CY., Chen, N., Li, H. et al. Kocuria rosea HN01, a newly alkaliphilic humus-reducing bacterium isolated from cassava dreg compost. J Soils Sediments 14, 423–431 (2014). https://doi.org/10.1007/s11368-013-0679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0679-1