Abstract
Purpose
Soil contamination by multiple organic and inorganic contaminants is common but its remediation by hyperaccumulator plants is rarely reported. The growth of a cadmium (Cd) hyperaccumulator Sedum alfredii and removal of contaminants from Cd and polycyclic aromatic hydrocarbons (PAHs) co-contaminated soil were reported in this study.
Materials and methods
Soil slightly contaminated by Cd (0.92 mg kg−1 DW) was collected from a vegetable field in Hangzhou and was spiked with two levels (0 and 6 mg kg−1 DW) of Cd and three levels (0, 25, and 150 mg kg−1 DW) of phenanthrene (PHE) or pyrene (PYR). A pot experiment was conducted in a greenhouse using S. alfredii with unplanted controls for 60 days. Shoot and root biomass of plants, dehydrogenase activity (DHA), and microbial biomass carbon in the soil were measured. Concentrations of Cd and PAHs in the plant and soil were determined.
Results and discussion
Elevated Cd level (6.38 mg kg−1 DW) increased S. alfredii growth. The presence of PAHs decreased the stimulatory effects of Cd on plant biomass and Cd concentrations in shoots in Cd spiked soil, thus decreasing Cd phytoextraction efficiency. Cadmium removal by S. alfredii after 60 days of growth varied from 5.8% to 6.7% and from 5.7% to 9.6%, in Cd unspiked and spiked soils, respectively. Removal rate of PAHs in the soil was similar with or without the plants. Removal rate of PYR decreased at the elevated Cd level in the soil. This appears to be due to a decrease in soil microbial activity. This is confirmed by a decrease in DHA, which is a good indicator of soil microbial activity.
Conclusions
Our results demonstrate that S. alfredii could effectively extract Cd from Cd-contaminated soils in the presence of PHE or PYR; however, both PAHs exhibited negative effects on phytoextraction of Cd from Cd spiked soil (6.38 mg kg−1 DW). S. alfredii is not suitable for remediation of PAHs. The effects of Cd and PAHs concentrations on the removal rate of PAHs appear to be attributed to the changes in microbial activities in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil contamination by cadmium (Cd) and polycyclic aromatic hydrocarbons (PAHs) has been accelerated in China during the past decade because of rapid urbanization and industrialization (Sun et al. 2011). Accumulation of these pollutants in the soil negatively influences the soil health, food chain safety, and public health (Lu and Zhu 2009; Murakami et al. 2009). Increasing environmental Cd load represents a threat to plant, animal, and human due to its high mobility and toxicity (Bertin and Averbeck 2006). Polycyclic aromatic hydrocarbons (PAHs), a group of priority pollutants suggested by the US Environmental Protection Agency (US EPA), are frequently detected in varying concentrations in agricultural and urban soils worldwide (Tao et al. 2004; Li et al. 2006; Honda et al. 2007; Morillo et al. 2007). Forty percent of the hazardous waste sites currently on the National Priority List of the US EPA are contaminated by multiple pollutants (Sandrin et al. 2000).
Remediation of soils co-contaminated by metals and organic pollutants is a complex problem, since the chemical processes and remediation technologies are different for each group of pollutants (Sandrin and Maier 2003). The feasibility of phytoremediation for soils contaminated by multiple organic and inorganic contaminants has been recently investigated (Lin et al. 2006, 2008; Singer et al. 2007; Zhang et al. 2009; Rengel et al. 2011; Sun et al. 2011). The presence of multiple contaminants influences remediation processes because of their interactive effects on soil processes, plant growth, and rhizosphere biota (Almeida et al. 2008). The presence of PAHs may influence the mechanisms of metal uptake, thus kinetics and extent of metal phytoextraction (Almeida et al. 2008). For instance, the presence of pyrene in copper (Cu) contaminated soils decreased phytoextraction efficiency of Cu by maize (Zea mays L.; Lin et al. 2008). However, extraction of nickel (Ni) by hyperaccumalator plant Alyssum lesbiacum was not influenced by co-contamination of PAHs (Singer et al. 2007). Uptake of zinc (Zn) by Indian mustard (Brassica juncea) was greater from a soil co-contaminated by pyrene and Zn as compared to that from only Zn-contaminated soil (Batty and Anslow 2008). On the other hand, excess metals in the soil may influence biodegradation of organic pollutants in aerobic or anaerobic co-contaminated systems (Sandrin and Maier 2003).
Hyperaccumulating plants are valuable for phytoextraction of metals in contaminated soils (Salt et al. 1998; McGrath and Zhao 2003). Sedum alfredii is a Cd hyperaccumulator native to China (Lu et al. 2008; Tian et al. 2009). It has been studied extensively with respect to its hyperaccumulating characteristics (Lu et al. 2008, 2009, 2011; Tian et al. 2009, 2010). The plant exhibited high translocation of soil Cd from roots to shoots (Yang et al. 2004). Roots of this plant could excrete high amounts of dissolved organic matter that can complex and detoxify heavy metals in the rhizosphere, hence could be beneficial to PAHs degrading microorganisms in the soil (Li et al. 2011).
Several researchers focused mainly on the potential of crops or wetland plants for phytoremediation of soils or sediments co-contaminated by metals and organic contaminants (Lin et al. 2008; Zhang et al. 2009; Rengel et al. 2011). The growth and phytoextraction potential of Cd hyperaccumulator S. alfredii in metal and PAHs co-contaminated soils are not fully investigated. The objectives of this study were to investigate: (1) the growth of S. alfredii in soil co-contaminated by Cd and PAHs, (2) phytoextraction of Cd by S. alfredii in the presence of PAHs and vice versa, (3) PAHs removal in co-contaminated soils, and (4) the interactive effects of Cd, PAHs and plant on soil dehydrogenase activity (DHA) and microbial biomass carbon, which are good indicators of soil microbial activity.
2 Materials and methods
2.1 Chemicals
Phenanthrene (PHE) and pyrene (PYR) as representative PAHs, were purchased from Aldrich Chemical Co. with a purity > 98%. Molecular weights (M w, g mol−1) were 178.23 for PHE and 202.26 for PYR; solubility in water at 25°C (S w, mg L−1) were 1.18 for PHE and 0.12 for PYR and octanol-water partition coefficients (log K ow) were 4.46 for PHE and 4.88 for PYR (Yaws 1999).
2.2 Soil treatments
Soil was collected (0-20 cm depth) from a vegetable field in Hangzhou, Zhejiang province, China, which was PAHs free but slightly contaminated with Cd (0.92 mg kg−1 DW). Selected physicochemical properties of the soil are presented in Table 1. The bulk soil was air-dried and sieved through a 5-mm sieve prior to spiked with Cd (6 mg kg−1 DW) and PAHs (25 or 150 mg kg−1 DW). With inclusion of unspiked soil, there were two Cd levels and three PHE/PYR levels. The soil, as per treatment, was first spiked with aqueous solution of Cd(NO3)2 as a source of Cd and incubated moist for 8 weeks. The unspiked soil was also incubated under the same conditions. The subsamples were air-dried, fully homogenized and sieved through a 5-mm sieve again and then spiked with PHE or PYR in acetone. After acetone was evaporated, the treated soils were progressively mixed with PAHs free soil and homogenized. The treated soils were then packed into pots (1 kg soil per pot), and equilibrated in a greenhouse for 3 days at 60% water-holding capacity (WHC). The initial concentrations of contaminants in soils of different treatments were measured and data are shown in Table 2.
2.3 Plant material
Healthy and uniform sized shoots of S. alfredii were chosen from seedlings grown in Cd-free soil for 4 months and grown first in distilled water for initiation of new roots, then transferred to one fourth strength of Hoagland solution for 4 weeks, and then transplanted (five plants per pot) to the soil pots with different treatments.
2.4 Pot experiment
Each treatment shown in Table 2 was maintained with or without plants (each with three replicate pots). The experiment was conducted in the greenhouse (Huajiachi campus, Zhejiang University) with natural light and day/night temperature of 30/24°C and humidity of 70/85%. The water content of the soil in the pots was regularly adjusted to about 60% WHC by weighing the pots.
2.5 Soil and plant sampling and sample preparation
Soil and plants were sampled 60 days after transplanting. Plant shoots and roots separated from soil were carefully washed first with tap water, and then distilled water for several times. Shoots and roots were freeze-dried (LABCONCO, USA). Dry biomass weights were recorded, ground to powder (<0.25 mm) using a horizontal grinder (Retsch RS-100, Germany) and stored in a deep freezer at –80°C prior to analysis.
The whole soil from each pot was homogenized and divided into two subsamples. One subsample was air dried, sieved through 2 mm screen and stored at 4°C prior to analysis of microbial biomass carbon according to the method described below and DHA following the procedure described by Lee et al. (2008). A mixture of 1 mL of triphenyltetrazolium chloride solution (3%) and 1.5 mL of distilled water were added to 10 g of soil mixed with 0.1 g of CaCO3. After 24 h of incubation at 37°C, the reaction product was extracted with methanol, and the absorbance was measured at 485 nm. The second subsample was freeze dried and ground to powder (<0.25 mm) and stored in the deep freezer at –80°C prior to PAHs and Cd analyses.
2.6 Soil microbial biomass carbon analysis
The fumigation-extraction method described by Vance et al. (1987) with some modifications was used. The sample, after remoistened and incubated for a week, was fumigated in the dark in a vacuum desiccator filled with gasified ethanol-free CHCl3 for 24 h at 25°C. The CHCl3 was removed and the soil samples were extracted by shaking with 20 mL 0.5 M K2SO4 for 30 min on a rotary shaker. The suspension was then filtered through filter paper. Triplicate samples of unfumigated control soils were extracted for dissolved organic carbon under the same conditions. Dissolved organic C in the extract was measured using an automated TOC analyzer (Analytik Jena multi N/C 3100, Germany). Biomass C (B C ) was calculated as: B C = E C /0.45, where E C = [(organic C extracted from fumigated soil) − (organic C extracted from non-fumigated soil)], 0.45 is the conversion coefficient from carbon flush to microbial biomass carbon.
2.7 PAHs analyses in soil and plant
The procedure reported by Gao and Zhu (2004) with some modifications was used for soil samples. Two grams soil was weighed into a 25-mL glass tube; 10 mL dichloromethane was added, followed by ultrasonication for 1 h and centrifuged at 3,000 rpm for 10 min. Then 2 mL of the supernatant was filtered through 2.5 g of silica gel column with 15 mL hexane and dichloromethane mixture (1/1, v/v). The solvent fraction was then evaporated using a rotary evaporator and dissolved in methanol for a final volume of 5 mL.
For plant tissue, the procedure described by Zhu and Zhang (2008) with some modifications was used. Freeze-dried plant sample was weighed 0.2 g (for shoot) or 0.05 g (for root) into 25-mL glass tubes, 10 mL acetone and dichloromethane mixture (1/1, v/v) was added, followed by ultrasonication for 30 min and centrifuged at 3,000 rpm for 10 min. The supernatants from three successive extractions were collected, evaporated and dissolved in 2 mL of hexane, followed by the same clean up and concentration procedure as described above for soil samples.
The above extract was filtrated through a 0.22-μm filter, PAHs concentration was determined using Agilent 1200 HPLC with a 4.6 × 150 mm reverse phase XDB-C18 column and both UV and FLD detectors with methanol/water (85/15, v/v) as the mobile phase at a flow rate of 1 mL min−1. Chromatography was performed at 30°C. PHE and PYR were detected at 245 and 234 nm, respectively, using UV detector. The excitation wavelengths of PHE and PYR were 246 and 270 nm, while the emission wavelengths were 370 and 390 nm for FLD detector.
The average recoveries obtained by spiked soil samples with known concentrations of PHE and PYR were 96.8% (n = 4, RSD < 2.13%) and 93.2% (n = 4, RSD < 1.98%), respectively. The average recoveries obtained by spiked plant samples with PHE and PYR were 94.3% (n = 4, RSD < 2.87%) and 91.9% (n = 4, RSD < 2.22%), respectively.
PAHs removal rate in soils was calculated as:
2.8 Cd analyses in soil and plant samples
Ground plant samples were digested in HNO3-HClO4, while the soil samples were digested in HF-HNO3-HClO4. Cadmium concentrations were determined using ICP-MS (Agilent 7500a, USA).
The translocation factor (TF) was calculated as:
The percent of removal of Cd by plants was calculated as:
2.9 Statistical analyses
Statistical two-way analysis of variance was used to compare the effects of Cd, PAHs, and their interactions on plant growth, biomass, contaminant concentrations, and accumulation in plant tissues, Cd removal by plants, PAHs removal rate in soils, soil dehydrogenase activity, and microbial biomass carbon. All data were statistically analyzed using the SPSS package (version 16.0) and least significant difference was applied to test for significance between the means.
3 Results and discussion
3.1 Plant growth and biomass
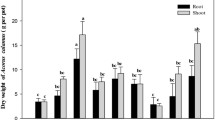
After 60 days of growth, no visible symptoms of contaminant toxicity were observed in S. alfredii across all the treatments. The growth of shoot was significantly influenced by the presence of Cd but not by PAHs and their interactions; however, the growth of root was also influenced by PAHs (Fig. 1). Plant biomass weight was significantly greater in Cd spiked soil without PAHs, as compared to that in all other treatments, except shoot biomass in Cd spiked soil with 25 mg kg−1 PYR. The effects of Cd toxicity have been studied for various plants across a wide range of production systems (Clemens 2006). Shentu et al. (2008) reported no inhibitory effects of Cd (up to 7.00 mg kg−1 DW) on shoot growth of three vegetables. Relatively low levels of Cd (≤3.5 mg kg−1 DW) had promotion effects on plant growth. Similar findings were reported for a broad range of species (Liu et al. 2003; Nyitrai et al. 2003). In the present study, shoot biomass in 60 days was significantly greater with Cd addition alone as compared to control. Significant shoot growth enhancement of S. alfredii was noted at relatively low Cd levels (25-100 μmol L−1) under hydroponic condition (Yang et al. 2004). It seems that low to moderate Cd level in growth medium promotes S. alfredii growth, while it can also tolerate higher level of Cd.
Dry weight of Sedum alfredii (five plants per pot) influenced by Cd and PAHs treatments after 60 days of growth. Means with different letters by shoot and root are significantly different based on least significant difference (P < 0.05). The results of two-way analysis of variance (ANOVA) are shown below the graph, *P < 0.05, **P < 0.01 and n.s. not significant. Cd1 and Cd2 represent unspiked and 6.00 mg kg−1 DW Cd-spiked soils, P0 represents PAHs free soil, PHE1 and PHE2 represent 25 and 150 mg kg−1 DW spiked with phenanthrene, PYR1 and PYR2 represent 25 and 150 mg kg−1 DW spiked with pyrene. Error bars standard deviation (n = 3)
Low sensitivities to PAHs have been reported on some grasses and vegetables (Gao and Zhu 2004; Batty and Anslow 2008; Lu and Zhu 2009). The effect of PAHs on wetland plant growth could be largely depending on plant species regardless of PAHs types and media (Zhang et al. 2010). Concentrations of PHE and PYR above certain levels (133 and 172 mg kg−1 DW) decreased dry weight of 12 plant species (Gao and Zhu 2004). However, shoot biomass of Z. mays L. was not negatively influenced by high initial concentration of 500 mg kg−1 DW PYR (Lin et al. 2008). In the present study, PAHs at both concentrations showed no significant influence on plant growth in Cd unspiked treatments. However, in Cd spiked treatments, addition of PAHs decreased the shoot and root biomass. These results suggest that interactions of Cd and PAHs in Cd-spiked soil might exacerbate the phytotoxicities of the contaminants, thus resulting in a decrease in biomass weight. Both antagonistic and synergistic effects of metals and PAHs on plant growth have been reported in co-contaminated soils. For instance, the shoot biomass of Z. mays L. increased with increasing concentration of PYR from 50 to 500 mg kg−1 DW, suggesting mitigation of Cu toxicity effects by PYR (Lin et al. 2008). Rengel et al. (2011) also reported mitigation of Cd toxicity to a wetland plant (Juncus subsecundus) with addition of PHE and PYR. However, PYR did not alleviate Cd toxicity to Z. mays L. (Zhang et al. 2009).
3.2 Cd accumulation, translocation, and extraction by plant
The concentration of Cd in the shoot and root were significantly influenced by Cd, PAHs, and their interactions. The concentrations of Cd in shoot and root were greater in the plants grown in Cd spiked soil as compared to those of the plants in unspiked soil (Fig. 2), which agreed with the reports by Yang et al. (2004). In the Cd unspiked soil, the shoot and root Cd concentration were similar regardless of types and concentrations of PAHs. The shoot Cd concentrations in Cd spiked soil decreased in the order: PHE or PYR unspiked > both levels of PHE spiked > both levels of PYR spiked treatments. Cadmium concentration of shoot in Cd spiked soil decreased by the presence of either PHE or PYR at both levels. This response was similar to that reported by Lin et al. (2008), i.e., Cu concentration in shoot of Z. mays L. grown in soil containing 400 mg Cu kg−1 DW soil was lower with co-contamination of PYR as compared to that without PYR. Almerida et.al (2008) reported that PAHs can increase Cu sorption by salt marsh plants by way of increasing Cu solubility; however, such phenomenon only occurred when plants were present, indicating that PAHs-Cu interaction may be related to plants. Fractionation of Cd in Cd contaminated soil showed no difference with or without co-contamination of PYR (Zhang et al. 2009). Rengel et al. (2011) reported a decrease in water-extractable Cd with PAHs additions, however no effect on ethylenediaminetetraacetic acid-extractable Cd (which is more related to plant growth). In the present study, the shoot Cd concentrations of plants grown in Cd spiked soil were lower with additional spiking of PYR than that of PHE (see Fig. 2). This differential effect between two species of PAHs on Cd uptake by S. alfredii may be related to the difference in the phytotoxicities of PHE and PYE. In addition, PHE and PYR have different water solubility which leads to lower bioavailability of PYR. As a result, PHE is easier to degrade than PYR, with less negative influence on the uptake of Cd by S. alfredii. Further research is needed.
Cd concentrations and translocation factors of Sedum alfredii influenced by Cd and PAHs treatments after 60 days of growth. The translocation factor (TF) was calculated as: TF = Cd concentration in shoot/Cd concentration in root. Means with different letters by shoot and root are significantly different based on least significant difference (P < 0.05). The results of two-way analysis of variance (ANOVA) are shown below the graph, *P < 0.05, **P < 0.01, and n.s. not significant. Refer to Fig. 1 legend for explanation of treatments abbreviations. Error bars standard deviation (n = 3)
The TFs of Cd varied from 1.51 to 3.32 across all the treatments, and were greater for the plants grown in Cd spiked soils than those in Cd unspiked soils (see Fig. 2). This is in agreement with the characteristics of Cd hyperaccumulator (Salt et al. 1998). In the Cd spiked soil, TF for Cd was lower in PYR spiked treatment as compared to those in PHE spiked or in soil without PAHs. In the Cd unspiked soil, TF was not influenced by PAHs co-contamination (except 150 mg kg−1 DW PYR spiked treatment).
Cadmium accumulation in shoot and root of plants across different Cd and PAHs treatments exhibited similar trend as that of Cd concentration (Fig. 3). The percentage of Cd removal from soil was significantly greater by the plants grown in Cd spiked soil without PAHs as compared to that for all other treatments. Cadmium removal percentage varied from 5.8% to 6.7%, and from 5.7% to 9.6%, in Cd unspiked and Cd spiked soils, respectively. Presence of PAHs at both concentrations in Cd spiked soil decreased the percentage of Cd removal by S. alfredii.
Cd accumulation in plant shoot and root (a), and the percentage of Cd removal from soils by whole plants (b) influenced by Cd and PAHs treatments after 60 days of growth. Means with different letters are significantly different based on least significant difference (P < 0.05). The results of two-way analysis of variance (ANOVA) are shown below the graph, *P < 0.05, **P < 0.01, and n.s. not significant. Refer to Fig. 1 legend for explanation of treatments abbreviations. Error bars standard deviation (n = 3)
3.3 PAHs removal from soil
Numerous literatures have pointed out that PAHs could be degraded by soil indigenous microorganisms (Reilley et al. 1996; Gao and Zhu 2004; Lee et al. 2008; Lu et al. 2008; Sun et al. 2011). In the present study, the range of percentage of PHE removal was greater than that of PYR (Table 3). These results agreed with other reports (Gao and Zhu 2004; Lee et al. 2008). The removal of PAHs could be due to biotransformation, biodegradation, plant uptake and metabolism, or abiotic dissipation including photodegradation, volatilization, and incorporation into soil organic material (Reilley et al. 1996; Lin et al. 2008). The magnitudes of abiotic and biotic losses of PAHs were not separated in the present study. Sun et al. (2010) indicated that the contribution of abiotic losses of soil PHE (amounting to 83% of the initial amount) and PYR (57%) was the greatest among the four pathways including abiotic losses, microbial degradation, plants uptake, and promotion from root exudates. In this study, the abiotic losses including volatilization and photodegradation might be also high due to the labile nature of PHE and PYR.
Polycyclic aromatic hydrocarbons accumulation by S. alfredii was quantified in the present study. There was a significant positive correlation between PHE or PYR concentrations in plant tissues and the concentrations of PHE or PYR spiked to soil (Fig. 4). These results were similar to those reported by Gao and Zhu (2004). However, the accumulation of PAHs in the plant was not significantly influenced by soil Cd levels. The contribution of PAHs uptake by plant to the total loss of PAHs from the soil was quite small, which was consistent with previous studies (Ke et al. 2003; Gao and Zhu 2004; Lu and Zhu 2009).
Concentrations of phenanthrene (a) and pyrene (b) in shoot and root of Sedum alfredii influenced by Cd and PAHs treatments after 60 days of growth. Means with different letters by shoot and root are significantly different based on least significant difference (P < 0.05). The results of two-way analysis of variance (ANOVA) are shown below the graph, *P < 0.05, **P < 0.01, and n.s. not significant. Refer to Fig. 1 legend for explanation of treatments abbreviations. Error bars show standard deviation (n = 3)
Many studies have demonstrated that numbers of plant species can enhance PAHs removal (Reilley et al. 1996; Lee et al. 2008; Lin et al. 2008; Sun et al. 2011; Teng et al. 2011) as compared to that from unplanted soil due to phenomena such as increased microbial activity and degradation mediated by plant-secreted enzymes in the root zone (Lee et al. 2008). In contrast, there were no significant effects of S. alfredii on enhancement of PAHs removal as compared to the unplanted treatments (see Table 3). Previous studies also reported small difference in PAHs removal between planted and unplanted soil, if the root system of plant was not very active and developed (Ke et al. 2003; Lee et al. 2008). Plant species vary widely with respect to root parameters and rhizosphere characteristics (Gill and Jackson 2000; Smalla et al. 2001), and the remediation potential of PAHs may vary with plant species and ecotypes (Lee et al. 2008).
In the present study, the removal rate of PHE was not influenced by either Cd or PHE levels in unplanted treatments (see Table 3). However, PHE loss in planted treatment was significantly lower in Cd spiked soil in the presence of low concentration of PHE as compared to that in the rest of the treatments. The removal rate of PYR was significantly influenced by PYR and Cd levels but not by their interactions. The removal rate of PYR decreased significantly with the addition of Cd and an increase in PYR concentration in the soil (see Table 3). Effect of metals on the degradation of PAHs can be negative or positive depending on type and concentration of both metals and PAHs (Rengel et al. 2011). In the study of lead (Pb) and PYR remediation, PYR removal was accelerated by Pb in both rhizosphere and nonrhizosphere soils (Khan et al. 2009). The presence of plant also played an important role on influence of metals on PAHs removal, i.e., Z. mays L. inhibited PYR removal in the presence of high concentration of Cu, implying that the change in microbial activity or modified root physiology under Cu stress was probably unfavorable to PYR removal (Lin et al. 2008). In this study, negative effects of Cd on PYR removal were significant (see Table 3); however, this effect was quite similar between planted and unplanted soil. It is well documented that the presence of metals such as Cd (as low as 2 mg kg−1 DW soil) can inhibit a broad range of microbial processes (Baath 1989). Rengel et al. (2011) reported that the treatments planted with J. subsecundus significantly increased PHE removal when 10 mg kg−1 DW Cd was added, but that was not the case in the treatment mixed with 50 mg kg−1 DW Cd and 500 mg kg−1 DW PAHs. The additions of Cd and PAHs could influence the plant-microbe interactions in the rhizosphere. The high Cd and PAHs concentrations might be toxic to specific groups of microorganisms, and the presence of PAHs may exasperate Cd toxicity to microorganisms (Shen et al. 2005).
The biological parameters measured during the experiment were significantly correlated with PAHs concentration; the DHA was negatively correlated with both PAHs concentration, whereas the microbial biomass C was only positively correlated with PYR concentration. Dehydrogenase activity in the soil is a good index of biological activity in the soil (Gunther et al. 1996). Strong correlations between PAHs removal and dehydrogenase activity are frequently reported (Lee et al. 2008; Teng et al. 2011). In the present study, an increase in Cd concentration in the soil decreased the PYR removal at both PYR concentrations (see Table 3). This also corresponds to a decrease in DHA. However, Cd did not affect soil microbial biomass carbon at the end of 60 days, which was only affected by PAHs addition as a carbon resource. The effects of Cd on PAHs removal and biological parameters in soil need to be investigated within a broad range of Cd levels in the future. Soil microbial community responses to Cd and PAHs co-contamination are also important to reveal the mechanisms of effects of Cd on PAHs removal.
4 Conclusions
The results of this study demonstrated that S. alfredii could effectively extract Cd from Cd-contaminated soil (Cd aged and spiked) in the presence of PHE or PYR; however both exhibited negative effects on phytoextraction of Cd from the Cd-spiked soil. Dissipation of both PHE and PYR was similar in the bare soil as well as that planted with S. alfredii. Removal rate of pyrene and soil DHA were negatively impacted by an increase in Cd concentration. Hence, remediation of heavy metals and organic contaminants co-contaminated soils by metal hyperaccumulator plants is influenced by interactions of contaminants in the soil. Additional strategies are needed to accomplish the coremoval of Cd and PAHs from co-contaminated soils by S. alfredii.
References
Almeida CMR, Mucha AP, Delgado MFC, Cacador MI, Bordalo AA, Vasconcelos MTSD (2008) Can PAHs influence Cu accumulation by salt marsh plants? Mar Environ Res 66(3):311–318
Baath E (1989) Effects of heavy-metals in soil on microbial processes and populations (a review). Water Air Soil Pollut 47(3–4):335–379
Batty LC, Anslow M (2008) Effect of a polycyclic aromatic hydrocarbon on the phytoremediation of zinc by two plant species (Brassica juncea and Festuca arundinacea). Int J Phytorem 10(3):236–251
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88(11):1549–1559
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11):1707–1719
Gao YZ, Zhu LZ (2004) Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 55(9):1169–1178
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147(1):13–31
Gunther T, Dornberger U, Fritsche W (1996) Effects of ryegrass on biodegradation of hydrocarbons in soil. Chemosphere 33(2):203–215
Honda K, Mizukami M, Ueda Y, Hamada N, Seike N (2007) Residue level of polycyclic aromatic hydrocarbons in Japanese paddy soils from 1959 to 2002. Chemosphere 68(9):1763–1771
Ke L, Wang WQ, Wong TWY, Wong YS, Tam NFY (2003) Removal of pyrene from contaminated sediments by mangrove microcosms. Chemosphere 51(1):25–34
Khan S, Hesham AE, Qing G, Shuang L, He JZ (2009) Biodegradation of pyrene and catabolic genes in contaminated soils cultivated with Lolium multiflorum L. J Soils Sediments 9(5):482–491
Lee SH, Lee WS, Lee CH, Kim JG (2008) Degradation of phenanthrene and pyrene in rhizosphere of grasses and legumes. J Hazard Mater 153(1–2):892–898
Li XH, Ma LL, Liu XF, Fu S, Cheng HX, Xu XB (2006) Polycyclic aromatic hydrocarbon in urban soil from Beijing, China. J Environ Sci 18(5):944–950
Li TQ, Di ZZ, Islam E, Jiang H, Yang XE (2011) Rhizosphere characteristics of zinc hyperaccumulator Sedum alfredii involved in zinc accumulation. J Hazard Mater 185(2–3):818–823
Lin Q, Wang ZW, Ma S, Chen YX (2006) Evaluation of dissipation mechanisms by Lolium perenne L, and Raphanus sativus for pentachlorophenol (PCP) in copper co-contaminated soil. Sci Total Environ 368(2–3):814–822
Lin Q, Shen KL, Zhao HM, Li WH (2008) Growth response of Zea mays L. in pyrene-copper co-contaminated soil and the fate of pollutants. J Hazard Mater 150(3):515–521
Liu D, Jiang W, Gao X (2003) Effects of cadmium on root growth, cell division and nucleoli in root tip cells of garlic. Biol Plant 47(1):79–83
Lu L, Zhu LZ (2009) Reducing plant uptake of PAHs by cationic surfactant-enhanced soil retention. Environ Pollut 157(6):1794–1799
Lu LL, Tian SK, Yang XE, Wang XC, Brown P, Li TQ, He ZL (2008) Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J Exp Bot 59(11):3203–3213
Lu LL, Tian SK, Yang XE, Li TQ, He ZL (2009) Cadmium uptake and xylem loading are active processes in the hyperaccumulator Sedum alfredii. J Plant Physiol 166(6):579–587
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14(3):277–282
Morillo E, Romero AS, Maqueda C, Madrid L, Ajmone-Marsan F, Grcman H, Davidson CM, Hursthouse AS, Villaverde J (2007) Soil pollution by PAHs in urban soils: a comparison of three European cities. J Environ Monit 9(9):1001–1008
Murakami M, Nakagawa F, Ae N, Ito M, Arao T (2009) Phytoextraction by rice capable of accumulating Cd at high levels: reduction of Cd content of rice grain. Environ Sci Technol 43(15):5878–5883
Nyitrai P, Boka U, Gaspar L, Savari E, Lenti K, Keresztes A (2003) Characterization of the stimulating effect of low-dose stressors in maize and bean seedlings. J Plant Physiol 160(10):1175–1183
Reilley KA, Banks MK, Schwab AP (1996) Dissipation of polycyclic aromatic hydrocarbons in the rhizosphere. J Environ Qual 25(2):212–219
Rengel Z, Zhang ZH, Meney K, Pantelic L, Tomanovic R (2011) Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. J Hazard Mater 189(1–2):119–126
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Ann Rev Plant Physiol Plant Mol Biol 49:643–668
Sandrin TR, Maier RM (2003) Impact of metals on the biodegradation of organic pollutants. Environ Health Persp 111(8):1093–1101
Sandrin TR, Chech AM, Maier RM (2000) A rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation. Appl Environ Microbiol 66(10):4585–4588
Shen GQ, Cao LK, Lu YT, Hong JB (2005) Influence of phenanthrene on cadmium toxicity to soil enzymes and microbial growth. Environ Sci Pollut Res 12(5):259–263
Shentu JL, He ZL, Yang XE, Li TQ (2008) Accumulation properties of cadmium in a selected vegetable-rotation system of southeastern China. J Agric Food Chem 56(15):6382–6388
Singer AC, Bell T, Heywood CA, Smith JAC, Thompson IP (2007) Phytoremediation of mixed-contaminated soil using the hyperaccumulator plant Alyssum lesbiacum: Evidence of histidine as a measure of phytoextractable nickel. Environ Pollut 147(1):74–82
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67(10):4742–4751
Sun TR, Cang L, Wang QY, Zhou DM, Cheng JM, Xu H (2010) Roles of abiotic losses, microbes, plant roots, and root exudates on phytoremediation of PAHs in a barren soil. J Hazard Mater 176(1–3):919–925
Sun YB, Zhou QX, Xu YM, Wang L, Liang XF (2011) Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J Hazard Mater 186(2–3):2075–2082
Tao S, Cui YH, Xu F, Li BG, Cao J, Liu W, Schmitt G, Wang XJ, Shen W, Qing BP, Sun R (2004) Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci Total Environ 320(1):11–24
Teng Y, Shen YY, Luo YM, Sun XH, Sun MM, Fu DQ, Li ZG, Christie P (2011) Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J Hazard Mater 186(2–3):1271–1276
Tian SK, Lu LL, Yang XE, Labavitch JM, Huang YY, Brown P (2009) Stem and leaf sequestration of zinc at the cellular level in the hyperaccumulator Sedum alfredii. New Phytol 182(1):116–126
Tian SK, Lu LL, Yang XO, Webb SM, Du YH, Brown PH (2010) Spatial imaging and speciation of lead in the accumulator plant sedum affredii by microscopically focused synchrotron X-ray investigation. Environ Sci Technol 44(15):5920–5926
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19(6):703–707
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259(1–2):181–189
Yaws CL (1999) Chemical properties handbook. McGraw-Hill, New York, pp 340–389
Zhang H, Dang Z, Zheng LC, Yi XY (2009) Remediation of soil co-contaminated with pyrene and cadmium by growing maize (Zea mays L.). Int J Environ Sci Technol 6(2):249–258
Zhang ZH, Rengel Z, Meney K (2010) Polynuclear aromatic hydrocarbons (PAHs) differentially influence growth of various emergent wetland species. J Hazard Mater 182(1–3):689–695
Zhu LZ, Zhang M (2008) Effect of rhamnolipids on the uptake of PAHs by ryegrass. Environ Pollut 156(1):46–52
Acknowledgments
This study was financially supported by “863” Target Goal Project from Ministry of Science of China (#2009AA06Z316), a key project from Ministry of Education of China (#310003), and a project from Ministry of Environmental Protection of China (#2011467057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Wang, K., Zhu, Z., Huang, H. et al. Interactive effects of Cd and PAHs on contaminants removal from co-contaminated soil planted with hyperaccumulator plant Sedum alfredii . J Soils Sediments 12, 556–564 (2012). https://doi.org/10.1007/s11368-012-0471-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0471-7