Abstract
In this study, pot-culture experiments were conducted to investigate the single effect of Cd, PCBs, and the combined effect of Cd-PCBs with Tagetes patula L. The study highlights that the minimum concentration of PCBs (100 µg kg−1) could enable the growth of the plant with an increase in biomass by 27.76% when compared with the control. In all the experiments performed, the Cd concentrations over the surface parts were found to be above 100 mg kg−1. Significant positive correlations were observed between the Cd and PCBs concentrations accumulated in tissues of the soil and plants (p < 0.05). T. patula exhibited high tolerance to Cd and PCBs, and the plant promoted the removal rate of PCBs. The removal rates of PCB18 and PCB28 were up to 42.72 and 42.29%, respectively. The study highlights the potential and suitability of T. patula for phytoremediation of Cd and PCBs in contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) and polychlorinated biphenyls (PCBs) are very harmful, hazardous substances in the world (Weyens et al. 2015). Trichlorodiphenyl is the most common type of PCBs, especially 2,2′,5-Trichlorobiphenyl (PCB18) and 2,4,4′-Trichlorobiphenyl (PCB28) (Borja et al. 2005; Mackova et al. 1997; Safe 1994). Research has shown the feasibility of T. patula for phytoremediation (Marrugo-Negrete et al. 2017; Phusantisampan et al. 2016; Sun et al. 2011). Therefore, T. patula was selected for phytoremediation studies using pot-culture experiments. Previous research has demonstrated the remediation effects of herbaceous plants on HMs and organic pollutants concentrations in soil (Khandare et al. 2017). However, only a few reports have been documented on the processes and effectiveness of remediation of Cd and PCBs and its co-contamination with flowering plants (Teng et al. 2010; Wu et al. 2012). The rationale of this work is to examine the increasing responses and likely hyperaccumulation capacity of T. patula under single contamination of Cd and PCBs, and co-contamination of Cd and PCBs. Furthermore, the study also explores and evaluates the plant’s uptake, translocation, accumulation, and removal behaviors of Cd and PCBs under co-contamination soil treatment. This study will provide detailed insights into the feasibility and portability of using T. patula for phytoremediation of Cd and PCBs as well as its co-contamination.
Materials and Methods
The soil sample was collected from Tanggu Forest Park (117°41′N and 38°65′E) located at Binhai New in Tianjin Economic-Technological Development Area, China. The soil was found to be yellow soil. The soil chemical analysis revealed the total P, N, and Cd content to be 5.32%, 0.19%, and 0.025 mg/kg respectively. The pH of the soil was found to be 4.11. The moisture content was observed as 7.60%. The initial concentration of polychlorinated biphenyls was non-detectable. The collected surface soil samples (0–20 cm) were ground using a mortar and pestle to pass through a 2 mm mesh and were used during pot-culture experiments. Seeds of T. patula were procured from Tianjin Agriculture Science Institution, China and the damaged seeds were discarded.
Chemicals: CdCl2·2.5H2O (analytical pure), nitric acid (analytical pure), hydrogen peroxide solution(analytical pure), hydrofluoric acid (analytical pure), Trichloro biphenyl PCB18, PCB28 (fisher chemicals), acetone, and n-hexane (analytical pure).
For the experiments using different concentration gradients, the Cd and PCB concentrations for soil treatment were determined according to the National Soil-Environmental Quality Standard of China (Wang 2005; Xia 1996). The components and concentrations of Cd and PCBs in each treatment are shown in Table 1. The standard samples of PCB18 and PCB28 were dissolved separately in 100 mL of acetone solution for soil treatment. According to the concentrations of PCBs depicted in Table 1, PCB18 and PCB28 in acetone solution were mixed thoroughly in a 1:1 ratio and were added into the clean soil in different culture dishes representing the miniature of pot culture experimental set up. The soil treated with PCBs was kept for 24 h until the acetone solution in the culture dishes was completely volatilized. Next, the soil in the culture dishes was mixed with the soil in the plastic pots.
For Cd treatment, CdCl2·2.5H2O powder corresponding to the respective mg planned for the experiment was directly added to the soil in pots as shown in Table 1. 1 kg of sample soil was placed in each plastic pot (17 cm in diameter and 13 cm in height). After treating the soil with the appropriate amount of CdCl2·2.5H2O and Trichloro biphenyls (PCB18, PCB28), the pots were equilibrated completely for 30 days. The seeds were germinated in a germination plate. A sterile gauze treated with warm water was placed over the germination plate. The seeds were evenly spread on the gauze and germinated for 2–3 days in a constant temperature incubator. The seeds with a good germination effect were selected and transferred to the seedling tray. When the seedlings grew to a height of about 4 cm in the seedling tray, healthy plants were selected for transplanting. 30 days old seedlings of T. patula with similar biomass were transferred to the plastic pots. Based on the plant size, three seedlings were placed in each pot. The soil sample was water quenched and was then allowed to attend equilibrium in outdoor conditions under a waterproof tarpaulin for about three months. Afterward, the pots were organized in a randomized complete block design along with three replicates.
In each experimental pot, the plant samples were harvested and properly separated into leaves, stems, and roots. After washing, the samples were placed in a freeze dryer for freeze-drying at 50 ± 10 Pa and − 60 ± 2°C (BYK FD-1) and then were weighed to examine the biomass content. The collected soil samples were air-dried at room temperature and ground using 100-mesh sieves with mortar and pestles. The soil and plant samples were acid digested using a solution of 5:2:3 HNO3: H2O2: HF (v/v) and the Cd concentration was analyzed using the atomic absorption spectrophotometry (WFX-120). The PCBs concentration from both plant and soil was determined as per the method suggested by Tu et al. (2011). Furthermore, the evaporation of n-hexane under a stream of nitrogen was performed to concentrate the extract, and the residue was dissolved in hexane with a final volume of 1.0 mL for GC analysis.
The analysis was performed using Varian 3800 GC (Varian, USA) equipped with an auto-injector and electron capture detector. Triplicate analysis was performed to analyze all the collected samples, and the isooctane certified standards (J&K Company, two PCB congeners comprising PCB 18, PCB 28) were used during standard curves preparation for gas chromatography. The concentration of PCBs was measured using an external standard, and all the results are presented on a dry weight basis. The PCBs recoveries from spiked samples were in the range (85.6%–97.8%), and the limit of detection ranged from 1.33 to 3.45 μg kg−1, with a linear range of 0.5 to 50 ng mL−1 (Xu et al. 2010).
All experiments and treatment methods were performed in triplicates. The standard deviations (SD) and mean values were calculated using Origin Pro 8.5 and Microsoft Office Excel 2010 respectively. The SPSS19.0 was used for the one-way analysis of variance. During the study, if a significant (p < 0.05) difference was observed between treatments, then several comparisons were made by the ANOVA test (Zak et al. 1994).
Results and Discussion
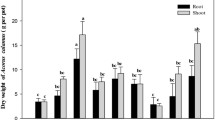
The growth condition of the plants in all the treatments is shown in Fig. 1. The T. patula dry matter yield at various soil pollution rates is shown in Fig. 2. The basic characteristics for a potential remediation plant are its high tolerant capability and exhibit diverse responses to mechanical stresses that enable them to evolve and tolerate resistance to adverse conditions which are potentially toxic to most plants (Sun et al. 2008). During the time of harvest, plant biomass could be an end-point of a bioassay. In this study, the biomass of T. patula did not decrease significantly under 100 µg PCBs kg−1 and 200 µg PCBs kg−1 compared with control. The addition of PCBs at a low level could enable the growth of plants, resulting in an increased growth at the rate of 11.47% to 30.82% when compared with the group where there are no PCBs. As shown in Fig. 1, the dry weight of shoot under low concentrations of PCBs was significantly higher than control. Especially for the application of 100 µg PCBs kg−1, the statistical analysis reveals that there is a significant increase in shoots of dry biomass yield (p < 0.05) and found the maximum value of 3.65 g kg−1. Particularly, the plant weight under T4 was the greatest among all the treatments. On the contrary, the plant weight under T12 was significantly lower than the control. These results suggest that a low concentration of PCBs could promote plant growth whereas a high concentration of PCBs restrains its growth to some extent. Regarding the shoot growth responses, compared with CK and T1 treatment groups, the dry weight of T4 and T5 increased respectively (Fig. 2). PCBs act as plant growth promoters, stimulate the release of auxin and play a positive role in the improvement of plant biomass. Regarding the root growth responses, no significant differences were identified among treatments under T4, T8, and CK, indicating that T. patula has a high tolerance to Cd and PCBs. The plant biomass decreased with the increased concentrations of Cd, showing that Cd inhibits the growth of T. patula, especially under the treatment of 30 mg Cd kg−1. This confirms that Cd has an inhibiting effect on plant growth (Liu et al. 2010). The existence of Cd affects the normal release of plant growth hormones and metabolites, resulting in the decrease of biomass. The biomass of CK, T1, T2, and T3 treatment groups decreased in sequence in both shoot and root growth responses. For this reason, T. patula cannot tolerate high concentrations of Cd and lacks phytoremediation ability. There is no significant difference between the plant biomass under T5 and CK, indicating the tolerance characteristics of a plant as a hyperaccumulator for low concentration co-contamination of Cd-PCBs (Gisbert et al. 2006; Peralta-Videa et al. 2004). In addition, the plant biomass tends to decrease under a high concentration of co-contaminated soil (Fig. 2), which indirectly affects the plant absorption of pollutants. Recent studies on metabolic pathway analysis have shown that plant root exudates have an important influence on the absorption of pollutants by plants. Hence, the root exudates may promote the absorption of contaminants through roots, and plant metabolites may also play an additional role in the process (Han et al. 2021).
Table 2 shows the distribution and amount of Cd absorption in the tissues of T. patula. With an increase in metal concentrations in soils, the concentration of Cd in plants also increased. The accumulation of Cd in T. patula under low concentration of contamination was in the order of leaf > stem > root and under high concentration the order was root > leaf > stem, indicating that high concentration of Cd could limit the ability of T. patula to transfer Cd from roots to stems. Statistical analysis showed that Cd accumulation in roots increased significantly under the combined stress of PCBs. Figure 3 shows the accumulation of Cd in T. patula under the combined stress of Cd and PCBs. The Cd concentration under 400 µg PCBs kg−1 was higher than other treatments, up to 996.9 µg kg−1, indicating that PCBs could promote the ability of T. patula to absorb Cd.
The criterion to define a Cd- hyperaccumulating plant is that the Cd concentration in overground parts is over 100 mg kg−1 with the translocation factor (TF) and bioaccumulation factor (BF) values higher than 1.0 (Zhou and Song 2004). The concentrations of Cd in T. patula different tissue parts were presented in Table 2. During the study, Cd concentration values of both TF and BF were greater than 1.0, up to 1.16 and 1.62 respectively under the co-contamination of 30 mg Cd kg−1 and 200 µg PCBs kg−1. The concentrations of Cd in this plant increased with the increasing soil concentrations of Cd. For all the treatments except T1 and T5, and the Cd concentrations in the plant were more than 100 mg kg−1. The Cd concentrations in shoots and roots increased with the increasing soil PCBs concentrations, but the values of TF were decreased with the increasing soil PCBs concentration. They were lower than 1.0 under 400 µg PCBs kg−1, indicating that high concentrations of PCBs could limit the ability to transfer Cd from roots to shoots.
The concentration of accumulated Cd by T. patula was shoot > root, and 81.46%–92.63% of Cd was absorbed by shoots, which is high compared with the concentration of Cd in the soil. Therefore, T. patula shows a very high capability of phytoextraction and could be a useful and feasible option for remediation of Cd-PCBs contamination. The accumulation of the heavy metal Cd in plants generally occurs by absorption through roots, and then transferred to above-ground parts (Table 3) (Sun et al. 2011).
The PCBs removal rate in soils with and without T. patula planting is presented in Fig. 4. With the increase in the concentration of PCBs, T. patula showed a significantly higher removal rate of PCBs than that of the unplanted control (C1 and C2), indicating that T. patula could promote the degradation of PCBs. Low concentrations of Cd also promote the degradation of PCBs. The efficiency was improved over 20%, especially for T13, the PCB18 and PCB28 removal rates were up to 42.72 and 42.29%, respectively. These results suggest that low concentrations of Cd could promote the degradation of PCBs by the plant. As shown in Fig. 4, the removal rates of PCBs by T. patula were significantly higher than that of natural degradation, indicating that T. patula could promote the degradation of PCBs. Compared with C1 and C2, the removal percentages of PCB18 and PCB28 in the plant increased by 6.92%–27.53% and 6.28%–21.97%, respectively. The removal rate increased with the increasing soil PCBs concentrations with the removal percentage of PCBs under 400 µg PCBs kg−1 11.09%–17.17% higher than that under 100 µg PCBs kg−1. That means high concentrations of Cd would promote the degradation of PCBs. The presence of pollutants affects plant root exudates and plant metabolites and improves the degradability of plants to pollutants. Recent studies have shown that plant root exudates and metabolites are significantly correlated with phytoremediation efficiency (Han et al. 2021). Therefore, in future experiments, we can analyze the correlation between plant root exudates and plant metabolites, the removal rate of heavy metals, and the degradation rate of organic pollutants.
The removal rates of PCBs were greater under co-contamination of 5 mg Cd kg−1, 15 mg Cd kg−1 than that of single PCBs contamination, except for T14. The removal percentages of PCB18 and PCB28 increased by 4.32%–14.90% and 1.43%–9.30% respectively. However, the removal rates of PCBs were inhibited under co-contamination of 30 mg Cd kg−1 with the removal percentages of PCB18 and PCB28 decreasing by 4.58%–15.23% and 2.65%–13.41% respectively. These results suggest that high concentrations of Cd would interfere with the removal rate of PCBs because of their toxicity. The degradation mechanism of PCBs is mainly the degradation of pollutants by root exudates and plant metabolites. A certain concentration of Cd will also stimulate the degradation of PCBs by plants.
In this study, the biomass of T. patula did not decrease significantly under 100 µg PCBs kg−1 and 200 µg PCBs kg−1 compared with control. Besides, low concentrations of PCBs could facilitate plant growth, especially for T4. Even under co-contamination of Cd-PCBs, the treatment of T5 and the biomass of root and shoot were not substantially different from CK. These findings suggest that this plant has a potentially strong tolerance level to single PCBs and Cd-PCBs stress. In addition, the promotion of PCBs degradation during the present work highlights that T. patula needs to be considered as a species having the tremendous potential for phytoremediation application specifically during co-contamination soils (Dmuchowski et al. 2014).
French marigolds have strong adaptability to the environment and are resistant to high temperatures. It can be sown in all seasons and can be seen everywhere in China. Polluted soils with PCBs typically contain a high concentration of Cd because they were discharged from e-waste emissions (Miao and Zhou 2016). There are many electronic waste landfills in China’s coastal areas, and French marigolds can be applied to these contaminated sites. The interplanting method can be used in the restoration process, harvesting before the withering of french marigolds and planting the next batch of plants that can not only improve the restoration efficiency of French marigold but also effectively avoid the secondary pollution caused by the withering leaves of French marigolds (Manousaki and Kalogerakis 2011). As the biomass of French marigolds is high, it can be harvested and burned in an incinerator after soil remediation, which will not have any impact on the environment. These results indicate that T. patula has potential feasibility and application to remediate combined contamination of Cd-PCBs at the field scale.
The analysis results that T. patula has a high tolerance to Cd and PCBs, especially to highly accumulated Cd. T. patula not only promoted Cd accumulation but also promoted Cd translocation from roots to shoots. Although the growth of T. patula was inhibited with the increased concentration of Cd in soil, the Cd concentrations in the polluted area were not more than 5 mg kg−1. Furthermore, the removal rate of PCBs was elevated with the increase of soil PCBs concentrations. The study explored the potential of phytoremediation of co-contamination of Cd and PCBs with T. patula, which is an ornamental plant. T. patula has a dual advantage, i.e. it could remediate contaminated soils as well as beautify the surrounding environment, especially in urban areas. Therefore, the study encourages and emphasizes the use of T. patula for phytoremediation of Cd and PCBs contaminated soils.
References
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated biphenyls and their biodegradation. Process Biochem 40(6):1999–2013
Dmuchowski W, Gozdowski D, Brągoszewska P, Baczewska AH, Suwara I (2014) Phytoremediation of zinc contaminated soils using silver birch (Betula pendula Roth). Ecol Eng 71:32–35
Gisbert C, Clemente R, Navarro-Avinó J et al (2006) Tolerance and accumulation of heavy metals by Brassicaceae species grown in contaminated soils from Mediterranean regions of Spain. Environ Exp Bot 56(1):19–27
Han T, Wang B, Wu Z et al (2021) Providing a view for toxicity mechanism of tetracycline by analysis of the connections between metabolites and biologic endpoints of wheat. Ecotoxicol Environ Safety 212:111998
Khandare RV, Desai SB, Bhujbal SS, Watharkar AD, Biradar SP, Pawar PK, Govindwar SP (2017) Phytoremediation of fluoride with garden ornamentals Nerium oleander, Portulaca oleracea, and Pogonatherum crinitum. Environ Sci Pollut Res 24(7):6833–6839
Liu J, Zhou Q, Wang S (2010) Evaluation of chemical enhancement on phytoremediation effect of Cd-contaminated soils with Calendula officinalis L. Int J Phytoremediation 12:503–515
Mackova M, Macek T, Kucerova P, Burkhard J, Pazlarova J, Demnerova K (1997) Degradation of polychlorinated biphenyls by hairy root culture of Solanum nigrum. Biotech Lett 19(8):787–790
Manousaki E, Kalogerakis N (2011) Halophytes present new opportunities in phytoremediation of heavy metals and saline soils. Ind Eng Chem Res 50(2):656–660
Marrugo-Negrete J, Pinedo-Hernández J, Díez S (2017) Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin. Colombia Environ Res 154:380–388
Miao X, Zhou Q (2016) Accumulation and pollution trends of heavy metals in the topsoil from an Industrial Park located in a typical new Chinese developing area. Fresen Environ Bull 25(12):5440–5448
Peralta-Videa JR, De la Rosa G, Gonzalez JH, Gardea-Torresdey JL (2004) Effects of the growth stage on the heavy metal tolerance of alfalfa plants. Adv Environ Res 8(3–4):679–685
Phusantisampan T, Meeinkuirt W, Saengwilai P, Pichtel J, Chaiyarat R (2016) Phytostabilization potential of two ecotypes of Vetiveria zizanioides in cadmium-contaminated soils: greenhouse and field experiments. Environ Sci Pollut Res 23(19):20027–20038
Safe SH (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24(2):87–149
Sun Y, Zhou Q, Diao C (2008) Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Biores Technol 99(5):1103–1110
Sun Y, Zhou Q, Xu Y, Wang L, Liang X (2011) Phytoremediation for co-contaminated soils of benzo [a] pyrene (B [a] P) and heavy metals using ornamental plant Tagetes patula. J Hazard Mater 186(2–3):2075–2082
Teng Y, Luo Y, Sun X et al (2010) Influence of arbuscular mycorrhiza and rhizobium on phytoremediation by alfalfa of an agricultural soil contaminated with weathered PCBs: a field study. Int J Phytorem 12(5):516–533
Tu C, Teng Y, Luo Y et al (2011) PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. J Soils Sediments 11(4):649–656
Wang X (2005) Resource potential analysis of ornamentals applied in contaminated soil remediation. A dissertation in Graduate School of Chinese Academy of Sciences, Beijing
Weyens N, Beckers B, Schellingen K et al (2015) The potential of the Ni-resistant TCE-degrading Pseudomonas putida W619-TCE to reduce phytotoxicity and improve phytoremediation efficiency of poplar cuttings on a Ni-TCE co-contamination. Int J Phytorem 17(1):40–48
Wu L, Li Z, Han C et al (2012) Phytoremediation of soil contaminated with cadmium, copper and polychlorinated biphenyls. Int J Phytorem 14(6):570–584
Xia J (1996) Detail explanation on the state soil-environment quality standard of China. Chinese Environmental Science Press, Beijing
Xu L, Teng Y, Li ZG, Norton JM, Luo YM (2010) Enhanced removal of polychlorinated biphenyls from alfalfa rhizosphere soil in a field study: the impact of a rhizobial inoculum. Sci Total Environ 408(5):1007–1013
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26(9):1101–1108
Zhou QX, Song YF (2004) Principles and methods of contaminated soil remediation. Science, Beijing
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (Grant No. 41907329) and Tianjin Key Laboratory of Hazardous Waste Safety Disposal and Recycling Technology, College of Environmental Science and Engineering, Tianjin University of Technology, Tianjin 300384, China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miao, X., Kumar, R.R., Shen, Q. et al. Phytoremediation for Co-contaminated Soils of Cadmium and Polychlorinated Biphenyls Using the Ornamental Plant Tagetes patula L.. Bull Environ Contam Toxicol 108, 129–135 (2022). https://doi.org/10.1007/s00128-021-03392-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03392-4