Abstract
Emerging evidence from both clinical and preclinical studies underscores the role of aging in potentiating the detrimental effects of hypertension on cerebral microhemorrhages (CMHs, or cerebral microbleeds). CMHs progressively impair neuronal function and contribute to the development of vascular cognitive impairment and dementia. There is growing evidence showing accumulation of senescent cells within the cerebral microvasculature during aging, which detrimentally affects cerebromicrovascular function and overall brain health. We postulated that this build-up of senescent cells renders the aged cerebral microvasculature more vulnerable, and consequently, more susceptible to CMHs. To investigate the role of cellular senescence in CMHs’ pathogenesis, we subjected aged mice, both with and without pre-treatment with the senolytic agent ABT263/Navitoclax, and young control mice to hypertension via angiotensin-II and L-NAME administration. The aged cohort exhibited a markedly earlier onset, heightened incidence, and exacerbated neurological consequences of CMHs compared to their younger counterparts. This was evidenced through neurological examinations, gait analysis, and histological assessments of CMHs in brain sections. Notably, the senolytic pre-treatment wielded considerable cerebromicrovascular protection, effectively delaying the onset, mitigating the incidence, and diminishing the severity of CMHs. These findings hint at the potential of senolytic interventions as a viable therapeutic avenue to preempt or alleviate the consequences of CMHs linked to aging, by counteracting the deleterious effects of senescence on brain microvasculature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of sophisticated magnetic resonance imaging (MRI) techniques, such as T2* gradient-recall echo and susceptibility-weighted imaging MRI sequences, has revolutionized the detection of cerebral microhemorrhages (CMHs) — small intracerebral hemorrhages once considered undetectable, which are particularly prevalent in elderly populations [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. These seemingly inconspicuous brain hemorrhages, typically under 5 mm in diameter, originate from ruptures in cerebral microvessels like arterioles and capillaries, leading to blood leakage into neighboring brain tissue [1, 25,26,27]. Gradually compromising neuronal functionality, CMHs are acknowledged contributors to vascular cognitive impairment and dementia (VCID) [3, 21].
Advanced age and hypertension emerge as the primary risk factors for CMHs [1, 20]. The prevalence of CMHs significantly increases with advanced age, from a modest ~ 6.5% in individuals aged 45 to 50 years to a staggering 50% or more in older adults [20]. These clinical observations are corroborated by recent rodent model data that highlight the synergistic interplay between aging and high blood pressure in the genesis of CMHs [28]. Besides the well-known role of arterial stiffening [29,30,31,32,33] and increased penetration of high-pressure waves into the susceptible distal portion of the cerebral microcirculation [34,35,36,37,38,39,40,41,42,43,44,45], aging is supposed to foster CMH development by exacerbating brain microvascular fragility [28]. However, the specific mechanisms through which aging amplifies microvascular fragility remain elusive, and effective preventive interventions are currently absent.
One of the most prominent hallmarks of aging is the accumulation of senescent cells, which are characterized by an irreversible cell cycle arrest and the establishment of an inflammatory environment termed the senescence-associated secretory phenotype (SASP) [46,47,48,49,50,51]. This phenotype involves the secretion of cytokines, chemokines, and extracellular matrix-degrading enzymes, including matrix metalloproteinases (MMPs) [52]. The emergence of cellular senescence is a multifaceted process, influenced by factors such as DNA damage, oxidative stress, and telomere attrition, which are known to increase with advancing age [50, 51]. The mounting presence of senescent cells has far-reaching implications, as it is associated with tissue dysfunction and paves the way for a plethora of age-related diseases including cancer, cardiovascular disorders, and neurodegenerative conditions [46,47,48,49,50,51]. Our recent investigations furnish compelling evidence that senescent cells, including endothelial cells, astrocytes, and microglia, accumulate within the neurovascular unit as aging progresses, contributing to compromised neurovascular function, cerebral blood flow dysregulation, and blood–brain barrier disruption [53,54,55,56,57,58,59,60,61]. Motivated by these findings, senolytics — a class of therapeutics proficient in selectively eliminating senescent cells — have piqued interest for their capacity to rejuvenate tissue function and mitigate age-related pathologies in preclinical settings [62,63,64]. However, the potential of senolytics in counteracting CMH remains an uncharted territory.
This study aims to investigate the hypothesis that the accumulation of senescent cells and the associated SASP may render the cerebral microvasculature more susceptible to rupture, hence predisposing the aging brain to CMHs, and to evaluate whether employing senolytics can prevent the formation of CMHs. To probe this hypothesis, we administered ABT263 (Navitoclax), a BCL-2/BCL-xL inhibitor endowed with potent senolytic activity [62,63,64], to old mice. This was followed by the induction of hypertension through the administration of angiotensin II and the NO synthase inhibitor L-NAME. This protocol is recognized for inducing CMHs in mice, with the severity displaying a notable escalation in conjunction with advancing age [28, 65,66,67,68,69,70]. Rigorous analysis was conducted to assess the occurrence, size, and distribution of CMHs, and to evaluate the neurological outcomes and gait irregularities in both ABT263-treated and untreated aged mice, as well as in young control mice.
Methods
Experimental animals, senolytic treatment
All experimental procedures involving animals, as well as animal husbandry practices, were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Oklahoma Health Sciences Center (OUHSC), OK, USA. In this study, we utilized a cohort of young C57BL/6 mice (6 months old, n = 25, 13 males and 12 females) and aged C57BL/6 mice (18 months old, n = 50, equally divided between males and females), procured from Charles River Laboratories, Wilmington, MA. Mice were housed in the Rodent Barrier Facility at OUHSC, which is maintained under stringent specific-pathogen-free conditions. Furthermore, a subset of mice was housed in the conventional rodent housing facilities of the Division of Comparative Medicine at OUHSC. The light–dark cycle was meticulously controlled at 12-h intervals, and the mice were provided unrestricted access to standard rodent chow (sourced from Purina Mills, Richmond, IN, USA) and water.
Within the aged cohort, mice were randomly allocated into two groups of 25 mice each (Fig. 1). One subset of this aged cohort underwent treatment with the BCL-2/BCL-xL inhibitor, ABT263/Navitoclax, a potent senolytic agent. The drug was administered through oral gavage at a dosage of 151.5 mg/kg/day ABT, dissolved in 60% Phosal/PG, 30% PG400, and 10% ethanol diluent. The solution was incubated on 35 °C for 30 min before administration. This treatment regimen consisted of two cycles, each spanning 5 days, with a 1-week intermission between cycles. Following this intensive course, the mice were granted a recuperation period of 1 month in their original housing environments before the subsequent phases of the study. The second group of animals received vehicle instead of ABT263 (hereafter referred to as aged or aged control). This treatment regimen was shown to effectively eliminate senescent cells from the murine cerebral circulation in previous studies [62, 64]. Notably, by the time of hypertension induction, the aged mice had reached ~ 20 months of age, while their younger counterparts were ~ 8 months old.
Experimental timeline and workflow. A Illustrates the experimental timeline. Aged mice were subjected to treatment with the senolytic agent ABT263/Navitoclax. Subsequently, in young and aged control mice and ABT263-treated aged ice, hypertension is induced by angiotensin II (Ang II) plus L-NAME treatment. Daily neurological testing and gait analysis were conducted to assess the neurological signs of CMHs. Upon neurological manifestations of CMHs animals were euthanized, and their brains were collected for further analysis. B provides an overview of the workflow for the histological analysis of CMHs. The collected brain tissue is processed through various steps, including fixation, sectioning, staining, and microscopy, to examine and quantify CMHs within the brain parenchyma. This histological analysis helps evaluate the effectiveness of the senolytic treatment in preventing CMHs in the aging mice
Hypertension induction and blood pressure measurements
To investigate the potential of senolytic treatment as a protective measure against hypertension-induced cerebral microbleeds, we induced hypertension in each cohort of mice (Fig. 1) by administering a combination treatment of the nitric oxide inhibitor ω-nitro-l-arginine-methyl ether (L-NAME, 100 mg/kg/day) in drinking water, along with subcutaneous administration of angiotensin II (Ang-II; s.c. via osmotic mini-pumps [Alzet Model 2006, 0.15 µl/h, 42 days; Durect Co, Cupertino, CA]). The dosage of L-NAME and Ang-II used was based on the results of previous studies using the same protocol [28, 66]. Pumps were filled either with saline vehicle or solutions of angiotensin II (Sigma Chemical Co., St. Louis, Missouri, USA) delivered subcutaneously at 1 µg/min/kg of angiotensin II thus generating three experimental groups: (1) aged mice + Ang-II + L-NAME, (2) ABT263-treated aged mice + Ang-II + L-NAME, (3) young mice + Ang-II + L-NAME. The pumps were implanted under isoflurane anesthesia into the subcutaneous space of mice through a small incision in the interscapular area. The incision was closed with surgical sutures using aseptic techniques, and buprenorphine analgesia (0.1 mg/kg of body weight, s.c.) was administered immediately after closing the incisions. The incision sites healed quickly without the need for additional medication. Ang II-dependent hypertension serves as a clinically relevant model for studying age-related cerebrovascular alterations [37]. This is particularly relevant since that aging is associated with heightened activity of the vascular renin-angiotensin system, and Ang II-dependent hypertension is prevalent among older individuals [71]. Blood pressure of the animals was measured during the treatment period using a tail-cuff blood pressure machine (CODA Non-Invasive Blood Pressure System, Kent Scientific Co., Torrington, CT) to ensure successful hypertension induction, as described [28, 35, 37]. Each experimental group was closely monitored, and mice were sacrificed upon the occurrence of clinical signs of intracerebral hemorrhages.
Standardized neurological examination of mice
We conducted a standardized neurological examination on mice to evaluate the presence of clinical indicators of hemorrhages. The examination involved assessing the animals’ spontaneous activity, symmetry in the movement of all four limbs, forelimb outstretching, climbing ability, body proprioception, response to vibrissae touch, and gait coordination. Each animal was assigned a daily score, which was calculated by summing up the scores from all individual tests. If a consistent decline in the neurological score was observed, the mice were humanely euthanized, and the brains were collected after transcardial perfusion (see below).
Analysis of gait function
To evaluate gait function and analyze the spatial and temporal aspects of interlimb coordination, we employed the CatWalk System (Noldus Information Technology Inc., Leesburg, VA) following the methodology as reported [28, 67, 68, 72,73,74,75,76]. Briefly, animals from each group were acclimatized and trained to voluntarily walk across the illuminated walkway in a dark and quiet room dedicated for behavioral experimentation. Mice gait function was acquired for over 20 consecutive runs, producing over 200 steps for each animal. The resulting data was averaged across the ~ 20 runs in which mice maintained a relatively constant speed across the walkway. Subsequently, computer-aided analysis of the gait data and manual paw identification and labeling of each footprint was carried out blindly, and spatial and temporal gait parameters were calculated [67]. Gait in each mouse was analyzed on the day before hypertension induction and each day thereafter. This allowed us to assess changes in various gait parameters associated with CMHs.
The variability of the data was assessed using quartile dispersion. We adopted a common outlier definition, labeling points more than 1.5 interquartile ranges away from the sample median as extreme values. Mean gait characteristics calculated included speed, stride length, stride time, and base of support. Stride length is the distance (in centimeter) between successive placements of the same paw. Stride time is the interval lapsed (s) between each successive paw contact. Base of support is the average width between the paws. Variability characteristics and measures of gait coordination were also acquired using the CatWalk system.
The regularity index (%) is a fractional measure of inter-paw coordination, which expresses the number of normal step sequence patterns relative to the total number of paw placements:
In healthy, fully coordinated mice, the regularity index value is closer to 100%.
Investigating gait variability, the stride-to-stride fluctuations in gait parameters, offers a sensitive, novel method of quantifying subtle changes in locomotion in mice [67, 77, 78]. Stride time and stride length variability were analyzed by computing the median absolute deviation (MAD) for each dataset obtained in consecutive runs at similar speeds. MAD is a robust measure of variability that indicates the average distance between observations and their median.
Analysis of gait symmetry provides an additional approach in the characterization of gait coordination. Importantly, as location of CMHs is asymmetric, it can be expected that assessment of gait symmetry can reveal sub-clinical alterations in gait coordination [67]. This study investigated the effects of CMHs on gait symmetry by quantifying the symmetry index (SI). The symmetry index (SI) is a very sensitive and commonly used assessment of gait symmetry on the basis of spatial–temporal gait characteristics [75, 79]. The equation for the stride length (SL) symmetry index is displayed. Similar calculations were conducted for stride time and print area symmetry indices:
The symmetry index is a method of percentage assessment of the relative inter-limb differences between the kinematic and kinetic parameters during locomotion. The value of SI = 0 indicates full symmetry, while SI ≥ 100% indicates lack of symmetry in the gait characteristic of interest.
Histological analysis of CMHs
Mice were anesthetized and transcardially perfused with ice-cold heparinized PBS for 10 min and subsequently decapitated as reported [28, 67]. Then, the brains were removed from the skull and fixed in 4% formalin at room temperature for 1 day. The next day, the brains were placed in fresh 4% formalin (at 4 °C, for 2 days), then in 70% ethanol (at 4 °C, for 2 days), followed by embedding in paraffin. The brains were serially sectioned at 8-μm thickness yielding approximately 800 sections per brain. The sections were stained with hematoxylin to reveal the brain structure and diaminobenzidine (DAB) to highlight the presence of hemorrhages. DAB turns into dark brown when it undergoes a reaction with peroxidases present in red blood cells therefore allowing precise detection of extravasated blood cells in the parenchyma of the brain. All stained sections were screened by a reader blinded to the treatment groups, and images were acquired in regions with positive DAB staining. Digital images were analyzed with ImageJ 1.52p (NIH, USA) software to identify the location and quantify the number and size of hemorrhages. Images captured with same magnification were color deconvolved and thresholded uniformly, and then the pixel intensity integrated density was measured on the selected bleed area. The volumetric reconstruction of individual hemorrhages was estimated based on the sum of CMH volumes on consecutive sections (CMH area x slice thickness) as described [28, 68].
Statistical analysis
Two-tailed t-test was used for comparison of two groups. Two-way analysis of variance followed by Fisher LSD method or Kruskal–Wallis one-way analysis of variance on ranks was used for comparison of multiple groups as needed based on the sample distribution. The incidence curves were compared with Mantel-Cox log-rank test. A P value < 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
Exacerbation of hypertension-induced spontaneous CMHs in aged mice: prevention by senolytic treatment
Treatment with Ang II plus L-NAME resulted in comparable increases in blood pressure in all three groups, from ~ 115 to ~ 155 mmHg. Given that aging is often accompanied by heightened activity of the vascular renin-angiotensin system, and Ang II-dependent hypertension is prevalent in the elderly population. Ang II-dependent hypertension serves as a clinically highly relevant model for investigating age-related cerebrovascular alterations [37]. Previous investigations conducted by the Heistad laboratory [54, 55], as well as subsequent studies carried out by our own laboratories [28, 68], have established models of spontaneous hypertension-induced hemorrhages in mice. These studies have consistently demonstrated that co-administration of L-NAME leads to an additional increase of approximately 15 mmHg in blood pressure. This elevation in blood pressure is associated with a significant rise in the incidence of CMHs, particularly in the presence of increased microvascular fragility.
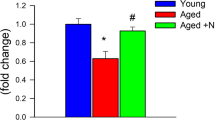
Throughout the experimental period, daily neurological assessments were conducted, revealing that 28% of young mice exhibited signs indicative of hypertension-induced CMHs. In stark contrast, a significant 91% of aged mice displayed clinically manifest signs of CMHs, and notably, these manifestations occurred much earlier than in young mice. The cumulative distribution curves depicting the time-to-event in the two groups are illustrated in Fig. 2A. Remarkably, pre-treatment with ABT263 yielded promising results. The incidence of clinical manifestations of CMHs in aged hypertensive mice was significantly reduced to 62% following ABT263 administration. Furthermore, the onset of symptoms was significantly delayed in comparison to age-matched controls, as depicted in Fig. 2A. The differences among the cumulative incidence curves proved to be statistically highly significant (P < 0.0001 for both comparisons: hypertensive senolytic-treated aged mice versus hypertensive aged mice, and hypertensive aged mice versus hypertensive young mice). These statistical analyses were conducted using the Mantel-Cox log-rank test (Fig. 2A).
Prevention of hypertension-induced spontaneous CMHs in aged mice by senolytic treatment with ABT263/Navitoclax. A Cumulative incidence curves for neurological signs of hypertension-induced intracerebral hemorrhages in young control mice (n = 25), aged control mice (n = 25), and aged mice treated with the senolytic drug ABT263/Navitoclax (n = 25). In aged control mice, there was a significant increase in CMH incidence compared to young control mice. Senolytic treatment significantly reduced CMH incidence in aged mice (log-rank test; Mantel-Cox). B Representative images of CMHs stained by diaminobenzidine in the cortex of hypertensive aged mice. Right: workflow showing analysis of CMHs. Brightfield images of CMHs were captured using a 10 × and a 4 × objective. The images were batch-processed for color deconvolution, thresholding, binarization, and area measurement. C Panels are depicting a single CMH that spreads across multiple sections. The volumetric reconstruction of individual hemorrhages was estimated based on the sum of CMH volumes on consecutive sections. (CMH area x slice thickness)
Histological analysis confirmed that mice with neurological signs developed CMHs (Fig. 2B, C) that were distributed widely in the brain. No normotensive mice developed neurologic signs or histologically detectable CMHs (data not shown). In hypertensive young mice, only a few CMHs were observed (Fig. 3A). Figure 3 A reveals a significant increase in the number of CMHs observed in hypertensive aged mice when compared to their young hypertensive counterparts. These findings further support previous studies, which have consistently demonstrated that aging exacerbates the development of CMHs by augmenting microvascular fragility [28]. The administration of senolytic treatment using ABT263 yielded a significant reduction in the number of CMHs observed in the brains of hypertensive aged mice, as demonstrated in Fig. 3A.
ABT263 treatment mitigates hypertension-induced CMHs in aging mice. A Total number of hypertension-induced cerebral microhemorrhages (CMHs) across the entire brain in young mice, aged mice, and aged mice treated with ABT263/navitoclax. Data presented as mean ± SEM. **P < 0.01 vs. Aged. B Distribution of CMHs based on location. ABT263 treatment significantly reduces the number of CMHs in the cerebral cortex and hindbrain. Data represented as mean ± SEM. *P < 0.05. ****P < 0.001 C Distribution of CMHs by volume in aged control mice and ABT263-treated aged mice. Smaller bleeds (volume < 10−4 mm.3) predominate in aged mice, and ABT263 senolytic treatment effectively prevents these. **P < 0.01. D Total CMH volume per mouse reveals that ABT263 treatment significantly reduces the overall volume of hemorrhages in the brain (n = 3 mice analyzed in each group). ***P < 0.001
The results depicted in Fig. 3B indicate that aging primarily contributes to an elevated incidence of CMHs located in the cortex. Furthermore, the analysis of the volume distribution of CMHs, as illustrated in Fig. 3C, suggests that aging plays a significant role in increasing the occurrence of the smallest hemorrhages, which are known to originate from the distal portion of the microcirculation. When the cerebral vessels associated with the CMHs were clearly distinguishable, their internal diameter was observed to fall within the approximate range of 10–20 µm. Notably, the administration of senolytic treatment with ABT263 proved effective in preventing the formation of these small CMHs specifically in the cerebral cortex, as illustrated in Fig. 3B, C. Senolytic treatment with ABT263 also significantly reduced CMH burden, expressed as total CMH volume, in aged mice (Fig. 3C). This finding highlights the potential of ABT263 as a senolytic treatment to mitigate the development of small CMHs in the cerebral cortex.
Reduced incidence of CMHs is associated with improved gait function in ABT263-treated aged hypertensive mice
In line with previous investigations conducted in both human studies [80] and laboratory mice [28, 67, 68] that link gait with CMHs, we conducted a comprehensive analysis of gait in the present study’s cohorts. Our observations showed that gait abnormalities were markedly more pronounced in hypertensive aged mice when compared to their hypertensive young counterparts, as illustrated in Fig. 4. Specifically, the regularity index, serving as a robust measure of inter-paw coordination, exhibited a notable decline in hypertensive aged mice relative to hypertensive young mice (Fig. 4A). Importantly, the administration of senolytic treatment with ABT263 effectively mitigated gait dysfunction associated with hypertension-induced genesis of CMHs, as depicted in Fig. 4A.
Impact of reduced CMH incidence on gait coordination in hypertensive aged mice treated with ABT263/Navitoclax. A Regularity index, B base of support (hind paws), C stride length symmetry index, D stride time symmetry index, E paw print area symmetry index, F stride time variability (MAD), and G stride length variability (MAD) in spontaneously walking young mice, aged mice, and aged mice treated with ABT263/navitoclax before and after hypertension-mediated CMH induction. Data are presented as mean ± SEM. *P < 0.05 vs. young, #P < 0.05 vs. aged
Gait encompasses a cyclical and laterally alternating stride progression to maintain balance. A comparison of left versus right stride lengths and stride time asymmetries in hypertensive mice allows for the assessment of potential functional deficits arising from asymmetric CMHs that may otherwise remain unnoticed. We conducted this assessment by calculating symmetry indices [67, 79] for hind limb stride length, stride time, and paw print areas in hypertensive aged mice and aged mice treated with ABT263 (Fig. 4C–E). While our findings indicated a tendency for hypertensive aged mice to exhibit a more asymmetric stride pattern during gait in comparison to hypertensive senolytic-treated aged mice, particularly in terms of increased asymmetry in both stride time and stride length (Fig. 4C–E), these differences did not reach statistical significance.
The study of gait variability, which involves examining stride-to-stride fluctuations during walking, provides a sensitive means of quantifying and characterizing locomotion. Prior studies in older adults have suggested that measures of gait variability are more closely linked to cognitive decline or falls than measures based solely on mean values of other gait characteristics [81,82,83,84,85,86,87,88,89,90]. In our study, we did not observe statistically significant differences in either stride time variability or stride length variability between the two groups (Fig. 4F, G). Gait parameters in normotensive aged and young mice did not change significantly during the experimental period (data not shown).
Discussion
This study aimed to investigate the potential of senolytic treatment as a protective measure against hypertension-induced CMHs in aged mice. Our findings revealed that aged mice exhibited a significantly higher incidence of CMHs compared to young mice, consistent with previous studies highlighting the exacerbating effects of aging on CMH development. This further supports the notion that aging contributes to increased microvascular fragility, making aged individuals more susceptible to CMHs [1, 28]. Our ongoing studies employing single-cell RNA sequencing reveal that by the time mice reach 18 to 20 months of age, there is a notable increase in the number of senescent cerebromicrovascular endothelial cells. Intriguingly, our study demonstrated that senolytic treatment with ABT263 had a significant effect in reducing the number of CMHs in the brains of hypertensive aged mice. This finding suggests that senolytic treatment holds promise in attenuating CMH development, particularly in the context of hypertension and aging. The observed reduction in CMH incidence and delayed onset of symptoms in senolytic-treated aged mice further emphasize the potential therapeutic benefits of senolytic treatment.
The mechanisms by which senescent cells may promote microvascular fragility and the genesis of CMHs are likely multifaceted and involve various interconnected pathways (Fig. 5). Cellular senescence induces a state of chronic inflammation, known as senescence-associated secretory phenotype (SASP), which involves the secretion of numerous pro-inflammatory cytokines, chemokines, growth factors, and extracellular matrix degrading enzymes (i.e., matrix metalloproteinases) [53]. This chronic inflammatory microenvironment and MMP activation can contribute to the remodeling of the extracellular matrix (ECM) and impair the integrity and stability of blood vessels [57, 58]. Oxidative stress, a hallmark of cellular senescence, can activate inflammatory pathways and lead to the activation of MMPs [28, 68]. MMPs degrade components of the ECM, including collagen and elastin, compromising the structural integrity of blood vessels [28, 68]. Previous studies also demonstrate an age-related exacerbation of hypertension-induced cerebrovascular ROS production and redox-sensitive activation of MMPs in the pathogenesis of CMHs [28].
Proposed mechanisms underlying the exacerbation of hypertension-induced microvascular damage and CMHs by age-related endothelial senescence. This schematic illustrates the proposed model elucidating how age-related endothelial senescence may intensify hypertension-induced microvascular damage, ultimately leading to cerebral microhemorrhages (CMHs). In this model, senescent cells express a highly pro-inflammatory senescence-associated secretory phenotype (SASP), characterized by the secretion of matrix-degrading matrix metalloproteinases (MMPs) [54]. Age-related oxidative stress amplifies the activation of MMPs [28], resulting in increased microvascular fragility in the vicinity of senescent cells. Furthermore, age-related circulating insulin-like growth factor-1 (IGF-1) deficiency contributes to microvascular oxidative stress [105] and impairs the microvascular structural adaptation to hypertension, further worsening microvascular fragility [68]. Additionally, age-related circulating IGF-1 deficiency disrupts the functional adaptation of small arteries and arterioles to hypertension, leading to autoregulatory dysfunction and the transmission of high pulsatile pressure waves to the vulnerable distal portion of the cerebral microcirculation [35, 37, 38]. Collectively, these factors promote the genesis of CMHs in the aging cerebral microvasculature. Furthermore, senescent cells also play a role in blood–brain barrier (BBB) disruption [62, 106], leading to neuroinflammation, and endothelial dysfunction, which impairs neurovascular coupling (NVC) and disrupts cerebral blood flow (CBF) regulation [56]. These combined effects contribute to the pathogenesis of vascular cognitive impairment
Recent findings have demonstrated that age-related exacerbation of hypertension-induced activation of MMPs can be mitigated by antioxidative treatments, which also provide partial protection against the genesis of CMHs [28]. The increased oxidative stress observed in aged vessels during hypertension has been attributed to the upregulation of NOX oxidases, elevated mitochondrial ROS generation, and impaired antioxidant defense mechanisms [28, 91,92,93]. Consequently, further studies are warranted to investigate the effects of inhibitors targeting NOX oxidases and mitochondria-derived ROS production as combination treatments with senolytics on CMH development in the context of aging.
The mechanisms through which cellular senescence promotes CMHs likely also involve the impaired structural and functional adaptation of the cerebral circulation to hypertension (Fig. 5). During hypertension, healthy cerebral arterioles undergo structural remodeling, including media hypertrophy, which reduces circumferential stress and prevents mechanical damage to the vascular wall [94]. It is plausible that the presence of senescent cells in the microvasculature disrupts arteriolar remodeling processes by impairing hypertension-induced adaptive media hypertrophy and ECM remodeling. Future studies should be conducted to test this hypothesis and evaluate the effects of senolytic treatments on these processes.
Another mechanism through which senescent cells may exacerbate microvascular fragility is through the induction of paracrine senescence [57, 58]. SASP factors secreted from senescent cells in the neurovascular unit can propagate senescence to neighboring cells, thereby amplifying the detrimental effects on microvascular function and integrity. Taken together, these senescence-related mechanisms are likely interconnected and work in concert to contribute to the increased susceptibility of microvessels to rupture and the subsequent development of CMHs.
In addition to promoting CMHs, senescent cells are also implicated in the pathogenesis of VCID through their contribution to cerebromicrovascular functional impairment [57, 58]. Senescent cells within the cerebrovascular system can lead to various detrimental effects on microvascular function, including endothelial dysfunction, impaired neurovascular coupling, compromised blood–brain barrier integrity, and reduced capillarization [56, 62, 64]. Understanding the mechanisms by which senescent cells influence both the structural and functional integrity of the cerebral microcirculation will pave the way for developing targeted interventions to mitigate the detrimental effects of senescence on the brain and preserve cognitive function in aging individuals.
Multiple cell types within the neurovascular unit may undergo senescence and contribute to the pathogenesis of VCID and microvascular fragility [56, 62, 64]. Endothelial cells, as key regulators of vascular function, are particularly susceptible to senescence-associated dysfunction [56]. Perivascular microglia, resident immune cells in the brain, can also undergo senescence [54, 55], leading to chronic neuroinflammation and impairing their beneficial roles in maintaining vascular homeostasis. Vascular smooth muscle cells, pericytes, and astrocytes, all essential components of the neurovascular unit, may also be affected by senescence [47, 95,96,97,98,99,100,101,102]. The specific contribution of each cell type to VCID and microvascular fragility warrants further investigation. Understanding the involvement of distinct senescent cell populations in these processes will provide valuable insights for developing targeted therapeutic strategies aimed at mitigating senescence-related vascular complications.
ABT263/Navitoclax is an orally active experimental anti-cancer drug that targets apoptosis regulator proteins Bcl-2 and Bcl-XL, selectively inducing apoptosis in senescent cells while sparing non-senescent cells [103]. Of note, senescent endothelial cells exhibit heightened susceptibility to ABT263/Navitoclax-induced apoptosis due to decreased Bcl-2 expression and increased BAX expression [104]. Further studies are needed to determine the specific senescent cell types relevant for the pathogenesis of CMHs (including endothelial cells, pericytes, vascular smooth muscle cells, and perivascular microglia) that can be eliminated in the aged mouse brain using ABT263/Navitoclax as a senolytic treatment. The development of novel, translatable senolytic approaches is essential to address the growing burden of age-related CMHs and other CNS pathologies and improve clinical outcomes. While the potential of senolytic treatments has been demonstrated in preclinical studies, the translation of these findings into effective therapies for human patients remains a crucial challenge. Advancing the field of senolytics requires the identification of safe and specific senolytic agents, optimization of treatment regimens, and rigorous evaluation of their efficacy and safety in human clinical trials. Furthermore, the development of strategies targeting specific senescent cell populations and understanding the interplay between cellular senescence and the surrounding tissue microenvironment will be paramount for the success of future senolytic interventions.
Our study has several important limitations that should be acknowledged. First, our study focused on a mouse model, and further investigations are needed to determine the translational potential of senolytic treatment in human populations. Second, the specific mechanisms by which senolytic treatment exerts its protective effects on CMH development and gait dysfunction remain to be fully elucidated. Future studies should delve into the cellular and molecular pathways and mechanisms involved and investigate potential cellular senescence targets for combination treatments. Another limitation of this study is that we did not conduct a direct comparison between male and female mice, potentially overlooking sex-specific differences in the observed outcomes.
In conclusion, our study provides compelling evidence that senolytic treatment with ABT263/Navitoclax significantly reduces the incidence of hypertension-induced CMHs and effectively prevents neurological consequences in aged mice. These findings highlight the potential of senolytic treatment as a promising therapeutic approach to mitigate CMHs and preserve neuronal function in hypertensive aging individuals. However, further research is warranted to validate the effects of different senolytic regimens in human populations and to elucidate the underlying mechanisms responsible for senolytic-mediated protection of the cerebromicrovasculature. These endeavors will pave the way for the development of novel interventions aimed at preventing age-related cerebromicrovascular complications and the onset of VCID.
References
Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–43.
Akoudad S, Portegies ML, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, Vernooij MW. Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam Study. Circulation. 2015;132:509–16.
Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–43.
Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, Sattar N, Westendorp RG, van Buchem MA, van der Grond J. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42:638–44.
Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging. 2010;31:142–8.
Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM. Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL Study. JAMA Neurol. 2015;72:539–45.
Caplan LR. Microbleeds. Circulation. 2015;132:479–80.
Caunca MR, Del Brutto V, Gardener H, Shah N, Dequatre-Ponchelle N, Cheung YK, Elkind MS, Brown TR, Cordonnier C, Sacco RL and Wright CB. Cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: the Northern Manhattan Study. J Am Heart Assoc 2016;5.
Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003.
Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–60.
De Reuck JL, Deramecourt V, Auger F, Durieux N, Cordonnier C, Devos D, Defebvre L, Moreau C, Capparos-Lefebvre D, Pasquier F, Leys D, Maurage CA, Bordet R. The significance of cortical cerebellar microbleeds and microinfarcts in neurodegenerative and cerebrovascular diseases. A post-mortem 7.0-tesla magnetic resonance study with neuropathological correlates. Cerebrovasc Dis. 2015;39:138–43.
Ding J, Sigurethsson S, Jonsson PV, Eiriksdottir G, Meirelles O, Kjartansson O, Lopez OL, van Buchem MA, Gudnason V, Launer LJ. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88:2089–97.
Fluri F, Jax F, Amort M, Wetzel SG, Lyrer PA, Katan M, Hatz F, Engelter ST. Significance of microbleeds in patients with transient ischaemic attack. Eur J Neurol. 2012;19:522–4.
Gregoire SM, Scheffler G, Jager HR, Yousry TA, Brown MM, Kallis C, Cipolotti L, Werring DJ. Strictly lobar microbleeds are associated with executive impairment in patients with ischemic stroke or transient ischemic attack. Stroke. 2013;44:1267–72.
Hilal S, Saini M, Tan CS, Catindig JA, Koay WI, Niessen WJ, Vrooman HA, Wong TY, Chen C, Ikram MK, Venketasubramanian N. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord. 2014;28:106–12.
Mesker DJ, Poels MM, Ikram MA, Vernooij MW, Hofman A, Vrooman HA, van der Lugt A, Breteler MM. Lobar distribution of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2011;68:656–9.
Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, Kitagawa K. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83:646–53.
Nannoni S, Ohlmeier L, Brown RB, Morris RG, MacKinnon AD, Markus HS, investigators DNAL. Cognitive impact of cerebral microbleeds in patients with symptomatic small vessel disease. Int J Stroke. 2022;17:415–24.
Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, Kwak KC, Yoon U, Lee JM, Lee JW, Shin JS, Kim CH, Noh Y, Cho H, Kim HJ, Yoon CW, Oh SJ, Kim JS, Choe YS, Lee KH, Lee JH, Ewers M, Weiner MW, Werring DJ, Na DL. Pathogenesis of cerebral microbleeds: in vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol. 2013;73:584–93.
Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–61.
Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–33.
Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75:2221–8.
Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jager HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–75.
Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–5.
Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol. 2003;24:88–96.
Yates PA, Sirisriro R, Villemagne VL, Farquharson S, Masters CL, Rowe CC. Cerebral microhemorrhage and brain beta-amyloid in aging and Alzheimer disease. Neurology. 2011;77:48–54.
Blitstein MK, Tung GA. MRI of cerebral microhemorrhages. AJR Am J Roentgenol. 2007;189:720–5.
Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–8.
Babici D, Kudej RK, McNulty T, Zhang J, Oydanich M, Berkman T, Nishimura K, Bishop SP, Vatner DE, Vatner SF. Mechanisms of increased vascular stiffness down the aortic tree in aging, premenopausal female monkeys. Am J Physiol Heart Circ Physiol. 2020;319:H222–34.
DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol. 2021;320:H169–80.
Toth L, Czigler A, Szarka N, Toth P. The role of transient receptor potential channels in cerebral myogenic autoregulation in hypertension and aging. Am J Physiol Heart Circ Physiol. 2020;319:H159–61.
Charlton PH, Mariscal Harana J, Vennin S, Li Y, Chowienczyk P, Alastruey J. Modeling arterial pulse waves in healthy aging: a database for in silico evaluation of hemodynamics and pulse wave indexes. Am J Physiol Heart Circ Physiol. 2019;317:H1062–85.
Pagoulatou SZ, Bikia V, Trachet B, Papaioannou TG, Protogerou AD, Stergiopulos N. On the importance of the nonuniform aortic stiffening in the hemodynamics of physiological aging. Am J Physiol Heart Circ Physiol. 2019;317:H1125–33.
Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–30.
Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–708.
Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–20.
Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–42.
Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–97.
Kim DH, Choi JH, Moon JS, Kim HJ, Cha JK. Association between the severity of cerebral small vessel disease, pulsatility of cerebral arteries, and brachial ankle pulse wave velocity in patients with lacunar infarction. Eur Neurol. 2010;64:247–52.
Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23.
Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age. Gene/Environ Susceptibility-Reykjavik Study Brain. 2011;134:3398–407.
O’Sullivan C, Duggan J, Lyons S, Thornton J, Lee M, O’Brien E. Hypertensive target-organ damage in the very elderly. Hypertension. 2003;42:130–5.
Seo WK, Lee JM, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovasc Dis. 2008;26:618–23.
Shimoyama T, Iguchi Y, Kimura K, Mitsumura H, Sengoku R, Kono Y, Morita M, Mochio S. Stroke patients with cerebral microbleeds on MRI scans have arteriolosclerosis as well as systemic atherosclerosis. Hypertens Res. 2012;35:975–9.
Thorin-Trescases N, de Montgolfier O, Pincon A, Raignault A, Caland L, Labbe P, Thorin E. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol. 2018;314:H1214–24.
Tuttle CSL, Waaijer MEC, Slee-Valentijn MS, Stijnen T, Westendorp R, Maier AB. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell. 2020;19:e13083.
Cohen J, Torres C. Astrocyte senescence: evidence and significance. Aging Cell. 2019;18:e12937.
Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–16.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705.
Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL, Jurk D. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20:e13296.
Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: relevance for clearance of senescent cells. Aging Cell. 2019;18:e12841.
Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118.
Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–21.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–44.
Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, Wiley G, Garman L, Ungvari Z, Csiszar A. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience. 2022;44:661–81.
Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–40.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–41.
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67.
Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–8.
Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–72.
Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–61.
Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, Tarantini A, Nagaraja R, Yan F, Tang Q, Mukli P, Csipo T, Yabluchanskiy A, Campisi J, Ungvari Z, Csiszar A. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 2023; e13832.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83.
Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, Ungvari Z. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience. 2020;42:409–28.
Miller LR, Tarantini S, Nyul-Toth A, Johnston MP, Martin T, Bullen EC, Bickel MA, Sonntag WE, Yabluchanskiy A, Csiszar A, Ungvari ZI, Elliott MH, Conley SM. Increased susceptibility to cerebral microhemorrhages is associated with imaging signs of microvascular degeneration in the retina in an insulin-like growth factor 1 deficient mouse model of accelerated aging. Front Aging Neurosci. 2022;14:788296.
Tarantini S, Yabluchanskiy A, Lindsey ML, Csiszar A, Ungvari Z. Effect of genetic depletion of MMP-9 on neurological manifestations of hypertension-induced intracerebral hemorrhages in aged mice. Geroscience. 2021;43:2611–9.
Nyul-Toth A, Tarantini S, Kiss T, Toth P, Galvan V, Tarantini A, Yabluchanskiy A, Csiszar A, Ungvari Z. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer's disease. Geroscience 2020.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–79.
Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke. 2010;41:790–7.
Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69.
Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev. 2010;6:266–81.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 2018;17.
Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–14.
Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Perz A, O’Connor D, Johnson E, Sorond F, Ungvari ZI, Csiszar A. Age-related alterations in gait function in freely moving male C57BL/6 mice: translational relevance of decreased cadence and increased gait variability. J Gerontol A Biol Sci Med Sci. 2019;74:1417–21.
Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42.
Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Perz A, O'Connor D, Johnson E, Sorond F, Ungvari Z, Csiszar A. Age-related alterations in gait function in freely moving male C57BL/6 mice: translational relevance of decreased cadence and increased gait variability. J Gerontol A Biol Sci Med Sci 2018.
Nyul-Toth A, DelFavero J, Mukli P, Tarantini A, Ungvari A, Yabluchanskiy A, Csiszar A, Ungvari Z, Tarantini S. Early manifestation of gait alterations in the Tg2576 mouse model of Alzheimer’s disease. Geroscience. 2021;43:1947–57.
Blazkiewicz M, Wiszomirska I, Wit A. Comparison of four methods of calculating the symmetry of spatial-temporal parameters of gait. Acta Bioeng Biomech. 2014;16:29–35.
Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, Chong W, Srikanth V. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke. 2012;43:1505–10.
Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology. 1996;42:108–13.
Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, Muir-Hunter SW. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–21.
Rosso AL, Olson Hunt MJ, Yang M, Brach JS, Harris TB, Newman AB, Satterfield S, Studenski SA, Yaffe K, Aizenstein HJ, Rosano C. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40:225–30.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–8.
Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901.
Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, Lipton RB. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56:1244–51.
Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–35.
Verlinden VJ, van der Geest JN, Hoogendam YY, Hofman A, Breteler MM, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture. 2013;37:500–5.
Visser H. Gait and balance in senile dementia of Alzheimer’s type. Age Ageing. 1983;12:296–301.
Wittwer JE, Webster KE, Hill K. Reproducibility of gait variability measures in people with Alzheimer’s disease. Gait Posture. 2013;38:507–10.
Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–75.
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363-72.
Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–9.
Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–72.
Burton DGA, Faragher RGA. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19:447–59.
Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu TT, Tang X, Fu WY, Lu J, Yan YF, Wang XM, Han L, Zhang ZQ, Zhang R, Zou MH, Liu DP. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ Res. 2016;119:1076–88.
Reagan AM, Gu X, Paudel S, Ashpole NM, Zalles M, Sonntag WE, Ungvari Z, Csiszar A, Otalora L, Freeman WM, Stout MB, Elliott MH. Age-related focal loss of contractile vascular smooth muscle cells in retinal arterioles is accelerated by caveolin-1 deficiency. Neurobiol Aging. 2018;71:1–12.
Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594:2115–24.
Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909–19.
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–82.
Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. Geroscience. 2020;42:51–5.
Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD and Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2015.
Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–53.
Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–44.
Gulej R, Nyul-Toth A, Ahire C, DelFavero J, Balasubramanian P, Kiss T, Tarantini S, Benyo Z, Pacher P, Csik B, Yabluchanskiy A, Mukli P, Kuan-Celarier A, Krizbai IA, Campisi J, Sonntag WE, Csiszar A, Ungvari Z. Elimination of senescent cells by treatment with navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience 2023 https://doi.org/10.1007/s11357-023-00990-4.
Funding
This work was supported by grants from the American Heart Association (ANT: AHA834339) and (ST: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528), the NCI Cancer Center Support Grant (P30 CA225520) and the Oklahoma Tobacco Settlement Endowment Trust. ZS was supported by Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1–21-2022–00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/EUniWell/EAC-A02-2019/EAC-A02-2019–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience. Dr. Stefano Tarantini, Dr. Shannon Conley, Dr. Peter Mukli, Dr. Peter Toth and Dr. Andriy Yabluchanskiy serve as Associate Editors for GeroScience.
Disclaimer
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation. The 3.5 version of ChatGPT, developed by OpenAI, was used as a language tool to refine our writing, enhancing the clarity of our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Faakye, J., Nyúl-Tóth, Á., Muranyi, M. et al. Preventing spontaneous cerebral microhemorrhages in aging mice: a novel approach targeting cellular senescence with ABT263/navitoclax. GeroScience 46, 21–37 (2024). https://doi.org/10.1007/s11357-023-01024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-01024-9