Abstract
The harmful effects of microplastics and Cd on the testicular activity of sexually mature rats are here documented. Oral treatment with both substances caused testicular impairment that was evidenced by histological and biomolecular alterations, such as MP accumulation in the seminiferous epithelium, imbalance of oxidative status, and reduced sperm quality. Importantly, the cytoarchitecture of the blood-testis barrier was compromised, as revealed by the down-regulation of protein levels of structural occludin, Van Gogh-like protein 2, and connexin 43 and activation of regulative kinases proto-oncogene tyrosine-protein kinase and focal adhesion kinase. Interestingly, for the first time, MPs are reported to activate the autophagy pathway in germ cells, to reduce damaged organelles and molecules, probably in an attempt to avoid apoptosis. Surprisingly, the results obtained with the simultaneous Cd + MPs treatment showed more harmful effects than those produced by MPs alone but less severe than with Cd alone. This might be due to the different ways of administration to rats (oral gavage for MPs and in drinking water for Cd), which might favor the adsorption, in the gastrointestinal tract, of Cd by MPs, which, by exploiting the Trojan horse effect, reduces the bioavailability of Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of spermatozoa (SPZ) that can successfully fertilize an oocyte is the basis of an efficient reproduction and, therefore, of primary importance in ensuring the survival of a species.
In contrast, the decline in male fertility, accompanied by a deterioration of sperm quality, is a growing phenomenon worldwide (Levine et al. 2017, 2022), the causes of which are not always identifiable. Indeed, besides to the “classic” reasons contributing to male infertility (genetic and anatomical factors, hormonal imbalances among them), almost half of the cases are idiopathic (Venditti et al. 2020a).
It has been demonstrated that the significant deterioration in reproductive rate we are witnessing nowadays, confirmed by the decrease in gamete quality, is associated with the intensification of industrialization, which contributes to a substantial release of synthetic, often toxic, xenobiotics into the environment (Selvaraju et al. 2021). Indeed, many studies have considered the testis as a major target of environmental pollutants, which can act at the molecular, cellular, and histological levels, perturbing several physiological pathways (Krzastek et al. 2020). Increasing evidence also emphasizes the importance of using in vivo and in vitro models, not only to accurately predict the state of reproductive welfare in humans after exposure to environmental pollutants, but also to be used as advantageous tools to unravel the mechanisms regulating the cellular and molecular events that occur during the spermatogenic process (Li et al. 2016; Gao et al. 2021; Wang et al. 2022a).
Among the plethora of harmful substances that affect human, animal, and plant health, microplastics (MPs) are attracting more and more attention, as all organisms are continuously exposed to them (EFSA 2016; Lehner et al. 2019). In fact, the global plastic production and use has so much grown (from 1.5 to 335 M t) (Alimba and Faggio 2019) that the current historical era has been called the “age of plastics” (Avio et al. 2017). Considering the widespread overuse of disposable items, and the durable nature of plastic, the spread of MPs in the environments and their impact on wildlife and human health are inevitable. MPs can be distinguished into primaries (small particles designed for commercial use, such as cosmetics) or tiny particles derived from any type of plastic that, once discharged, can be degraded into fragments < 5 mm in diameter, through a variety of physical, chemical, and biological ways (Webb et al. 2013). MPs are widely distributed in many environmental compartments and, in hydrosphere, are easily ingested by the marine and freshwater fauna, entering and accumulating through the food web, with dangerous consequences for all the species, including humans (Bhagat et al. 2021), at both tissue and cellular levels (Banerjee and Shelver 2021). Although ingestion of food and water is the most representative mode of exposure to MPs for humans, other ways, such as inhalation of polluted air and dermal contact with contaminated water, air, tissue, and cosmetics, cannot be excluded (Amato-Lourenço et al. 2020; Prata et al. 2020; Revel et al. 2018; Sangkham et al. 2022).
Regarding the effects of MPs on testicular physiopathology in mammals, they have been shown to induce inflammation, oxidative stress, alteration of the seminiferous epithelium (SE) cytoarchitecture, and, ultimately, abnormal differentiation of mature gametes (D’Angelo and Meccariello 2021; Hou et al. 2021; Jin et al. 2021). In addition, it has been suggested that disturbance in spermatogenesis may be caused by the disruption of blood-testis barrier (BTB) integrity, through the unbalanced expression of actin-binding proteins and junctional proteins in the BTB (Wei et al. 2021) and, consequently, to the enhanced germ cell (GC) apoptosis through the activation of the MAPK-Nrf2 pathway (Li et al. 2021).
Additionally, to their intrinsic toxicity, MPs, because of their high surface area-to-volume ratio and hydrophobicity, have the potential to interact with and absorb other molecules/particles in the environment, acting as carriers of these chemicals and releasing them into new sites, as well as into living beings. This effect is defined “Trojan horse effect” and is based on the characteristics of MPs (composition, size, shape, color, and functional groups) (Hildebrandt et al. 2021). Sorption of organic compounds and heavy metals on MPs can actually increase the risk of exposure and bioavailability of these contaminants to the organisms, with synergistic, antagonistic, or enhancing effects (Bhagat et al. 2021; He et al. 2021).
Many pollutants act as endocrine disrupting chemicals (EDCs), molecules that mimic endogenous hormones and interfere with physiological endocrine systems (Rehman et al. 2018). The effects of EDCs can be directed on the gonads, altering their physiology, or on the components of the hypothalamic-pituitary–gonadal axis, interfering with the hormonal feedback and resulting in the modification of gonadotropin release (Frye et al. 2012). Our recent research is focusing on the molecular mechanisms that regulate the cellular and differentiation processes that occur during spermatogenesis, which are perturbed by the use of Cd, with two purposes: (1) to unravel the pathways involved, we aim to discover/propose the use of molecules to ameliorate/mitigate these harmful effects (Chemek et al. 2018; Venditti et al. 2020b, 2021a, 2021b; Kechiche et al. 2021) and (2) its use may be helpful in expanding knowledge about the mechanisms of spermatogenesis; as such, chemicals have disruptive effects on the testis via signaling proteins and pathways used to support spermatogenesis under physiological conditions (Gao et al. 2021).
To date, information on the combined effects of MPs and EDCs on mammalian male gametogenesis is limited (Deng et al. 2021). Therefore, the combined effects of MPs and Cd on the physiopathology of the rat testis were evaluated in this study.
Cd has damaging effects due to its action as an EDC that have been well studied in terms of induced autophagy and apoptosis, unbalanced testosterone production, somatic and germ cell cytoskeleton disorders, and, finally, on sperm quality (Venditti et al. 2021a, b).
We recently demonstrated, for the first time in rat testis, the combined impact of MPs and Cd, at the cellular and molecular level, on the progression of GC differentiation events in mature gametes (Venditti et al. 2023), and here we extended the analysis on additional parameters, such as autophagy (microtubule-associated proteins 1A/1B light chain 3B (LC3B) and sequestosome 1 (p62), BTB alteration (occludin (OCN), connexin 43 (Cx43), Van Gogh-Like protein 2 (VANGL2), proto-oncogene tyrosine-protein kinase (Src), Focal adhesion kinase (FAK)), and on SPZ parameters and quality.
Materials and methods
Polystyrene microplastic particles
In this study, two kinds of polystyrene microplastics (PS-MPs) were employed: fluorescent particles of polystyrene (FPS-MPs) with excitation and emission wavelengths of 502 and 518 nm, respectively (PS-FluoGreen-Fi199; GmbH, Berlin, Germany) to assess the accumulation and the distribution of MPs in rat testis, and pristine PS-MPs (PS/Q-R-KM491; GmbH, Berlin, Germany) for the remaining experiments (Deng et al. 2017). Both types were spherical particles of 5 µm diameter. Particle size was measured with non-invasive backscatter optics. The ENM ζ-potential was also measured via Zetasizer Nano ZS. Particle size was independently monitored by our partner in CNRS, ICMPE, University of Creteil, Paris, France. An in-house assessment of the polystyrene spheres revealed an average particle agglomerate size of 4.98 μm ± 0.15 and a ζ-potential of − 0.0934 ± 0.172.
Animals, experimental design, and sample collection
Two-month-old male Wistar rats (n = 40), weighting 222 ± 18.97 g, were individually housed in stainless steel cages under controlled conditions of temperature (22 ± 2 °C), light (hours light/dark schedule), and humidity (55 ± 20%). Animals had free access to food and water ad libitum. Rats, randomly divided into five groups (n = 8 each), were treated as follows: (1) control, (2) 0.1 mg FPS-MP-treated, (3) 0.1 mg PS-MP-treated, (4) Cd-treated (50 mg CdCl2/L in drinking water; Sigma‐Aldrich, Milan, Italy), and (5) Cd + MP-treated (50 mg CdCl2/L + 0.1 mg PS-MPs). The MP solution was prepared as follows: l mg of PS-MPs was diluted in 5 mL of Millipore Milli-Q water and processed by ultrasonic vibration and 0.5 mL of the resulting solution was given by oral gavage once daily (0.1 mg/day which corresponds to 1.5 × 106 particles/day). The chosen MPs used concentration according to Deng et al. (2017) and Haddadi et al. (2022) while that used for Cd was the same previously utilized in our studies (Chemek et al. 2016, 2018; Messaoudi et al. 2010; Venditti et al. 2020b). The two different ways of exposure (drinking water for Cd and oral gavage for MPs) were chosen to avoid the early adsorption of Cd by MPs.
The rats were treated for 30 days, and they were weighted every 5 days. At the 31st day, the animals were euthanized by 4% chloral hydrate (i.p. 10 mL/Kg). The testes were removed and weighted; for each rat, left testis was immersed in formol 10% buffer for histological studies, while the right was kept at − 80 °C for biomolecular studies. Rats were housed in accordance with the EEC 609/86 Directives regulating the welfare of the experimental animals. The experimental protocol was approved by the ethics committee of the Institute of Biotechnology, University of Monastir (Ref: CER-SVS/ISBM022/2020).
Biochemical analysis
Evaluation of testicular CAT and SOD activities
Enzymatic activities of catalase (CAT) and superoxide dismutase (SOD) were assayed using the methods of Claiborne (1985) and of Marklund and Marklund (1974), respectively. The measurement of each activity was performed in triplicate and indicated as units per milligram of protein (U/mg of protein).
Testicular PSH and TBARS level assay
Protein thiol (PSH) levels were determined following the method of Sedlak and Lindsay (1968), while thiobarbituric acid reactive species (TBARS) levels were evaluated in accordance with our previous paper (Satoh 1978; Venditti et al. 2021c). Results were expressed as micrograms per milligram of proteins (µg/mg of protein) and as TBARS µM/µg of proteins. Each measurement was performed in triplicate.
Sperm parameter evaluation
Epididymal sperm count, mobility, viability, morphology, and DSP assessment
From each animal, sperm parameters were analyzed using SPZ obtained by cutting the right epididymis in 1 mL of RPMI culture medium (R0883, Sigma-Aldrich) pre-heated to 32 °C, while the left was minced in 1 mL of 0.9% saline + 1 mL 10% neutral buffer formalin for microscopic studies.
All the main sperm parameters were assayed in accordance with previous works (Robb et al. 1978; Jeyendran et al. 1984; Linder et al. 1986; Chemek et al. 2018). For morphological analysis, the SPZ suspension obtained from the left epididymis was diluted with water to an appropriate volume (20 mL). One to two milliliters of eosin (1%) was added to 20 mL of the suspension and incubated at room temperature for 1 h. A drop of the suspension was placed on a slide, and a smear was prepared and observed under a light microscope (Axiostar Plus Zeiss) at × 40 magnification. A total number of 200–300 SPZ were examined on each slide, and the rate of abnormal SPZ was expressed as a percentage.
For motility evaluation, SPZ obtained from the right epididymis were diluted 1:10, 20 µL of the solution was added to a Malassez cell, and the mobile and non-motile spermatozoa were counted using an optical microscope (Axiostar Plus Zeiss) at × 40 magnification. Motility was expressed as the percentage of motile SPZ to their total number.
SPZ DNA integrity
Acridine orange (AO) staining was employed on formalin-fixed SPZ to evaluate the DNA integrity rate, following Tejada et al. (1984). The slides were observed under a fluorescent microscope with a UV lamp (Leica DM5000 B + CTR 5000; Leica Microsystems, Wetzlar, Germany) and saved using the IM 1000 software (version 4.7.0; Leica Microsystems, Wetzlar, Germany). Photographs were taken using the Leica DFC320 R2 digital camera. SPZ with normal DNA showed green staining, whereas those with abnormal DNA showed yellow to red staining, depending on the degree of the damage (Tejada et al. 1984). A total of about 300 SPZ/slide was counted, and the parameter was expressed as a percentage of yellow/orange SPZ (positive AO).
Protein extraction and WB analysis
Total proteins were extracted from testis and SPZ using the RIPA lysis buffer (TCL131; HiMedia Laboratories GmbH, Einhausen, Germany) supplemented with 10 μl/mL of protease inhibitor mix (39,102; SERVA Electrophoresis GmbH, Heidelberg, Germany) and subsequently processed as described in Pariante et al. (2016).
SPZ extracts were used to perform a Western blot (WB) analysis for prolyl endopeptidase (PREP), a protein implicated in sperm motility (Venditti and Minucci 2019). Table S1 reported details about all the antibodies used. All the bands were analyzed using ImageJ software (version 1.53 g; NIH, Bethesda, MD, USA). WB was performed in triplicate.
IF analysis
IF analysis on rat testis and SPZ
For OCN, VANGL2, Cx43, and LC3B localization, 5 µm serial sections were processed as previously described (Venditti et al. 2019; Venditti and Minucci 2022). For the assessment of acrosome integrity, PNA lectin staining was performed on SPZ fixed with 10% formol. SPZ showing a complete red staining of the acrosome were considered intact, while those showing irregularities and/or perforations were considered abnormal (Lybaert et al. 2009). To determine PREP localization in SPZ, samples were processed as described in Ergoli et al. (2020). The slides were observed under a fluorescent microscope, as previously described. Table S1 reported details about all the used antibodies. IF was performed in triplicate.
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was done by using one‐way ANOVA followed by a Tukey post hoc t-test performed with Prism 5.0, GraphPad Software (San Diego, CA, USA). Differences between the groups were considered statistically significant at p < 0.05.
Results
FPS-MP accumulation in the rat testis
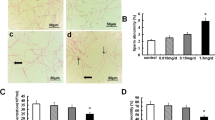
The accumulation of FPS-MPs in rat testis is shown in Fig. 1. MPs appeared as distinctive fluorescent points distributed throughout the seminiferous epithelium. The FPS-MPs particularly accumulated in at level of GC (with arrow and lower inset) and many other also in the tubular lumen (upper inset), indicating that FPS-MPs deeply penetrate in the testicular tissue.
Oxidative stress markers
MPs, when given alone or together with Cd, induced a significant reduction of the antioxidant activities of SOD and CAT as compared to that of the control (p < 0.05 and p < 0.01, respectively; Table 1). Cd alone induced the most pronounced decrease in both the considered enzyme activities, as compared to that of the control (p < 0.001), MP (p < 0.01), and Cd + MP (p < 0.05) groups.
The oxidative stress was also analyzed by measuring the PSH and lipid peroxidation level of rat testis (Table 1). MP exposure, alone or together with Cd, decreased the level of protein thiol groups, as compared to that of the control (p < 0.05 and p < 0.01, respectively; Table 1). Cd group showed the most evident decrease in protein thiol groups as compared to that of the control (p < 0.001), MP (p < 0.05), and Cd + MP (p < 0.05) groups.
Concurrently, MPs, alone or combined with Cd, increased the level of lipid peroxidation, as compared to that of the control (p < 0.05). Once again, the most drastic increase in the level of TBARS was observed in the Cd group, as compared to the control (p < 0.001), MP (p < 0.05), and Cd + MP (p < 0.05) groups. Interestingly, for all the analyzed parameters, except for the TBARS levels, the alterations induced by the combined treatment with Cd + MPs were higher than MPs alone but lower than Cd alone.
Effect of MPs and/or Cd on specific markers of BTB
Cd exposure caused a significant reduction in the OCN (p < 0.001; Fig. 2 A, B), VANGL2 (p < 0.01; Fig. 2 A, C), and Cx43 (p < 0.001; Fig. 2 A, D), so as in the phosphorylation status of p-Src (p < 0.001; Fig. 2 A, E) and p-FAK (p < 0.01; Fig. 5 A, F) as compared to the control. Similarly, treatment with MPs induced a decrease in the levels of all the analyzed structural (p < 0.05) and regulatory (p < 0.01 for p-Src and p < 0.05 for p-FAK) proteins, as compared to the control. The contemporaneous treatment of MPs and Cd also induced a decrease in the level of these BTB markers, as compared to the MP group, but not as drastic as those seen in the Cd group. However, no differences were observed for VANGL2 and Cx43 as compared to the MP group and in p-FAK as compared to the Cd group.
WB analysis of BTB markers in C-, MP-, and/or Cd-treated rat testis. A WB analysis showing the expression of OCN (59 kDa), VANGL2 (60 kDa), Cx43 (43 kDa), p-Src (60 kDa), Src (60 kDa), p-FAK (120 kDa), FAK (120 kDa), and β-actin (44 kDa) in the testis of animals treated with MPs and/or Cd. B–F Histograms showing OCN, VANGL2, Cx43, p-Src, and p-FAK relative protein levels. Protein levels were normalized with β-actin or the relative non-phosphorylated form and reported as OD ratio. All the values are expressed as means±SEM from 8 animals in each group. Statistical significance was evaluated by ANOVA (at least p < 0.05) followed by Tukey’s test for multigroup comparison. Same letter indicates no significative difference; different letters indicate significative differences. All the WB experiments were performed in triplicate
For a more detailed characterization of the effects exerted by MPs and/or Cd on OCN, VANGL2, and Cx43 localization, an IF analysis was carried out (Fig. 3). OCN, one of the most representative components of the BTB, specifically localized in the Sertoli cell (SC) cytoplasm (arrowheads; Fig. 3; insets), in all the groups, however, the signal intensity decreased drastically in the three treated groups.
IF analysis of OCN, VANGL2, and Cx43 in C-, MP-, and/or Cd-treated rat testis. IF staining of OCN (green; upper part), VANGL2 (middle part), and Cx43 (lower part). Slides were counterstained with DAPI-fluorescent nuclear staining (blue) and with PNA lectin (red) which marks the acrosome. Scale bars represent 20 μm and 10 μm in the insets. Arrowheads: SC; dotted arrows: SPC; arrows: SPT; asterisk: LC. All the IF experiments were performed in triplicate
VANGL2 is a protein acting on microtubules and actin microfilament organization, localized in the SC cytoplasm (arrowheads; Fig. 3; insets), and in its extensions, surrounding the differentiating spermatids (SPT)/SPZ (arrows; Fig. 3), showing a well-defined striped conformation. In the MP- and/or Cd-treated groups, a fainter signal was observed, while, just in the Cd + MP and Cd groups, VANGL2 distribution pattern appeared more diffused and disorganized, especially in SC protrusions (arrows; Fig. 3). Finally, a positive signal was also detected in the Leydig cells (LC) of all groups (asterisks; Fig. 3).
Cx43 is the principal testicular component composing gap junction between adjacent SC and at Sertoli-GC interface, localized just in these cell types, namely in spermatocytes (SPC) (dotted arrows; Fig. 3), and cytoplasmic protrusions of SC (arrowheads; Fig. 3; insets) surrounding SPT (arrows; Fig. 3). Furthermore, an evident localization in the LC (asterisks; Fig. 3) was noticeable. Also, in this case, MP and/or Cd treatment induced a pronounced decrease of staining intensity in GC, SC cytoplasm (arrowheads; Fig. 3 and insets), and in the LC (asterisk; Fig. 3).
Effect of MPs and/or Cd on autophagy
As in our previous work, we demonstrated that Cd treatment activates autophagy (Venditti et al. 2021b); here, we analyzed two typical markers of autophagy, LC3B and p62, to verify whether this mechanism is also induced by MPs (Fig. 4). Actually, WB analysis revealed that MP treatment induced a significant increase of LC3B-II and decrease of p62 protein levels, as compared to that of the control (p < 0.05; Fig. 4 a–c). Moreover, the co-administration of Cd further increased LC3B-II protein level as compared to the control (p < 0.001) and MP (p < 0.05) groups; however, the level of p62 decreased as compared to the control (p < 0.01), but it was similar to the MP group. Cd treatment induced the highest increase of LC3B-II and decrease of p62 protein levels, as compared to all the other groups, confirming the activation of the autophagic pathway. To establish which cells exhibited the most prominent activation of autophagy, a LC3B immunofluorescence staining was performed (Fig. 4 d). In all the groups, a positive signal was specifically observed in the cytoplasm of SC (arrowheads) but with a more extension and intensity in the testis of Cd-treated rats. Interestingly, in MP and Cd-MP groups, the staining was also present in GC cytoplasm, namely SPC (dotted arrow; Fig. 4 d) and SPT (arrow; Fig. 4 d), but with a lower intensity than that seen in the SC.
Autophagy rate analysis of C-, MP-, and/or Cd-treated rat testis. a WB analysis showing the expression of LC3B-I (14 kDa), LC3B-II (16 kDa), p62 (62 kDa), and β-actin (44 kDa) in the testis of animals treated with MPs and/or Cd. b, c Histograms showing LC3B-II and p62 relative protein levels. Protein levels were normalized with LC3B-I and β-actin, respectively, and reported as OD ratio. All the values are expressed as means ± SEM from 8 animals in each group. Statistical significance was evaluated by ANOVA (at least p < 0.05) followed by Tukey’s test for multigroup comparison. Same letter indicates no significative difference; different letters indicate significative differences. d IF analysis of LC3B (green) in the testis of animals treated with MPs and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue) and with PNA lectin (red) which marks the acrosome. Scale bars represent 20 μm and 10 μm in the insets. Arrowheads: SC; dotted arrows: SPC; arrows: SPT. All the WB and IF experiments were performed in triplicate
Effect of MPs and/or Cd on sperm
Sperm parameters, lipid peroxidation, and DNA and acrosome integrity
The effects of MP and/or Cd treatment were extended on gamete physiology. Firstly, the analysis of the main sperm parameters, shown in Table 2, revealed that MP and Cd + MP treatment induced substantial modifications in DSP, efficiency, sperm concentration, viability, motility, and morphology, as compared to the control group (p < 0.1 and p < 0.01, respectively). Moreover, when given alone, Cd induced the most pronounced alterations in all the analyzed parameters, as compared to the control (p < 0.001), MP (p < 0.01), and Cd + MP (p < 0.05) groups.
To investigate the oxidative stress, the sperm lipid peroxidation rate was analyzed via the TBARS assay (Fig. 5 A).
Sperm physiology analysis of C-, MP-, and/or Cd-treated rats. A Histogram showing TBARS levels of animals exposed to MPs and/or Cd. B AO staining that highlights the SPZ with damaged DNA (yellow/orange) respect to those with intact DNA (green). The insets showed the sperm acrosome morphology, highlighted with PNA lectin staining. C Histogram showing the percentage of SPZ with damaged DNA. Scale bars represent 20 μm. In A and C, all the values are expressed as means ± SEM from 8 animals in each group. Statistical significance was evaluated by ANOVA (at least p < 0.05) followed by Tukey’s test for multigroup comparison. Same letter indicates no significative difference; different letters indicate significative differences. All the experiments were performed in triplicate
As for the previous data, MPs induced a slight increase in TBARS level (p < 0.05), Cd + MPs an average increase (p < 0.01), and Cd alone a marked increase (p < 0.001) as compared to the control.
By AO staining, the negative effects of MPs and/or Cd on SPZ DNA integrity were indicated by a significant increase in the percentage of SPZ showing a yellow/orange head (indicating damaged DNA) as compared to the control (p < 0.01; Fig. 5 B, C). It should be pointed out that there were no differences in the number of SPZ with damaged DNA between the MP and Cd + MP groups; however, in the latter, more SPZ presenting dark yellow/orange heads were observed, indicating the induction of deeper damages. Cd group exhibited the highest number of damaged SPZ, in terms of number (p < 0.001 as compared to the control) and severity, since many dark orange/red SPZ heads were observed (Fig. 5 B, C).
Finally, in the insets in Fig. 5 B, PNA lectin staining allowed us to identify sperm acrosome. Control SPZ showed an acrosome of the correct shape, and the red signal was quite intense. In the three treated groups, the staining revealed an abnormal acrosome, in terms of both shape and staining intensity, with the Cd-treated group exhibiting the worse situation.
Analysis on PREP
Finally, the analysis of the effects of MP and/or Cd treatment on sperm physiology was extended on PREP, a microtubule-associated protein involved in the sperm motility (Fig. 6). As also observed in many other parameters, the three treatments induced a significant decrease in PREP, with MPs showing a slight decrement (p < 0.05), Cd alone the highest (p < 0.0001), and Cd + MPs an “intermediate” (p < 0.01) as compared to the control (Fig. 6 A, B).
WB and IF analysis of PREP in C-, MP-, and/or Cd-treated rat SPZ. A WB analysis showing the expression of PREP (80 kDa) and α-tubulin (50 kDa) in SPZ of animals treated with MPs and/or Cd. B Histograms showing the relative protein levels of PREP. Data were normalized with α-tubulin and reported as OD ratio. All the values are expressed as means ± SEM from 8 animals in each group. Statistical significance was evaluated by ANOVA (at least p < 0.05) followed by Tukey’s test for multigroup comparison. Same letter indicates no significative difference; different letters indicate significative differences. C IF analysis of PREP in SPZ of animals treated with MPs and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue) and with PNA lectin (red). Scale bars represent 20 μm. All the WB and IF experiments were performed in triplicate
This data was then confirmed by IF analysis performed on SPZ (Fig. 6 C), showing that PREP was clearly localized in the tail. The lower signal intensity observed in the sperm of the experimental groups confirmed the trend previously described.
Discussion
In this paper, we evaluated, for the first time, the combined effect of MPs and Cd on the rat testis. We used 60-day-old rats that, at this time point, they cannot be defined as adult yet but in the period of the late puberty/early adulthood. However, it has been well recognized that rats are sexually mature at this stage, as the first wave of the spermatogenic cycle is completed, and they can produce mature SPZ. In fact, the two signs of sexual maturity are the presence of complete spermiogenesis in the seminiferous tubules and mature SPZ in the epididymis (Picut et al. 2018).
It has been proposed that induced oxidative stress is one of the main adverse effects provoked by pollutants, MPs (Wen et al. 2022) and Cd (Venditti et al. 2021a) included. Our data confirmed that, after the exposure to MPs and/or Cd, the antioxidant activity of SOD and CAT and the PSH level decreased, while the concentration of TBARS increased, suggesting that the treatments caused oxidative stress. It should be remembered that testicular cells, due to their high unsaturated fatty acid content, are extremely sensitive to reactive oxygen/nitrogen species (Dutta et al. 2019).
Recent studies highlighted the ability of MPs to penetrate testicular tissue, here confirmed by the presence of FS-MPs in the SE, and promote the BTB destruction, both in mice (Wei et al. 2021) and in rats (Li et al. 2021), through the imbalance of the mTORC1/mTORC2 and MAPK-Nrf2 signaling pathways, respectively. Furthermore, we have previously demonstrated that Cd alters the BTB, by acting on structural (OCN, VANGL2, and Cx43) and regulative (Src and FAK) proteins (Venditti et al. 2021b). Here, we also confirm that MPs downregulated the protein level of OCN and Cx43, integral membrane proteins that form the tight junctions and gap junctions between adjacent SC, respectively.
However, to our knowledge, this is the first paper to report the effects of MPs on the phosphorylation status of Src and FAK, kinases that regulate the dynamics of adjacent and tight junction, as well as on the protein level of VANGL2, a planar cell polarity protein that, acting on actin microfilament and microtubule organization, plays a central role in regulating BTB integrity and SPT transport. IF data revealed that MP treatment induced an altered spatial distribution of VANGL2 in the SC cytoplasm, at the interface with SPT, suggesting that MPs may also act on the cytoskeletal organization of testicular cells. Further studies are in progress to better elucidate this point.
The combined data, present in the literature and in the current paper, not only suggest that by inducing oxidative stress and altering the mTORC1/mTORC2 (Wei et al. 2021) and MAPK-Nrf2 (Li et al. 2021) signaling pathways, MPs can affect the integrity of BTB and, consequently, the whole spermatogenic process, but also confirm, once again, the importance of the harmonious cooperation among all the components of BTB to ensure the proper differentiation of GC into SPZ.
Remarkably, many stressful circumstances (namely the absence of survival factors or excessive oxidative stress) can activate the autophagic pathway, an adaptive mechanism that leads damaged molecules and organelles to lysosomal degradation. The present data, in addition to confirm previous studies indicating that Cd induces autophagy, especially in SC (Venditti et al. 2021b; Wang et al. 2022a), demonstrated, for the first time, that MPs can also stimulate autophagy not only in SC but also in GC, contrarily to Cd. Autophagy in SC could be a consequence of the loss of BTB component integrity, as these proteins, impaired by overproduced ROS (or by the withdrawal of testosterone, induced either by MPs or Cd (Ilechukwu et al. 2022)), are degraded via autophagy (Venditti et al. 2021b; Zhou et al. 2021). The occurrence of autophagy just in GC of MP-treated rats could be a consequence of the less damages produced by MPs compared to Cd: in fact, it is known that one of the purposes of autophagy is to avoid apoptosis (Mizushima et al. 2008); for this reason, we hypothesized that GC, to reduce the “limited” damages induced by MPs, activate the autophagy pathway rather than the entry into apoptosis, whereas Cd, which caused the most prominent injuries, directly provoked apoptosis in GC (Ijaz et al. 2021).
The induced oxidative stress and the loss of BTB integrity could be one of the causes of the decline in sperm quality, as evidenced by the increased number of positive sperm to AO staining (an index of DNA damage), other than by the downregulated PREP protein level. Since PREP, a serine protease, has been implicated in microtubule-associated processes (Venditti and Minucci 2019; Venditti et al. 2019), the altered sperm motility can probably be attributed specifically to its impaired expression and localization in the sperm tail. It is well known that, in the Wistar rats, spermatogenesis lasts about 63 days, including SPZ maturation during epididymal transit (Picut et al. 2018). Although to observe a more accurate impact of the used pollutants on sperm quality, the treatment should have lasted at least 63 days; in this paper, in any case, alterations were observed in all the cell types that compose the SE and in the mature SPZ. Thus, it can be assumed that all cells, even those that have already started mitotic and meiotic divisions, as well as spermiogenesis, are susceptible to MP and/or Cd effects, independently of the duration of treatment.
Interestingly, the combined effects of Cd + MPs were worse than those induced by MPs but less intense than those induced by Cd alone in most of the analyzed parameters. This antagonistic effect has been previously demonstrated, as MPs reduced Cd toxicity in plants (Lian et al. 2020; Wang et al. 2022b; Zhang et al. 2022), fish (Wang et al. 2022c; Yang et al. 2022), and mice (Wang et al. 2022d).
This result may be due to the different ways of exposure used to administer the two chemicals to rats: via oral gavage (MPs) and in drinking water (Cd); thus, the first “contact” between MPs and Cd occurred just in the gastrointestinal tract of the animals. Considering that MPs show the highest adsorption rate at neutral pH values and the highest release at acidic pH values of heavy metals (Hildebrandt et al. 2021), we can speculate that in the stomach of rats there was no adsorption/release due to acid pH, while, in the gut, in which the pH starts to increase reaching the neutrality (about 7), a favorable microenvironment is generated for Cd absorption by MPs, reducing its bioavailability (Chen et al. 2022).
Finally, corroborating our previous hypothesis (Venditti et al. 2023), we can propose a kind of “positive Trojan horse effect” of MPs, which can be the cause for the lower effects observed by the simultaneous treatment with Cd + MPs compared to MPs or Cd alone. For this reason, progress studies are focused on the use of MPs “pre-adsorbed” with Cd, to verify the presence of an effective “Trojan horse” effect on the rat testis.
Conclusions
This report demonstrates the combined effects of MPs and Cd on rat testicular physiology. A particular attention has been given to their effects on the BTB cytoarchitecture and on SPZ quality parameters, confirming that the structural and regulative proteins composing the BTB and GC are targeted by these pollutants. Furthermore, new data indicate that MPs, given alone or in combination with Cd, can induce autophagy in the GC as well. Interestingly, the simultaneous use of both pollutants produced less effects than Cd alone, probably because of the separate ways of administration to rats.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- ANOVA:
-
Analysis of variance
- AO:
-
Acridine orange
- BTB:
-
Blood-testis barrier
- CAT:
-
Catalase
- Cd:
-
Cadmium
- Cx43:
-
Connexin 43
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DSP:
-
Daily sperm production
- EDCs:
-
Endocrine disrupting chemicals
- FAK:
-
Focal adhesion kinase
- FPS-MPs:
-
Fluorescent particles of polystyrene
- GC:
-
Germ cells
- IF:
-
Immunofluorescence
- LC:
-
Leydig cells
- LC3B:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MAPK:
-
Mitogen-activated protein kinase
- MPs:
-
Microplastics
- mTORC:
-
Mechanistic target of rapamycin complex
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- OCN:
-
Occludin
- OD:
-
Optical density
- p62:
-
Sequestosome 1
- PNA:
-
Peanut agglutinin
- PREP:
-
Prolyl endopeptidase
- PSH:
-
Protein thiols
- PS-MPs:
-
Polystyrene microplastics
- RIPA:
-
Radioimmunoprecipitation assay buffer
- ROS:
-
Reactive oxygen species
- SC:
-
Sertoli cells
- SE:
-
Seminiferous epithelium
- SEM:
-
Standard error of the mean
- SOD:
-
Superoxide dismutase
- SPC:
-
Spermatocytes
- SPT:
-
Spermatids
- SPZ:
-
Spermatozoa
- Src:
-
Proto-oncogene tyrosine-protein kinase
- TBARS:
-
Thiobarbituric acid-reactive species
- VANGL2:
-
Van Gogh-like protein 2
- WB:
-
Western blotting
References
Alimba CG, Faggio C (2019) Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol 68:61–74. https://doi.org/10.1016/j.etap.2019.03.001
Amato-Lourenço LF, Dos Santos Galvão L, de Weger LA, Hiemstra PS, Vijver MG, Mauad T (2020) An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ 749:141676. https://doi.org/10.1016/j.scitotenv.2020.141676
Avio CG, Gorbi S, Regoli F (2017) Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar Environ Res 128:2–11. https://doi.org/10.1016/j.marenvres.2016.05.012
Banerjee A, Shelver WL (2021) Micro- and nanoplastic induced cellular toxicity in mammals: A review. Sci Total Environ 755:142518. https://doi.org/10.1016/j.scitotenv.2020.142518.
Bhagat J, Nishimura N, Shimada Y (2021) Toxicological interactions of microplastics/nanoplastics and environmental contaminants: current knowledge and future perspectives. J Hazard Mater 405:123913. https://doi.org/10.1016/j.jhazmat.2020.123913
Chemek M, Mimouna SB, Boughammoura S, Delbès G, Messaoudi I (2016) Protective role of zinc against the toxicity induced by exposure to cadmium during gestation and lactation on testis development. Reprod Toxicol 63:151–160. https://doi.org/10.1016/j.reprotox.2016.06.005
Chemek M, Venditti M, Boughamoura S, Mimouna SB, Messaoud I, Minucci S (2018) Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J Cell Physiol 233:630–640. https://doi.org/10.1002/jcp.25923
Chen XJ, Ma JJ, Yu RL, Hu GR, Yan Y (2022) Bioaccessibility of microplastic-associated heavy metals using an in vitro digestion model and its implications for human health risk assessment. Environ Sci Pollut Res Int 29:76983–76991. https://doi.org/10.1007/s11356-022-20983-8
Claiborne A (1985) Catalase activity. In handbook of methods for oxygen radical research, 1st ed., Greenwald RA, (Ed.), CRC Press, Boca Raton, FL, USA, pp. 283–284
D’Angelo S, Meccariello R (2021) Microplastics: a threat for male fertility. Int J Environ Res Public Health 18:2392. https://doi.org/10.3390/ijerph18052392
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep 7:46687. https://doi.org/10.1038/srep46687
Deng Y, Yan Z, Shen R, Huang Y, Ren H, Zhang Y (2021) Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J Hazard Mater 406:124644. https://doi.org/10.1016/j.jhazmat.2020.124644
Dutta S, Majzoub A, Agarwal A (2019) Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol 17:87–97. https://doi.org/10.1080/2090598X.2019.1599624
EFSA (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J 14(6). https://doi.org/10.2903/j.efsa.2016.4501
Ergoli M, Venditti M, Piccillo E, Minucci S, Politano L (2020) Study of expression of genes potentially responsible for reduced fitness in patients with myotonic dystrophy type 1 and identification of new biomarkers of testicular function. Mol Reprod Dev 87:45–52. https://doi.org/10.1002/mrd.23307
Frye CA, Bo E, Calamandrei G, Calzà L, Dessì-Fulgheri F, Fernández M, Fusani L, Kah O, Kajta M, Le Page Y et al (2012) Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol 24:144–159. https://doi.org/10.1111/j.1365-2826.2011.02229.x
Gao S, Wu X, Wang L, Bu T, Perrotta A, Guaglianone G, Silvestrini B, Sun F, Cheng CY (2021) Signaling proteins that regulate spermatogenesis are the emerging target of toxicant-induced male reproductive dysfunction. Front Endocrinol (Lausanne) 12:800327. https://doi.org/10.3389/fendo.2021.800327
Haddadi A, Kessabi K, Boughammoura S, Ben Rhouma M, Mlouka R, Banni M, Messaoudi (2022) Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ Sci Pollut Res Int 29:34594–34606. https://doi.org/10.1007/s11356-021-18218-3
He J, Yang X, Liu H (2021) Enhanced toxicity of triphenyl phosphate to zebrafish in the presence of micro- and nano-plastics. Sci Total Environ 756:143986. https://doi.org/10.1016/j.scitotenv.2020.143986
Hildebrandt L, Nack FL, Zimmermann T, Pröfrock D (2021) Microplastics as a Trojan horse for trace metals. J Hazard Mater Letters 2:100035. https://doi.org/10.1016/j.hazl.2021.100035
Hou B, Wang F, Liu T, Wang Z (2021) Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J Hazard Mater 405:124028. https://doi.org/10.1016/j.jhazmat.2020.124028
Ijaz MU, Shahzadi S, Samad A, Ehsan N, Ahmed H, Tahir A, Rehman H, Anwar H (2021) Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male Sprague Dawley rats. Dose Response 19: 15593258211019882.https://doi.org/10.1177/15593258211019882
Ilechukwu I, Ehigiator BE, Ben IO, Okonkwo CJ, Olorunfemi OS, Modo UE, Ilechukwu CE, Ohagwa NJ (2022) Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats. Environ Anal Health Toxicol 37:e2022015-0. https://doi.org/10.5620/eaht.2022015
Jeyendran R, Van der Ven H, Perez-Pelaez M, Crabo B, Zaneveld L (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70:219–228. https://doi.org/10.1530/jrf.0.0700219
Jin H, Ma T, Sha X, Liu Z, Zhou Y, Meng X, Chen Y, Han X, Ding J (2021) Polystyrene microplastics induced male reproductive toxicity in mice. J Hazard Mater 401:123430. https://doi.org/10.1016/j.jhazmat.2020.123430
Kechiche S, Venditti M, Knani L, Jabłońska K, Dzięgiel P, Messaoudi I, Reiter RJ, Minucci S (2021) First evidence of the protective role of melatonin in counteracting cadmium toxicity in the rat ovary via the mTOR pathway. Environ Pollut 270:116056. https://doi.org/10.1016/j.envpol.2020.116056
Krzastek SC, Farhi J, Gray M, Smith RP (2020) Impact of environmental toxin exposure on male fertility potential. Transl Androl Urol 9:2797–2813. https://doi.org/10.21037/tau-20-685
Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B (2019) Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol 53:1748–1765. https://doi.org/10.1021/acs.est.8b05512
Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH (2017) Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 23:646–659. https://doi.org/10.1093/humupd/dmx022
Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, Pinotti R, Swan SH (2022) Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update dmac035. https://doi.org/10.1093/humupd/dmac035
Li N, Mruk DD, Lee WM, Wong CK, Cheng CY (2016) Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis? Semin Cell Dev Biol 59:141–156. https://doi.org/10.1016/j.semcdb.2016.01.003
Li S, Wang Q, Yu H, Yang L, Sun Y, Xu N, Wang N, Lei Z, Hou J, Jin Y et al (2021) Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ Sci Pollut Res Int 28:47921–47931. https://doi.org/10.1007/s11356-021-13911-9
Lian J, Wu J, Zeb A, Zheng S, Ma T, Peng F, Tang J, Liu W (2020) Do polystyrene nanoplastics affect the toxicity of cadmium to wheat (Triticum aestivum L.)? Environ Pollut 263(Part A):114498. https://doi.org/10.1016/j.envpol.2020.114498
Linder RE, Strader LF, McElroy WK (1986) Measurement of epididymal sperm motility as a test variable in the rat. Bull Environ Contam Toxicol 36:317–324. https://doi.org/10.1007/BF01623514
Lybaert P, Danguy A, Leleux F, Meuris S, Lebrun P (2009) Improved methodology for the detection and quantification of the acrosome reaction in mouse spermatozoa. Histol Histopathol 24:999–1007. https://doi.org/10.14670/HH-24.999
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Messaoudi I, Banni M, Saïd L, Saïd K, Kerkeni A (2010) Evaluation of involvement of testicular metallothionein gene expression in the protective effect of zinc against cadmium-induced testicular pathophysiology in rat. Reprod Toxicol 29:339–345. https://doi.org/10.1016/j.reprotox.2010.01.004
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. https://doi.org/10.1038/nature06639
Pariante P, Dotolo R, Venditti M, Ferrara D, Donizetti A, Aniello F, Minucci S (2016) Prothymosin alpha expression and localization during the spermatogenesis of Danio rerio. Zygote 24:583–593. https://doi.org/10.1017/S0967199415000568
Picut CA, Ziejewski MK, Stanislaus D (2018) Comparative aspects of pre- and postnatal development of the male reproductive system. Birth Defects Res 110:190–227. https://doi.org/10.1002/bdr2.1133
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Rehman S, Usman Z, Rehman S, AlDraihem M, Rehman N, Rehman I, Ahmad G (2018) Endocrine disrupting chemicals and impact on male reproductive health. Transl Androl Urol 7:490–503. https://doi.org/10.21037/tau.2018.05.17
Revel M, Châtel A, Mouneyrac C (2018) Micro(nano)plastics: a threat to human health? Curr Opin Environ Sci Health 1:17–23. https://doi.org/10.1016/j.coesh.2017.10.003
Robb GW, Amann RP, Killian GJ (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil 54:103–107. https://doi.org/10.1530/jrf.0.0540103
Sangkham S, Faikhaw O, Munkong N, Sakunkoo P, Arunlertaree C, Chavali M, Mousazadeh M, Tiwari A (2022) A review on microplastics and nanoplastics in the environment: their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar Pollut Bull 181:113832. https://doi.org/10.1016/j.marpolbul.2022.113832
Satoh K (1978) Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 90:37–43. https://doi.org/10.1016/0009-8981(78)90081-5
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Selvaraju V, Baskaran S, Agarwal A, Henkel R (2021) Environmental contaminants and male infertility: effects and mechanisms. Andrologia 53:e13646. https://doi.org/10.1111/and.13646
Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril 42:87–91. https://doi.org/10.1016/s0015-0282(16)47963-x
Venditti M, Minucci S (2019) Subcellular localization of prolyl endopeptidase during the first wave of rat spermatogenesis and in rat and human sperm. J Histochem Cytochem 67:229–243. https://doi.org/10.1369/0022155418810064
Venditti M, Minucci S (2022) Differential expression and localization of EHBP1L1 during the first wave of rat spermatogenesis suggest its involvement in acrosome biogenesis. Biomedicines 10:181. https://doi.org/10.3390/biomedicines10010181
Venditti M, Aniello F, Santillo A, Minucci S (2019) Study on PREP localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. Zygote 27:160–165. https://doi.org/10.1017/S0967199419000194
Venditti M, Fasano C, Minucci S, Serino I, Sinisi AA, Dale B, Di Matteo L (2020a) DAAM1 and PREP are involved in human spermatogenesis. Reprod Fertil Dev 32:484–494. https://doi.org/10.1071/RD19172
Venditti M, Chemek M, Minucci S, Messaoudi I (2020b) Cadmium-induced toxicity increases prolyl endopeptidase (PREP) expression in the rat testis. Mol Reprod Dev 87:565–573. https://doi.org/10.1002/mrd.23345
Venditti M, Ben Rhouma M, Romano Z, Messaoudi I, Reiter RJ, Minucci S (2021a) Altered expression of DAAM1 and PREP induced by cadmium toxicity is counteracted by melatonin in the rat testis. Genes (basel) 12:1016. https://doi.org/10.3390/genes12071016
Venditti M, Ben Rhouma M, Romano MZ, Messaoudi I, Reiter RJ, Minucci S (2021b) Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol Environ Saf 226:112878. https://doi.org/10.1016/j.ecoenv.2021.112878
Venditti M, Romano MZ, Aniello F, Minucci S (2021c) Preliminary investigation on the ameliorative role exerted by D-aspartic acid in counteracting ethane dimethane sulfonate (EDS) toxicity in the rat testis. Animals (basel) 11:133. https://doi.org/10.3390/ani11010133
Venditti M, Ben Hadj Hassine M, Messaoudi I, Minucci S (2023) The simultaneous administration of microplastics and cadmium alters rat testicular activity and changes the expression of PTMA, DAAM1 and PREP. Front Cell Dev Biol. In Press. https://doi.org/10.3389/fcell.2023.1145702
Wang L, Bu T, Wu X, Gao S, Li X, De Jesus AB, Wong CKC, Chen H, Chung NPY, Sun F, Cheng CY (2022a) Cell-cell interaction-mediated signaling in the testis induces reproductive dysfunction—lesson from the toxicant/pharmaceutical models. Cells 11:591. https://doi.org/10.3390/cells11040591
Wang L, Lin B, Wu L, Pan P, Liu B, Li R (2022b) Antagonistic effect of polystyrene nanoplastics on cadmium toxicity to maize (Zea mays L.). Chemosphere 307(Pt 1):135714. https://doi.org/10.1016/j.chemosphere.2022.135714
Wang S, Xie S, Wang Z, Zhang C, Pan Z, Sun D, Xu G, Zou J (2022c) Single and combined effects of microplastics and cadmium on the cadmium accumulation and biochemical and immunity of Channa argus. Biol Trace Elem Res 200:3377–3387. https://doi.org/10.1007/s12011-021-02917-6
Wang L, Xu M, Chen J, Zhang X, Wang Q, Wang Y, Cui J, Zhang S (2022d) Distinct adverse outcomes and lipid profiles of erythrocytes upon single and combined exposure to cadmium and microplastics. Chemosphere 307(Pt 2):135942. https://doi.org/10.1016/j.chemosphere.2022.135942
Webb HK, Arnott J, Crawford RJ, Ivanova EP (2013) Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 5:1–18. https://doi.org/10.3390/polym5010001
Wei Y, Zhou Y, Lon C, Wu H, Hong Y, Fu Y, Wang J, Wu Y, Shen L, Wei G (2021) Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-mediated imbalance of mTORC1 and mTORC2. Environ Pollut 289:117904. https://doi.org/10.1016/j.envpol.2021.117904
Wen S, Chen Y, Tang Y, Zhao Y, Liu S, You T, Xu H (2022) Male reproductive toxicity of polystyrene microplastics: Study on the endoplasmic reticulum stress signaling pathway. Food Chem Toxicol 172:113577. https://doi.org/10.1016/j.fct.2022.113577
Yang H, Zhu Z, Xie Y, Zheng C, Zhou Z, Zhu T, Zhang Y (2022) Comparison of the combined toxicity of polystyrene microplastics and different concentrations of cadmium in zebrafish. Aquat Toxicol 250:106259. https://doi.org/10.1016/j.aquatox.2022.106259
Zhang Z, Li Y, Qiu T, Duan C, Chen L, Zhao S, Zhang X, Fang L (2022) Microplastics addition reduced the toxicity and uptake of cadmium to Brassica chinensis L. Sci Total Environ 852:158353. https://doi.org/10.1016/j.scitotenv.2022.158353
Zhou GX, Zhu HL, Shi XT, Nan Y, Liu WB, Dao LM, Xiong YW, Yi SJ, Cao XL, Xu DX, Wang H (2021) Autophagy in Sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ Pollut 270:116241. https://doi.org/10.1016/j.envpol.2020.116241
Acknowledgements
We want to thank the project Erasmus+ KA107 (Napoli-Monastir) of the Università della Campania “Luigi Vanvitelli”, and the Ministry of Higher Education and Scientific Research of Tunisia.
Funding
This work was supported by the Italian Ministry of University and Research (Grant No. PRIN to S.M., 2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: Sergio Minucci and Imed Messaoudi. Formal analysis: Majida Ben Hadj Hassine and Massimo Venditti. Funding acquisition: Sergio Minucci. Investigation: Majida Ben Hadj Hassine, Massimo Venditti, and Mariem Ben Rhouma. Supervision: Imed Messaoudi, Visualization: Majida Ben Hadj Hassine and Massimo Venditti, Writing—original draft: Massimo Venditti. Writing—review and editing: Sergio Minucci and Imed Messaoudi.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedure was approved by the Ethics Committee for Research in life science and health of the Higher Institute of Biotechnology of Monastir (CER-SVS/ISBM- protocol 022/2020) and was carried out accordingly to the UNESCO Recommendation Concerning Science and Scientific Research (1974, 2017).
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassine, M.B.H., Venditti, M., Rhouma, M.B. et al. Combined effect of polystyrene microplastics and cadmium on rat blood-testis barrier integrity and sperm quality. Environ Sci Pollut Res 30, 56700–56712 (2023). https://doi.org/10.1007/s11356-023-26429-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26429-z