Abstract
Microplastics (MPs) are defined as plastic particles or fragments with a diameter of less than 5 mm. These particles have been identified as causing male reproductive toxicity, although the precise mechanism behind this association is yet to be fully understood. Recent research has found that exposure to polystyrene microplastics (PS-MPs) can disrupt spermatogenesis by impacting the integrity of the blood-testis barrier (BTB), a formidable barrier within mammalian blood tissues. The BTB safeguards germ cells from harmful substances and infiltration by immune cells. However, the disruption of the BTB leads to the entry of environmental pollutants and immune cells into the seminiferous tubules, resulting in adverse reproductive effects. Additionally, PS-MPs induce reproductive damage by generating oxidative stress, inflammation, autophagy, and alterations in the composition of intestinal flora. Despite these findings, the precise mechanism by which PS-MPs disrupt the BTB remains inconclusive, necessitating further investigation into the underlying processes. This review aims to enhance our understanding of the pernicious effects of PS-MP exposure on the BTB and explore potential mechanisms to offer novel perspectives on BTB damage caused by PS-MPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics (MPs) are available in various forms, including polystyrene (PS), polyethylene (PE), polypropylene (PP), and other variants (Song et al., 2023). These MPs can be found in commonly used items, including takeaway boxes and textile fibers in clothing, cosmetics, and other products (Moita Neto & Silva, 2023). Recent evidence suggests that MPs are found in significant quantities in various human organs and tissues, including the lung (Amato-Lourenço et al., 2021), testis (Gao et al., 2023), blood (Leslie et al., 2022), intestinal tract (Zhang et al., 2022a, 2022b, 2022c), brain (Prüst et al., 2020), etc. Among MPs, PS-MPs are the most commonly studied type regarding their infaust effects on human health (Gan et al., 2023; Wu et al., 2019). Currently, particular attention is being paid to PS-MPs impact on the aquatic environment (Xu et al., 2020), whereas limited knowledge exists regarding their health risks, particularly in mammals. MPs can enter the human body through food and water consumption, accumulating in human tissues and posing a potential hazard to human health (Chang et al., 2022).
Plastic pollution was listed as a severe environmental concern on a global scale at the Fifth United Nations Environment Assembly, and an internationally legally binding agreement was forged to end plastic pollution (Landrigan et al., 2023). Because of the limited standardization and quality control measures, particularly regarding more prominent MPs (> 10 to 50 μm), existing research on MPs-induced male reproductive function impairment has primarily concentrated on more minor MPs (< 10 μm). Recent research suggested that exposure to PS-MPs exhibit a detrimental influence on male fertility and sperm health, potentially compromising the reproductive system (D’Angelo & Meccariello, 2021). In vitro and in vivo studies have shown that PS-MPs can reduce sperm motility and male fertility (Fard et al., 2023).
Male infertility is a serious problem in human reproduction, and studies have shown that MPs can lead to reduced sperm numbers, especially PS-MPs (Zhao et al., 2023). MPs are widely available in water and food (Leslie et al., 2022), and the internal exposure of plastic particles in human body fluids and tissues is still in its infancy. To further discuss the toxicity of MPs, the research on MPs toxicity in recent years mainly includes internal and external studies, and different mechanisms may coexist and interact with each other (Liu et al., 2023a, 2023b, 2023c, 2023d; Weis & Alava, 2023). BTB is indispensable in protecting sperm from toxic substances and providing appropriate microenvironments (O’Donnell et al., 2022). However, there are not enough studies on the reproductive toxicity of PS-MPs in humans, and special attention should be paid to the toxic effects of PS-MPs on the reproductive system. Accordingly, this review comprehensively summarized the potential mechanisms of PS-MPs causing spermatogenic dysfunction and BTB damage and discussed the significance of PS-MPs in the field of toxicology research, which helps reveal the potential mechanism of MPs on the male reproductive system and public health.
Methods
We reviewed recent original articles from 191 published within the past 5 years (2019–2024) by searching the PubMed database in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria (Page et al., 2021). A keyword/abstract of articles containing information on PS-MPs, related mechanisms of toxicity, and reproductive system (Table 1). Only studies in English were included. The search strategy involved the utilization of the following search terms: (Group A) combined with (Group B), as well as (Group B) in conjunction with (Group C), the Boolean operator OR was employed for within-group analysis.
Exposure routes and proper characters
MPs can enter the body through various routes of exposure: (1) Food intake. The ingestion of microplastics by aquatic organisms into the food chain and the human through food may be one of the main pathways of human microplastic exposure (Kutralam-Muniasamy et al., 2024; Ojeda et al., 2023). Researchers estimated that adults' average annual exposure to particles in proteins was 11,000 ± 29,000 particles in America (Milne et al., 2023). (2) Drinking water. Microplastics present in water bodies such as rivers, lakes, self-service rehydration booths (Shruti et al., 2022) and even bottled water have become important sources of human microplastics exposure (Wu et al., 2019). (3) Air contact. Microplastics can be released into the air through various ways and then enter the body through the respiratory system. Research showed that MPs contribute to the effects of urban and industrial dusts (Abbasi et al., 2019), and higher MPs concentrations in indoor air and dust compared to outdoors (Ageel et al., 2022). (4) Personal care products use is the most common carrier of microplastics, and the MPs can directly cross the dermal barrier and enter the body. (Sripada et al., 2022). In short, humans are widely exposed to PS-MPs through water and food intake, inhalation of air and dust, and contact with pollution matrices.
MPs possess the following characteristics: (1) Small size and high penetration capability. Most MPs have micrometer or even nanometer diameters, enabling them to penetrate various barriers and directly or indirectly affect organisms (Wu et al., 2019). (2) Large surface area and high adsorption capacity. MPs possess different adsorption sites on their surface, allowing them to bind to metals, chemicals, and other pollutants (Xu et al., 2020). (3) Resistance to degradation. MPs are highly resistant to corrosion because of their inertness; (4) Low density and easy migration. With their low density and lightweight, MPs can easily migrate in air and water environments (Zhou et al., 2023). In summary, microplastics persist in the environment, exhibiting resistance to degradation, ease of migration, small size, strong adsorption capacity, and wide distribution in human living environments (Fig. 1).
Exposure doses and reproductive hazard
At present, there are limited population-based epidemiological studies on microplastics, but available data have confirmed the adverse effects of microplastics on humans. A meta-analysis showed that MPs decreased sperm concentration, motility, and viability (Hu et al., 2023). Traces of MPs have also been found in human placentas (Liu et al., 2023a, 2023b, 2023c, 2023d; Ragusa, Matta, et al., 2022), breastmilk (Ragusa, Notarstefano, et al., 2022), living lungs (Jenner et al., 2022), and blood (Leslie et al., 2022; D. Wu et al., 2023a, 2023b) in recent years. Notably, a team in the Netherlands was the first to discover the MPs in the blood of 17 healthy volunteers, with an average concentration of 1.6 μg/mL (Leslie et al., 2022). One study further provided the first photographic and Raman spectroscopic evidence of MPs in the thrombus (Wu et al., 2023a, 2023b). These reliable studies of circulating MPs concentrations in humans powerfully suggest that MPs can plausibly reach multiple organs throughout the body via the blood circulatory system. To search for evidence that MPs accumulate in men and cause damage to reproductive health, the researchers detected MPs in six testicular and thirty semen samples and found the mean abundance of MPs was 0.23–0.68 particles/mL in semen and 11.60–27.12 particles/g in testis. PS was the main MPs in the testis, and PE and PVC were the main MPs in the semen, most of which was 20–100 μm (Zhao et al., 2023).
According to the existing literature, the daily MPs exposure of adults is about 0.014–0.71 g/person (Senathirajah et al., 2021). The daily exposure of mice was 1.856–92.856 mg/g BW based on the body surface area of humans and mice. However, a study estimated human adult MP intake at 583 ng/person/day (Mohamed Nor et al., 2021). Over time, studies using elevated concentrations of MPs may become more meaningful. However, this blurs the field of real exposure and makes it difficult to understand which effects of MPs may actually pose real hazards to human health. Therefore, more comparative studies and real environmental exposure studies are needed. In this review, we mainly focused on male reproductive damage caused by PS-MPs. Notably, toxic mechanisms, toxic identification, and quantification of MPs are also relatively emerging areas of reproductive toxicology research (Liu et al., 2023a, 2023b, 2023c, 2023d), and the current trend is to shift the focus of research from aquatic animals to terrestrial mammals. Because the definition and detection methods of MPs have yet to be specified, there is a lack of sufficient epidemiological evidence. Still, there are more and more relevant experimental studies at the animal levels (Table 2).
The function of the blood-testis barrier
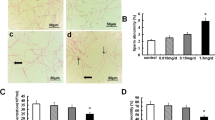
Sertoli cells (SCs), located at the basement of seminiferous tubules, play essential nourishing and supportive roles in the testicular microenvironment (Johnson et al., 2008), and they are responsible for maintaining the BTB integrity (Mruk & Cheng, 2015). The BTB is composed of tight junctions, gap junctions, and ectoplasmic specialization, which are formed between SCs (Wen et al., 2018a, 2018b). The junction protein represents a crucial component of the BTB (Gao et al., 2015), as it prevents harmful substances from accessing germ cells at all stages, thus ensuring the normal progression of the spermatogenic process (Chen et al., 2016). Researchers confirmed that high-dose PS-MPs exposure may lead to the destruction of BTB integrity and the apoptosis of spermatogenic cells through reactive oxygen species (ROS) -triggered p38 Mitogen-activated protein kinase (MAPK)/nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway. Their male Wistar rats were administered 0, 0.015, 0.15, and 1.5 mg/day PS-MPs (0.5 μm) for 90 days. Noteworthily, the expressions of BTB-related proteins, such as Occluding, Claudin-11, N-Cadherin, and Connexin-43 were decreased, especially at 0.15 and 1.5 mg/day groups (Li et al., 2021). Moreover, the reproductive function in male mice is more sensitive to the MPs toxicity in a meta-analysis (Liu et al., 2023a, 2023b, 2023c, 2023d). Some researchers found that PS-MPs in female ovaries were much more severe than those in male testes according to fluorescently labeled PS-MPs accumulation (Wei et al., 2022). It is reasonable to infer that gender differences exist in terms of the toxicity induced by PS-MPs exposure, with the BTB in male mice playing a protective role against PS-MPs. Given the unique structure and functioning of the BTB, there is an increasing research interest in exploring the reproductive toxicity resulting from PS-MPs that disrupt the BTB.
Potential mechanisms of PS-MPs induced BTB damage
Reactive oxygen species
Oxidative stress arises from an imbalance between ROS production and the antioxidant system, and this manifestation is primarily characterized by an increase in ROS levels and alterations in the expression of oxidative stress-related enzymes and metabolites (Yang & Lian, 2020). Numerous experimental studies indicated that ROS and the dysfunction of antioxidant defense are key contributors to the toxicity of PS-MPs (Ding et al., 2024; Yao et al., 2023). When cells are stimulated by oxidative damage signals, an excessive ROS leads to depletion of antioxidant substances or decreased activity, thus breaking the balance (Yang & Lian, 2020). An adverse outcome pathway analysis of MPs mediated mammalian male reproductive toxicity revealed that the increase of ROS is the molecular initiating event, triggering multiple key events (Hu et al., 2023). Research has identified that the nuclear factor-κB (NF-κB) is a pivotal molecule in the onset of senescence in testicular Sertoli cells of normal mice (TM4) induced by PS-MPs. By neutralizing ROS, the TM4 cells senescent can be inhibited (Wu, Zhang, et al., 2023a, 2023b). One representative study showed that rats exposed to PS-MPs diet for 90 days had increased catalase activity and decreased superoxide dismutase activity, contributing to reproductive dysfunction in male rats (Ilechukwu et al., 2022).
ROS are commonly linked to multiple signaling pathways. Researchers demonstrated that PS-MPs induced oxidative stress in a dose-dependent manner (2, 20, 200, and 2000 μg/L) (Ijaz et al., 2021). Previous studies demonstrated that PS-MPs can induce reproductive dysfunction through excessive ROS and activate the p38 MAPK signaling pathway (Xie et al., 2020). Interestingly, an experiment investigated the effects of PS-MPs exposure on porcine germ cells (Wang et al., 2022a, 2022b). PS-MPs induced excessive ROS in porcine spermatogenic cells (GCs), promoted the phosphorylation of MAPK pathway related genes, and activated the hypoxia inducible factor (HIF-1α). Similarly, another article found that PS-MPs induced ROS, and activated p38 MAPK pathway, which depleted Nrf2, caused a decrease in BTB junction proteins expression (Li et al., 2021). Previous studies showed that Wingless/Integrated (Wnt) signal pathway can be triggered by oxidative stress (Hou et al., 2022a, 2022b; Wang et al., 2022a, 2022b). Researchers found that PS-MPs could activate the Wnt/β-Catenin signaling pathway through ROS to cause ovarian fibrosis (An et al., 2021) and cardiac fibrosis (Li et al., 2020). PS-MPs exposure significantly increased the expression levels of Wnt/β-Catenin signaling pathways-related proteins and the primary fibrosis markers transforming growth factor (TGF)-β. TGF-β is a critical cytokine in the process of fibrotic disease development (An et al., 2021). The level of TGF-β increased significantly accompanied with the activation of Wnt pathway. Notably, TGF-β, a key regulator of BTB reconstruction (Lui et al., 2001; Xia et al., 2009), is believed to impede BTB function in SCs through its excessive expression, potentially resulting from increased endocytosis of BTB-related junction proteins (Alves et al., 2013). In addition, based on the close connection between the cytoskeleton and the maintenance of BTB integrity (Wen, Tang, Li, et al., 2018a, 2018b). The researchers have found that PS-MPs induce an imbalance in mechanistic target of rapamycin complex (mTORC) 1 and 2 through a burst of ROS, and alter the expression profile of actin-binding proteins, resulting in the fragmentation of f-actin and reduction in junction protein of BTB (Wei et al., 2021a, 2021b). Actin-related protein 3 in BTB is essential for spermatogenesis (Li et al., 2023a, 2023b; Wang et al., 2023a, 2023b, 2023c, 2023d), and this suggests that PS-MPs impair spermatogenesis through actin filament truncation and disruption of BTB integrity, which may be the vital cause of ROS increase.
In reality, MPs released into the environment undergo aging processes via physical, chemical, and biological processes, thus harming the environment and human health. Photoaging is one of the most common processes that accelerate the aging of MPs. Studies have suggested that ultraviolet oxidation accelerates aging and affects the structural properties and surface chemistry of MPs (Chen et al., 2023a, 2023b, 2023c, 2023d, 2023e). Researchers focused on the detriment of aged PS-MPs on reproductive function. Their mice were exposed to PS-MPs that had been aged by ultraviolet light. The superoxide dismutase and glutathione contents were significantly reduced in mice, suggesting that aged PS-MPs quickly interfere with the antioxidant capacity of the mice (Cui et al., 2023). Researchers speculated that ultraviolet irradiation may cause PS-MPs to develop a rough surface, fragment, and increase the presence of carbonyl groups. It also provides new insights for assessing the environmental risks of photoaging MPs. Compared with rodents, Caenorhabditis elegans provides a practical and fast detection system. Due to their short life span, Caenorhabditis elegans is an ideal model for studying the long-term effects of exposure to MPs throughout life. Chen et al. found that photoaged PE microbeads-induced toxicity and oxidative stress may be involved in regulating adverse reactions in Caenorhabditis elegans (Chen et al., 2023a, 2023b, 2023c, 2023d, 2023e). They subsequently studied aged PS microbeads and found that maternal exposure to aged PS induced transgenerational reproductive effects through H3K4 and H3K9 methylation (Chen et al., 2023a, 2023b, 2023c, 2023d, 2023e). Caenorhabditis elegans provides a favorable high-throughput model system to determine the effects of MPs on animal reproduction. This Photoaging makes the PS-MPs more prone to induce the production of ROS. Caenorhabditis elegans can provide insights into how exposure to MPs early in life can lead to detrimental consequences later in life.
Inflammatory response
The inflammatory responses in SCs are essential for the function of BTB (Fang et al., 2021). Increasing evidence suggests that increased inflammation and cytokines after MPs exposure are associated with the disruption of various barriers, including the blood–brain barrier (Kwon et al., 2022), lung epithelial barrier (Dong et al., 2020), intestinal mucosal barrier (Martel et al., 2022; Zeng et al., 2024), and vascular endothelial barrier (Lee et al., 2021). These barriers share a similar structure and function with the BTB. Researchers found that PS-MPs induce apoptosis in chicken testis via crosstalk between NF-κB and Nrf2 pathways. They exposed chickens to water containing PS-MPs (0, 1, and 100 mg/L), and they found that PS-MPs caused chicken testicular inflammatory infiltration and interstitial hemorrhage, resulting in testicular tissue damage. Notably, BTB-related proteins Claudin3 and Occludin decreased, and the integrity of BTB was damaged (Hou et al., 2022a, 2022b). Besides, Jin et al. confirmed that PS-MPs induced testicular inflammation and increased inflammatory factors in mice testis. Spermatogenetic disorder, testicular inflammation, and destruction of the BTB were observed in the mice testis following exposure to PS-MPs (Jin et al., 2021).
The inflammatory response is one of the significant phenotypes induced by exposure to exogenous pollutants (Germolec et al., 2018). Numerous studies have corroborated that MPs activate the immune system and elicit an inflammatory response by stimulating the release of cytokines such as Interleukin (IL)-6 and IL-1β, and by activating the NF-κB. This activation can damage cell membranes, necrosis, and structural and functional impairment of various tissues (Yin et al., 2023; Zeng et al., 2024). Also, exposure to PS-MPs can induce phosphorylation of Jun N-terminal kinase (JNK) and p38 MAPK in testicular tissue, leading to the promotion of inflammatory responses and structural damage (Li et al., 2021). An in vivo study proved that PS-MPs exposure significantly increase the expression of pro-inflammatory molecules NF-κB, inflammatory factors IL -1β and IL-6, with reduced anti-inflammatory molecules expression (Hou et al., 2021a, 2021b). Similarly, exposure to PS-MPs in another study significantly increased inflammatory marker levels (Ijaz et al., 2023). Moreover, Rizwan et al. and Hamza et al. found that PS-MPs were able to cause inflammatory markers IL-6, NF-κB, IL-1β, tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) activity increased in the rat testis. They demonstrated the anti-inflammatory effects of Rhamnetin (Rizwan et al., 2023) and Astilbin (Hamza et al., 2023) in PS-MPs-induced testicular injury, respectively. So far, numerous studies have highlighted the interplay between oxidative stress and inflammatory response resulting from exposure to MPs, which then influences toxic endpoints such as apoptosis and autophagy. Inflammatory response as a critical mechanism of PS-MPs-induced reproductive toxicity needs further study.
Apoptosis
Apoptosis can be categorized into exogenous and endogenous apoptosis. Exogenous apoptosis occurs when a cell receives an external signal instructing it to undergo cell death. The cell then undergoes a series of complex cascade reactions, ultimately leading to its demise. On the other hand, endogenous apoptosis takes place when a cell detects internal abnormalities or activates its suicide program, ultimately resulting in its own demise (Kopeina & Zhivotovsky, 2022). Apoptosis may occur due to various factors triggered by exposure to PS-MPs. A study confirmed that PS-MPs can induce apoptosis in germ cells through activating p53 signaling pathway, and finally lead to reproductive dysfunction in mice (Lu et al., 2023a, 2023b). Furthermore, a different study indicated a decline in sperm count and downregulation of anti-apoptotic-related protein Bcl-2 following exposure to PS-MPs, as well as the increase in apoptosis-related protein Bax and caspase-3 expression (Ijaz et al., 2023). Apoptosis, a crucial mechanism that cannot be overlooked in the context of PS-MPs-induced reproductive dysfunction (Hamza et al., 2023). Under redox system imbalance and inflammatory stress, exposure to PS-MPs led to apoptosis, and ultimately causing testicular damage (Hou et al., 2022a, 2022b).
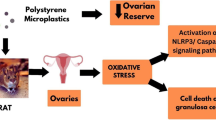
It is crucial to emphasize that apoptosis represents just one form of cell death (Bertheloot et al., 2021), and additional studies are required to elucidate pathways through which PS-MPs activate cell death. Here, we also discussed the relationship between some other cell death and PS-MPs (Fig. 2). Researchers found that PS-MPs activated the Wnt/β-catenin signaling pathway in rats and promoted apoptosis of cardiomyocytes (Li et al., 2020). Interestingly, other researchers further found that pyroptosis is crucial in PS-MPs-induced cardiotoxicity (Wei et al., 2021a, 2021b). Pyroptosis is also a pro-inflammatory cell death associated with caspases and cytokines (Vasudevan et al., 2023). The activation of Nod-like receptor pyrin domain 3 (NLRP3) inflammasome is one of the mechanisms that mediate PS-MPs-induced toxicity. Emerging data have suggested that PS-MPs cause pyroptosis via NLRP3/caspase-1 in various tissues and cells (Zeng et al., 2024; Zhang et al., 2022a, 2022b, 2022c). Researchers found that PS-MPs can induce pyroptosis and apoptosis of ovarian granulosa cells via the NLRP3/caspase-1 signaling pathway maybe triggered by ROS (Hou et al., 2021a, 2021b). Furthermore, PS-MPs can cause hepatocyte ferroptosis (Wang et al., 2023a, 2023b, 2023c, 2023d), a newly defined type of cell death characterized by iron accumulation and lipid oxidation (Ursini & Maiorino, 2020). In a combined exposure experiment, PS-MPs and cadmium (Cd) synergistically impeded the Keap1-Nrf2 pathway and its downstream genes, inducing the production of ferroptosis (Lan et al., 2024). Likewise, subchronic co-exposure to PS-MPs and Cd exacerbated reproductive damage in male mice. The testicular injury induced by PS-MPs alone or combined with Cd was correlated with the disruption of the miR-199a-5p/HIF-1α/ferroptosis pathway (Zhang et al., 2023a, 2023b). Besides, the epigenetic pathways involved in microRNA are poorly understood. The emerging types of cell death induced by PS-MPs led us to wonder whether other types of cell death exist in the testis. The programmed cell death caused by PS-MPs exposure alone or co-exposure needs further investigation.
Autophagy
Autophagy is a sophisticated cellular process involving the degradation and recycling of cellular components through the lysosomal machinery. The involvement of autophagy in the toxicity induced by PS-MPs is an area of active research, as it may influence the cellular response to these microplastics and contribute to their potentially harmful effects. The autophagy process comprises several vital steps, including phagophore formation, autophagosome completion, and fusion of the autophagosome with a lysosome to form autolysosome (Mizushima & Komatsu, 2011; Parzych & Klionsky, 2014). Autophagy is also considered a programmed death in a broad sense. Recently, studies have shown that autophagy is one of the mechanisms involved in the destruction of BTB integrity after exposure to toxicants, including cadmium, streptozotocin, zearalenone and di-(2-ethylhexyl) phthalate (DEHP) (Zheng et al., 2022). In the studied aquatic animals exposed to PS-MPs, PS-MPs could induce autophagy by regulating ROS levels (Lu et al., 2023a, 2023b). Previous studies have shown that PS-MPs cause autophagic cell death in bronchial epithelial cells, eventually leading to inflammatory impairment (Jeon et al., 2023). Autophagy is a dynamic process that is usually described using autophagic flux, including the formation and degradation of autophagosomes (Parzych & Klionsky, 2014). A recent study has shown that PS-MPs could impair BTB integrity via autophagy Inhibition (Ma et al., 2023), indicating that it is worth discussing the potential mechanisms of autophagy-relevant BTB damage.
Excessive autophagy can result in cellular damage. Prolonged autophagy and lysosomal activation can finally result in lysosomal rupture and calcium release following the phagocytosis of PS-MPs by macrophages (Yin et al., 2023). Not only may excessive autophagy cause damage, but insufficient autophagy also affects spermatogenesis. In an investigation focused on the effects of PS-MPs on mouse osteoblasts, investigators observed the accumulation of senescent osteoblasts in the bone trabeculae in mice, as well as impaired autophagy in senescent osteoblasts. Autophagy activator significantly reversed the senescent cell accumulation, and ameliorated PS-MPs induced bone growth arrest (Pan et al., 2023). Notably, researchers found the effect of polystyrene nanoplastics (0.05 μm) on sperm acrosome defects. Based on the expression of autophagy-related proteins, inhibition of autophagy was observed in the testes of PS-MPs exposed mice. Using autophagy inhibitors aggravates the injury, and the promotion of autophagy contributes to the recovery (Zhou et al., 2022). Likewise, In a co-exposure of nanoplastics and dibutyl phthalate, the researchers demonstrated a detrimental effect on male reproductive organs through disrupting BTB, which can alleviated by activation of autophagy (Ma et al., 2023). However, the variation of autophagy flux by PS-MPs and the impairment of BTB have not explicitly been studied so far.
Intriguingly, in one study of hepatocyte damage by PS-MPs (Wang et al., 2023a, 2023b, 2023c, 2023d), researchers found that the degree of damage to hepatocytes was closely correlated with particle diameter. Small particles (1–10 μm) induce cell death mainly as programmed necrosis, while large particles (50–100 μm) primarily induce apoptosis and affect autophagic flow. Inhibition of autophagy not only alleviated cell death triggered by PS-MPs but also altered the nature of death damage. PS-MPs-induced apoptosis and necroptosis may be related to different particle sizes, but further studies of the relationship between autophagy and cell death types are needed in Sertoli cells.
Sex hormone disorder
The hypothalamic-pituitary–gonadal (HPG) axle is momentous in regulating the male reproductive system and spermatogenesis. This axle primarily involves the secretion of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), as well as the synthesis of estrogen and testosterone (Oyola & Handa, 2017). Sex hormones, such as GnRH, FSH, LH, and androgens, regulate the disassembly and recombination of the BTB. FSH modulates the bioavailability of testosterone by altering the expression of sex hormone-binding globulin via SCs. (Koysombat et al., 2023). Furthermore, FSH also plays an important role in maintaining spermatogenic microenvironmental homeostasis by acting on SCs (Oduwole et al., 2021). The HPG axle is widely recognized as susceptible to various forms of environmental pollution (Xie et al., 2022). In a chronic PS-MPs exposure study, morphological changes in the testis and reduced testosterone in serum levels. Likewise, LH and FSH were concentrated in the testicular tissue. They subsequently demonstrated that PS-MPs reduce testosterone via the steroidogenic protein expression change (Jin et al., 2022). Similarly, several studies proved that the HPG axle was negatively regulated by MPs exposure (Li et al., 2022a, 2022b; Wang et al., 2019). Existing articles have also revealed that PS-MPs can downregulate the expression of steroidogenic genes within the HPG axle (Gupta et al., 2023). Moreover, transcriptome sequencing analysis provided unique insights into the effects on cellular processes (Gao et al., 2023). The analysis suggested that nanoparticles are mainly involved in steroid biosynthesis, while microparticles primarily affect amino acid metabolism. Therefore, it is urgent to define the size of MPs, which is conducive to more precise research in PS-MPs-induced reproductive dysfunction. Testosterone is synthesized by Leydig cells and acts on SCs via the classical or non-classical pathway (Zheng et al., 2022). Testosterone may promote the BTB-related proteins to endocytose and relocate to the SCs surface, while TGF-β3 promotes them to endocytose except relocate. TGF-β, a key regulator of BTB reconstruction (Lui et al., 2001; Xia et al., 2009), is believed to impede BTB function in SCs through its excessive expression, potentially resulting from increased endocytosis of BTB-related junction proteins (Alves et al., 2013). Growing evidence has shown that sex hormones, especially testosterone, could stimulate the expression and the correct localization of the BTB-relative proteins in vivo and in vitro models. However, the underlying mechanisms of the PS-MPs-induced disruption of the BTB associated with testosterone is unclear.
Mitochondrial dysfunction
Mitochondria are pivotal in cellular energy production and apoptosis. During energy production, a proton gradient across the inner mitochondrial membrane, established through nutritional metabolism, serves as the driving force for adenosine triphosphate (ATP) synthesis, but decreased mitochondrial membrane potential (MMP) will block the mitochondrial energy supply (Monzel et al., 2023). In an in vivo study, PS-MPs induced a decrease in mitochondrial membrane potential and ATP content in the testicular tissues of mice. ROS-induced mitophagy may be the cause of mitochondrial damage (Liu, Hou, Zhang, et al., 2022a, 2022b, 2022c). Researchers found that the PS-MPs induced Ca2+ overload caused the accumulation of mitochondrial ROS, which triggers premature testicular aging (Wu, Zhang, et al., 2023a, 2023b). In an in vitro study, results uncovered that PS-MPs reduced ATP and MMP levels, disrupted the integrity of the mitochondrial genome, and created an imbalance arises in mitochondrial fission–fusion homeostasis. The researchers further found that the mitophagy pathway was activated, and time series analysis showed that PS-MPs damage mitochondrial structure through ROS (Liu, Hou, Wang, et al., 2022a, 2022b). Likewise, in a co-exposure study of MPs with DEHP, a commonly used plasticizer, genes related to mitochondrial respiratory chain complex and ATP synthesis were differentially regulated (Li et al., 2022a, 2022b).
Mitochondrial damage can result in cell senescence, excessive ROS, and cellular energy depletion (Zorov et al., 2014). Spermatocytes and spermatids situated in the BTB are unable to access glucose from the bloodstream, necessitating reliance on lactate generated by SCs for energy production (Xu et al., 2022). PS-MPs were found to disrupt BTB and reduce the number of SCs in high-fat diet mice, especially the increased lactate by mitochondrial dysfunction (Cai et al., 2023). Similarly, the researchers also confirmed that PS-MPs induce gastric barrier damage via mitochondrial dysfunction (Ding et al., 2024). In general, mitochondria, as an essential organelle in the cell body, are closely related to the physiological state of cells. The study of apoptosis, autophagy, senescence, ROS, and other mechanisms closely related to mitochondria is the focus of future research. The exact mechanism by which PS-MPs damage SCs requires further subcellular exploration.
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) possesses the largest surface area among cellular organelles, pivotal in synthesizing, folding, and modifying secreted proteins (Groenendyk et al., 2021). Despite the highly refined regulation of ER protein folding capacity, various external factors can perturb this process. ER stress is accompanied by aggregation of unfolded or misfolded proteins within the ER lumen (Chen et al., 2023a, 2023b, 2023c, 2023d, 2023e). The activation of ER stress triggers the unfolded protein response, an adaptive mechanism aimed at reestablishing ER homeostasis. Significantly, inositol-requiring enzyme 1α (IRE1α), activating transcription factor 6 (ATF6), and ER kinase serve as critical transmembrane sensors of stress that start the adaptive response in mammalian cells (Ren et al., 2021). PS-MPs were found to upregulate ER stress-related factors and downstream regulators of apoptosis. Following the administration of ER stress inhibitors, PS-MPs-induced testicular damage were improved to near-normal levels (Wen et al., 2023). Similarly, researchers found that PS-MPs caused mitochondrial dysfunction and ER stress in the kidney cells of mice (Wang et al., 2021). Moreover, PS-MPs inhibited the functions of TM4, as evidenced by the decrease in the phosphorylation of ER kinase and protein kinase B (PKB) in the cell line. Researchers confirmed that mitochondrial dysfunction and activation of ER stress in PS-MPs-induced TM4 (Grillo et al., 2024). It is known that mitochondria are closely associated with ER to form the mitochondrial-associated ER membranes, the site of calcium ions transfer, as well as lipid biosynthesis-involved enzymes and cholesterol transport from ER to the mitochondria (Ge et al., 2022). And other researchers found that the accumulation of PS-MPs in the aquatic environment disturbs the gut microbiota of carp, and induces ER stress and apoptosis in the intestinal tissues (Wang et al., 2023a, 2023b, 2023c, 2023d). The above findings indicated that PS-MPs can result in reproductive toxicity by activating ER stress and apoptosis. Moderate ER stress contributes to the restoration of ER homeostasis, facilitating cellular adaptation to stress. Conversely, sustained and excessive ER stress can potentiate cell dysfunction and even death. Currently, studies on the mechanisms of ER stress are limited in PS-MPs-induced BTB damage.
Intestinal flora disorder
Exposure to MPs is associated with metabolic disorders, characterized by alterations in energy, lipid, glucose, and protein metabolism (Sun et al., 2022), closely related to oxidative stress, inflammation, cell death, and other factors. As the second largest genome of the host, the gut microbiota has been reported to be involved in various metabolic disorders (Dabke et al., 2019). MPs can be imported into the body and have been reported to be associated with intestinal toxicity (Hirt & Body-Malapel, 2020). Notably, researchers have found that MPs-induced gut microbiota imbalance affects the brain through the gut-brain axle (Chen et al., 2023a, 2023b, 2023c, 2023d, 2023e). In addition, researchers have also found that the interaction between gut and liver after MPs exposure eventually leads to insulin resistance and even diabetes (Shi et al., 2022). The two cardinal functions of barriers include preventing access to deleterious elements of the environment while facilitating the transport of essential ions, signaling molecules, and nutrients needed to maintain the internal milieu. The effects of microbial groups and the BTB were also reported (Al-Asmakh & Hedin, 2015). Interestingly, increased inflammation after exposure to PS-MPs in mice is mainly regulated by gut microbes (Fu et al., 2023). One study found that maintaining gut microbial homeostasis is crucial for the physiological function of the testis. The researchers found that the increased proportion of pro-inflammatory bacteria in PS-MPs exposed mice and corresponding recipient mice may drive the translocation of Th17 cells, leading to the excessive production of IL-17a and downstream inflammatory responses. Researchers demonstrated that intestinal microbiota related lipid metabolism disorder was the cause of PS-MPs exposed spermatogenesis dysfunction (Wen et al., 2022). The gut microbiota is diverse and numerous, and the exact mechanism of how PS-MPs affect the testis through the gut microbiota needs further study. The metabolic disorders caused by microplastics deserve further study, but the evidence is relatively lacking (Table 3).
Macrophage polarization
The BTB not only acts as a barrier against xenobiotics but also upholds an immune-privileged status to prevent the onset of an inflammatory response (Fang et al., 2021). C–C chemokine receptor-2 (CCR2) is expressed on a variety of immune cells, and its ligand monocyte chemoattractant protein-1 (MCP-1) is secreted by SCs (Zhang et al., 2022a, 2022b, 2022c). According to a study (Figueiredo et al., 2021), the activation of the CCR2 receptor exerts a regulatory influence on the number of macrophages in the testes, as well as steroidogenesis and spermatogenic progression. Similarly, a study has revealed macrophage in the development of testicular fibrosis (Peng et al., 2022). In physiological states, immune cells are confined to the interstitium, whereas in conditions of compromised BTB integrity, these cells can infiltrate the seminiferous epithelium (Archana et al., 2019), which suggests that immune cells may be involved in BTB damage. Researchers found that exposure to PS-MPs led to a substantial shift in the M1/M2 ratio among macrophages, resulting in dominance of the M2 subtype in pregnant mice (Hu et al., 2021). In the male mice reproductive system, researchers observed that PS-MPs led to the vacuolization of seminiferous tubules, concomitant with apoptosis of testicular tissue and the infiltration of M1 macrophages (Li et al., 2023a, 2023b). Under normal physiological conditions, the dominant type of macrophage in the testis is M2 (Zhang et al., 2022a, 2022b, 2022c). Studies have shown that PS-MPs exposure leads to an increase in M1 macrophages in the testis and the secretion of large amounts of pro-inflammatory cytokines (Li et al., 2023a, 2023b). Under testicular inflammation, the expression of Claudin3 and Occludin in the BTB decreased and damaged the integrity of the BTB (Hou et al., 2022a, 2022b). Moreover, in a co-culture system involving GC2 cells and macrophages, PS-MPs induced macrophage M1 polarization, activating the macrophage migration inhibitory factor and prompting GC2 cell apoptosis (Li et al., 2023a, 2023b). The mechanism by which PS-MPs induce testicular inflammation may involve the promotion of M1 macrophage infiltration. It has been reported that M1 polarization of macrophages can affect testosterone biosynthesis in Leydig cells (Yamauchi et al., 2022). However, the role of macrophage polarization in PS-MPs-induced SCs injury and BTB damage remains to be elucidated.
Conclusions and prospects
In conclusion, the BTB creates a microenvironment that safeguards spermatogenesis from the deleterious effects of toxicants and immune cell infiltration. Conversely, exposure to PS-MPs has been demonstrated to compromise the integrity of BTB via diverse toxicological mechanisms, including oxidative stress, inflammation, and autophagy (Fig. 3). Interest in the connection between MPs and the male reproductive system has been growing in the community. However, research into the specific mechanisms by which PS-MPs injure the BTB is currently limited. The purpose of this review is to compile the existing knowledge and explore potential mechanisms associated with PS-MPs-induced BTB damage, intending to stimulate more comprehensive and profound investigations into this topic.
Currently, the characteristics of MPs used in experimental studies differ from those found in the surrounding environment. It appears to be a massive publication bias in MPs research driven by hat is often unrealistically high-dose exposures. Therefore, the collection of MPs in the external environment and the preparation of standards contribute to the replication and accuracy of the study. The concentration, type, exposure time, and combined toxicity of MPs with other substances are all factors that influence the reproductive toxicity (Schmid et al., 2021). Importantly, while exposure to different sizes of MPs can cause reproductive toxicity, the specific mechanism may be dependent on specific particle sizes (Yang et al., 2022). Additionally, it is essential to elucidate the exposure pathways of MPs further and develop more effective detection methods and biomarkers, these efforts will contribute to a deep understanding of the relationship between MPs and reproductive risk.
References
Abbasi, S., Keshavarzi, B., Moore, F., Turner, A., Kelly, F. J., Dominguez, A. O., & Jaafarzadeh, N. (2019). Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County Iran. Environmental Pollution, 244, 153–164. https://doi.org/10.1016/j.envpol.2018.10.039
Ageel, H. K., Harrad, S., & Abdallah, M.A.-E. (2022). Occurrence, human exposure, and risk of microplastics in the indoor environment. Environmental Science. Processes & Impacts, 24(1), 17–31. https://doi.org/10.1039/d1em00301a
Al-Asmakh, M., & Hedin, L. (2015). Microbiota and the control of blood-tissue barriers. Tissue Barriers, 3(3), e1039691. https://doi.org/10.1080/21688370.2015.1039691
Alves, M. G., Rato, L., Carvalho, R. A., Moreira, P. I., Socorro, S., & Oliveira, P. F. (2013). Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cellular and Molecular Life Sciences, 70(5), 777–793. https://doi.org/10.1007/s00018-012-1079-1
Amato-Lourenço, L. F., Carvalho-Oliveira, R., Júnior, G. R., Dos Santos Galvão, L., Ando, R. A., & Mauad, T. (2021). Presence of airborne microplastics in human lung tissue. Journal of Hazardous Materials, 416, 126124. https://doi.org/10.1016/j.jhazmat.2021.126124
An, R., Wang, X., Yang, L., Zhang, J., Wang, N., Xu, F., Hou, Y., Zhang, H., & Zhang, L. (2021). Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology, 449, 152665. https://doi.org/10.1016/j.tox.2020.152665
Archana, S. S., Selvaraju, S., Binsila, B. K., Arangasamy, A., & Krawetz, S. A. (2019). Immune regulatory molecules as modifiers of semen and fertility: A review. Molecular Reproduction and Development, 86(11), 1485–1504. https://doi.org/10.1002/mrd.23263
Bertheloot, D., Latz, E., & Franklin, B. S. (2021). Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cellular & Molecular Immunology, 18(5), 1106–1121. https://doi.org/10.1038/s41423-020-00630-3
Cai, P., Wang, Y., Feng, N., Zou, H., Gu, J., Yuan, Y., Liu, X., Liu, Z., & Bian, J. (2023). Polystyrene nanoplastics aggravate reproductive system damage in obese male mice by perturbation of the testis redox homeostasis. Environmental Toxicology, 38(12), 2881–2893. https://doi.org/10.1002/tox.23923
Chang, X., Fang, Y., Wang, Y., Wang, F., Shang, L., & Zhong, R. (2022). Microplastic pollution in soils, plants, and animals: A review of distributions, effects and potential mechanisms. The Science of the Total Environment, 850, 157857. https://doi.org/10.1016/j.scitotenv.2022.157857
Chen, G., Xiong, S., Jing, Q., van Gestel, C. A. M., van Straalen, N. M., Roelofs, D., Sun, L., & Qiu, H. (2023a). Maternal exposure to polystyrene nanoparticles retarded Fetal growth and triggered metabolic disorders of placenta and Fetus in mice. The Science of the Total Environment, 854, 158666. https://doi.org/10.1016/j.scitotenv.2022.158666
Chen, H., Gu, Y., Jiang, Y., Yu, J., Chen, C., Shi, C., & Li, H. (2023b). Photoaged polystyrene nanoplastics result in transgenerational reproductive toxicity associated with the methylation of histone H3K4 and H3K9 in Caenorhabditis elegans. Environmental Science & Technology, 57(48), 19341–19351. https://doi.org/10.1021/acs.est.3c05861
Chen, H., Jiang, Y., Gu, Y., Ding, P., Wang, C., Pan, R., Shi, C., Zeng, L., Chen, X., & Li, H. (2023c). The generation of environmentally persistent free radicals on photoaged microbeads from cosmetics enhances the toxicity via oxidative stress. Environment International, 174, 107875. https://doi.org/10.1016/j.envint.2023.107875
Chen, H., Mruk, D., Xia, W., Bonanomi, M., Silvestrini, B., & Cheng, C.-Y. (2016). Effective delivery of male contraceptives behind the blood-testis barrier (BTB)—lesson from adjudin. Current Medicinal Chemistry, 23(7), 701–713. https://doi.org/10.2174/0929867323666160112122724
Chen, X., Shi, C., He, M., Xiong, S., & Xia, X. (2023d). Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduction and Targeted Therapy, 8(1), 352. https://doi.org/10.1038/s41392-023-01570-w
Chen, X., Xu, L., Chen, Q., Su, S., Zhuang, J., & Qiao, D. (2023e). Polystyrene micro- and nanoparticles exposure induced anxiety-like behaviors, gut microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicology and Environmental Safety, 259, 115000. https://doi.org/10.1016/j.ecoenv.2023.115000
Chen, X., Zhuang, J., Chen, Q., Xu, L., Yue, X., & Qiao, D. (2022). Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicology and Environmental Safety, 241, 113809. https://doi.org/10.1016/j.ecoenv.2022.113809
Cui, H., Yang, W., Cui, Y., Qi, L., Jiang, X., & Li, M. (2023). Adverse effects of pristine and aged polystyrene microplastics in mice and their Nrf2-mediated defense mechanisms with tissue specificity. Environmental Science and Pollution Research International, 30(14), 39894–39906. https://doi.org/10.1007/s11356-022-24918-1
D’Angelo, S., & Meccariello, R. (2021). Microplastics: A threat for male fertility. International Journal of Environmental Research and Public Health, 18(5), 2392. https://doi.org/10.3390/ijerph18052392
Dabke, K., Hendrick, G., & Devkota, S. (2019). The gut microbiome and metabolic syndrome. The Journal of Clinical Investigation, 129(10), 4050–4057. https://doi.org/10.1172/JCI129194
Deng, Y., Yan, Z., Shen, R., Wang, M., Huang, Y., Ren, H., Zhang, Y., & Lemos, B. (2020). Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environment International, 143, 105916. https://doi.org/10.1016/j.envint.2020.105916
Deng, Y., Zhang, Y., Qiao, R., Bonilla, M. M., Yang, X., Ren, H., & Lemos, B. (2018). Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). Journal of Hazardous Materials, 357, 348–354. https://doi.org/10.1016/j.jhazmat.2018.06.017
Ding, R., Chen, Y., Shi, X., Li, Y., Yu, Y., Sun, Z., & Duan, J. (2024). Size-dependent toxicity of polystyrene microplastics on the gastrointestinal tract: Oxidative stress related-DNA damage and potential carcinogenicity. The Science of the Total Environment, 912, 169514. https://doi.org/10.1016/j.scitotenv.2023.169514
Dong, C.-D., Chen, C.-W., Chen, Y.-C., Chen, H.-H., Lee, J.-S., & Lin, C.-H. (2020). Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. Journal of Hazardous Materials, 385, 121575. https://doi.org/10.1016/j.jhazmat.2019.121575
Fan, X., Li, X., Li, J., Zhang, Y., Wei, X., Hu, H., Zhang, B., Du, H., Zhao, M., Zhu, R., Yang, D., Oh, Y., & Gu, N. (2024). Polystyrene nanoplastics induce glycolipid metabolism disorder via NF-κB and MAPK signaling pathway in mice. Journal of EnvironmEntal Sciences, 137, 553–566. https://doi.org/10.1016/j.jes.2023.02.040
Fang, Y., Su, Y., Xu, J., Hu, Z., Zhao, K., Liu, C., & Zhang, H. (2021). Varicocele-mediated male infertility: From the perspective of testicular immunity and inflammation. Frontiers in Immunology, 12, 729539. https://doi.org/10.3389/fimmu.2021.729539
Fard, N. J. H., Mohammadi, M. J., & Jahedi, F. (2023). Effects of nano and microplastics on the reproduction system: In vitro and in vivo studies review. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 178, 113938. https://doi.org/10.1016/j.fct.2023.113938
Figueiredo, A. F. A., Wnuk, N. T., Vieira, C. P., Gonçalves, M. F. F., Brener, M. R. G., Diniz, A. B., Antunes, M. M., Castro-Oliveira, H. M., Menezes, G. B., & Costa, G. M. J. (2021). Activation of C–C motif chemokine receptor 2 modulates testicular macrophages number, steroidogenesis, and spermatogenesis progression. Cell and Tissue Research, 386(1), 173–190. https://doi.org/10.1007/s00441-021-03504-w
Fu, X., Liu, L., Han, H., Li, Y., Si, S., Xu, B., Dai, W., Yang, H., He, T., Du, X., & Pei, X. (2023). Integrated fecal microbiome and metabolome analysis explore the link between polystyrene nanoplastics exposure and male reproductive toxicity in mice. Environmental Toxicology, 38(6), 1277–1291. https://doi.org/10.1002/tox.23763
Gan, Q., Cui, J., & Jin, B. (2023). Environmental microplastics: Classification, sources, fates, and effects on plants. Chemosphere, 313, 137559. https://doi.org/10.1016/j.chemosphere.2022.137559
Gao, L., Xiong, X., Chen, C., Luo, P., Li, J., Gao, X., Huang, L., & Li, L. (2023). The male reproductive toxicity after nanoplastics and microplastics exposure: Sperm quality and changes of different cells in testis. Ecotoxicology and Environmental Safety, 267, 115618. https://doi.org/10.1016/j.ecoenv.2023.115618
Gao, Y., Mruk, D. D., & Cheng, C. Y. (2015). Sertoli cells are the target of environmental toxicants in the testis—a mechanistic and therapeutic insight. Expert Opinion on Therapeutic Targets, 19(8), 1073–1090. https://doi.org/10.1517/14728222.2015.1039513
Ge, X., He, Z., Cao, C., Xue, T., Jing, J., Ma, R., Zhao, W., Liu, L., Jueraitetibaike, K., Ma, J., Feng, Y., Qian, Z., Zou, Z., Chen, L., Fu, C., Song, N., & Yao, B. (2022). Protein palmitoylation-mediated palmitic acid sensing causes blood-testis barrier damage via inducing ER stress. Redox Biology, 54, 102380. https://doi.org/10.1016/j.redox.2022.102380
Germolec, D. R., Shipkowski, K. A., Frawley, R. P., & Evans, E. (2018). Markers of inflammation. Methods in Molecular Biology, 1803, 57–79. https://doi.org/10.1007/978-1-4939-8549-4_5
Grillo, G., Falvo, S., Latino, D., Chieffi Baccari, G., Venditti, M., Di Fiore, M. M., Minucci, S., & Santillo, A. (2024). Polystyrene microplastics impair the functions of cultured mouse Leydig (TM3) and Sertoli (TM4) cells by inducing mitochondrial-endoplasmic reticulum damage. Ecotoxicology and Environmental Safety, 274, 116202. https://doi.org/10.1016/j.ecoenv.2024.116202
Groenendyk, J., Agellon, L. B., & Michalak, M. (2021). Calcium signaling and endoplasmic reticulum stress. International Review of Cell and Molecular Biology, 363, 1–20. https://doi.org/10.1016/bs.ircmb.2021.03.003
Gupta, P., Mahapatra, A., Suman, A., Ray, S. S., Malafaia, G., & Singh, R. K. (2023). Polystyrene microplastics disrupt female reproductive health and fertility via sirt1 modulation in zebrafish (Danio rerio). Journal of Hazardous Materials, 460, 132359. https://doi.org/10.1016/j.jhazmat.2023.132359
Hamza, A., Ijaz, M. U., & Anwar, H. (2023). Rhamnetin alleviates polystyrene microplastics-induced testicular damage by restoring biochemical, steroidogenic, hormonal, apoptotic, inflammatory, spermatogenic and histological profile in male albino rats. Human & Experimental Toxicology, 42, 9603271231173378. https://doi.org/10.1177/09603271231173378
Hirt, N., & Body-Malapel, M. (2020). Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Particle and Fibre Toxicology, 17(1), 57. https://doi.org/10.1186/s12989-020-00387-7
Hou, B., Wang, F., Liu, T., & Wang, Z. (2021a). Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. Journal of Hazardous Materials, 405, 124028. https://doi.org/10.1016/j.jhazmat.2020.124028
Hou, J., Lei, Z., Cui, L., Hou, Y., Yang, L., An, R., Wang, Q., Li, S., Zhang, H., & Zhang, L. (2021b). Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicology and Environmental Safety, 212, 112012. https://doi.org/10.1016/j.ecoenv.2021.112012
Hou, L., Wang, D., Yin, K., Zhang, Y., Lu, H., Guo, T., Li, J., Zhao, H., & Xing, M. (2022). Polystyrene microplastics induce apoptosis in chicken testis via crosstalk between NF-κB and Nrf2 pathways. Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP, 262, 109444. https://doi.org/10.1016/j.cbpc.2022.109444
Hou, X., Wei, Z., Zouboulis, C. C., & Ju, Q. (2022b). Aging in the sebaceous gland. Frontiers in Cell and Developmental Biology, 10, 909694. https://doi.org/10.3389/fcell.2022.909694
Hu, J., Qin, X., Zhang, J., Zhu, Y., Zeng, W., Lin, Y., & Liu, X. (2021). Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reproductive Toxicology, 106, 42–50. https://doi.org/10.1016/j.reprotox.2021.10.002
Hu, Y., Shen, M., Wang, C., Huang, Q., Li, R., Dorj, G., Gombojav, E., Du, J., & Ren, L. (2023). A meta-analysis-based adverse outcome pathway for the male reproductive toxicity induced by microplastics and nanoplastics in mammals. Journal of Hazardous Materials, 465, 133375. https://doi.org/10.1016/j.jhazmat.2023.133375
Huang, D., Zhang, Y., Long, J., Yang, X., Bao, L., Yang, Z., Wu, B., Si, R., Zhao, W., Peng, C., Wang, A., & Yan, D. (2022). Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. The Science of the Total Environment, 838(Pt 1), 155937. https://doi.org/10.1016/j.scitotenv.2022.155937
Ijaz, M. U., Najam, S., Hamza, A., Azmat, R., Ashraf, A., Unuofin, J. O., Lebelo, S. L., & Simal-Gandara, J. (2023). Pinostrobin alleviates testicular and spermatological damage induced by polystyrene microplastics in adult albino rats. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 162, 114686. https://doi.org/10.1016/j.biopha.2023.114686
Ijaz, M. U., Shahzadi, S., Samad, A., Ehsan, N., Ahmed, H., Tahir, A., Rehman, H., & Anwar, H. (2021). Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male Sprague Dawley rats. Dose-Response: A Publication of International Hormesis Society, 19(2), 15593258211019882. https://doi.org/10.1177/15593258211019882
Ilechukwu, I., Ehigiator, B. E., Ben, I. O., Okonkwo, C. J., Olorunfemi, O. S., Modo, U. E., Ilechukwu, C. E., & Ohagwa, N. J. (2022). Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats. Environmental Analysis, Health and Toxicology, 37(2), e2022015–e2022010. https://doi.org/10.5620/eaht.2022015
Jenner, L. C., Rotchell, J. M., Bennett, R. T., Cowen, M., Tentzeris, V., & Sadofsky, L. R. (2022). Detection of microplastics in human lung tissue using μFTIR spectroscopy. The Science of the Total Environment, 831, 154907. https://doi.org/10.1016/j.scitotenv.2022.154907
Jeon, M. S., Kim, J. W., Han, Y. B., Jeong, M. H., Kim, H. R., Sik Kim, H., Park, Y. J., & Chung, K. H. (2023). Polystyrene microplastic particles induce autophagic cell death in BEAS-2B human bronchial epithelial cells. Environmental Toxicology, 38(2), 359–367. https://doi.org/10.1002/tox.23705
Jin, H., Ma, T., Sha, X., Liu, Z., Zhou, Y., Meng, X., Chen, Y., Han, X., & Ding, J. (2021). Polystyrene microplastics induced male reproductive toxicity in mice. Journal of Hazardous Materials, 401, 123430. https://doi.org/10.1016/j.jhazmat.2020.123430
Jin, H., Yan, M., Pan, C., Liu, Z., Sha, X., Jiang, C., Li, L., Pan, M., Li, D., Han, X., & Ding, J. (2022). Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Particle and Fibre Toxicology, 19(1), 13. https://doi.org/10.1186/s12989-022-00453-2
Jin, Y., Lu, L., Tu, W., Luo, T., & Fu, Z. (2019). Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Science of the Total Environment, 649, 308–317. https://doi.org/10.1016/j.scitotenv.2018.08.353
Johnson, L., Thompson, D. L., & Varner, D. D. (2008). Role of Sertoli cell number and function on regulation of spermatogenesis. Animal Reproduction Science, 105(1–2), 23–51. https://doi.org/10.1016/j.anireprosci.2007.11.029
Kopeina, G. S., & Zhivotovsky, B. (2022). Programmed cell death: Past, present and future. Biochemical and Biophysical Research Communications, 633, 55–58. https://doi.org/10.1016/j.bbrc.2022.09.022
Koysombat, K., Dhillo, W. S., & Abbara, A. (2023). Assessing hypothalamic pituitary gonadal function in reproductive disorders. Clinical Science, 137(11), 863–879. https://doi.org/10.1042/CS20220146
Kutralam-Muniasamy, G., Shruti, V. C., & Pérez-Guevara, F. (2024). Microplastic contamination in commercially packaged edible seaweeds and exposure of the ethnic minority and local population in Mexico. Food Research International (Ottawa, Ont.), 176, 113840. https://doi.org/10.1016/j.foodres.2023.113840
Kwon, W., Kim, D., Kim, H.-Y., Jeong, S. W., Lee, S.-G., Kim, H.-C., Lee, Y.-J., Kwon, M. K., Hwang, J.-S., Han, J. E., Park, J.-K., Lee, S.-J., & Choi, S.-K. (2022). Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. The Science of the Total Environment, 807(Pt 2), 150817. https://doi.org/10.1016/j.scitotenv.2021.150817
Lan, Y., Hu, L., Feng, X., Wang, M., Yuan, H., & Xu, H. (2024). Synergistic effect of PS-MPs and Cd on male reproductive toxicity: Ferroptosis via Keap1-Nrf2 pathway. Journal of Hazardous Materials, 461, 132584. https://doi.org/10.1016/j.jhazmat.2023.132584
Landrigan, P. J., Raps, H., Cropper, M., Bald, C., Brunner, M., Canonizado, E. M., Charles, D., Chiles, T. C., Donohue, M. J., Enck, J., Fenichel, P., Fleming, L. E., Ferrier-Pages, C., Fordham, R., Gozt, A., Griffin, C., Hahn, M. E., Haryanto, B., Hixson, R., & Dunlop, S. (2023). The minderoo-monaco commission on plastics and human health. Annals of Global Health, 89(1), 23. https://doi.org/10.5334/aogh.4056
Lee, H.-S., Amarakoon, D., Wei, C.-I., Choi, K. Y., Smolensky, D., & Lee, S.-H. (2021). Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 154, 112356. https://doi.org/10.1016/j.fct.2021.112356
Leslie, H. A., van Velzen, M. J. M., Brandsma, S. H., Vethaak, A. D., Garcia-Vallejo, J. J., & Lamoree, M. H. (2022). Discovery and quantification of plastic particle pollution in human blood. Environment International, 163, 107199. https://doi.org/10.1016/j.envint.2022.107199
Li, D., Sun, W., Jiang, X., Yu, Z., Xia, Y., Cheng, S., Mao, L., Luo, S., Tang, S., Xu, S., Zou, Z., Chen, C., Qiu, J., & Zhou, L. (2022a). Polystyrene nanoparticles enhance the adverse effects of di-(2-ethylhexyl) phthalate on male reproductive system in mice. Ecotoxicology and Environmental Safety, 245, 114104. https://doi.org/10.1016/j.ecoenv.2022.114104
Li, S., Liu, L., Luo, G., Yuan, Y., Hu, D., & Xiao, F. (2023a). The crosstalk between M1 macrophage polarization and energy metabolism disorder contributes to polystyrene nanoplastics-triggered testicular inflammation. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 180, 114002. https://doi.org/10.1016/j.fct.2023.114002
Li, S., Wang, Q., Yu, H., Yang, L., Sun, Y., Xu, N., Wang, N., Lei, Z., Hou, J., Jin, Y., Zhang, H., Li, L., Xu, F., & Zhang, L. (2021). Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environmental Science and Pollution Research International, 28(35), 47921–47931. https://doi.org/10.1007/s11356-021-13911-9
Li, X., Feng, L., Kuang, Q., Wang, X., Yang, J., Niu, X., Gao, L., Huang, L., Luo, P., & Li, L. (2024). Microplastics cause hepatotoxicity in diabetic mice by disrupting glucolipid metabolism via PP2A/AMPK/HNF4A and promoting fibrosis via the Wnt/β-catenin pathway. Environmental Toxicology, 39(2), 1018–1030. https://doi.org/10.1002/tox.24034
Li, Y., Yang, G., Wang, J., Lu, L., Li, X., Zheng, Y., Zhang, Z., & Ru, S. (2022b). Microplastics increase the accumulation of phenanthrene in the ovaries of marine medaka (Oryzias melastigma) and its transgenerational toxicity. Journal of Hazardous Materials, 424(Pt D), 127754. https://doi.org/10.1016/j.jhazmat.2021.127754
Li, Z.-F., Qi, H.-Y., Wang, J.-M., Zhao, Z., Tan, F.-Q., & Yang, W.-X. (2023b). mTORC1/rpS6 and mTORC2/PKC regulate spermatogenesis through Arp3-mediated actin microfilament organization in Eriocheir sinensis. Cell and Tissue Research, 393(3), 559–575. https://doi.org/10.1007/s00441-023-03795-1
Li, Z., Zhu, S., Liu, Q., Wei, J., Jin, Y., Wang, X., & Zhang, L. (2020). Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environmental Pollution, 265(Pt A), 115025. https://doi.org/10.1016/j.envpol.2020.115025
Liu, H., Jin, H., Pan, C., Chen, Y., Li, D., Ding, J., & Han, X. (2023a). Co-exposure to polystyrene microplastics and microcystin-LR aggravated male reproductive toxicity in mice. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 181, 114104. https://doi.org/10.1016/j.fct.2023.114104
Liu, M., Liu, J., Xiong, F., Xu, K., Pu, Y., Huang, J., Zhang, J., Pu, Y., Sun, R., & Cheng, K. (2023b). Research advances of microplastics and potential health risks of microplastics on terrestrial higher mammals: A bibliometric analysis and literature review. Environmental Geochemistry and Health, 45(6), 2803–2838. https://doi.org/10.1007/s10653-022-01458-8
Liu, S., Guo, J., Liu, X., Yang, R., Wang, H., Sun, Y., Chen, B., & Dong, R. (2023c). Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. The Science of the Total Environment, 854, 158699. https://doi.org/10.1016/j.scitotenv.2022.158699
Liu, T., Hou, B., Wang, Z., & Yang, Y. (2022a). Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicology and Environmental Safety, 237, 113520. https://doi.org/10.1016/j.ecoenv.2022.113520
Liu, T., Hou, B., Zhang, Y., & Wang, Z. (2022). Determination of biological and molecular attributes related to polystyrene microplastic-induced reproductive toxicity and its reversibility in male mice. International Journal of Environmental Research and Public Health, 19(21), 14093. https://doi.org/10.3390/ijerph192114093
Liu, W., Zhang, B., Yao, Q., Feng, X., Shen, T., Guo, P., Wang, P., Bai, Y., Li, B., Wang, P., Li, R., Qu, Z., & Liu, N. (2023d). Toxicological effects of micro/nano-plastics on mouse/rat models: A systematic review and meta-analysis. Frontiers in Public Health, 11, 1103289. https://doi.org/10.3389/fpubh.2023.1103289
Lu, C., Liang, Y., Cheng, Y., Peng, C., Sun, Y., Liu, K., Li, Y., Lou, Y., Jiang, X., Zhang, A., Liu, J., Cao, J., & Han, F. (2023a). Microplastics cause reproductive toxicity in male mice through inducing apoptosis of spermatogenic cells via p53 signaling. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 179, 113970. https://doi.org/10.1016/j.fct.2023.113970
Lu, H., Hou, L., Zhang, Y., Guo, T., Wang, Y., & Xing, M. (2023b). Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environmental Toxicology. https://doi.org/10.1002/tox.24068
Lui, W.-Y., Lee, W. M., & Cheng, C. Y. (2001). Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on Occludin, Zonula Occludens-1, and Claudin-111. Endocrinology, 142(5), 1865–1877. https://doi.org/10.1210/endo.142.5.8116
Luo, T., Zhang, Y., Wang, C., Wang, X., Zhou, J., Shen, M., Zhao, Y., Fu, Z., & Jin, Y. (2019). Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environmental Pollution, 255, 113122. https://doi.org/10.1016/j.envpol.2019.113122
Ma, T., Liu, X., Xiong, T., Li, H., Zhou, Y., & Liang, J. (2023). Polystyrene nanoplastics aggravated dibutyl phthalate-induced blood-testis barrier dysfunction via suppressing autophagy in male mice. Ecotoxicology and Environmental Safety, 264, 115403. https://doi.org/10.1016/j.ecoenv.2023.115403
Martel, J., Chang, S.-H., Ko, Y.-F., Hwang, T.-L., Young, J. D., & Ojcius, D. M. (2022). Gut barrier disruption and chronic disease. Trends in Endocrinology and Metabolism: TEM, 33(4), 247–265. https://doi.org/10.1016/j.tem.2022.01.002
Milne, M. H., De Frond, H., Rochman, C. M., Mallos, N. J., Leonard, G. H., & Baechler, B. R. (2023). Exposure of U.S. adults to microplastics from commonly-consumed proteins. Environmental Pollution, 343, 123233. https://doi.org/10.1016/j.envpol.2023.123233
Mizushima, N., & Komatsu, M. (2011). Autophagy: Renovation of cells and tissues. Cell, 147(4), 728–741. https://doi.org/10.1016/j.cell.2011.10.026
Mohamed Nor, N. H., Kooi, M., Diepens, N. J., & Koelmans, A. A. (2021). Lifetime accumulation of microplastic in children and adults. Environmental Science & Technology, 55(8), 5084–5096. https://doi.org/10.1021/acs.est.0c07384
Moita Neto, J. M., & da Silva, E. A. (2023). Sources of microplastic generation in the environment. International Journal of Environmental Research and Public Health, 20(13), 6202. https://doi.org/10.3390/ijerph20136202
Monzel, A. S., Enríquez, J. A., & Picard, M. (2023). Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction. Nature Metabolism, 5(4), 546–562. https://doi.org/10.1038/s42255-023-00783-1
Mruk, D. D., & Cheng, C. Y. (2015). The mammalian blood-testis barrier: Its biology and regulation. Endocrine Reviews, 36(5), 564–591. https://doi.org/10.1210/er.2014-1101
O’Donnell, L., Smith, L. B., & Rebourcet, D. (2022). Sertoli cells as key drivers of testis function. Seminars in Cell & Developmental Biology, 121, 2–9. https://doi.org/10.1016/j.semcdb.2021.06.016
Oduwole, O. O., Huhtaniemi, I. T., & Misrahi, M. (2021). The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. International Journal of Molecular Sciences, 22(23), 12735. https://doi.org/10.3390/ijms222312735
Ojeda, M., Rimondino, G. N., Fraysse, C. P., Cossi, P. F., Boy, C. C., & Pérez, A. F. (2023). Microplastic ingestion in key fish species of food webs in the Southwest Atlantic (Marine protected area Namuncurá/Burdwood Bank). Aquatic Toxicology, 267, 106827. https://doi.org/10.1016/j.aquatox.2023.106827
Oyola, M. G., & Handa, R. J. (2017). Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress, 20(5), 476–494. https://doi.org/10.1080/10253890.2017.1369523
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., & Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Pan, C., Wu, Y., Hu, S., Li, K., Liu, X., Shi, Y., Lin, W., Wang, X., Shi, Y., Xu, Z., Wang, H., & Chen, H. (2023). Polystyrene microplastics arrest skeletal growth in puberty through accelerating osteoblast senescence. Environmental Pollution, 322, 121217. https://doi.org/10.1016/j.envpol.2023.121217
Parzych, K. R., & Klionsky, D. J. (2014). An overview of autophagy: Morphology, mechanism, and regulation. Antioxidants & Redox Signaling, 20(3), 460–473. https://doi.org/10.1089/ars.2013.5371
Peng, W., Kepsch, A., Kracht, T. O., Hasan, H., Wijayarathna, R., Wahle, E., Pleuger, C., Bhushan, S., Günther, S., Kauerhof, A. C., Planinić, A., Fietz, D., Schuppe, H.-C., Wygrecka, M., Loveland, K. L., Ježek, D., Meinhardt, A., Hedger, M. P., & Fijak, M. (2022). Activin A and CCR2 regulate macrophage function in testicular fibrosis caused by experimental autoimmune orchitis. Cellular and Molecular Life Sciences: CMLS, 79(12), 602. https://doi.org/10.1007/s00018-022-04632-4
Prüst, M., Meijer, J., & Westerink, R. H. S. (2020). The plastic brain: Neurotoxicity of micro- and nanoplastics. Particle and Fibre Toxicology, 17(1), 24. https://doi.org/10.1186/s12989-020-00358-y
Ragusa, A., Matta, M., Cristiano, L., Matassa, R., Battaglione, E., Svelato, A., De Luca, C., D’Avino, S., Gulotta, A., Rongioletti, M. C. A., Catalano, P., Santacroce, C., Notarstefano, V., Carnevali, O., Giorgini, E., Vizza, E., Familiari, G., & Nottola, S. A. (2022). Deeply in plasticenta: Presence of microplastics in the intracellular compartment of human placentas. International Journal of Environmental Research and Public Health, 19(18), 11593. https://doi.org/10.3390/ijerph191811593
Ragusa, A., Notarstefano, V., Svelato, A., Belloni, A., Gioacchini, G., Blondeel, C., Zucchelli, E., De Luca, C., D’Avino, S., Gulotta, A., Carnevali, O., & Giorgini, E. (2022). Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers, 14(13), 2700. https://doi.org/10.3390/polym14132700
Ren, J., Bi, Y., Sowers, J. R., Hetz, C., & Zhang, Y. (2021). Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nature Reviews. Cardiology, 18(7), 499–521. https://doi.org/10.1038/s41569-021-00511-w
Rizwan, A., Ijaz, M. U., Hamza, A., & Anwar, H. (2023). Attenuative effect of astilbin on polystyrene microplastics induced testicular damage: Biochemical, spermatological and histopathological-based evidences. Toxicology and Applied Pharmacology, 471, 116559. https://doi.org/10.1016/j.taap.2023.116559
Schmid, C., Cozzarini, L., & Zambello, E. (2021). Microplastic’s story. Marine Pollution Bulletin, 162, 111820. https://doi.org/10.1016/j.marpolbul.2020.111820
Senathirajah, K., Attwood, S., Bhagwat, G., Carbery, M., Wilson, S., & Palanisami, T. (2021). Estimation of the mass of microplastics ingested—a pivotal first step towards human health risk assessment. Journal of Hazardous Materials, 404, 124004. https://doi.org/10.1016/j.jhazmat.2020.124004
Shi, C., Han, X., Guo, W., Wu, Q., Yang, X., Wang, Y., Tang, G., Wang, S., Wang, Z., Liu, Y., Li, M., Lv, M., Guo, Y., Li, Z., Li, J., Shi, J., Qu, G., & Jiang, G. (2022). Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environment International, 164, 107273. https://doi.org/10.1016/j.envint.2022.107273
Shruti, V. C., Kutralam-Muniasamy, G., Pérez-Guevara, F., Roy, P. D., & Elizalde-Martínez, I. (2022). Free, but not microplastic-free, drinking water from outdoor refill kiosks: A challenge and a wake-up call for urban management. Environmental Pollution, 309, 119800. https://doi.org/10.1016/j.envpol.2022.119800
Song, X., Du, L., Sima, L., Zou, D., & Qiu, X. (2023). Effects of micro(nano)plastics on the reproductive system: A review. Chemosphere, 336, 139138. https://doi.org/10.1016/j.chemosphere.2023.139138
Sripada, K., Wierzbicka, A., Abass, K., Grimalt, J. O., Erbe, A., Röllin, H. B., Weihe, P., Díaz, G. J., Singh, R. R., Visnes, T., Rautio, A., Odland, J. Ø., & Wagner, M. (2022). A children’s health perspective on nano- and microplastics. Environmental Health Perspectives, 130(1), 15001. https://doi.org/10.1289/EHP9086
Sun, J., Fang, R., Wang, H., Xu, D.-X., Yang, J., Huang, X., Cozzolino, D., Fang, M., & Huang, Y. (2022). A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environment International, 158, 106941. https://doi.org/10.1016/j.envint.2021.106941
Ursini, F., & Maiorino, M. (2020). Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radical Biology & Medicine, 152, 175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
Vasudevan, S. O., Behl, B., & Rathinam, V. A. (2023). Pyroptosis-induced inflammation and tissue damage. Seminars in Immunology, 69, 101781. https://doi.org/10.1016/j.smim.2023.101781
Wang, F., Zhang, Q., Cui, J., Bao, B., Deng, X., Liu, L., & Guo, M.-Y. (2023). Polystyrene microplastics induce endoplasmic reticulum stress, apoptosis and inflammation by disrupting the gut microbiota in carp intestines. Environmental Pollution, 323, 121233. https://doi.org/10.1016/j.envpol.2023.121233
Wang, J., Li, Y., Lu, L., Zheng, M., Zhang, X., Tian, H., Wang, W., & Ru, S. (2019). Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environmental Pollution, 254(Pt B), 113024. https://doi.org/10.1016/j.envpol.2019.113024
Wang, J.-M., Li, Z.-F., Qi, H.-Y., Zhao, Z., & Yang, W.-X. (2023b). Es-Arp3 and es-Eps8 regulate spermatogenesis via microfilaments in the seminiferous tubule of Eriocheir sinensis. Tissue & Cell, 81, 102028. https://doi.org/10.1016/j.tice.2023.102028
Wang, L., Zhang, X., Xu, M., Zheng, G., Chen, J., Li, S., Cui, J., & Zhang, S. (2023). Implication of ferroptosis in hepatic toxicity upon single or combined exposure to polystyrene microplastics and cadmium. Environmental Pollution, 334, 122250. https://doi.org/10.1016/j.envpol.2023.122250
Wang, S., Wu, H., Shi, X., Wang, Y., & Xu, S. (2023d). Polystyrene microplastics with different sizes induce the apoptosis and necroptosis in liver through the PTEN/PI3K/AKT/autophagy axis. The Science of the Total Environment, 899, 165461. https://doi.org/10.1016/j.scitotenv.2023.165461
Wang, X., Zhang, X., Sun, K., Wang, S., & Gong, D. (2022a). Polystyrene microplastics induce apoptosis and necroptosis in swine testis cells via ROS/MAPK/HIF1α pathway. Environmental Toxicology, 37(10), 2483–2492. https://doi.org/10.1002/tox.23611
Wang, Y.-L., Lee, Y.-H., Hsu, Y.-H., Chiu, I.-J., Huang, C.C.-Y., Huang, C.-C., Chia, Z.-C., Lee, C.-P., Lin, Y.-F., & Chiu, H.-W. (2021). The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environmental Health Perspectives, 129(5), 57003. https://doi.org/10.1289/EHP7612
Wang, Y., Zheng, L., Shang, W., Yang, Z., Li, T., Liu, F., Shao, W., Lv, L., Chai, L., Qu, L., Xu, Q., Du, J., Liang, X., Zeng, J., & Jia, J. (2022b). Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death and Differentiation, 29(11), 2190–2202. https://doi.org/10.1038/s41418-022-01008-w
Wei, J., Wang, X., Liu, Q., Zhou, N., Zhu, S., Li, Z., Li, X., Yao, J., & Zhang, L. (2021a). The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environmental Toxicology, 36(5), 935–944. https://doi.org/10.1002/tox.23095
Wei, Y., Zhou, Y., Long, C., Wu, H., Hong, Y., Fu, Y., Wang, J., Wu, Y., Shen, L., & Wei, G. (2021). Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2. Environmental Pollution, 289, 117904. https://doi.org/10.1016/j.envpol.2021.117904
Wei, Z., Wang, Y., Wang, S., Xie, J., Han, Q., & Chen, M. (2022). Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology, 465, 153059. https://doi.org/10.1016/j.tox.2021.153059
Weis, J. S., & Alava, J. J. (2023). (Micro) Plastics are toxic pollutants. Toxics, 11(11), 935. https://doi.org/10.3390/toxics11110935
Wen, Q., Tang, E. I., Gao, Y., Jesus, T. T., Chu, D. S., Lee, W. M., Wong, C. K. C., Liu, Y.-X., Xiao, X., Silvestrini, B., & Cheng, C. Y. (2018a). Signaling pathways regulating blood-tissue barriers—lesson from the testis. Biochimica Et Biophysica Acta. Biomembranes, 1860(1), 141–153. https://doi.org/10.1016/j.bbamem.2017.04.020
Wen, Q., Tang, E. I., Li, N., Mruk, D. D., Lee, W. M., Silvestrini, B., & Cheng, C. Y. (2018). Regulation of blood-testis barrier (BTB) dynamics, role of actin-, and microtubule-based cytoskeletons. Methods in Molecular Biology, 1748, 229–243. https://doi.org/10.1007/978-1-4939-7698-0_16
Wen, S., Chen, Y., Tang, Y., Zhao, Y., Liu, S., You, T., & Xu, H. (2023). Male reproductive toxicity of polystyrene microplastics: Study on the endoplasmic reticulum stress signaling pathway. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 172, 113577. https://doi.org/10.1016/j.fct.2022.113577
Wen, S., Zhao, Y., Liu, S., Yuan, H., You, T., & Xu, H. (2022). Microplastics-perturbed gut microbiota triggered the testicular disorder in male mice: Via fecal microbiota transplantation. Environmental Pollution, 309, 119789. https://doi.org/10.1016/j.envpol.2022.119789
Wu, D., Feng, Y., Wang, R., Jiang, J., Guan, Q., Yang, X., Wei, H., Xia, Y., & Luo, Y. (2023a). Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. Journal of Advanced Research, 49, 141–150. https://doi.org/10.1016/j.jare.2022.09.004
Wu, D., Zhang, M., Bao, T. T., & Lan, H. (2023b). Long-term exposure to polystyrene microplastics triggers premature testicular aging. Particle and Fibre Toxicology, 20(1), 35. https://doi.org/10.1186/s12989-023-00546-6
Wu, P., Huang, J., Zheng, Y., Yang, Y., Zhang, Y., He, F., Chen, H., Quan, G., Yan, J., Li, T., & Gao, B. (2019). Environmental occurrences, fate, and impacts of microplastics. Ecotoxicology and Environmental Safety, 184, 109612. https://doi.org/10.1016/j.ecoenv.2019.109612
Xia, W., Wong, E. W. P., Mruk, D. D., & Cheng, C. Y. (2009). TGF-β3 and TNFα perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Developmental Biology, 327(1), 48–61. https://doi.org/10.1016/j.ydbio.2008.11.028
Xie, Q., Kang, Y., Zhang, C., Xie, Y., Wang, C., Liu, J., Yu, C., Zhao, H., & Huang, D. (2022). The role of Kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Frontiers in Endocrinology, 13, 925206. https://doi.org/10.3389/fendo.2022.925206
Xie, X., Deng, T., Duan, J., Xie, J., Yuan, J., & Chen, M. (2020). Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicology and Environmental Safety, 190, 110133. https://doi.org/10.1016/j.ecoenv.2019.110133
Xu, S., Ma, J., Ji, R., Pan, K., & Miao, A.-J. (2020). Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. The Science of the Total Environment, 703, 134699. https://doi.org/10.1016/j.scitotenv.2019.134699
Xu, Y., Jiang, S., Hu, Y., Zhang, Q., & Su, W. (2022). TGF-β3 induces lactate production in Sertoli cell through inhibiting the Notch pathway. Andrology, 10(8), 1644–1659. https://doi.org/10.1111/andr.13288
Yamauchi, S., Yamamoto, K., & Ogawa, K. (2022). Testicular macrophages produce progesterone de novo promoted by cAMP and inhibited by M1 polarization inducers. Biomedicines, 10(2), 487. https://doi.org/10.3390/biomedicines10020487
Yang, S., & Lian, G. (2020). ROS and diseases: Role in metabolism and energy supply. Molecular and Cellular Biochemistry, 467(1–2), 1–12. https://doi.org/10.1007/s11010-019-03667-9
Yang, Z. S., Bai, Y. L., Jin, C. H., Na, J., Zhang, R., Gao, Y., Pan, G. W., Yan, L. J., & Sun, W. (2022). Evidence on invasion of blood, adipose tissues, nervous system and reproductive system of mice after a single oral exposure: Nanoplastics versus microplastics. Biomedical and Environmental Sciences: BES, 35(11), 1025–1037. https://doi.org/10.3967/bes2022.131
Yao, F. C., Gu, Y., Jiang, T., Wang, P. F., Song, F. B., Zhou, Z., Sun, J. L., & Luo, J. (2023). The involvement of oxidative stress mediated endoplasmic reticulum pathway in apoptosis of Golden Pompano (Trachinotus blochii) liver under PS-MPs stress. Ecotoxicology and Environmental Safety, 249, 114440. https://doi.org/10.1016/j.ecoenv.2022.114440
Yin, K., Wang, D., Zhang, Y., Lu, H., Hou, L., Guo, T., Zhao, H., & Xing, M. (2023). Polystyrene microplastics promote liver inflammation by inducing the formation of macrophages extracellular traps. Journal of Hazardous Materials, 452, 131236. https://doi.org/10.1016/j.jhazmat.2023.131236
Zeng, G., Li, J., Wang, Y., Su, J., Lu, Z., Zhang, F., & Ding, W. (2024). Polystyrene microplastic-induced oxidative stress triggers intestinal barrier dysfunction via the NF-κB/NLRP3/IL-1β/MCLK pathway. Environmental Pollution, 345, 123473. https://doi.org/10.1016/j.envpol.2024.123473
Zhang, D., Yu, Y., Duan, T., & Zhou, Q. (2022a). The role of macrophages in reproductive-related diseases. Heliyon, 8(11), e11686. https://doi.org/10.1016/j.heliyon.2022.e11686
Zhang, Q., Xia, W., Zhou, X., Yang, C., Lu, Z., Wu, S., Lu, X., Yang, J., & Jin, C. (2023). PS-MPs or their co-exposure with cadmium impair male reproductive function through the miR-199a-5p/HIF-1α-mediated ferroptosis pathway. Environmental Pollution, 339, 122723. https://doi.org/10.1016/j.envpol.2023.122723
Zhang, X., He, Y., Xie, Z., Peng, S., Xie, C., Wang, H., Liu, L., Kang, J., Yuan, H., & Liu, Y. (2022b). Effect of microplastics on nasal and gut microbiota of high-exposure population: Protocol for an observational cross-sectional study. Medicine, 101(34), e30215. https://doi.org/10.1097/MD.0000000000030215
Zhang, Y., Hou, B., Liu, T., Wu, Y., & Wang, Z. (2023b). Probiotics improve polystyrene microplastics-induced male reproductive toxicity in mice by alleviating inflammatory response. Ecotoxicology and Environmental Safety, 263, 115248. https://doi.org/10.1016/j.ecoenv.2023.115248
Zhang, Y., Yin, K., Wang, D., Wang, Y., Lu, H., Zhao, H., & Xing, M. (2022c). Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. The Science of the Total Environment, 840, 156727. https://doi.org/10.1016/j.scitotenv.2022.156727
Zhao, Q., Zhu, L., Weng, J., Jin, Z., Cao, Y., Jiang, H., & Zhang, Z. (2023). Detection and characterization of microplastics in the human testis and semen. The Science of the Total Environment, 877, 162713. https://doi.org/10.1016/j.scitotenv.2023.162713
Zheng, S., Jiang, L., & Qiu, L. (2022). The effects of fine particulate matter on the blood-testis barrier and its potential mechanisms. Reviews on Environmental Health. https://doi.org/10.1515/reveh-2022-0204
Zhou, L., Yu, Z., Xia, Y., Cheng, S., Gao, J., Sun, W., Jiang, X., Zhang, J., Mao, L., Qin, X., Zou, Z., Qiu, J., & Chen, C. (2022). Repression of autophagy leads to acrosome biogenesis disruption caused by a sub-chronic oral administration of polystyrene nanoparticles. Environment International, 163, 107220. https://doi.org/10.1016/j.envint.2022.107220
Zhou, X., Xiao, C., Li, X., Chen, T., & Yang, X. (2023). Microplastics in coastal blue carbon ecosystems: A global Meta-analysis of its distribution, driving mechanisms, and potential risks. The Science of the Total Environment, 878, 163048. https://doi.org/10.1016/j.scitotenv.2023.163048
Zorov, D. B., Juhaszova, M., & Sollott, S. J. (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews, 94(3), 909–950. https://doi.org/10.1152/physrev.00026.2013
Acknowledgements
The authors acknowledge that there are no conflicts of interest or financial disclosures related to this paper.
Funding
This review was supported by the National Natural Science Foundation of China (No. 81302452), Natural Science Foundation of Jiangsu Province (BK20221374), Major Projects of Natural Sciences of University in Jiangsu Province of China (18KJB330004), Basic Scientific Research Program of Nantong City (JC2019021, JC2020032), Scientific Research Starting Foundation for The Doctoral Researcher of Nantong University (No. 14B14) and Innovation project of graduate student scientific research in Jiangsu province (KYCX22_3377).
Author information
Authors and Affiliations
Contributions
J.C. reviewed the literature and wrote the manuscript. Z.H. help to make tables and figures. L.Q. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors acknowledge that there are no conflicts of interest or financial disclosures related to this paper.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, J., Shu, Z. & Qiu, L. Adverse effects and potential mechanisms of polystyrene microplastics (PS-MPs) on the blood-testis barrier. Environ Geochem Health 46, 238 (2024). https://doi.org/10.1007/s10653-024-02033-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-024-02033-z