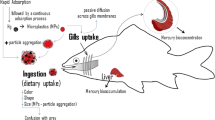
Abstract
Microplastics can accumulate residual drugs and heavy metals in the environment and accumulate through the layers of the food chain, ultimately causing harm to human health. The pollution of microplastics in the freshwater environment is becoming more and more serious, which directly affects the safety of aquatic organisms. This experiment studied the effects of single and composite microplastics and Cd on the tissue damage, antioxidant, and immune response of juvenile Channa argus. Microplastics with different diameters of 80 nm, 0.5 μm (200 μg/L), and Cd (50 μg/L) were used for exposure, and four sampling points were set for 24 h, 48 h, 96 h, and clear 48 h. Under different treatments, a certain degree of gill tissue damage can be found in 96 h. Microplastics and Cd can cause oxidative stress and affect the antioxidant status, and the impact of 0.5-μm microplastics is stronger than that of 80-nm microplastics. There is an antagonistic effect between the two microplastics and Cd during compound exposure, but the activity of CAT shows a synergistic effect. Microplastics and Cd affect the expression of immune-related genes to varying degrees. When exposed together, the expression of HSP70 gene all showed mutual antagonism, while the expression of IL-1β gene was different. The expression of the MT gene can infer the ability of microplastics to accumulate Cd, and microplastics with a small diameter of 80 nm have stronger enrichment capabilities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At the beginning of the twentieth century, modern plastic products were mass-produced and applied due to their portability, durability, and low-cost characteristics [1, 2]. At present, the application of plastics in human society involves all aspects, from industrial production to animal husbandry, from high-tech products to daily life. However, humans have gradually discovered that plastic waste degraded slowly in the environment and posed a serious threat to the stability of the ecological environment [3]. Once plastic materials were discarded in the environment, they would gradually be broken down into smaller plastics. Then, it would be broken into smaller pieces under the influence of physical and chemical factors as well as ultraviolet rays, and finally become microplastics (MPs) with a particle size of ≤ 5 mm [4]. Since the 1960s, plastic production has grown rapidly, reaching 335 million tons in 2016 [5]. The generation of plastic waste has also increased proportionally, and poor management causes most waste to end up in landfills or dispersed in the form of marine waste [6]. An estimated 1.15–2.41 million tons of plastic are discharged into the world’s oceans from rivers every year [7]. When plastic waste enters the ocean, it poses a serious threat to the marine environment, not only affecting the photosynthesis of certain marine plants [8] and the growth and development of certain marine organisms [9] but also leading to even severe malnutrition and death of certain marine organisms [10]. As plastics decompose into microplastics in the environment, the reduction in size can substantially increase the bioavailability of plastics for various aquatic organisms with different feeding habits, especially for filter-feeder animals.
Microplastics can be found in almost all parts of ecosystems, such as soil, oceans, freshwater rivers, lakes, and even in polar ice [11, 12]. Some studies have shown that the metal content of microplastics in different natural environments was at a high level in some times. The reason was that in the production process, these metals were incorporated into plastic in the form of additives, which can enhance the properties of the product [13]. Fe, Cu, Cd, or Mn were the most common metals in microplastics from sediment and seawater of the British, Egyptian, the Persian Gulf, and Iran [14, 15]. The presence of zinc and copper, which are toxic at high concentrations, and other more dangerous metals such as lead or cadmium, has also been demonstrated at various points along the coast of China [16, 17]. Compared with larger forms of plastics, microplastics have the characteristics of large specific surface area, strong hydrophobicity, and easy interaction with microorganisms. These physicochemical properties of microplastics enhance the concentration of certain pollutants, such as persistent organic pollutants and heavy metals [18, 19], and as a carrier to make it easier to accumulate in the body. Recent publications revealed the adverse impacts of ingestion of MPs by mammals, fish, and invertebrates [20, 21]. Studies have shown that microplastics can have a more toxic impact than warming on the actions, predation, and metabolism of S. aequifasciatus [22, 23].
Cadmium, by virtue of its toxicity, persistence, and bioaccumulation, appears on the “black list” of all the international conventions aimed at protecting the aquatic environment from pollution. There is no doubt that cadmium is both persistent and toxic to aquatic life at relatively low concentrations [24]. When fish are exposed to Cd, there will be changes in behavior and metabolism [25, 26]. Oxidative stress and immunosuppression [27, 28], disruption of blood biochemical homeostasis [29], changes in some growth and reproductive parameters [30, 31], and genetic damage were also reported in fish exposed to Cd. Environmental pollutants, including microplastics and heavy metals, often appear in aquatic ecosystems, and these pollutants could cause single or multiple effects on aquatic organisms. At present, most research was concentrated in coastal areas, and there was few related research on the freshwater environment. As the freshwater environment was more closely related to human life, its pollution was also more serious. Guangdong Province was the seat of the Pearl River system, one of the three major freshwater river systems in China. Special geographical conditions and environmental factors have made the aquaculture industry develop rapidly. Once the water environment was polluted, it would directly affect the safety of the breeding industry and threaten human health.
Therefore, investigating the impact of two or more environmental pollutants, including microplastics, on the health of aquatic organisms is one of the main goals of future microplastics research. The toxicity of microplastics or cadmium alone has been established; however, the effects of fish exposed to a combination of microplastics and cadmium are still relatively lacking [32]. Consider the potential risks of microplastics, cadmium, and susceptibility at early life stages. The aim of the present study was to evaluate the effects of microplastics and cadmium alone and in combination on the influence of tissue damage, oxygen stress, and immune response using the Channa argus as bio-indicator species.
Materials and Methods
Chemical Materials
Virgin polystyrene-MPs (PS-MPs; 80 nm and 0.5-μm diameter, spherical) were used as the MP model in this study. This kind of engineered MP was also used in other toxicity studies on MPs [33,34,35]. The PS-MPs were purchased from Big Goose (Tianjin) Technology (www.tjdaekj.com). The cadmium chloride (CdCl2; analytical grade; 98% purity) was purchased from Macklin Biochemical Technology (Shanghai, China) (www.macklin.cn). Fifty milligrams per liter of Cd stock solution in ultrapure water was prepared.
Ethics Statement
The animals used in the present study were cultured and sacrificed following the terms of use of the animals Management System of Laboratory Animal Center of South China Agricultural University.
Sample Fish
Juvenile Channa argus (3.63 ± 0.98 g) were purchased from a local fish farm in Shunde, Guangdong Province (July 2020). The fish were packed in aerated plastic bags and transported to the aquaculture circulating aquaculture system of the School of Animal Science of South China Agricultural University. These fish were temporarily kept in a circulating water tank with a capacity of 150 L (60 cm × 50 cm × 50 cm). The test fish were kept temporarily in dechlorinated tap water and oxygenated conditions for 2 weeks. During the acclimatization, fish were fed twice a day at 9:00 and 17:00 at a daily ratio of 5% of weight. During the acclimatization, the water conditions in the tanks were as follows: water temperature = 25.0 ± 2 °C, pH = 7.0 ± 0.5, and 24 h of aeration.
Experimental Protocol
Stop feeding for 24 h before the start of the experiment, and then randomly assign the Juvenile Channa argus to 18 glass tanks (3L), with 5 individuals per tank. The glass tanks were further divided into six groups, with three tanks, i.e., 15 individuals for each treatment. Group (C) was maintained in tap water as a control group. Specimens of groups M 0.08 and M 0.5 were exposed to 80 nm and 0.5-μm PS-MPs at a concentration of 200 μg/L, respectively. Group (Cd) was exposed to 50 μg/L cadmium chloride. Group (Cd + M 0.08) was exposed to a combined dose of 50 μg/L Cd and 200 μg/L 80 nm PS-MPs. Group (Cd + M 0.5) was exposed to a combined dose of 50 μg/L Cd and 200 μg/L 0.5-μm PS-MPs. During the experiment, each glass tank did not perform aeration and water change to reduce the stress caused by external factors on the experimental fish and affect the experimental results. Three sampling time points were set during the experiment, namely 24 h, 48 h, and 96 h. At each sampling time, a fish was randomly selected from each glass tank for dissection and took the liver, gills, and intestines of each fish. The liver was placed in a sterile enzyme-free 2.0-mL centrifuge tube, placed in liquid nitrogen for 2 h, and then stored at − 80 °C for subsequent molecular experiments; the fish’s intestines were placed in a sterile enzyme-free sample 2.0 mL centrifuge stored in the tube at − 20 °C for the determination of the anti-oxidation and digestive enzyme activity of the tissue; and collected a part of the gills of the sample fish and store it in 4% paraformaldehyde for making paraffin sections. After the experiment, the remaining sample fish were transferred to a clean water body for a clearing experiment. After 48 h, samples of each tissue were collected again for subsequent analysis and comparison. The microplastic concentrations employed in the present study were determined according to the microplastic levels used in other toxicological studies [22, 23, 36, 37]. The cadmium concentration was selected according to the water environmental concentration [22, 23, 38].
Tissue Section Analysis
The sample was fixed with 4% paraformaldehyde. According to conventional tissue sectioning procedures, perform dehydration, transparency, paraffin embedding, sectioning, and H&E staining, to observe whether fish gills in different treatment groups were affected.
Biochemical Parameter Analysis
Collect the intestinal tissues of the sample fish at each time point, weigh them separately, and add 9 times the volume of homogenization medium (0.9% normal saline). Centrifuge for 10 min at 2500 r/min in a refrigerated centrifuge (4 °C), and take the supernatant for subsequent enzyme activity index determination. In addition, it was necessary to determine the protein content in the tissue sample before determining the enzyme activity-related indicators of the sample.
Antioxidant indicators included in this experiment: total antioxidant capacity (T-AOC), superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT) were assayed by using corresponding commercial kits (Nanjing Jiancheng, Bioengineering Institute, Nanjing, China). The assay is according to the steps and methods suggested by the kit manufacturer.
Gene Expression Assays
After the experiment, the collected liver tissue samples would be used to detect the expression of interleukin (IL-1β), heat shock protein (HSP70), metallothionein (MT), and other related genes. RNA from livers of exposed fish was extracted with TRIzol, and all centrifuge tubes and tips during the extraction process were processed by DEPC. RNA concentration and quality were determined with a micro-analysis analyzer.
Reverse transcription reactions were carried out on 10 ng of total RNA using an Evo M-MLV RT Kit with gDNA clean for qPCR II (Accurate Biotechnology, Hunan, China). RT-PCR assayed by SYBR Green Premix Pro Tap HS qPCR Kit (Accurate Biotechnology, Hunan, China). Refer to previous research [39]; the gene β-actin was used as a constitutive control to normalize all samples. The relative expression of the target gene was calculated by the 2−△△CT methods. The primer sequence design is shown (Table 1).
Statistical Analysis
Microsoft Office Excel, SPSS 20, and GraphPad Prism were used to conduct the statistical analysis and drawing. A one-way analysis of variance and Duncan method multiple comparisons was used to determine interspecies and spatial differences (P < 0.05) in the data.
Results
Tissue Section Analysis
Observing the gill tissue of the Channa argus in the control group at each time point (Fig. 1-A). The gill filament epithelial cells structure is intact; the secondary lamellae were evenly distributed and smoothly stretched, and various cells were not damaged or desquamated.
Gills of Channa argus in different treatment groups. Control group (A); M 0.08 group (B); M 0.5 group (C); cadmium group (D); Cd + M 0.08 group (E); Cd + M 0.5 group (F). 1 = 24 h; 2 = 48 h; 3 = 96 h; 4 = removal 48 h. Eh, Epithelial hyperplasia; Es, Epithelial swell; Bp, Basal proliferation; Ef, Epithelial fall; Lb, Lamellae bending
Compared with the control group, after 24 h of exposure, the gill tissue of each treatment group had no obvious lesions, and the structure was intact. After 48 h, some epithelial cell proliferation was found in the Cd + M 0.08 group (Fig. 1-E2); the secondary lamellae in the Cd + M 0.5 group (Fig. 1-F2) were enlarged, and the epithelial cell proliferation was obvious. After 96 h, each treatment group suffered different degrees of damage. In the M 0.08 group (Fig. 1-B3), secondary lamellae were enlarged, and epithelial cells proliferated; in the M 0.5 group (Fig. 1-C3), secondary lamellae were bent, and epithelial cells desquamated; the Cd group (Fig. 1-D3) was found to have epithelial cell proliferation and even shedding; the secondary lamellae in the Cd + M 0.08 group (Fig. 1-E3) were swollen more obvious, and the proportion increased; Cd + M 0.5 group (Fig. 1-F3) has secondary lamellae bending, and the base cell proliferation is obvious. Compared to 48 h, there was a certain degree of deterioration. After 48 h of removal, the condition of each treatment group was improved, the proliferation of epithelial cells was reduced, and the structure of the gill lamellae showed signs of recovery.
Antioxidant Status
The results show that the T-AOC content was affected by MPs and Cd (Fig. 2A). The T-AOC activity of 80 nm and Cd groups at each time point was significantly higher than that of the control group. When microplastics and cadmium co-exist, 80-nm microplastics promote the influence of cadmium on T-AOC, while 0.5-μm microplastics will be the opposite. However, the T-AOC of the two co-exposure groups both tended to increase over time.
The SOD activity was affected by MPs and Cd (Fig. 2B). The SOD activity increases with the diameter of the microplastics. At 24 and 48 h, the effect of the microplastics on SOD is significantly different from that of the control group. When microplastics and cadmium co-exist, there was an antagonistic effect, which reduced the impact on SOD. The SOD activity of the Cd + M 0.5 group was lower than that of the Cd + M 0.08 group at all time points.
The CAT activity was influenced by MPs and Cd and the interaction between the two stressors (Fig. 2C). Compared with the control group, the treatment group was significantly higher than the control group at almost all time points. The effect of the M 0.5 group on CAT activity was higher than that of the M 0.08 group. The effect of cadmium on CAT activity is higher than that of microplastics alone. Co-exposure will promote the influence of microplastics and Cd on CAT activity. The CAT activity of the Cd + M 0.5 group and Cd + M 0.08 group were higher than that of the microplastic and Cd treatment groups alone at each time point. The effect of each treatment group on CAT activity increased with time.
The MDA content was affected by 80 nm MPs and Cd (Fig. 2D). The 0.5-μm microplastic has little effect on MDA. When the microplastics and Cd were exposed together, the microplastics and Cd antagonize each other and reduce the impact on MDA. There was no trend of change in MDA content between each time point.
Real-time PCR Analysis
Relative Expression of IL-1β Gene
Under different treatment conditions and different sampling time points, the relative expression of IL-1β gene in liver tissue was shown in the figure (Fig. 3), where 24 h, 48 h, 96 h, and D-48 h represent exposure 24 h, 48 h, 96 h, and removal 48 h, respectively. The analysis found that the expression of IL-1β gene in the M 0.08 group, M 0.5 group, and Cd + M 0.5 group was significantly higher than that in the C, Cd, and Cd + M 0.08 group at 24-h exposure, while the IL-1β gene expression in the M 0.5 group. The expression of IL-1β gene was significantly higher than that of M 0.08 group, and the expression of IL-1β gene of Cd + M 0.5 group was significantly higher than that of M 0.5 group. The expression of IL-1β gene in the M 0.08 group, M 0.5 group, and Cd + M 0.5 group was significantly higher than that in the C, Cd, and Cd + M 0.08 group at 48-h exposure and IL-1β in the Cd + M 0.5 group. The gene expression level was significantly higher than that of the M 0.08 group and the M 0.5 group. Although the expression level of the M 0.5 group was higher than that of the M 0.08 group, there was no difference. The IL-1β gene expression in the M 0.5 group and Cd + M 0.5 group was significantly higher than that in the C group, M 0.08 group, Cd group, and Cd + M 0.08 group at 96-h exposure, and the IL-1β of the Cd + M 0.5 group. The expression of IL-1β gene was significantly higher than that of M 0.5 group; the expression of M 0.08 group was significantly higher than that of C group and Cd + M 0.08 group, and compared with the Cd group, although it was higher than the Cd group, there was no difference. After being cleared for 48 h, the expression of IL-1β gene in the M 0.08 group, M 0.5 group, and Cd + M 0.5 group was significantly higher than that in the C group, Cd group, and Cd + M 0.08 group, while the IL-1β in the M 0.5 group. The gene expression level was significantly higher than that of the M 0.08 group, and the IL-1β gene expression level of the Cd + M 0.5 group was significantly higher than that of the M 0.5 group. However, compared with the experimental period, the expression of the IL-1β gene in all treatment groups had a downward trend.
Relative Expression of HSP70 Gene
The expression of HSP70 gene in liver tissue is shown in the figure (Fig. 4). The analysis found that the expression of HSP70 gene in all treatment groups was significantly higher than that of the C group at 24-h exposure, while the expression of the M 0.5 group was significantly higher than that of other treatment groups. At 48 h of exposure, the expression of HSP70 gene in the M 0.5 group was significantly higher than that of the C group and Cd + M 0.08 group, while the expression of the M 0.08 group, Cd group, and Cd + M 0.5 group was higher than that of the C group and Cd + M 0.08 group, but there is no difference. At 96-h exposure, the expression of HSP70 gene in the M 0.05 group, Cd group, and Cd + M 0.5 group was significantly higher than that in the C group and Cd + M 0.08 group. After 48 h of clearance, there were differences in the expression of HSP70 gene between each treatment group and group C, but they were not significant.
Relative Expression of MT Gene
The expression of the MT gene in liver tissue is shown in the figure (Fig. 5). The analysis found that the expression of the MT gene in the Cd + M 0.08 group was significantly higher than that in the other treatment groups at 24-h exposure; the expression of the Cd + M 0.5 group was significantly higher than that of the C, M 0.08, and M 0.5 groups. At 48 h of exposure, the expression of MT gene in all treatment groups was significantly higher than that in group C; the expression of MT gene in Cd group, Cd + M 0.08 group, and Cd + M 0.5 group was significantly higher than that of M 0.08 group and M 0.5. The expression level of Cd + M 0.08 group was significantly higher than that of Cd group. The expression of the MT gene in Cd group, Cd + M 0.08 group, and Cd + M 0.5 group was significantly higher than that of C group at 96-h exposure, while the expression of the Cd group was significantly higher than other treatment groups. After 48 h of removal, the expression of MT gene in the Cd group, Cd + M 0.08 group, and Cd + M 0.5 group was significantly higher than that in the C and M 0.08 group, but compared with the M 0.5 group, although there was a difference, there was no difference.
Discussion
Damage to Gill Tissue Under Different Exposure Conditions
From the histopathological results, it could be found that both microplastics and Cd would cause different degrees of damage to the gill tissue. At 24 h and 48 h, the M 0.08 group and M 0.5 group were the same as group C, and there was no obvious tissue damage. At 96 h, certain tissue damage can be observed, and the damage in the M 0.5 group is more serious than that in the M 0.08 group. Speculation that the microplastics adsorbed on the gill tissue increased over time; no damage was observed in the early stages. The 0.5-μm microplastics were more difficult to remove than 80-nm microplastics, so the damage was more serious.
Gill tissue damage was observed in both the Cd group and the co-exposure group at 48 h, and it became serious over time. It showed that the impact of Cd on gill tissue was more serious than that of microplastics. However, microplastics have the characteristics of enriching heavy metals in the environment. Existing studies have shown that smaller microplastics have a larger specific surface area and have a stronger ability to enrich heavy metals [40]. The results of the two composite groups showed that the damage was more severe than that of the Cd group alone. At 48 h and 96 h, the degree of gill tissue damage in the Cd + M 0.5 group was more severe than in the Cd + M 0.08 group. It showed that when Cd and microplastics were exposed together, the impact of the larger microplastics group was greater than that of the smaller group. This might be because the larger microplastics are more difficult to remove when they are adsorbed on the gill tissue, and the accumulated Cd has a continuous impact on the gills.
The Antioxidant Status Under Different Exposure Conditions
Microplastics and Cd could cause oxidative damage to the body. A series of antioxidant indicators could be used to investigate the body’s antioxidant status. In the antioxidant system, the total antioxidant capacity (T-AOC) is composed of two parts: the enzyme system and the non-enzyme system, which can be used as a measure of the total antioxidant level composed of various antioxidant substances and antioxidant enzymes. The SOD-CAT system is usually regarded as the first line of defense towards the production of reactive oxygen species (ROS) under environmental stressors [41]. The superoxide radical can be turned into H2O2 by SOD, while H2O2 is metabolized by CAT. MDA is a by-product of LPO; generally, increased MDA content in tissues/organs of organisms can be considered a sign of oxidative damage.
The results showed that 80-nm microplastics and Cd have a greater impact on T-AOC than 0.5-μm microplastics, but when microplastics with different diameters and Cd were exposed together, the impact on T-AOC is different. It might be that the presence of microplastics has a different ability to accumulate Cd, and microplastics with larger diameters were more difficult to remove and are likely to remain in the body. The SOD results showed that exposure to microplastics could cause oxidative stress, but the impact of 0.5 μm was greater than that of 80-nm microplastics. The research on red tilapia (Oreochromis niloticus) [34, 35], it was also found that SOD activity is affected by microplastics. Our research found that the co-exposure of microplastics and Cd would produce antagonistic effects and reduce the activity of SOD. In this study, the activity of CAT was affected by both microplastics and Cd. When exposed together, microplastics of different diameters and Cd all have a synergistic effect, which promotes the activity of CAT. This was also proven in studies on different fish [42,43,44]. The results of MDA further explained the degree of oxidative damage in the body, but the activity of MDA did not change significantly between different time points.
The Expression of Related Genes Under Different Exposure Conditions
Cytokine IL-1β is a protein that regulates signals between various cells, causes an immune response, and plays an important role in regulating an immune mechanism [45]. HSP70 has critical functions of intracellular transport, chaperoning, and tumor-specific antigen production by activating the immune function of T cells and NK cells. In addition, HSP70 induces the secretion of a cytokine such as ILs and TNFs [46]. MT plays a vital role in protecting fish tissue from toxic heavy metals [47]. The expression of related genes in the liver shows that both 80-nm and 0.5-μm microplastics will affect the immunity and antioxidant capacity of the fish, but the 0.5-μm microplastics have a higher impact than 80-nm microplastics. From this figure (Fig. S1), it can be found that the effect of the M 0.08 group on the fish body increases first and then decreases over time, while 0.5 μm continues to increase. It may be that 80-nm microplastics are less likely to accumulate in the body than 0.5-μm microplastics, so the impact will gradually decrease.
Microplastics can accumulate heavy metals in the environment, and the expression of the MT gene also shows that the presence of microplastics will cause more Cd to enter the fish body. The data at 96 h shows that as the microplastics are excreted, a part of Cd will also be excreted, thereby reducing the impact on the fish. Compared with the 0.5-μm microplastics, 80-nm microplastics have a stronger ability to enrich Cd (Fig. S2).
From the expression of the IL-1β gene and HSP70 gene (Fig. S3), it is found that the effects of microplastics of different diameters and Cd are different in effect on the immunity and antioxidant capacity of fish. The presence of Cd will not affect the expression of the IL-1β gene, but when there are 80-nm microplastics in the water, it will have an antagonistic effect, thereby reducing the impact of 80-nm microplastics on the body’s immunity; when there are 0.5-μm microplastics in the water, it will promote the influence of 0.5-μm microplastics on the body’s immunity. The expression of the HSP70 gene showed that 0.5-μm microplastics > Cd > 80-nm microplastics. But when microplastics and Cd exist at the same time, Cd and 80-nm microplastics inhibit each other, and the expression of the HSP70 gene continues to decrease over time, while Cd and 0.5-μm microplastics also inhibit each other, but the expression of HSP70 gene continues over time elevated.
It is speculated that there should be an antagonistic effect between Cd and 80-nm microplastics, and there should be a mutual promotion effect between Cd and 0.5-μm microplastics in terms of the impact on fish immunity. In terms of the effect on the antioxidant capacity of fish, there is an antagonistic effect between Cd and microplastics, but the 0.5-μm microplastics are not easily removed by the fish, so the impact will continue to rise. The results of this study also verified from the side that microplastics would enrich the heavy metals in the environment, and the smaller the microplastics, the stronger the enrichment ability.
Conclusion
In this study, we analyzed the effects of single and composite microplastics and Cd on the juvenile Channa argus from the aspects of tissue damage, antioxidant, and immune response. The results show that the effects of different stressors are different. Under the conditions of this study, 0.5 μm has a greater impact on juvenile fish than 80-nm microplastics. However, 80-nm microplastics were stronger than 0.5-μm microplastics in the ability to enrich Cd. The co-exposure of microplastics and Cd has both antagonistic and synergistic effects. It reflects the complex interaction between microplastics and Cd, which requires further research to understand.
Data Availability
The datasets used and/or analyzed under the current study are available from the corresponding author upon reasonable request.
References
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci 364:1977–1984
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Xu S, Ma J, Ji R, Pan K, Miao A (2020) Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Sci Total Environ 703:134699
Smith M, Love DC, Rochman CM, Neff RA (2018) Microplastics in seafood and the implications for human health. Curr Environ Health Rep 5:375–386
PlasticsEurope (2018) Plastics-the facts 2017 an analysis of European plastics production, demand and waste data
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782
Eriksen M, Liboiron M, Kiessling T, Charron L, Alling A, Lebreton L, Richards H, Roth B, Ory NC, Hidalgo-Ruz V, Meerhoff E, Box C, Cummins A, Thiel M (2018) Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ Pollut 232:430–439
Gao F, Li J, Sun C, Zhang L, Jiang F, Cao W, Zheng L (2019) Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar Pollut Bull 144:61–67
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A (2016) Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci 113:2430
Bond AL, Provencher JF, Elliot RD, Ryan PC, Rowe S, Jones IL, Robertson GJ, Wilhelm SI (2013) Ingestion of plastic marine debris by common and thick-billed Murres in the northwestern Atlantic from 1985 to 2012. Mar Pollut Bull 77:192–195
Auta HS, Emenike CU, Fauziah SH (2017) Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int 102:165–176
Avio CG, Gorbi S, Regoli F (2017) Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar Environ Res 128:2–11
Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA (2019) The potential of microplastics as carriers of metals. Environ Pollut 255:113363
Dobaradaran S, Schmidt TC, Nabipour I, Khajeahmadi N, Tajbakhsh S, Saeedi R, Javad Mohammadi M, Keshtkar M, Khorsand M, Faraji Ghasemi F (2018) Characterization of plastic debris and association of metals with microplastics in coastline sediment along the Persian Gulf. Waste Manag 78:649–658
Gunatilake SK, Chandrajith R (2017) Removal of Pb(II) from contaminated water using low-temperature pyrolyzed agricultural and forest waste biochars: a comparative study. Desalin Water Treat 62:316–324
Wang J, Liu RH, Yu P, Tang AK, Xu LQ, Wang JY (2012) Study on the pollution characteristics of heavy metals in seawater of Jinzhou Bay. Procedia Environ Sci 13:1507–1516
Zhao B, Wang X, Jin H, Feng H, Shen G, Cao Y, Yu C, Lu Z, Zhang Q (2018) Spatiotemporal variation and potential risks of seven heavy metals in seawater, sediment, and seafood in Xiangshan Bay, China (2011–2016). Chemosphere 212:1163–1171
Rochman CM, Hoh E, Hentschel BT, Kaye S (2013) Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ Sci Technol 47:1646–1654
Wang F, Shih KM, Li XY (2015) The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 119:841–847
Barboza LGA, Vieira LR, Guilhermino L (2018) Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): changes in behavioural responses and reduction of swimming velocity and resistance time. Environ Pollut 236:1014–1019
Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51:4714–4721
Wen B, Jin S, Chen Z, Gao J, Liu Y, Liu J, Feng X (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243:462–471
Wen B, Zhang N, Jin S, Chen Z, Gao J, Liu Y, Liu H, Xu Z (2018) Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat Toxicol 195:67–76
Taylor D (1983) The significance of the accumulation of cadmium by aquatic organisms. Ecotoxicol Environ Saf 7:33–42
Li D, Pi J, Wang J, Zhu P, Liu D, Zhang T (2017) Effects of cadmium exposure on metal accumulation and energy metabolism of silver carp (Hypophthalmichthys molitrix). Bull Environ Contam Toxicol 99:567–573
McRae NK, Gaw S, Glover CN (2018) Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat Toxicol 194:1–9
McRae NK, Gaw S, Brooks BW, Glover CN (2019) Oxidative stress in the galaxiid fish, Galaxias maculatus, exposed to binary waterborne mixtures of the pro-oxidant cadmium and the anti-oxidant diclofenac. Environ Pollut 247:638–646
Zhang Z, Zheng Z, Cai J, Liu Q, Yang J, Gong Y, Wu M, Shen Q, Xu S (2017) Effect of cadmium on oxidative stress and immune function of common carp (Cyprinus carpio L.) by transcriptome analysis. Aquat Toxicol 192:171–177
Pi J, Li X, Zhang T, Li D (2016) Effects of acute exposure to sublethal waterborne cadmium on energy homeostasis in silver carp (Hypophthalmichthys molitrix). Bull Environ Contam Toxicol 97:497–503
Drąg-Kozak E, Socha M, Gosiewski G, Łuszczek-Trojnar E, Chyb J, Popek W (2018) Protective effect of melatonin on cadmium-induced changes in some maturation and reproductive parameters of female Prussian carp (Carassius gibelio B.). Environ Sci Pollut Res 25:9915–9927
Driessnack MK, Matthews AL, Raine JC, Niyogi S (2016) Interactive effects of chronic waterborne copper and cadmium exposure on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Comp Biochem Physiol C Toxicol Pharmacol 179:165–173
Banaee M, Soltanian S, Sureda A, Gholamhosseini A, Haghi BN, Akhlaghi M, Derikvandy A (2019) Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236:124335
de Sá LC, Luís LG, Guilhermino L (2015) Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ Pollut 196:359–362
Ding J, Ma T, Cui M, Shao R, Guan R, Wang P (2018) Gas phase dehydration of glycerol to acrolein over Cs2.5H0.5PW12O40/Zr-MCM-41 catalysts prepared by supercritical impregnation. Mol Catal 461:1–9
Ding J, Zhang S, Razanajatovo RM, Zou H, Zhu W (2018) Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ Pollut 238:1–9
Jin Y, Xia J, Pan Z, Yang J, Wang W, Fu Z (2018) Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ Pollut 235:322–329
Karami A, Romano N, Galloway T, Hamzah H (2016) Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ Res 151:58–70
Matsuo AYO, Wood CM, Val AL (2005) Effects of copper and cadmium on ion transport and gill metal binding in the Amazonian teleost tambaqui (Colossoma macropomum) in extremely soft water. Aquat Toxicol 74:351–364
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA (2019) The potential of microplastics as carriers of metals. Environ Pollut 255:113363
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115
Choi JS, Jung Y, Hong N, Hong SH, Park J (2018) Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar Pollut Bull 129:231–240
Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50:4054–4060
Pretto A, Loro VL, Baldisserotto B, Pavanato MA, Moraes BS, Menezes C, Cattaneo R, Clasen B, Finamor IA, Dressler V (2011) Effects of water cadmium concentrations on bioaccumulation and various oxidative stress parameters in Rhamdia quelen. Arch Environ Contam Toxicol 60:309–318
Savan R, Sakai M (2006) Genomics of fish cytokines. Comp Biochem Physiol Part D Genomics Proteomics 1:89–101
Johnson JD, Fleshner M (2006) Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79:425–434
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci. 14(3):6044–66
Acknowledgements
This study was funded by the Key Realm R&D Program of Guangdong Province (No. 2020B0202080005) and the China Agriculture Research System of MOF and MARA (CARS-45-50).
Author information
Authors and Affiliations
Contributions
Shaodan Wang: writing—original draft preparation
Shaolin Xie: software, validation
Zhenlu Wang: software, validation
Chaonan Zhang: software, validation
Zhengkun Pan: validation
Di Sun: validation
Guohuan Xu: visualization, investigation, and editing
Jixing Zou: methodology, writing—reviewing and editing, supervision
Corresponding authors
Ethics declarations
Ethics Approval
Ethical approval was obtained from the South China Agricultural University Ethics Committee.
Consent to Participate
Not applicable. This study does not contain any individual person’s data in any form.
Consent to Publish
Not applicable. This study does not contain any individual person’s data in any form.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Xie, S., Wang, Z. et al. Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation and Biochemical and Immunity of Channa argus. Biol Trace Elem Res 200, 3377–3387 (2022). https://doi.org/10.1007/s12011-021-02917-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02917-6