Abstract
Improving nitrogen use efficiency (NUE) under salt stress has become crucial for rice as it is increasingly facing two major environmental constraints: excessive nitrogen fertilization and soil salinization. However, the interaction between salinity and N levels is very complex and has not yet been considered from the perspective of reduced nitrogen input. We conducted a hydroponic experiment at the early tillering stage on the Yoshida solution to evaluate the impact of rising NaCl and decreasing N application on NUE of four rice cultivars cultivated under three NaCl (0, 56, and 113 mM) and four N (2.86, 1.43, 0.72, and 0.36 mM) concentrations. After 4 weeks, physiological NUE (pNUE), absorption NUE (aNUE), agronomical NUE (agNUE), N transport efficiency (NTE), and physiological traits were evaluated. Significant interactions between N and NaCl-applied concentrations were found in all measured parameters. In all cultivars, increasing the NaCl-applied concentration markedly decreased aNUE and agNUE. For each NaCl treatment, lowering the N applied sharply increased aNUE and agNUE, and this effect was stronger when the NaCl applied was higher. The effect of N lowering on pNUE depended on the NaCl treatment: it enhanced pNUE in the absence of NaCl but had no influence under the highest NaCl-applied concentration. Cultivars largely differed in response to NaCl. The aNUE—but not pNUE—differed between salt-tolerant and salt-sensitive cultivars: aNUE markedly decreased with NaCl concentration in the most salt-sensitive cultivar, whereas it was the highest at the intermediate NaCl concentration in the most salt-tolerant cultivar, especially under low N levels. This finding suggests that under salt conditions, the use of salt-tolerant rice genotypes combined with reducing N level application is necessary to improve NUE. The study of NUE in rice should be focused on the improvement of aNUE with a strong emphasis on the salt tolerance of cultivars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is a staple food for more than half of the world’s population, and its cultivation is increasingly facing soil salinization and overapplication of nitrogen fertilizer (FAO 2012; Reddy et al. 2017; IFA 2019). Soil salinity has been increasing due to global climate change and is expected to continue increasing in the coming decades: a predicted sea-level rise of 30 cm by the year 2050 will lead to salt intrusion into coastal areas (Pachauri et al. 2014; Smajgl et al. 2015; Wassmann et al. 2019). In addition, salinity alters nitrogen utilization by crops (Hu and Schmidhalter 2005; Wang et al. 2012), adding to the environmental problems linked to the overuse of this element (Ashraf et al. 2018).
In the past 50 years, nitrogen (N) fertilization has increased considerably, especially in developing countries, from 4.7 Mt in 1966 to 71.0 Mt in 2016 (IFA 2019). Excess N fertilization in rice cultivation causes a large proportion of N2O, one of two main greenhouse gases apart from CH4, which is also produced by rice paddies (Khalil et al. 1991; Cai et al. 1997; FAO 2012; Li et al. 2015). However, less than 50% of the N applied is actually absorbed and used by plants, and additional N is lost into water and the atmosphere by leaching, surface runoff, volatilization, and denitrification (Ladha et al. 2005). In addition to its detrimental environmental effects, excess N causes financial losses and detrimental effects on human health (Ladha et al. 2005; Anjana and Iqbal 2007). Therefore, improving nitrogen use efficiency (NUE) by reducing N rates, especially under saline conditions, is necessary. However, NUE is a complex phenomenon, and the mechanism of NUE improvement in rice under salinity has been poorly documented from the perspective of lowering N fertilization.
NUE can be defined as the yield (grain, starch, or biomass, depending on the study) per N-applied unit (agronomical NUE—agNUE). NUE involves N sensing, uptake, translocation, assimilation, and remobilization and is governed by multiple interacting genetic and environmental factors (Ladha et al. 2005). agNUE is the combination of two components: absorption NUE (aNUE—also known as recovery NUE or nitrogen uptake efficiency—NUpE), which is the ratio between N absorbed and N applied, and physiological NUE (pNUE—also known as internal NUE—iNUE or utilization efficiency—NUtE), which is the rate of grain, starch, or biomass production per unit of N absorbed. Whereas aNUE is the capacity of the plant to absorb applied N and pNUE is the efficiency of a plant to use N for metabolic purposes (Ladha et al. 2005; Murtaza et al. 2013; Nguyen et al. 2014). The importance of distinguishing these two components from each other in rice has been reported previously (Castilo et al. 2006; Nguyen et al. 2014; Wang et al. 2016). However, information about NUE components in rice under saline conditions is lacking, although the agNUE of rice decreases with either decreasing N levels or increasing salinity levels alone (Murtaza et al. 2013; Nguyen et al. 2014; Mandal et al. 2018).
Salinity affects all processes of N metabolism in rice, including N uptake, N assimilation, and N mobilization, thus causing a severe decline in crop production. Salt reduces N uptake by reducing water absorption, inhibiting NO3− uptake due to the antagonism between NO3− and Cl−, and reducing plant N demand (Abdelgadir et al. 2005; Munns and Tester 2008; Ashraf et al. 2018). Moreover, salt alters the activities of enzymes related to N assimilation and amino acid synthesis in plants: salt weakens the activities of nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), and glutamate synthase (GOGAT) and increases the activity of glutamate dehydrogenase (GDH), thus decreasing amino acid synthesis (Lea and Miflin 2003; Nguyen et al. 2005; Wang et al. 2012). In addition, salinity stress generally causes an elevated level of NH4+ content in plants (Nguyen et al. 2005), which could become toxic (Britto and Kronzucker 2002). Furthermore, salt influences N mobilization and remobilization in tissues and N-containing compounds (Lutts et al. 1996; Mansour 2000). Salt induces higher NO3− concentrations in old leaves than in young leaves of rice (Wang et al. 2012). Salt also alters N metabolism with more accumulation of amino acids as a compatible solute rather than its use as a component of proteins (Lutts et al. 1996; Xu et al. 2016; Cui et al. 2019). Thus, the effects of salinity on N metabolism are complicated and involve complex regulatory mechanisms.
The interaction between salinity and N is complex and is influenced not only by salinity and N levels but also by plant species, genotype, plant age, and environmental conditions (Murtaza et al. 2013; Ashraf et al. 2018; Mondal et al. 2020). To date, few studies on NUE-related traits, such as DW, yield, and N concentration of rice under the interaction of N and salinity levels, have been reported. Furthermore, most of these studies focused on high N levels (Abdelgadir et al. 2005; Mondol et al. 2014). No research on the interaction between N and NaCl in rice has been conducted to date from the perspective of reducing N fertilization, while the current environmental concern is improving NUE under salinity conditions with lower N fertilization. Our preliminary research on reducing N rates under saline conditions was conducted to evaluate the growth and yield of rice, but it was conducted under severe saline conditions in a paddy field with a peak salinity level of 8 dS m−1, and the effect of N was not clear (Phan et al. 2017). Additionally, we did not study NUE components. Thus, further studies on the effect of reducing the N level on the growth and NUE of rice under saline conditions should be conducted. Understanding how growth and NUE may change in rice under NaCl stress is indispensable for planning desirable N inputs under the rising salinization of rice paddy fields. Therefore, the present study was conducted to determine the effects and interactions between NaCl and low N concentrations on NUE components, growth, and development parameters of different rice cultivars. Hydroponics has been used extensively in rice research because it allows precise control of the treatment applied. Therefore, we chose hydroponics to perform the experiments to precisely control the concentrations of the studied parameters—applied N and NaCl—as well as those of other elements.
Materials and Methods
Plant Materials and Growing Conditions
Four rice cultivars (Oryza sativa L.)—Cuom, Ngoi, FL478, and IR28—were provided by Plant Resources Center, Vietnam. FL478, also known as IR66946-3R-178-1-1, is a salt-tolerant recombinant-inbred line created by crossing Pokkali and IR29 (Ferreira et al. 2015). IR28 is salt sensitive (Wang et al. 2018). Cuom (Cườm dạng 1) and Ngoi are two traditional Vietnamese varieties. Cuom is described as being as salt tolerant as FL478, while Ngoi is described as being as salt sensitive as IR28 at the early tillering stage in our previous research (Phan et al. 2017).
A hydroponic experiment was performed in a phytotron at the Université catholique de Louvain, Belgium, from September to October 2016 and was repeated identically and independently from November to December 2016 to assess the reproducibility of the results. In each experiment, three seeds were sown directly into holes in extruded polystyrene plates floating on the Yoshida nutrient solution (Yoshida et al. 1976) in 25 L tanks (36.5 cm length × 26.5 cm width × 31.5 cm height) as described by Dufey et al. (2015). The holes were spaced 4.5 to 5.0 cm apart, with 40 holes per tank and 10 holes for each cultivar. The solution contained 0.32 mM NaH2PO4, 0.5 mM K2SO4, 1.0 mM CaCl2, 1.7 mM MgSO4, 9.1 µM MnCl2, 0.52 µM (NH4)6Mo7O24, 18.0 µM H3BO3, 0.15 µM ZnSO4, 0.16 µM CuSO4, 36.0 µM FeCl3, 70 µM citric acid, and N in NH4NO3 form with adjusted concentrations depending on the treatment. The nutrient solution was renewed weekly. The pH was adjusted daily to 5.0–5.5 using 2 M KOH or 1 M HCl. The climatic conditions in the phytotron were maintained at 30 °C/25 °C (day/night), 85–95% relative humidity, 12-h photoperiod, and 210 µmol m−2 s−1 photosynthetic photon flux density (PPFD) at the top of each tank.
At the fully expanded three-leaf stage (i.e., 14 days after sowing), uniform seedlings were kept at a density of one seedling per hole. Then, four different concentrations of N (as NH4NO3) in the Yoshida solution were applied: 1 N (standard Yoshida concentration with 2.86 mM N), 1/2 N (1.43 mM N), 1/4 N (0.72 mM N), and 1/8 N (0.36 mM N), and all other nutrients were kept at their original concentrations.
NaCl treatments were established to create a gradient of salt stress—heavy, moderate, and no stress. The electrical conductivity (EC) threshold for rice soil solutions has been established as 3 dS m−1 in indica rice (Grattan et al. 2002), and salinities higher than 6.6 dS m−1 caused 50% yield losses (Van Genuchten and Gupta 1993; Zeng and Shannon 2000). A salinity level of 12 dS m−1 has often been used to study the salt tolerance of rice (Castillo et al. 2007; Pang et al. 2017). Therefore, we chose two salinity levels: moderate, 6.5 dS m−1 (equivalent to 56 mM NaCl) and high, 11.5 dS m−1 (equivalent to 113 mM NaCl), for this study. Three NaCl concentrations were crossed with the four N concentrations at the same time as the N treatments: no NaCl added, 56 mM NaCl, and 113 mM NaCl. NaCl treatment was applied in a single step as described in previous studies (Lutts et al. 1996; Wang et al. 2012). According to Shavrukov (2013), the lower concentration is within the range of mild stress and should not cause plasmolysis in root cells, while the second is close to the range of intermediate stress, which may or may not cause an osmotic shock. A concentration above 150 mM should always cause plasmolysis.
Thus, in each of the independent replications, 12 treatments were conducted, amounting to 12 tanks with 480 plants, consisting of 120 of each cultivar. The experimental design was a completely randomized design by rearranging the tank positions weekly, with three fixed and crossed factors consisting of cultivar, N, and NaCl concentrations. Plant growth and biological processes were analyzed after 4 weeks of treatment.
Agronomical Parameters
After 4 weeks of treatment, shoot length, maximum root length (length of the longest root), number of tillers, and number of crown roots were measured on five plants of each cultivar in each treatment (240 plants in total).
Stomatal Conductance and Gas Exchange Rates
After 4 weeks of treatment, the photosynthetic characteristics, including the CO2 gas exchange rate (CER, µCO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), and instantaneous transpiration rate (Tr, mmol H2O m−2 s−1), were recorded in the middle of the youngest fully expanded leaf of three plants of each cultivar in each treatment (144 plants) using an Infra-Red Gas Analyzer (IRGA, LCi-SD, UK). The climatic conditions for photosynthetic measurement were approximately 400 mmol mol−1 CO2 concentration, 210 µmol m−2 s−1 PPFD, and 30 °C leaf temperature.
Osmotic Potential
Leaf osmotic potential (Ψs) was determined according to Lutts et al. (1996). Another set of 144 fresh plants was used and included three plants per treatment and cultivar. Leaf blades of each plant were cut into small segments (5 mm length), placed in 2.5 ml Eppendorf tubes perforated with four small holes, and immediately frozen in liquid nitrogen. After being individually encased in a second intact Eppendorf tube, the leaves were thawed for 30 min and centrifuged at 15,000 rpm for 15 min at 4 °C. The collected sap was analyzed for Ψs determination. Osmolarity (C, mOsmol kg−1) is assessed with a vapor pressure osmometer (VAPRO 5520, Wescor, Logan, USA) and converted from mOsmol kg−1 to MPa using Formula (1) according to the Van’t Hoff equation:
Plant Dry Weight
Five other plants from each cultivar within each treatment—a total of 240 plants—were collected to determine the fresh and dry weight (DW) of the shoots and roots. DW was determined by drying the samples in an oven at 65 °C until reaching a constant weight (approximately 96 h). The water percent in the shoots (SW%) was then determined. The ratio of the shoot and root dry weights (SRratio) of each plant was calculated as the shoot dry weight (SDW) per root dry weight (RDW).
Na+ and K+ Concentrations in Plant Tissue
After measuring dry weights, 192 of the 240 plants mentioned above and four plants from each treatment and each cultivar were ground to determine Na+ and K+ concentrations in the shoot (SNa+ and SK+) and root tissue (RNa+ and RK+). A ground sample of 0.3 mg was diluted with 40 ml of 0.5% HNO3 in a 50 ml sterile tube. The tube was shaken for 24 h before centrifuging at 4000 rpm for 15 min. Subsequently, the solution was filtrated and kept at 6 °C before analysis. Na+ and K+ concentrations were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) using an iCAP 6500 (Thermo Scientific).
N Concentration and Content
The dried shoots and roots of four plants from each cultivar and each treatment—the same 192 plants used for DW and ionic determinations—were ground separately. The N concentrations of shoots (SN) and roots (RN) were determined using the FLASH NC Analyzer (Model AE1112, CE Instruments, UK). The N content in the shoot (SNC) and root tissue was calculated by multiplying SN times SDW and RN times RDW, respectively.
Nitrogen Use Efficiency
NUE and nitrogen transport efficiency (NTE) are calculated according to Nguyen et al. (2014) and Huang et al. (2018):
where total N applied refers to the total N quantity available per plant during the 4 weeks of treatment.
Statistical Analysis
Both independent experiments led to similar results for CER and DW. However, only the data from the first experiment (September to October 2016) are presented. Data were analyzed using “R” software ver. 3.4.1 (R Development Core Team 2019).
A principal component analysis was conducted by combining data for CER, gs, Tr, SDW, RDW, SRratio, and Na+ concentration in the shoot tissue (SNa+), SN, SNC, Ψs, SW%, aNUE, pNUE, agNUE, and NTE. Each component was determined by the variables and their eigenvalues according to Eq. (6). Pearson’s correlation coefficients among these parameters were determined.
where x1 to x15 are the eigenvalues of the variables.
The complete generalized linear Model (7) was used to test whether the factors and the interactions were significant for each variable. The restrained Model (8) was used when interactions were not significant. An F test was performed to test the suitability of the restrained model.
where Yi represents the tested dependent variable and β are the coefficients. The explained variables are NaCl concentration (NaCl), N-applied concentration (N), and genotypic response (Gen). εi is the residual error. A type III ANOVA was used. When significant interactions were found, multiple comparisons were performed using the Tukey test for least square means at a significance level of p < 0.05.
Results
Effect of NaCl and N Concentrations on the Growth and Development of Rice Plantlets
The principal component analysis revealed that 84.28% of the total variability was explained by the first two principal components (PC). PC1 accounted for 58.14% of the variance and was highly related to salinity and its negative effects on gs, gas exchanges (CER and Tr), Ψs, SNa+, and all three dry weights (Eq. 9, Fig. 1). The contributions of SN and pNUE to this first axis were negligible. PC2 explained 26.14% of the total variance and was related to the effect of N-applied concentrations on both SN and SNC, pNUE, agNUE, and NTE. The contributions of the parameters related to NaCl treatment listed above and DW were negligible on this second axis (Eq. 10, Fig. 1). PC3 explained only 6.65% of the variability, and all other PCs accounted for less than 3% each.
PCA plot of four rice cultivars under different N and NaCl-applied levels in the Yoshida et al. (1976) solution. C, N, F, and I: Cuom, Ngoi, FL478, and IR28 cultivars, respectively. CER photosynthesis rate, gs stomatal conductance, Tr transpiration rate, SDW shoot dry weight, RDW root dry weight, SRratio ratio of shoot dry weight and root dry weight, SNa Na+ concentration in shoot, pNUE physiological NUE, agNUE agronomical NUE, aNUE absorption NUE, NTE nitrogen transport efficiency, OsP osmotic potential, SW% percentage of water in shoot, SN nitrogen concentration in shoot, SNC nitrogen content in shoot
Several interesting relationships were found between the studied parameters. First, the orthogonality of the eigenvectors revealed that both SN and pNUE were independent of SNa+. Second, SRratio was positively correlated with NTE (0.88, p < 0.001) but negatively correlated with both aNUE (− 0.77, p < 0.001) and agNUE (− 0.82, p < 0.001). SRratio, NTE, aNUE, and agNUE were partially linked to both SNa+ and SN. Third, SNC was positively correlated with SDW (0.93, p < 0.001) and to a lesser extent to SN (0.54, p < 0.01).
Examining the locations of each cultivar and the N and Na+ treatment in Fig. 1 indicated that increasing NaCl concentration caused an increase in SNa+—as expected—and SRratio. However, gs, gas exchanges—CER and Tr –, Ψs, and DWs were reduced. Cuom tended to be located on the left-hand side of the graph—low SNa+—while Ngoi was on the right-hand side—high SNa+. Regarding N effects, increasing N levels led to increases in SN, SRratio, and NTE but decreases in pNUE and agNUE.
In summary, pNUE was opposed to SN, and both were independent of SNa+ and Ψs—which were mainly influenced by NaCl concentrations—whereas both aNUE and agNUE were partially linked to both SN and SNa+. Cuom tended to maintain a low SNa+, as opposed to Ngoi.
Agronomical Parameters
The effect of N on shoot length, maximum root length, number of tillers, and number of crown roots per plant changed depending on the salinity level. As expected, under non-saline treatment, increasing N stimulated the formation of new tissue and organs: shoot length, the number of tillers per plant, and the number of crown roots per plant increased, although the maximum root length was reduced. However, this stimulating effect of N was not observed under saline conditions (Fig. 2a, b).
Number of tillers per plant (a), number of crown roots per plant (b), shoot dry weight—SDW (c), root dry weight—RDW (d), Na+ concentration in shoot tissue—SNa+ (e), osmotic potential in leaves—Ψs (f), stomatal conductance—gs (g), and rate of photosynthesis CER (h) of rice grown in Yoshida et al. (1976) solution under different N and salinity levels. Data are the means ± SE (n = 12) of four cultivars. Bars with the same lower case letter are not significantly different according to Tukey’s test at the 5% level between treatments—nitrogen concentrations and salinity levels
Dry Weights
Both SDW and RDW were significantly influenced by NaCl and N applied and their interaction (p < 0.001) and decreased drastically with increasing NaCl concentration (Fig. 2c, d). However, the effects of N concentrations differed between roots and shoots, and trends went in opposite directions according to the NaCl treatments. In the 0 mM NaCl treatment, SDW decreased with decreasing N concentrations, while RDW generally increased. In the 56 mM NaCl treatment, the largest DWs were found under intermediate N concentrations in both tissues. Finally, in the 113 mM NaCl treatment, trends were the opposite of those found in the 0 mM NaCl treatment: DWs tended to increase moderately with decreasing N-applied concentration, although the effect was significant only in roots.
Na+ Concentration in Tissue and Osmotic Potential of the Blade
SNa+ and RNa+ were influenced by NaCl treatment (p < 0.01) and the interaction between NaCl, N, and cultivar (p < 0.001, Supplement Table S1). As expected, both SNa+ and RNa+ always increased with increasing applied NaCl. Again, the effect of N-applied concentration on Na+ in the tissue depended on NaCl treatment and the organs: in the 56 mM NaCl treatment, the highest SNa+ was observed under 1/8 N, while in the 113 mM NaCl treatment, it was found under 1 N (Fig. 2e). However, in the root tissue, RNa+ in the 113 mM NaCl treatment was the lowest under 1 N, while no significant difference appeared among the other treatments (Supplement Fig. S2).
The evolution of Ψs was clearly related to SNa+, which became more negative with increasing NaCl treatment (Fig. 2f). Ψs was not influenced by N treatment in the absence of NaCl but was significantly affected by N deficiency (1/8 N) under salinity conditions. Interestingly, at the intermediate NaCl concentration (56 mM), although SNa+ and Ψs were measured in different plants, the effects of N treatment on Ψs and SNa+ were related to each other, with SNa+ being significantly higher and Ψs being significantly lower under 1/8 N than under the other N treatments. However, in the 113 mM NaCl treatment, the highest Ψs was found under 1/8 N, while the Ψs in the other three N treatments were not significantly different from each other.
Stomatal Conductance, Gas Exchanges, and Percentage of Water in Shoots
The ANOVA results indicated that NaCl treatment, N treatment, and their interactions influenced gs and gas exchanges—CER and Tr—and SW% (Supplement Table S1). These parameters markedly decreased with increasing NaCl concentrations. However, they were again differentially influenced by N levels under each NaCl treatment (Fig. 2g, h, Supplement Fig. S1). In the absence of NaCl, the measured parameters showed significantly lower values under 1/8 N than under the other N levels. In contrast, under a high concentration of NaCl applied, the lowest values were recorded with high concentrations of N applied.
N and K+ Concentrations and N Content in the Tissue
The tendencies for N concentration according to NaCl and N-applied levels were similar in the shoot and root tissues; thus, for clarity, we focused on the concentration in the shoot tissue only (SN). The effect of NaCl treatment on SN depended on N input: NaCl enhanced SN under low N input (1/8 N and 1/4 N) and had no influence under 1/2 N but reduced it under high N levels (1 N) (Fig. 3a). Similarly, the effect of N input on SN depended on NaCl treatment. Under 0 and 56 mM NaCl-applied concentrations, SN increased with increasing N applied. However, under the 113 mM NaCl-applied concentration, increasing N to 1/2 N or 1 N caused a significant decrease in SN.
N concentration in shoot tissue—SN (a), N content in shoot tissue—SNC (b), Nitrogen transport efficieny—NTE (c), absorption NUE—(d), physiological NUE—pNUE (e), and agronomical NUE—agNUE (f) in rice grown in Yoshida et al. (1976) solution under different N and salinity levels. Data are means ± SE (n = 12) of four cultivars. Bars with the same lower case letter are not significantly different according to Tukey’s test at the 5% level between treatments—nitrogen concentrations and salinity levels
SNC followed the same trends as SDW and was strongly influenced by N treatment (p < 0.001) and the interaction between NaCl and N concentration (p < 0.001). SNC was differentially affected by N treatment depending on NaCl treatment (Fig. 3b). Indeed, under 0 mM NaCl, SNC markedly increased with increasing N applied. However, under the intermediate NaCl treatment (56 mM), the highest SNC was observed under an intermediate N concentration (1/2 N). Finally, under the highest NaCl treatment (113 mM), there was no significant effect of N, although the lowest SNC was found under 1 N applied.
Both SK+ and RK+ were influenced by NaCl, N applied, and their interaction (Supplement Table S1). SK+ and RK+ decreased with increasing external NaCl concentrations and was influenced by N depending on the salinity levels (Supplement Fig. S1). Under no or moderate salinity, increasing N levels from 1/8 N to 1/2 N increased SK+ and RK+. Then, increasing N from 1/2 N to 1 N maintained or decreased SK+ and RK+ significantly (under non-saline and moderate salinity, respectively). Under severe salt conditions, the N concentration did not significantly influence the K+ concentration in the tissue.
N Transport and Use Efficiency
NTE and all three NUEs were influenced by NaCl, N-applied concentrations, and their interaction (Supplement Table S1). Increasing NaCl or N concentrations increased NTE but reduced all three NUEs (Fig. 3c–f). The aNUE increased significantly with decreasing N concentrations under all three NaCl treatments, and more so as the NaCl treatment was stronger: decreasing N from 1 to 1/8 N increased aNUE by 48.0%, 76.8%, and 91.1% under 0, 56, and 113 mM NaCl treatments, respectively, the slope of the regression being significantly different between 0 on one hand and 56 and 113 mM on the other hand (data not shown). A high NaCl concentration caused no significant effect on aNUE in the 1/8 N treatment but significantly reduced aNUE in all three other N concentrations. Thus, aNUE was highly positively affected by reducing N treatment under high NaCl treatment.
Salt drastically reduced pNUE under low N levels (1/8 N and 1/4 N) but had no effect on pNUE at higher N levels (1/2 N and 1 N). Thus, when NaCl-applied levels increased, pNUE was less influenced by changing N rates. Decreasing N concentrations led to a marked increase in pNUE in the 0 mM NaCl treatment but had a smaller effect in the presence of NaCl: decreasing N from 1 to 1/8 N caused an increase in pNUE by 59.0% in the 0 mM treatment, 40.6% in the 56 mM NaCl treatment, and only 6.7% in the 113 mM NaCl treatment. agNUE followed the same trend as aNUE and was generally reduced by increasing NaCl applied and, under each NaCl-applied concentration, was drastically increased by decreasing N concentrations: decreasing N from 1 to 1/8 N caused an increase in agNUE by 78.9%, 87.6%, and 91.6% under 0 mM NaCl, 56 mM NaCl, and 113 mM NaCl, respectively.
In summary, NaCl treatment reduced aNUE and pNUE differently depending on N levels. This decrease caused higher aNUE reductions under higher N-applied concentrations. In contrast, NaCl did not reduce pNUE under higher N levels (1/2 N and 1 N) but significantly reduced pNUE under lower N levels (1/8 N and 1/4 N).
Response of Different Cultivars to NaCl and N Concentrations
Dry Weights
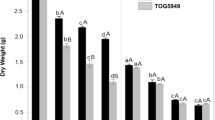
SDW and RDW generally decreased with the increasing application of NaCl from 0 to 113 mM in all cultivars, with marked differences between cultivars (Fig. 4a, Supplement Fig. S3). Interestingly, SDW in Cuom was not affected by the 56 mM NaCl treatment, whereas it drastically decreased in Ngoi (64.0%) and moderately in the other cultivars (22.9% and 43.3% for FL478 and IR28, respectively). Under 113 mM NaCl, Cuom also performed the best, with a reduction in SDW of only 50.9% compared to the 0 mM NaCl treatment, whereas it was approximately 70% in all other cultivars. Reductions in RDW were greater than those in SDW. Again, Cuom and Ngoi were the least and most affected by the NaCl treatment, respectively.
Shoot dry weight—SDW (a), Na+ concentration in shoots—SNa+ (b), osmotic potential—Ψs (c), N content in shoots—SNC (d), nitrogen tranport efficiency—NTE (e), absorption NUE—aNUE (f), physiological NUE—pNUE (g), and agronomical NUE—agNUE (h) of different cultivars grown in Yoshida et al. (1976) solution under three salinity levels. Data are means ± SE (n = 12) of four N treatments (0.36, 0.72, 1.43, and 2.86 mM). Bars with the same lower case letter are not significantly different according to Tukey’s test at the 5% level between different interactions of cultivars and salinity levels
Na+ in Shoot Tissues and Osmotic Potential in Leaf Tissue
SNa+ but not RNa+ at the same NaCl levels varied greatly according to the cultivar; thus, we focused on SNa+ (Fig. 4b, Supplement Fig. S5). SNa+ was significantly higher in Ngoi than in all other cultivars in the 56 mM NaCl treatment and higher in Ngoi and IR28 than in the two remaining cultivars with 113 mM NaCl treatment. Indeed, under 56 mM NaCl treatment, SNa+ of Ngoi and Cuom increased 3149 and 815%, respectively, compared to SNa+ in non-saline conditions. Under 113 mM NaCl, the increase in SNa+ of the two salt-sensitive cultivars—Ngoi (6293%) and IR28 (6606%)—was significantly higher than that of the two salt-tolerant cultivars—Cuom (3114%) and FL478 (3498%).
NaCl caused a dramatic decrease in Ψs, which differed according to the cultivar. Again, Cuom, and Ngoi were the least and most affected by the salt treatments, respectively (Fig. 4c).
Stomatal Conductance, Gas Exchanges, and Percentage of Water in Shoots
gs, CER, and SW% decreased with increasing NaCl concentration, with levels of reductions depending on the cultivar. Under both concentrations of NaCl applied, Cuom was the least affected, followed by FL478, IR28, and Ngoi (Supplement Fig. S5). Under the highest NaCl concentration, the CER and SW% of Ngoi and IR28 were strongly reduced. Their gs, CER, Tr, and SW% decreased by 99.4%, 98.5%, 98.0%, and 13.7% in Ngoi and 97.3%, 93.8%, 94.8%, and 13.7% in IR28, respectively.
N Concentration, N Content, and K+ Concentration in Tissues
NaCl concentrations caused no significant effect on RN but enhanced SN of all cultivars. Ngoi showed the smallest SN under all salinity levels (Supplement Fig. S4). Depending upon the cultivar, SNC was influenced by the applied NaCl levels: FL478, IR28, and Ngoi had significant reductions in SNC with increasing NaCl concentrations from 0 to 113 mM (Fig. 4d). However, in Cuom, applying 56 mM NaCl resulted in an increase in SNC compared to 0 mM NaCl under all three reduced N levels. In Cuom again, increasing NaCl application to 113 mM resulted in a lower SNC, but it remained significantly higher than in the three other cultivars.
The tendencies observed for K+ were the opposite of those for Na+ concentrations in the shoot tissues. Ngoi showed the lowest SK+. Under both salt conditions, Ngoi had the highest Na+/K+ ratio, followed by IR28, FL478, and finally Cuom (Supplement Fig. S5).
N Transport and Use Efficiency
NTE was slightly enhanced and pNUE was slightly reduced by applied NaCl concentrations, with few differences between cultivars. Ngoi showed the highest NTE and lowest pNUE under salinity conditions (Fig. 4e and g). The effect of cultivar on aNUE and agNUE was more obvious. Regardless of NaCl concentration, the salt-tolerant cultivars, mainly Cuom, showed higher aNUE than the salt-sensitive cultivars (Fig. 4f and h). aNUE and agNUE were always highest in Cuom, followed by FL478, IR28, and finally Ngoi. Interestingly, aNUE and agNUE in Cuom were even higher at intermediate NaCl concentrations than under 0 mM NaCl and were of the same order under high NaCl concentrations than at 0 mM NaCl. In contrast, in the other cultivars, aNUE and agNUE values were lowered when the NaCl concentrations increased.
In summary, aNUE and agNUE but not pNUE differed between salt-tolerant and salt-sensitive cultivars. Cuom was the most salt tolerant and showed the highest aNUE and agNUE values, followed by FL478, IR28, and finally the most salt-sensitive Ngoi. Increasing NaCl application resulted in lower values of SDW, RDW, Ψs, gs, CER, Tr, and SNC in all cultivars, including Cuom, but all of these values remained higher in this last cultivar than in the three other cultivars.
Discussion
In the present study, the evolution of NUE components under saline conditions and reducing N application was highlighted. Whereas, agNUE decreased with increasing NaCl or N levels, its two components—aNUE and pNUE—behaved differently: when NaCl-applied levels increased, aNUE was more affected than pNUE; for any given level of NaCl, aNUE was more affected than pNUE by the level of N. This finding could be explained as follows.
aNUE was low and further reduced with increasing NaCl concentration due to a reduction in both DW and N uptake under high N-applied concentrations. First, salt reduced water absorption and the SW% and Tr of the leaves and then decreased the mass flow of nutrients, including N, in our experiment. This result was consistent with those of Munns and Tester (2008). Second, under saline conditions, NO3− uptake decreases due to the antagonism between NO3− and Cl− and by downregulating some genes related to N uptake (Abdelgadir et al. 2005; Wang et al. 2012; Ashraf et al. 2018). Third, in our experiment, salinity reduced CER, gs, Tr, protein concentration (data not shown), SNC, and DW. Salinity can reduce plant N demand, thereby restricting N uptake (Lutts et al. 1996; Chen et al. 2010; Ashraf et al. 2018). Furthermore, salt markedly reduced the number of roots per plant and RDW, especially under high N levels such as 1 N, thus reducing the N uptake capacity of the plant (Fig. 2b, d). Therefore, aNUE under saline conditions was low due to N absorption inhibition by the presence of salt.
However, aNUE was not reduced by increasing salinity levels under very low N application levels, unlike under higher N treatments. DW, but neither SN nor NTE, was reduced by NaCl under low N treatment. Therefore, we confirmed that salt limited growth but did not reduce N transport under such conditions (Fig. 2c, 3c). This finding suggests that growth inhibition may be explained by an impairment of N uptake under salt stress (Supplement Fig. S9). However, salt induces the activity of the high-affinity transport system (HATS), which mediates N uptake activity at low N concentrations (0.2–0.5 mM N) (Crawford and Glass 1998; Bao et al. 2015; Mandal et al. 2018). Some genes coding for NH4+ and NO3− transporters belong to HATS, such as NRT2 and AMT1, which are upregulated under saline conditions (Wang et al. 2012). As a result, the N uptake under low N concentrations in the presence of NaCl increases. Therefore, although salt reduced DW, the total N content in rice under low N conditions was maintained regardless of the NaCl treatment.
Regarding pNUE, the negative effect of the N-applied concentration that was observed in the absence of NaCl diminished and finally disappeared with increasing NaCl-applied concentration. The trend of pNUE under non-saline conditions was consistent with the results of Nguyen et al. (2014), who reported that pNUE increased with decreasing N application concentration. When NaCl-applied levels increased, pNUE was less influenced by N-applied concentrations. A similar finding was described in oat, where it was reported that pNUE in a salt-tolerant genotype was not influenced by changing N rates (Song et al. 2019). This finding was attributed to the photosynthesis rate and N absorption. Indeed, when NaCl increased, these two last parameters were reduced by salt but were less influenced by changing N rates than in plants under non-saline treatment, meaning that under salt treatment, all N absorbed is used equally to allow for the synthesis of organic compounds.
agNUE was positively and more strongly correlated with aNUE than with pNUE under all levels of NaCl (0.83, 0.96, and 0.98, p < 0.001 in the 0 mM, 56 mM, and 113 mM NaCl treatments, respectively). However, although agNUE showed a positive correlation with pNUE in the absence of NaCl (0.90, p < 0.001), a weaker correlation was observed under the intermediate NaCl treatment (0.68, p < 0.01), but no correlation was observed under severe salt stress (− 0.04). In addition, NTE was enhanced by both increasing N and NaCl concentrations. Overall, under salt treatments, the contribution of aNUE to agNUE was higher than that of pNUE, and the variation in aNUE was higher than that of both pNUE and NTE. In other words, the reduction in NUE under salinity conditions mainly resulted from the reduction in N uptake capacity.
We highlighted the negative effect of high N (1 N—standard N under non-saline conditions) and the advantages of reducing N rates for growing rice under saline conditions. As expected, the standard N concentration in the Yoshida solution—1 N—resulted in the highest growth under non-saline conditions. In contrast, under NaCl treatments, reducing the N-applied concentration led to higher growth than under the standard N-applied concentration (Fig. 5). Indeed, under intermediate NaCl treatment (56 mM NaCl, equivalent to 6.5 dS m−1), reducing N-applied concentrations led to significantly higher dry matter accumulation—PDW, SDW, RDW –, gs, CER, Tr, SNC, SK+, aNUE, and agNUE. Thus, the positive effect of N under non-saline conditions became negative under saline conditions. This effect was caused mainly by a marked reduction in the number of tillers, the number of root tips, root length, gs, Tr, and subsequently SDW and RDW. In other words, salt inhibited the role of N in the formation of new tissues and organs and reduced the photosynthetic capacity. Moreover, the negative effect of 1 N concentration under saline conditions might be related to the high concentration of NH4+ induced by the presence of NaCl in rice (Hoai et al. 2003; Nguyen et al. 2005). In hydroponic systems using NH4NO3, NH4+ is absorbed faster than NO3−, with a high concentration of NH4+ disturbing NO3− uptake (Sasakawa and Yamamoto 1978). At high concentrations, NH4+ becomes toxic to rice by inhibiting photosynthesis and growth (Britto and Kronzucker 2002). Moreover, NH4+ assimilation is reduced by salt stress (Nguyen et al. 2005; Wang et al. 2012). Excess NaCl weakens GS/GOGAT pathways and increases the GDH pathway in leaves, causing less protein to be synthesized (Lea and Miflin 2003; Nguyen et al. 2005; Wang et al. 2012). Our study also indicated that changing the N application concentration under a high NaCl concentration (113 mM) showed few effects on the growth of rice plants. Similar findings were observed in chili pepper (Villa-Castorena et al. 2003), cotton (Chen et al. 2010), wheat (Hu and Schmidhalter 2005), and canola (Belouchrani et al. 2020). The intermediate NaCl concentration applied in our study was equivalent to the salinity that rice may experience in paddy fields while still provides a certain yield (Zeng and Shannon 2000; Phan et al. 2017). Based on this study, reducing N levels is necessary for growing rice under salt stress.
Phenotypes of rice under different conditions of N and NaCl concentrations after 4 weeks of growth on the Yoshida et al. (1976) solution with different N and NaCl concentrations. 1 N: normal N concentration of the Yoshida solution (2.86 mM). 1/2 N, 1/4 N, and 1/8 N: reduced N concentration at 1/2, 1/4, and 1/8 of the normal concentration, respectively (1.43, 0.72, and 0.36 mM)
Vietnam is highly exposed to salinity stress, and according to a recent study, the salinity-prone rice areas in Vietnam account for 44% of the total rice area (Smajgl et al. 2015; Wassmann et al. 2019). Some local rice varieties are expected to exhibit a high level of salinity resistance. Among the four tested cultivars, Cuom was the most salt tolerant, followed by FL478, IR28, and Ngoi. Under both salt treatments, Cuom showed the highest values for tiller number, root number, SNC, SK+, Ψs, CER, SDW, RDW, aNUE, and agNUE. In contrast, its SNa+ and ratio Na+/K+ in the shoot and root tissues were the lowest. Some studies have reported that salt-tolerant cultivars reduce salt accumulation so that they maintain higher antioxidant activities and subsequently grow (Lutts et al. 1996; Dionisio-Sese and Tobita 1998; Islam et al. 2016). Regarding the NUE of the four cultivars, Cuom always showed the highest aNUE and agNUE, followed by FL478, IR28, and finally Ngoi, regardless of the salinity level (Fig. 4f, h). This finding resulted from less SNa+ accumulation and good maintenance of Ψs of Cuom under intermediate salinity, allowing this cultivar to maintain water uptake as well as N uptake and accumulation in the tissue, thereby exhibiting higher aNUE than the other cultivars. Thus, there was a link between aNUE and the salt tolerance of rice cultivars. This result was consistent with the result of Song et al. (2019) in oat. However, our results revealed that the difference in pNUE between salt-tolerant and salt-sensitive cultivars was weak (Fig. 4g).
When an external essential element is present in a limited quantity, the most adapted plants can upregulate genes coding for specific transporters involved in the absorption of the element to compensate for its reduced bioavailability (Hoang et al. 2016; Islam et al. 2016). Hence, Cuom may be able to trigger transcriptomic adaptations related to N absorption. Moreover, Walia et al. (2005) demonstrated that salt-tolerant rice cultivars such as FL478 did not consume N to synthesize flavonoids and other phenolic protecting compounds because the low accumulation of Na+ did not trigger this protection strategy. Thus, absorbed N remains available for normal plant metabolism because it is not consumed as a response to stress. Interestingly, the intermediate NaCl treatment enhanced the aNUE of Cuom under all of the lower N concentrations but not under the standard N application (Supplement Fig. S6). Proper reduction of N concentration might improve the photosystem II activity, RuBP carboxylase activity, and gs, thus reducing salt damage in salt-tolerant cultivars, which maintained higher antioxidant activities leading to less damage than the sensitive ones (Munns and Tester 2008; Islam et al. 2016; Xu et al. 2019). These findings suggest that under moderate salt stress, cultivating a salt-tolerant cultivar with high aNUE and agNUE accompanied by reduced N input could limit NUE reductions in rice.
Findings about NUE components and the advantages of reducing N input under saline conditions have been highlighted in this study. These findings could help improve NUE by changing the level of N fertilizer under saline conditions. However, this research was conducted hydroponically with N applied as NH4NO3 at the early tillering stage. Further information is needed at the field scale during the entire rice life cycle to gain information about the grain yield and yield components of rice.
Conclusion
This study revealed that under different NaCl treatments, (1) the physiological parameters gs, CER, Tr, Ψs, DWs, SNC, and NUE, including aNUE, pNUE, and agNUE, were significantly influenced by the interactions between NaCl and N-applied concentrations; (2) aNUE and agNUE were more affected than pNUE by reducing N application; (3) lower N-applied concentrations caused lower aNUE reductions; (4) reducing N concentrations under moderate NaCl treatment is indispensable for the growth of rice; and (5) large differences between salt-tolerant and salt-sensitive cultivars were found for aNUE and agNUE but not pNUE.
Abbreviations
- N:
-
Nitrogen
- NUE:
-
Nitrogen use efficiency
- aNUE:
-
Absorption nitrogen use efficiency
- agNUE:
-
Agronomical nitrogen use efficiency
- pNUE:
-
Physiological nitrogen use efficiency
- NTE:
-
Nitrogen transport efficiency
- DW:
-
Dry weight
- SDW:
-
Shoot dry weight
- RDW:
-
Root dry weight
- PDW:
-
Plant dry weight
- SRratio:
-
Ratio of shoot dry weight and root dry weight
- SW%:
-
Water percent in the shoots
- CER:
-
CO2 exchange rate
- g s :
-
Stomatal conductance
- Tr:
-
Transpiration rate
- Ψs:
-
Osmotic potential
- SN:
-
Nitrogen concentration in the shoot tissue
- RN:
-
Nitrogen concentration in the root tissue
- SNC:
-
Nitrogen content in the shoot tissue
- SNa+ :
-
Na+ concentration in the shoot tissue
- RNa+ :
-
Na+ concentration in the root tissue
- SK+ :
-
K+ concentration in the shoot tissue
- RK+ :
-
K+ concentration in the root tissue
- EC:
-
Electrical conductivity
- PC:
-
Principal component
References
Abdelgadir EM, Oka M, Fujiyama H (2005) Nitrogen nutrition of rice plants under salinity. Biol Plant 49:99–104. https://doi.org/10.1007/s10535-005-0104-8
Anjana SU, Iqbal M (2007) Nitrate accumulation in plants, factors affecting the process, and human health implications. A Review. Agron Sustain Dev 27:45–57. https://doi.org/10.1051/agro:2006021
Ashraf M, Shahzad SM, Imtiaz M, Rizwan MS (2018) Salinity effects on nitrogen metabolism in plants—focusing on the activities of nitrogen metabolizing enzymes: a review. J Plant Nutr 41:1065–1081. https://doi.org/10.1080/01904167.2018.1431670
Bao A, Liang Z, Zhao Z, Cai H (2015) Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int J Mol Sci 16:9037–9063. https://doi.org/10.3390/ijms16059037
Belouchrani AS, Bouderbala A, Drouiche N, Lounici H (2020) The interaction effect to fertilization on the mineral nutrition of canola under different salinity levels. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10155-8
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159:567–584. https://doi.org/10.1078/0176-1617-0774
Cai Z, Xing G, Yan X et al (1997) Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil 196:7–14. https://doi.org/10.1023/A:1004263405020
Castilo EG, Tuong TP, Singh U et al (2006) Drought response of dry-seeded rice to water stress timing and N-fertilizer rates and sources. Soil Sci Plant Nutr 52:496–508. https://doi.org/10.1111/j.1747-0765.2006.00064.x
Castillo EG, Tuong TP, Ismail A, Inubushi K (2007) Response to salinity in rice: comparative effects of osmotic and ionic stresses. Plant Prod Sci 10:159–170. https://doi.org/10.1626/pps.10.159
Chen W, Hou Z, Wu L et al (2010) Effects of salinity and nitrogen on cotton growth in arid environment. Plant Soil 326:61–73. https://doi.org/10.1007/s11104-008-9881-0
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395. https://doi.org/10.1016/S1360-1385(98)01311-9
Cui G, Zhang Y, Zhang W et al (2019) Response of carbon and nitrogen metabolism and secondary metabolites to drought stress and salt stress in plants. J Plant Biol 62:387–399. https://doi.org/10.1007/s12374-019-0257-1
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Dufey I, Draye X, Lutts S et al (2015) Novel QTLs in an interspecific backcross Oryza sativa × Oryza glaberrima for resistance to iron toxicity in rice. Euphytica 204:609–625. https://doi.org/10.1007/s10681-014-1342-7
FAO (2012) FAOSTAT Database. http://www.fao.org/faostat/en/#data/QC. Accessed 18 Nov 2019
Ferreira LJ, Azevedo V, Maroco J et al (2015) Salt tolerant and sensitive rice varieties display differential methylome flexibility under salt stress. PLoS ONE 10:e0124060
Grattan SR, Zeng L, Shannon MC, Roberts SR (2002) Rice is more sensitive to salinity than previously thought. Calif Agric 56:189–198. https://doi.org/10.3733/ca.v056n06p189
Hoai NTT, Shim IS, Kobayashi K, Kenji U (2003) Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 41:159–164. https://doi.org/10.1023/A:1027305522741
Hoang MT, Tran NT, Nguyen KT et al (2016) Improvement of salinity stress tolerance in rice: challenges and opportunities. Agronomy. https://doi.org/10.3390/agronomy6040054
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549. https://doi.org/10.1002/jpln.200420516
Huang S, Zhao C, Zhang Y, Wang C (2018) Nitrogen use efficiency in rice. In: Amanullah, Fahad S (eds) Nitrogen in agriculture-updates. IntechOpen, London, p 189
IFA (2019) IFASTAT. https://www.ifastat.org/databases/plant-nutrition. Accessed 20 Sept 2019
Islam F, Ali B, Wang J et al (2016) Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol Biochem 107:82–95. https://doi.org/10.1016/j.plaphy.2016.05.027
Khalil MAK, Rasmussen RA, Wang MX, Ren L (1991) Methane emissions from rice fields in China. Environ Sci Technol 25:979–981. https://doi.org/10.1021/es00017a023
Ladha JK, Pathak H, Krupnik TJ et al (2005) Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Academic Press, Cambridge, pp 85–156
Lea PJ, Miflin BJ (2003) Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol Biochem 41:555–564. https://doi.org/10.1016/S0981-9428(03)00060-3
Li B, Fan CH, Zhang H et al (2015) Combined effects of nitrogen fertilization and biochar on the net global warming potential, greenhouse gas intensity and net ecosystem economic budget in intensive vegetable agriculture in Southeastern China. Atmos Environ 100:10–19. https://doi.org/10.1016/j.atmosenv.2014.10.034
Lutts S, Kinet JM, Bouharmont J (1996) Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regul 19:207–218. https://doi.org/10.1007/BF00037793
Mandal VK, Sharma N, Raghuram N (2018) Molecular targets for improvement of crop nitrogen use efficiency: current and emerging options BT—engineering nitrogen utilization in crop plants. In: Zayed A, Lightfoot DA (eds) Shrawat A. Springer, Cham, pp 77–93
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500. https://doi.org/10.1023/A:1002873531707
Mondal S, Hasan J, Biswas PL et al (2020) Nitrogen use efficiency in rice under abiotic stress: plant breeding approach. In: Mahmood-ur-Rahman A (ed) Recent advances in rice research. IntechOpen, London
Mondol M, Chamon A, Hood-Nowotny R, Blum W (2014) Use of 15N labelled urea to quantify nitrogen recovery and effect of salinity in rice. Bangladesh J Sci Ind Res 49:69–78
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Murtaza G, Azooz MM, Murtaza B et al (2013) Nitrogen-use-efficiency (NUE) in plants under NaCl stress. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants. Springer, Berlin, pp 415–437
Nguyen HTT, Shim IS, Kobayashi K, Usui K (2005) Regulation of ammonium accumulation during salt stress in rice (Oryza sativa L.) seedlings. Plant Prod Sci 8:397–404. https://doi.org/10.1626/pps.8.397
Nguyen HTT, Pham CV, Bertin P (2014) The effect of nitrogen concentration on nitrogen use efficiency and related parameters in cultivated rices (Oryza sativa L. subsp. indica and japonica and O. glaberrima Steud.) in hydroponics. Euphytica 198:137–151. https://doi.org/10.1007/s10681-014-1101-9
Pachauri RK, Allen MR, Barros VR et al (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
Pang Y, Chen K, Wang X et al (2017) Simultaneous improvement and genetic dissection of salt tolerance of rice (Oryza sativa L.) by designed QTL pyramiding. Front Plant Sci 8:1275
Phan THN, Tang TH, Bertin P, Pham VC (2017) Effect of inorganic nitrogen forms and concentration on growth of rice genotypes under severe saline condition. Vietnam J Agri Sci 15:189–197
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reddy INBL, Kim BK, Yoon IS et al (2017) Salt tolerance in rice: focus on mechanisms and approaches. Rice Sci 24:123–144. https://doi.org/10.1016/j.rsci.2016.09.004
Sasakawa H, Yamamoto Y (1978) Comparison of the uptake of nitrate and ammonium by rice seedlings: influences of light, temperature, oxygen concentration, exogenous sucrose, and metabolic inhibitors. Plant Physiol 62:665–669. https://doi.org/10.1104/pp.62.4.665
Shavrukov Y (2013) Salt stress or salt shock: which genes are we studying? J Exp Bot 64:119–127. https://doi.org/10.1093/jxb/ers316
Smajgl A, Toan TQ, Nhan DK et al (2015) Responding to rising sea levels in the Mekong Delta. Nat Clim Change 5:167–174. https://doi.org/10.1038/nclimate2469
Song X, Zhou G, Ma B-L et al (2019) Nitrogen application improved photosynthetic productivity, chlorophyll fluorescence, yield and yield components of two oat genotypes under saline conditions. Agronomy 9:115. https://doi.org/10.3390/agronomy9030115
Van Genuchten MT, Gupta SK (1993) A reassessment of the crop tolerance response function. J Indian Soc Soil Sci 41:730–737
Villa-Castorena M, Ulery AL, Catalán-Valencia EA, Remmenga MD (2003) Salinity and nitrogen rate effects on the growth and yield of chile pepper plants. Soil Sci Soc Am J 67:1781–1789. https://doi.org/10.2136/sssaj2003.1781
Walia H, Wilson C, Condamine P et al (2005) Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol 139:822–835. https://doi.org/10.1104/pp.105.065961
Wang H, Zhang M, Guo R et al (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol 12:194. https://doi.org/10.1186/1471-2229-12-194
Wang Z, Zhang W, Beebout S et al (2016) Grain yield, water and nitrogen use efficiencies of rice as influenced by irrigation regimes and their interaction with nitrogen rates. Field Crop Res 193:54–69. https://doi.org/10.1016/j.fcr.2016.03.006
Wang J, Zhu J, Zhang Y et al (2018) Comparative transcriptome analysis reveals molecular response to salinity stress of salt-tolerant and sensitive genotypes of indica rice at seedling stage. Sci Rep 8:2085. https://doi.org/10.1038/s41598-018-19984-w
Wassmann R, Phong ND, Tho TQ et al (2019) High-resolution mapping of flood and salinity risks for rice production in the Vietnamese Mekong Delta. Field Crop Res 236:111–120. https://doi.org/10.1016/j.fcr.2019.03.007
Xu J, Huang X, Lan H et al (2016) Rearrangement of nitrogen metabolism in rice (Oryza sativa L.) under salt stress. Plant Signal Behav. https://doi.org/10.1080/15592324.2016.1138194
Xu C, Li Q, Liu X et al (2019) Effects of nitrogen supply level on photosynthesis and chlorophyll fluorescence characteristics of rice under salt stress. Emir J Food Agric. https://doi.org/10.9755/ejfa.2019.v31.i10.2014
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Routine procedure for growing rice plants in culture solution. Lab Man Physiol Stud Rice, pp 61–66
Zeng L, Shannon MC (2000) Salinity effects on seedling growth and yield components of rice. Crop Sci 40:996–1003. https://doi.org/10.2135/cropsci2000.404996x
Acknowledgements
This research received support from the Belgian Académie de Recherche et d’Enseignement Supérieur—Commission de la Coopération au Développement (ARES-CCD: www.ares-ac.be).
Author information
Authors and Affiliations
Contributions
PTHN, PVC, and PB designed the project. PTHN, AH, and MB conducted the experiments and analyzed the data. AH and SL analyzed the parameters in the laboratory. PTHN and PB wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phan, N.T.H., Heymans, A., Bonnave, M. et al. Nitrogen Use Efficiency of Rice Cultivars (Oryza sativa L.) Under Salt Stress and Low Nitrogen Conditions. J Plant Growth Regul 42, 1789–1803 (2023). https://doi.org/10.1007/s00344-022-10660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10660-y