Abstract
The release of toxic metals in the water creates an adverse condition for the living organisms (e.g., fish). The aim of this research was to learn more about the spatiotemporal variations and toxicity of heavy metals (As, Cr, Cd, and Pb) among fish species that are economically important (Tenualosa ilisha, Gudusia chapra, Otolithoides pama, Setipinna phasa, Mystus vittatus, Glossogobius giuris, Harpadon nehereus, Pseudapocryptes elongatus, Polynemus paradiseus, and Sillaginopsis panijus) collected from Pasur River. Heavy metal (HMs) concentrations were evaluated using the atomic absorption spectrometry (AAS) technique. Most of the metals showed no significant variation spatiotemporally (p ˃ 0.05) except As and Cr showed substantial variation in terms of seasons (p ˂ 0.05). All fish species’ Cr and Pb concentrations, as well as As and Cd values, were estimated to be greater than FAO/WHO tolerable concentrations, implying that these metals pose danger to humans. HM has a total hazard quotient (THQ) value in individual fish species reported to be greater than 1, whereas an individual metal, arsenic, exceeds the standard value (THQ > 1), causing a significant noncarcinogenic issue in the study region. The target hazard (TR) value for As and Pb exceeds the USEPA norm (10−4) suggesting that long-term consumption of fish poses a chronic cancer risk to the people in the study field. According to the findings, the fish in the Pasur River are unfit for human consumption. The correlation matrix (CM) indicates that sources of metals are similar (e.g., industries, ships, agricultural inputs, etc.).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
HMs’ high poisonousness, endurance, and bioavailability in the river water ecosystem are regarded as the most harmful chemical pollutants (Han et al. 2021; Lao et al. 2019; Ali et al. 2018; Bhuyan and Bakar 2017a; Islam et al. 2012). Since their increased trend in sediments, water, and fish, metal contamination has become a worldwide problem (Ahsan et al. 2018; Bhuyan et al. 2017, 2019; Burger and Gochfeld 2005; Santos et al. 2004). River water pollution has been exacerbated by rapid industrialization, haphazard urbanization, and indiscriminate population development (Bhuyan and Bakar 2017b; Hajeb et al. 2009; Islam et al. 2017). The examined river receives immense amounts of untreated effluents from crop fields, agrochemicals, industrial waste, sewage treatment, and industries such as power plants, cotton, fertilizers, oil refineries, and others. Moreover, some brickfields, feeding sites, and entertainment areas directly discharge chemical wastes to the river. The rising population increased water withdrawal and agriculture and other industrial practices all significantly affect the river. The river environment faces significant problems from overexploitation and the dumping of unprocessed textile wastewaters into the river (Bebbington et al. 1977; Singh and Kumar, 2017). Toxic metals discharged by these sources damage marine ecosystems (Bhuyan and Bakar 2017b; FAO/WHO 2002; Habibullah-Al-Mamun et al. 2017). Toxic metal pollution of fish due to intake of contaminated water and food (zooplankton and phytoplankton) are considered bioindicators of toxic metal contamination (Burger et al. 2002; Karunanidhi et al. 2017; Kuklina et al. 2014; Saha and Zaman 2013; Svobodova et al. 2004). The fish’s membrane and branchiae could be a good substrate of HM buildup. Because of their bioaccumulation origin in marine environments, these HMs are harmful to aquatic animals and humans (Bhuyan et al. 2016a; Islam et al. 2018). Metal poisoning poses a concern to human health since it enters the human food chain through the consumption of a range of aquatic species (e.g., fish) (Alhashemi et al. 2012; Habibullah-Al-Mamun et al. 2017; Islam et al. 2016). Adulteration of fish is a big growing issue because of the health risks involved with eating fish. Moreover, the livelihood of communities (use river water for their daily activities) living near or adjacent to the river are more prone to threat (Ali et al. 2016; Bhuyan and Islam, 2016; Bhuyan et al. 2016a; Osman et al. 2016).
Fish consumption has increased over the world at the same time as concerns about its nutritious and medicinal benefits have developed. Fish are considered the source house of high protein, minerals, vitamins, and unsaturated lipid (Bhuyan et al. 2016a; DoF 2019; Hossen et al. 2018; Medeiros et al. 2012). Ingestion of poisonous metal-contaminated fish, on the other hand, has been linked to a number of serious disorders. Chromium (Cr) causes anuria, nephritis, and severe lesions in infected fish, including kidney lesions (Proshad et al. 2018). Cadmium toxicity results in impaired reproductive ability, kidney illness, malignancies, hypertension, and hepatic dysfunction, among other things (Al-Busaidi et al. 2011; Ali et al. 2018; Bhuyan et al. 2016a). Lead poisoning damages the liver and produces renal failure (Bhuyan et al. 2019; Lee et al. 2011).
With a yearly demand of 42.38 lakh metric tons, fish accounts for a major component of the Bangladeshi population’s daily diet (DoF 2019; Hossen et al. 2018). Bangladeshis eat fish on a regular basis (annual ingesting 21.90 kg/person), and it is one of their key sources of protein (DoE, 2019). However, toxic metal concentrations in fish bodies enter into the human body (FAO/WHO, 2002), either directly or indirectly, and have an effect on human health (Islam et al. 2016). Bangladeshis tend to eat river fish as part of their regular diet. Industrial effluents pollute the Pasur River, resulting in large concentrations of HMs (e.g., As, Cr, Cd, and Pd) being dumped into the river. However, there is little scientific evidence of HM pollution in the fish of the study river. As a result, the current research looked into HMs in various commercial species from the Pasur River.
Materials and methods
Sampling sites
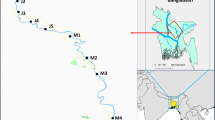
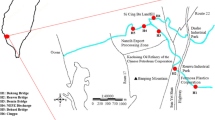
The study took place along the Pasur River, which is surrounded by various industries. It is in Khulna City, right next to the Sundarbans. The river flows into Bangladesh’s Bay of Bengal (Fig. 1). The Pasur River is a river in southwestern Bangladesh and a distributary of the Ganges. It continues the Rupsa River. All its distributaries are tidal. It meets the Shibsa River within the Sundarbans, and near to the sea, the river becomes the Kunga River (Dara et al. 2004). It is the deepest river in Bangladesh. The maximum and minimum widths are 650 m and 322 m, respectively, with an average width of 486 m. It is a meandering, perennial river and a considerable number of fisheries, dockyards, shipyards, and industries that are located along this river’s bank. Various types of industrial wastes, solid waste, and hazardous pollutants are produced as a result of unrest production activities, and most of them are promptly discharged into the river without adequate treatment. Fe, Cu, Zn, Cd, Pb, Mn, and As are found in the telecommunication, oil, limestone, metallurgical, plating, and battery industries’ by-products, respectively (Bhuyan et al. 2016b; Dara and Mishra 2004; Hilal and Ismail 2008).
Fish sample collection
During the winter and summer seasons, fish were collected from several water sites along the Pasur River. The detail of the collected fish is documented in Table 1. The studied fish species are mostly consumed by the Bangladeshi population on a regular basis. The government of Bangladesh earns foreign currency by exporting the examined fish species. Therefore, we have selected these fish species for the present study. A total of 10 individuals were collected for the analysis.
Geological information
The Pasur River is a distributary of the Ganges and one of Khulna’s most influential rivers. It follows the Rupsa River and all of its tributaries, all of which are tidal. Inside the Sundarbans, it reaches the Shibsa River, and near the sea, it merges with the Kunga River to form the Kunga River. It is the country’s river with the greatest depth. Its source is the Madhumati River northeast of Khulna, and it runs south for 110 mi (177 km) to the Bay of Bengal, passing through the swampy Sundarbans area and the port of Mongla. Summers have more rainfall and water than winters, which can lead to changes in water and sediment metal concentrations (Ali et al. 2016; Islam et al. 2015). Changes in HM concentrations in fish species are possible as a result of this (Ali et al. 2016; Bhuyan et al. 2016b).
Fish sample preservation
Before being transferred to the lab, the fish were placed on ice in a thermos to preserve roughly at − 4 °C. The fish were washed, scales removed, viscera removed, bone removed, skull removed, and gills removed) before being left to air dry. Samples were pasted and homogenized with an ultrasonic homogenizer after air drying before being kept in a plastic bag at − 25 °C (Bhuyan et al. 2016a).
Fish species identification
The site has been used to identify fish species. Species that were difficult to identify at the moment were taken to a lab for further examination. The photographs of studied fish species are presented in Fig. 2.
Heavy metal determination
AAS used a common analytical technique to assess the HM content. For metal analysis, sample selection is important. To prevent contamination, samples were regularly treated with care. The reagents were of analytical grade, and the glassware had been thoroughly washed. Throughout the report, distilled water was used. To correct the instrument’s readability, blank reagent determinations were used.
Fish sample digestion
Merck Germany provided all target element standard solutions with the maximum level of purity (99.98%). For sample digestion, The HNO3 used was ultrapure. All of the other acids and substances were super pure and were sourced from Germany and Spain (JECFA, 2005). Fish that had been homogenized were taken out of the freezer and allowed to come to room temperature for 1 h. For As determination, 2 g of material was poured in a 100 ml beaker and placed 15-m strenuous nitric acid on the hotplate (Ali et al. 2020; Bhuyan et al. 2016a; Rahman et al. 2020). Heat the sample at 130 °C for 5 h or until just 1–2 ml solution remains after digestion. After cooling the sample, 1 ml of hydrogen peroxide was added and heated at 120 °C for another 30 min. The sample was cleaned with Whatman 41 filter paper, rinsed with distilled water, and converted to a weight of 100 ml for AAS investigation. In a 50 ml beaker, 1.5 ml HCl was added to 12.5-ml processed test aliquots. After that, 1 ml of potassium iodide suspension was transferred to the last volume of 50 ml, and the As reduction reaction was allowed to finish for 2 h (FAO, 2006; Ali et al. 2020; Bhuyan et al. 2016a; Rahman et al. 2020; Shaheen et al. 2015). The reading was taken after a 5 ml aliquot of the ready sample was poured into the reaction container. Cr, Cd, and Pb analysis: in a 100-ml Pyrex beaker, 2 g of standardized sample was inserted and burned in a muffle furnace for a minimum of 10 h at 150 °C for 1 h. After it has cooled, we added 5 ml of 6 M HCl and heated it until totally dry on the hotplate. Finally, 10 ml of 0.1 M HNO3 was added and then heated for another 30 min before being filtered with Whatman No. 41 and leveled with 0.1 M HNO3 in a 50-ml volumetric flask. Finally, using GF-AAS, the samples were tested for Cr, Cd, and Pb (Ali et al. 2020; Rahman et al. 2020).
Analytical technique and accuracy check
Using a graphite furnace atomic absorption spectrometry (GFAAS) and hydride generator method, all of the media were tested for Pb, Cd, Cr, and As using AAS. All of the procedures were tested in-house in accordance with EC567/2002. Table 2 summarizes the analytical conditions for measuring HMs in samples using AAS.
The calibration criteria for instruments were created using Sigma-Aldrich’s (Switzerland) diluting standard (1000 ppm); mg/kg was used to denote the fish weight. Deionized ultrapure (0.05 s) water was utilized during the experiment. All equipment and bottles were washed with 20% nitric acid before being treated with deionized water and placed in an oven to dry (Lakshmanan et al. 2009; Lao et al. 2019). To ensure that the analytical process was correct, fish protein-approved reference substances for trace elements were applied. NRC (Canada) processed and supplied these fish samples (NRC 1989). The certified and observed values were found to be very close to each other. Table 3 demonstrates that the recorded certified materials’ standard deviations of the means ranged from 0.65 to 8%, with a % of a return of 89 to 99%.
Data analysis
Estimated daily intakes (EDIs)
Using the formula below, the EDI for HMs was derived by multiplying the mean content in samples by the wt. of food item intake by a person (60 kg bw/adult in Bangladesh), as determined by the family income and expenditure survey (Shaheen et al. 2015):
FIR stands for food intake rate (g/person/day), C stands for the metal amount in food (mg/kg), and BW stands for the adult resident’s BW (considering 60 kg) (FAO 2004; Pintaeva et al 2011). Fish is consumed at a rate of 59.91 g per day on a fresh wt. basis (HIES 2011; Oguri et al. 2012; Kuklina et al. 2013).
Risk of noncarcinogenicity
The risk-based concentration table was provided by the USEPA (2010). Region III was applied to estimate the noncarcinogenicity of fish. The THQ was used to calculate the noncarcinogenic danger of each metal from fish ingestion (USEPA 1989):
The letters THQ stand for target danger quotient, EFr for contact times (365 days/year), ED for exposure period (70 years), FIR for food intake rate (g/day), C for the amount of metal in foods (mg/kg dw), RfD for oral reference dosage (mg/kg/day), and AT for average time for noncarcinogens (365 days/year amount of exposure years) (USEPA 2008). For Cr, As, Cd, and Pb, object reference levels of 1.5, 0.0003, 0.0005, and 0.0035 mg/kg/day were adopted accordingly (Nadal et al. 2008; Reddy et al. 2008). There may be a health hazard if the THQ is ≥ 1, and relevant measures and safeguards should be undertaken (Islam et al. 2014).
To quantify the total possible for noncarcinogenic impacts from several HMs, a hazard index (HI) was developed following the (USEPA 1999USEPA (2006).) standards for health risk evaluation of chemical combinations. HI is calculated from THQs using the number of (USEPA 2010). The following is the equation for calculating the HI:
Carcinogenic risks
To evaluate carcinogen risk, the incremental risk of acquiring cancer throughout a lifetime of exposure to a possible carcinogen was used (USEPA 1989). The predicted carcinogenic hazards originating from As and Pb intake were estimated following the USEPA standard. The following equation was applied to estimate target hazard (TR):
where EFr is for contact time (365 days per year), ED stands for the contact period (70 years) [65], and AT stands for carcinogenic average time (365 days per year, 70 years). The oral carcinogen slope factor for As and Pb was 1.5 and 8.5 × 10−3 (mg/kg/day)−1, respectively, as per the IRIS database (USEPA, 2006; USEPA 2010).
Statistical analysis
The data was analyzed with the SPSS V. 20 statistics software. The metal values in fish were measured, and the means, standard deviations, and correlation coefficients were calculated. Microsoft Excel 2013 was used for the rest of the calculations.
Results and discussion
Amount of toxic substances in species of fish
The levels of hazardous metals in twelve fish from the Pasur River are tabulated in Table 4. In the current study, the mean value of As in fish fluctuated from 0.79 to 3.817 mg/kg dry wt. in the summer and winter seasons, respectively (Table 4).
HMs were estimated from fish muscle since Bangladeshi people are more likely to eat fish muscles (edible component) than branchiae, liver, kidneys, sex gland, and other portions of the fish. Present HMs concentration in fish flesh compared with different national and international rivers (Table 5). Figures 3 and 4 show the spatial–temporal variation of HMs.
Arsenic is widely distributed due to both man-made and natural origins. At site 3, the maximum value of As (3.817 mg/kg) was discovered in Mystus vittatus. Lates calcarifer at site 2 had the lowermost As amount (0.79 mg/kg). As levels varied between 0.79 and 2.94 mg/kg in the summer to 0.85 and 3.82 mg/kg in the winter. As is a possibly poisonous metal that is found in approximately 90% of seafood and fish species (USFDA 1993). For human health protection, in freshwater fish samples, the USEPA establishes a reference limit of 1.3 mg/kg (Burger et al. 2004). The highest allowed As concentration in tissue residual was set at 2 mg/kg by the ANZFA (2011). According to the findings of this analysis, approximately 70% of fish species surpass the value of the 1.3 limit set by the USEPA. G. chapra, T. ilisha, S. phasa, P. paradiseus, O. pama, H. nehereus, and S. panijus are among the fish species that cause As pollution due to higher As concentrations than MTC (Table 4).
The mean Cr concentration in the examined fish were fluctuated from 0.012 and 1.015 mg/kg dw between the summer and winter seasons (Table 4). Cr concentrations fluctuated from 0.012 to 0.834 mg/kg dw in the summer and 0.044 to 1.015 mg/kg dw in the winter. Sillaginopsis panijus at site 5 had the maximum amount (0.834 mg/kg) in the summer. Tenualosa ilisha at site 2 had the lowest, while Tenualosa ilisha at site 1 had the highest. In the winter, Harpadon nehereus had the highest value (1.015 mg/kg), and Tenualosa ilisha had the lowest value (0.044 mg/g) at site 3. In reality, Cr accumulation in the body of fish is lower in the developed world. According to the reference, Plaskett and Potter (1979) established a recommended reference value for Cr of 5.5 mg/kg dw in Western Australia, which was greater than the mean Cr concentration detected in the study. This study’s Cr amount was greater than earlier studies in the Kichera River, Okumeshi River, Gumti River, and Kichera River, indicating increased Cr pollution in this study region is shown in Table 4 (Amin et al. 2011; Raphael et al. 2011).
The mean Cd value in fish was recorded between 0.06 and 0.82 mg/kg dw in the summer and 0.08 and 0.88 mg/kg dw in the winter (Table 4). During the winter, the maximum value (0.88 mg/kg) was found in Mystus vittatus at site 3. During the summer, the lowest amount (0.06 mg/kg) was found in Cynoglossus arel at site 2 (Table 4). O. pama, G. chapra, H. nehereus, S. phasa, S. panijus, P. chinensis, and P. paradiseus surpass the maximum permissible value (0.10 mg/kg dw) for Cr, which is regarded a risk to human health when consumed. Cd in seafood has an allowable value of 2.0 mg/kg set by the ANHMRC (Plaskett and Potter 1979). Cd is a lethal HM that can cause extreme toxicity at very low concentrations (less than 1 mg/kg), and its deadly characteristics are greater than other metallic elements (Friberg et al. 1971). Cd amounts are capped at 1 mg/kg in Spanish law (DoF 2019; JECFA 2004). The Cd levels in the fish were found above the MTC value. The amount of Cd was recorded below the limit set by ANHMRC. Long-term Cd buildup in fish could pose a major threat to public health.
In the summer, the mean Pb amount in the examined fish was 0.5–3.3 mg/kg dw, while in the winter, it was 0.49–3.89 mg/kg dw (Table 4). The maximum amount (3.89 mg/kg dw) of Pb was reported in Mystus vittatus during winter at site 4, and the lowest value (0.49 mg/kg dw) was reported in Tenualosa ilisha during winter at site 4. Tenualosa ilisha and Gudusia chapra are some of the fish species found in the region. Humans living in the study area may be exposed to chronic toxicity from eating the studied fish species. ANHMRC proposed a maximum permissible value of 9.6 mg/kg dw (Plaskett and Potter 1979), and the Spanish regulation sets a limit of 2 mg/kg for Pb (Bristi et all 2019; Ekeanyanwu et al. 2010). When comparing Pb concentrations in the current study to those in the Gulf of Cambay, Okumeshi River, Wadi Hanifah, and Kichera River (Abdel-Baki et al. 2011; Reddy et al. 2007), it was found that the current study had higher Pb concentrations, indicating Pb toxicity is higher in fish species from the Pasur River. HM led is extremely toxic.
Spatiotemporal variation of metals in fish (ANOVA analysis)
There was no substantial variance in As (F = 0.032; p = 0.998), Cr (F = 0.084; p = 0.987), Cd (F = 0.023; p = 0.999), and Pd (F = 0.042; p = 0.997) according to sites (p ˃ 0.05) (Fig. 3). While As (F = 4.354; p = 0.04) and Cr (F = 5.422; p = 0.02) showed substantial fluctuation in respect of seasons (p ˂ 0.05). Cd (F = 2.917; p = 0.09) and Pd (F = 1.842; p = 0.08) exhibited lower variations in metal concentrations during summer and winter seasons (Fig. 4).
Heavy metal source identification in fish
To better understand the relationships between the HMs tested and to know the sources/origin of metals, the correlation matrix was used. The correlation among the elements in fish is shown in Table 6. Associations between metals can serve as sources and ways for metals found in the fish (Ahsan et al. 2018; Avigliano et al. 2015; Bebbington et al. 2012; Bhuyan et al. 2016a). In the summer, there was a very good positive association in As Vs Pb. In the winter, As Vs Pb (r = 0.852) had a very strong positive correlation, and Cd Vs Cr (r = 0.725) and Pd Vs Cr (r = 0.706) had a strong relationship. Pb and Cd had a moderately positive correlation (r = 0.694) (Table 6). The parameters were correlated and may have derived from the same sources in the study field, as the correlation between the metals was found to be positive and significant (Abbasi et al. 2013; Bhuyan and Bakar 2017a). Strong connections between heavy metals suggest mutual dependence, similar influence activity, and release from the same sources (Bhuyan et al. 2017; Jiang et al. 2014).
Estimated daily intake (EDI)
Adults in the study region who consume fish species in their regular diet have their dietary exposure to HMs determined by estimated daily consumption. To estimate daily consumption, utilize the mean value of each harmful element and the individual ingestion frequency of that element (Santos et al. 2004). We will find out how much HM is consumed on a daily basis based on the average daily consumption. Table 7 displays the average daily intake of HMs from fish eating in the current analysis. Fish consumption resulted in a lower total daily intake of HMs than the permissible value. Due to Bangladeshi people’s low fish consumption rate, the EDI is lesser than the allowable limit. As, Cr, Cd, and Pb had mean EDI concentrations of 0.42, 1.09, 0.28, and 0.86 mg/day, respectively.
Despite the fact that Bangladesh’s total EDI is low due to limited fish intake, long-term ingesting of polluted fish from the research area could have lethal health consequences for the people of Bangladesh (Islam et al. 2016). For the formulation of numerous regulatory criteria for fish intake, periodic surveillance is required. In this scenario, the EDI was calculated using a 60 kg person eating 59.91 g fish/day dw basis. Table 7 shows that the average EDI of HMs from fish is reported below than the reference amount (JECFA 1989, 2000; NRC 1989) implying that these fish species may not create an immediate risk, but continuous eating of these fish species may have a chance to create adverse risks to the consumers.
Risks of noncarcinogenic (THQ) and carcinogenic substances
Table 8 shows the THQ and carcinogenic risks of four HMs (As, Cr, Cd, and Pb) when consumed with HM contaminated fish. Due to fish consumption, the THQ values in As, Cr, Cd, and Pb were 60.66, 0.0046, 1.53, and 4.08, correspondingly. THQ values for As, Cd, and Pb are higher than the permissible value (1) that is considered important for human consumption, and these fish should not be consumed (Table 8). Again, people are exposed to many noncarcinogenic risks from high exposure to toxic metals as a result of eating these fish. In fish species, the total THQ for individual metals ranged from 0.005 to 60.66. In this study, mainly As is a single metal that can pose a noncarcinogenic danger (THQ for As is > 1) (Table 8). Assuming a single metal THQ, the highest THQ was found in As in Mystus vittatus (10.89), followed by Sillaginopsis panijus (6.003). Each fish’s THQ and total individual factor (THQ > 1) has the potential to generate noncarcinogenic hazards such as cardiovascular, kidney, nervous, and bone diseases, according to the current investigation. In fish, As and Cd play the most important roles in HI (Table 8). Out of all fish species, Mystus vittatus (12.15) had the highest HI, followed by Sillaginopsis panijus (6.71) (Table 8). The HI for the fish declining in order of Mystus vittatus (12.15) > Sillaginopsis panijus (6.71) > Polynemus paradiseus (6.49) > Otolithoides pama (6.16) > Glossogobius giuris (6.09) > Setipinna phasa (5.08) > Harpadon nehereus (4.47) > Gudusia chapra (4.34) > Pseudapocryptes elongatus (4.14) > Tenualosa ilisha (3.73).
Consumption of the studied fish species in excess and on a regular basis could pose a number of noncarcinogenic risks. When individual metal THQ was considered, As had the maximum THQ due to its low RfD value relative to its amount, and As in Bangladesh’s Pasur River could create major human health problems. The carcinogenic risk was calculated using As and Pb concentrations in several fish. Depending on the exposure amount, As and Pb have both noncarcinogenic and cancer-posing effects. Obtained from animal experiments, Pb is a likely carcinogen and a possibly dangerous component classed as a carcinogen. Table 8 illustrates the As and Cr cancer risk for people in the study location who consume HMs from fish species. For all fish, the cancer hazard value for As was 0.81 to 2.45, and for Pb, it was 0.003 to 0.03. The reference value for cancer risk between 10−6 and 10−4 is the normal cancer risk number (Turkmen et al. 2009; Uneyama et al. 2007).
The cancer posing threat is insignificant if the target hazard (TR) value is less than 10−6, and TR values greater than 10−4 are not healthy for humans and may be responsible for causing cancer (USEPA 1989). As was associated with a significantly greater risk of cancer when the TR value in this investigation was compared to the typical value (10−4), and the risk for Pb was likewise higher than the standard value. People exposed to higher amounts of As and Pb from the present research fish species are at risk for cancer for the rest of their lives. In this analysis, the risk of cancer was assessed based on the ingestion of fish species. Other food sources are also available, but they are not included in this report.
Conclusion
According to the findings of this analysis, the majority of the fish species studied were found to be unsuitable for human consumption. As, Cr, Cd, and Pb contents in fish samples were greater than the allowable limit. Since (THQ > 1.0) was confirmed as a posing health hazard that is not carcinogenic individually and collectively, the analyzed HMs were documented powerful enough to be assumed chronic. As and Pb risk levels were found above the recommended threshold based on cancer risk (10−4). People who consume the infected fish on a daily basis are in danger of developing chronic cancer in the long run, according to the study.
Availability of data and materials
Though this research is not relevant with big data. On reasonable request, the first and second authors of this manuscript will provide the datasets created and/or evaluated during this investigation.
References
Abbasi AM, Iqbal J, Khan MA et al (2013) Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotox Environ Saf 92:237–244
Abdel-Baki AS, Dkhil MA, Al-Quraishy S (2011) Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. Afri J of Biotech 10:2541–2547
Ahsan MA, Siddique MAB, Munni MA, Akbor MA, Bithi UH, Mia MY (2018) Analysis of major heavy metals in the available fish species of the Dhaleshwari River, Tangail. Bangladesh Int J Fish Aquat Stud 6(4):349–354
Al-Busaidi M, Yesudhason P, Al-Mughairi S et al (2011) Toxic metals in commercial marine fish in Oman with reference to national and international standards. Chemosphere 85:67–73
Alhashemi AH, Sekhavatjou MS, Kiabi BH et al (2012) Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland. Iran Microchem J 104:1–6
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environmental Nanotechnology, Monitoring and Management 5:27–35
Ali MM, Ali ML, Islam MS, Rahman MZ (2018) Assessment of toxic metals in water and sediment of Pasur River in Bangladesh. Water Sci Technol 77(5):1418–1430
Ali MM, Ali ML, Proshad R, Islam S, Rahman Z, Kormoker T (2020) Assessment of trace elements in the demersal fishes of a coastal river in Bangladesh: a public health concern. Thalassas: An International Journal of Marine Sciences 36(2):641–655
Amin MN, Begum A, Mondal MGK (2011) Trace element concentrations present in five species of freshwater fish of Bangladesh. Bang J Scien Indus Res 46:27–32
ANZFA, 2011. Australian and New Zealand Food Standards Code, Standard 1.4.1- Contaminants and Natural Toxicants (F2011C00542). <http:// www.comlaw.gov.au/Details/F2011C00542> (accessed 20.11.11).
Avigliano E, Schenone NF, Volpedo AV, Goessler W, Fernández CA (2015) HMs and trace elements in muscle of silverside (Odontesthes bonariensis) and water from different environments (Argentina): aquatic pollution and consumption effect approach. Sci Total Environ 506–507:102–108
Avigliano E, Schenone N (2016) Water quality in Atlantic rainforest mountain rivers (South America): quality indices assessment, nutrients distribution, and consumption effect. Environ Sci Pollut Res 23(15):15063–15075
Bebbington GN, Mackay NJ, Chvojka R et al (1977) Heavy metals, selenium and arsenic in nine species of Australian commercial fish. Austra J Mar Freshwa Res 28:277–286
Bebbington A (2012) Underground political ecologies: the second annual lecture of the Cultural and Political Ecology Specialty Group of the Association of American Geographers. Geoforum 43(6):1152–1162
Bhuyan S, Islam S (2016) Present status of socio-economic conditions of the fishing community of the Meghna River adjacent to Narsingdi district. Bangladesh. Journal of Fisheries and Livestock Production 4:192
Bhuyan MS, Bakar MA, Akhtar A, Islam MS (2016a) Heavy metals status in some commercially important fishes of Meghna River adjacent to Narsingdi District, Bangladesh: Health Risk Assessment. American Journal of Life Sciences 4:60–70
Bhuyan MS, Islam MS, Akhtar A (2016b) Effects of industrialization on fish and fishing community. Scholars' Press: 104 pages; ISBN-10: 3659845469; ISBN-13: 978-3659845468, Karimganj, Assam, India.
Bhuyan MS, Bakar MA (2017a) Seasonal variation of heavy metals in water and sediments in the Halda River, Chittagong, Bangladesh. Environ Sci Pollut Res 35:27587–27600
Bhuyan MS, Bakar MA (2017b) Assessment water quality in Halda River (the major carp breeding ground) of Bangladesh. Pollution 3:429–441
Bhuyan MS, Bakar MA, Akhtar A, Hossain MB, Ali MM, Islam MS (2017) Heavy metals contamination in surface water and sediment of the Meghna River, Bangladesh. Environmental Nanotechnology, Monitoring & Management 8:273–279
Bhuyan MS, Bakar MA, Nabi MRN, Senapathi V, Chung SY, Islam MS (2019) Monitoring assessment of heavy metals contamination in surface water and sediment of the Old Brahmaputra River, Bangladesh. Appl Water Sci 9:1–13
Brishti PS, Islam MK, Sarkar S, Taskin F, Akter S, Salam MA, Nahar L, Billah MB (2018) Environmental contamination of heavy metals in fish and water samples of Shitalakkhya River, Dhaka, Bangladesh. Int J Sci Technoledge 6:86–91
Bristi WR, Zaman Z, Sultana N (2019) Predicting imdb rating of movies by machine learning techniques. In 2019 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT) (pp. 1–5). IEEE
Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99:403–412
Burger J, Gaines KF, Shane BC et al (2002) Metal levels in fish from the Savannah River: potential hazards to fish and other receptors. Environ Res 89:85–97
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Muzyczka N (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10(2):302–317
Dara SS, Mishra DD. 2004. A Text Book of Environmental Chemistry & Pollution Control. India: S Chand & Co Ltd Company; ISBN: 9788121908832, 7th Edition.
Demirak A, Yilmaz F, Tuna AL et al (2006) Heavy metals in water, sediment and tissues of Leciscus cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458
DoE (2019) Yearbook of Fisheries Statistics of Bangladesh, 2018–19. Fisheries Resources Survey System (FRSS), Department of Fisheries, Bangladesh: Ministry of Fisheries and Livestock 36:135
National Fish Week. 2019. Compendium (in Bangla) (pp.79-83pp) Publisher: DoF.2020. National Fish Week 2019 Compendium (in Bangla). Department of Fisheries, Ministry of Fisheries and Livestock, Bangladesh.160p.
Ekeanyanwu CR, Ogbuinyi CA, Etienajirhevwe OF (2010) Trace metals distribution in fish tissues, bottom sediments and water from Okumeshi River in Delta State, Nigeria. Ethiop J Environ Stud Manag 3:12–17
FAO/WHO (2004) Report of the Thirtieth Session of the Codex Committee on Food Labelling. 6–10 May 2002, Halifax, Canada. Available at https://ftp.fao.org/codex/alinorm03/Al03_22e.pdf. Accessed Mar 2004
FAO. 2006. Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. Food and agriculture organization of the United Nations regional office for Asia and the Pacific, Bangkok, Thailand
FAO/WHO. 2002. Codex Alimentarius- general standards for contaminants and toxins in food. Schedule 1 maximum and guideline levels for contaminants and toxins in food. Joint FAO/WHO food standards programme, Codex Committee, Rotterdam. Reference CX/FAC 02/16
Friberg L, Piscator M, Nordberg G (1971) Cadmium in the environment. The Chemical Rubber Co, Press, Cleveland, Ohio
Habibullah-Al-Mamun M, Ahmed MK, Raknuzzaman M, Islam MS, Ali MM, Tokumura M, Masunaga S (2017) Occurrence and assessment of perfluoroalkyl acids (PFAAs) in commonly consumed seafood from the coastal area of Bangladesh. Mar Pollut Bull 124:775–785
Hajeb P, Jinap S, Ismail A et al (2009) Assessment of mercury level in commonly consumed marine fishes in Malaysia. Food Con 20:79–84
Han JL, Pan XD, Chen Q, Huang BF (2021) Health risk assessment of heavy metals in marine fish to the population in Zhejiang. China Scientific Reports 11(1):1–9
HIES (Household Income and Expenditure Survey). 2011. Preliminary report on household income and expenditure survey-2010. Bangladesh Bureau of Statistics, Statistics division, Ministry of planning, Dhaka
Hilal AHA, Ismail NS (2008) Heavy metals in eleven common species of fish from the Gulf of Aqaba, Red Sea. Jordan J Biol Sci 1:13–18
Hossen S, Ali MM, Rahman MA, Shahabuddin AM, Islam MS, Chowdhury MTH, Islam MK (2018) A comprehensive analysis of socioeconomic structure and constraints of fishers community of the Tetulia River in Bangladesh. Australian Journal of Science and Technology 2(2):83–89
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Masunaga S (2015) Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environ Toxicol Pharmacol 39:347–357
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Raknuzzaman M, Ali MM, Eaton DW (2016) Health risk assessment due to heavy metals exposure from commonly consumed fish and vegetables. Environment Systems and Decisions 36(3):253–265
Islam MS, Han S, Masunaga S (2014) Assessment of trace metal contamination in water and sediment of some rivers in Bangladesh. J Water Environ Technol 12:109–121
Islam SR, Kwak D, Kabir MH, Hossain M, Kwak KS (2015b) The internet of things for health care: a comprehensive survey. IEEE access 3:678–708
Islam MS, Kormoker T, Ali MM, Proshad R (2018) Ecological risk analysis of heavy metals toxicity from agricultural soils in the industrial areas of Tangail District, Bangladesh. SF Journal of Environmental and Earth Science 1(2):1022
Islam MS, Akbar A, Akhtar A, Kibria MM, Bhuyan MS (2017) Water quality assessment along with pollution sources of the Halda River. Asiatic Society Journal 43:61–70
Islam MN, Kitazawa D, Kokuryo N, Tabeta S, Honma T, Komatsu N (2012) Numerical modeling on transition of dominant algae in Lake Kitaura, Japan, Ecological Modelling, 242: 146–163. Elsevier publication. https://doi.org/10.1016/j.ecolmodel.2012.05.013
JECFA. 1989. Evaluation of certain food additives and contaminants. Thirty-third report of the joint FAO/WHO Expert Committee on Food Additives. (WHO technical report series, No. 776), World Health Organization, Geneva
JECFA. 2000. Evaluation of certain food additives and contaminants. Fifty-third report of the joint FAO/WHO Expert Committee on Food Additives. (WHO technical report series, No. 896), World Health Organization, Geneva
JECFA. 2005. Codex general standard for contaminants and toxins in food and feeds. 64th meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), JECFA/64/CAC/RCP 49–2001
Jiang X, Teng A, Xu W, Liu X (2014) Distribution and pollution assessment of heavy metals in surface sediments in the Yellow Sea. Mar Pollut Bull 83:366–375
Karunanidhi K, Rajendran R, Pandurangan D, Arumugam G (2017) First report on distribution of heavy metals and proximate analysis in marine edible puffer fishes collected from Gulf of Mannar Marine Biosphere Reserve, South India. Toxicol Rep 4:319–327
Kuklina I, Kouba A, Kozak P (2013) Real-time monitoring of water quality using fish and crayfish as bio-indicators: a review. Environ Monit Assess 185:5043–5053
Kuklina I, Kouba A, Buřič M, Horká I, Ďuriš Z, Kozák P (2014) Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. BioMed research international 2014
Lakshmanan R, Kesavan K, Vijayanand P et al (2009) Heavy metals accumulation in five commercially important fishes of Parangipettai, southeast coast of India. Advan J Food Sci Tech 1:63–65
Lao Qibin SuQ, Liu G, Shen Y et al (2019) Spatial distribution of and historical changes in heavy metals in the surface seawater and sediments of the Beibu Gulf, China. Mar Pollut Bull 146:427–434
Lee K, Kweon H, Yeo J et al (2011) Characterization of tyrosine-rich Antheraea pernyi silk fibroin hydrolysate. Inter J Biologi Macromol 48:223–226
Medeiros RJ, Santos LM, Freire AS et al (2012) Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control 23:535–541
Nadal M, Ferre´-Huget N, Martı´-Cid R et al (2008) Exposure to metals through the consumption of fish and seafood by population living near the Ebro River in Catalonia, Spain: health risks. Hum Ecol Risk Assess Int J 14:780–795
NRC. 1989. National Research Council Recommended Dietary Allowances (10th ed., pp. 241–243). Washington, DC: National Academy of Sciences, PP
Oguri T, Yoshinaga J, Tao H, Nakazato T (2012) Daily intake of inorganic arsenic and some organic arsenic species of Japanese subjects. Food Chem Toxicol 50:2663–2667
Osman BMS, Akhtar A, Islam MS (2016) Socio-economic conditions of the fishing community of Rezu khal in Ukhiya, Cox’s Bazar. Discovery 52:1933–1946
Pintaeva ET, Bazarsadueva SV, Radnaeva LD et al (2011) Content and character of metal accumulation in fish of the Kichera River (a tributary of Lake of Baikal). Contem Prob Eco 4:64–68
Plaskett D, Potter IC (1979) Heavy metals concentrations in the muscle tissue of 12 species of teleost from Cockburn Sound, Western Australia. Aus J Mar Freshwater Res 30:607–616
Proshad R, Islam MS, Kormoker T, Masud MEM, Ali MM (2018) Assessment of toxic metals contamination with ecological risk of surface water and sediment of Korotoa River in Bangladesh. International Journal of Advanced Geoscience 6(2):214–221
Rahman MM, Shehzad MT, Nayak AK, Sharma S, Yeasmin M, Samanta S, Naidu R (2020) Health risks from trace elements in muscles of some commonly available fish in Australia and India. Environ Sci Pollut Res 27(17):21000–21012
Raphael EC, Augustina OC, Frank EO (2011) Trace metals distribution in fish tissues, bottom sediments and water from Okumeshi River in Delta State, Nigeria. Environ Res J 5:6–10
Reddy MS, Mehata B, Dave S et al (2007) Bioaccumulation of heavy metals in some commercial fishes and crabs of the Gulf of Cambay, India. Current Sci 92:1489–1491
Reddy DV, Elanchezhian N (2008) Evaluation of tropical tree leaves as ruminant feedstuff based on cell contents, cell wall fractions and polyphenolic compounds. Livest Res Rural Dev 20 (5)
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ Monit Assess 185:3867–3878
Santos EE, Lauri DC, Silveira PCL (2004) Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Total Environ 327:69–79
Shaheen N, Ahmed MK, Islam MS et al (2015) Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’ foodstuffs (meat, milk and egg) in Bangladesh. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-015-6013-2
Singh UK, Kumar B (2017) Pathways of heavy metals contamination and associated human health risk in Ajay River basin, India. Chemosphere 174:183–199
Svobodova Z, Celechovska O, Kolara J et al (2004) Assessment of metal contamination in the upper reaches of the Ticha Orlice River. Czech J Anim Sci 49:458–641
Turkmen M, Turkmen A, Tepe Y et al (2009) Determination of metals in fish species from Aegean and Mediterranean seas. Food Chem 113:233–237
Uneyama C, Toda M, Yamamoto M, Morikawa K (2007) Arsenic in various foods: cumulative data. Food Addit Contam 24(5):447–534
USEPA. 2006. United States, Environmental Protection Agency, Integrated Risk Information System, [http://www.epa.gov/iris/substS]
USEPA. 1989. Risk assessment guidance for superfund. Human health evaluation manual part A, interim final, vol. I. Washington DC, United States Environmental Protection Agency; EPA/540/1–89/002
USEPA. 1999. Screening level ecological risks assessment protocol for hazardous waste combustion facilities. Appendix E: Toxicity Reference Values
USEPA (U.S. Environmental Protection Agency) (2008) Provisional Peer Reviewed Toxicity Values (PPRTV) for Antimony Trioxide. Superfund Health Risk Technical Support Center, NCEA, ORD, Cincinnati, OH
USEPA. 2010. Risk-based concentration table. http://www.epa.gov/ reg3hwmd/risk/human/index.htm.\
USFDA (1993) Guidance document for arsenic in shellfish. US Food and Drug Administration, Washington, DC, pp 25–27
Acknowledgements
During the field sampling, we were grateful for the assistance of Mr. Shaharior Hossen, Department of Fisheries Science, Chonnam National University, Republic of Korea, and Mr. A. S. Shafiuddin Ahmed from the Department of Fisheries and Marine Science, Noakhali Science and Technology University, Bangladesh
Funding
The National Science and Technology Fellowship (NST-2014–15) from the Ministry of Science and Technology, Government People’s Republic of Bangladesh gave economic support to the writers.
Author information
Authors and Affiliations
Contributions
Mir Mohammad Ali (MMA) and Mohammad Lokman Ali (MLA) were theinvestigators of this study. They have drafted the preliminary manuscript. Md. Simul Bhuyan (MSB) and Md. Saiful Islam (MSI) were the supporting investigators who collected data. Md. Zillur Rahman (MZR) and Md. Wahidul Alam (MWA) validated the experimental and laboratory analysis. Monika Das (MD) and Sobnom Mustary (SM) supported the analysis of the data and compiled the manuscript with the first and second authors. Md. Nazrul Islam (MNI) supported the analysis of the data for the manuscript and provided technical support to improve the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In this investigation, there are no ethical problems, but we have included a statement certificate on ethics approval and for experimental studies involving local fish and others.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, M.M., Ali, M.L., Bhuyan, M.S. et al. Spatiotemporal variation and toxicity of trace metals in commercially important fish of the tidal Pasur River in Bangladesh. Environ Sci Pollut Res 29, 40131–40145 (2022). https://doi.org/10.1007/s11356-022-18821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18821-y