Abstract
Contamination of heavy metals in fish and vegetables is regarded as a major crisis globally, with a large share in many developing countries. In Bogra District of Bangladesh, concentrations of six heavy metals, i.e., chromium (Cr), nickel (Ni), copper (Cu), arsenic (As), cadmium (Cd) and lead (Pb), were evaluated in the most consumed vegetables and fish species. The sampling was conducted during February–March 2012 and August–September 2013. The levels of metals varied between different fish and vegetable species. Elevated concentrations of As, Cd and Pb were observed in vegetable species (Solanum tuberosum, Allium cepa and Daucus carota), and fish species (Anabas testudineus and Heteropneustes fossilis) were higher than the FAO/WHO permissible limits, indicating these three metals might pose risk from the consumption of these vegetable and fish species. The higher concentration of heavy metals in these vegetable species might be due to the higher uptake from soil and sediment ingestion behavior in fish species. Multivariate principal component analysis (PCA) showed significant anthropogenic contributions of Cr, Ni, Cu and Pb in samples as the PCA axis scores were correlated with scores of anthropogenic activities. Target hazard quotients showed that the intakes of Cu, As and Pb through vegetables and fish were higher than the recommended health standards, indicated non-carcinogenic risk. Therefore, intakes of these elements via fish and vegetables for Bangladeshi people are a matter of concern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal pollution has become a worldwide concern because of their ever increasing contamination in soil, vegetables, water and fish in developed and developing countries such as Bangladesh (Ahmad et al. 2010; Islam et al. 2014a). Heavy metals are not only the threat to public water supply but also they can pose risk to human health through the consumption of aquatic products especially fish (Terra et al. 2008). Fish is an aquatic animal that can be severely exposed to metal pollution from the contaminated environment. Essentially, fish assimilate metals by ingestion of particulates suspended material in water, by ingestion of food, by ion exchange of dissolved metals across the lipophilic membranes, e.g., gills, and by adsorption on tissue and membrane surfaces. However, contamination of heavy metals in fish species has become an important worldwide concern, not only because of the threat to fish but also human health (Yi et al. 2011; Alhashemi et al. 2012). Like fish, vegetables also can uptake heavy metals from contaminated soil which can cause health problems to both animals and human beings who consume them (Alam et al. 2003; Rahman et al. 2013; Saha and Zaman 2013). Therefore, metal contamination in fish and vegetables cannot be underestimated as these foods are important components for human diet. Nowadays, food safety is a major concern to the environmental scientist. The increasing demand of research related to food safety has been accelerated regarding the consumption of metal-contaminated foods that posed risk to human health (Mansour et al. 2009; Shaheen et al. 2015). Heavy metals that are of concern include Cr, Ni, Cu, As, Cd and Pb. Heavy metals are responsible to cause variety of pulmonary adverse health effects, such as lung inflammation, fibrosis, emphysema, tumors, liver and kidney damage (Forti et al. 2011; Fang et al. 2014).
From the food consumption point of view, fish and vegetables are commonly consumed by the Bangladeshi population on a regular basis. Dietary intake is considered as the main route of exposure, although inhalation can play an important role in the highly contaminated sites (Zhuang et al. 2009). Hence, intake of heavy metals from fish and vegetables by humans has been widely reported throughout the world (Muchuweti et al. 2006; Zhuang et al. 2009). It is well established that the daily intake of heavy metals such as Zn, Mn, Fe and Cu via diets is required to maintain normal body function (Bean et al. 2010), and the excessive intake of essential elements would affect the human health with resultant pathological consequences (Powers et al. 2003). Although metals can change their chemical form, they cannot be degraded or destroyed. Therefore, the assessment of health risk of these metals via dietary intake is very important (Marti-Cid et al. 2008). There are numerous pathways (soil ingestion, inhalation of dust/soil particles) for exposure of the metals to humans, but food chain is the most important and noteworthy route (Islam et al. 2014b, 2015b). For the assessment of health risk, target hazard quotients (THQs) are used (Shaheen et al. 2015).
It is necessary to have the baseline data of heavy metal concentration in the environmental media especially fish and vegetables in Bangladesh. Some studies have been focused on the contamination of As in vegetables of Bangladesh (Alam et al. 2003; Das et al. 2004; Karim et al. 2008), while studies on other metals in most consumable fish and vegetables are scarce in the study area. Therefore, the objectives of the present study are to evaluate the concentration of heavy metals in vegetables and fish species that are usually consumed by Bangladeshi adults and to assess the potential health risk based on dietary intake, target hazard quotient and target cancer risk for the local residents of Bogra district, Bangladesh.
2 Materials and methods
2.1 Study area and sampling
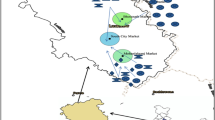
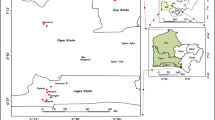
This study focused on the Korotoa River and its adjacent areas located at the northern part of Bangladesh. For the present study, sites of Korotoa River were selected that flows through the Bogra district urbanized area. The metropolitan area of Bogra district is about 71.56 square km, and total number of people is about 350,397. The study area is situated between 24°84′91.82″N and 89°37′29.57″E (Islam et al. 2015a). Thousands of villages, towns and commercial places such as Shibganj, Mohasthangarh and Sherpur have been built on both sides of Korotoa River. The sampling was conducted during February–March 2012 (winter season) and August–September 2013 (summer season). In Bangladesh, most of the vegetables are grown in winter and summer seasons, so we selected two seasons for our study. In total, 130 samples of thirteen different vegetable species, i.e., Phaseolus vulgaris, Brassica oleracea, Spinacia oleracea, Solanum melongena, Solanum lycopersicum, Solanum tuberosum, Allium cepa, Daucus carota, Capsicum annuum L, Lagenaria siceraria, Cucumis sativus, Musa acuminate and Momordica charantia, were collected from the agricultural fields adjacent to the Korotoa River, and 110 samples of eleven different fish species, i.e., Channa punctata, Awaous grammepomus, Anabas testudineus, Heteropneustes fossilis, Neotropius atherinoides, Colisa fasciata, Channa striata, Notopterus notopterus, Batasio batasio, Corica soborna and Puntius chola, were collected from the river (Fig. 1). After collection, samples were carefully washed with deionized water and the edible parts of vegetables and fish (muscles tissues) were cut into small pieces and then oven-dried at 70–80 °C to attain constant weight. The dried samples were then crumbled and pulverized with a porcelain mortar and pestle and stored in an airtight clean ziplock bag in freezing condition until chemical analysis.
2.2 Sample analysis
All chemicals were analytical grade reagents, and Milli-Q (Elix UV5 and MilliQ, Millipore, USA) water was used for the preparation of the solution. 0.5 g dried powdered of fish and vegetable samples were digested with 6 ml 69 % HNO3 and 2 ml 30 % H2O2 (Wako Chemical Co, Tokyo, Japan) in a Microwave Digestion System (Berghof speedwave®, Eningen, Germany). The extracted samples were then transferred into a Teflon beaker, and solution was filtered by using DISMIC®-25HP PTFE, pore size = 0.45 µm syringe filter(Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and stored in 50 ml polypropylene tubes.
2.3 Instrumental analysis and quality assurance
Metals were analyzed by using inductively coupled plasma mass spectrometer (ICP-MS). To prepare calibration curve multi-element Standard XSTC-13 (Spex CertiPrep® Metuchen, USA) solutions were used. Working standards, prepared daily in 5 % (V/V) HNO3 at 69 % ultrapure grade, were used. Multi-element solution (Agilent Technologies, USA) 1.0 µg/l was used as tuning solution covering a wide range of masses of elements. All test batches were evaluated using an internal quality approach and validated if they satisfied the defined internal quality controls (IQCs). The precision and analytical accuracy were checked by analysis of standard reference material (DORM-2—dogfish muscle from the National Research Council, Canada) and were analyzed to confirm analytical performance and good precision (relative standard deviation bellow 20 %) of the applied method (Table 1).
2.4 Data analysis
2.4.1 Estimated daily intake of heavy metals
The estimated daily intake (EDI) of heavy metals through fish and vegetables were measured using metal concentrations in samples, daily consumption and body weight. Following equation was used for the calculation of EDI (Ahmed et al. 2015a, b; Shaheen et al. 2015):
where FIR is the vegetable ingestion rate (g/person/day), C is the metal concentration in samples [mg/kg, fresh weight (fw)] and BW is the body weight assuming 60 kg for adult residents in the present study (FAO 2006). For adult residents, the daily consumption rate of vegetables and fish were 170.04 and 59.91 g, respectively, on a wet weight basis (HIES 2011).
2.4.2 Non-carcinogenic risk
Based on the USEPA (1989), we assumed that the ingestion dose is equal to the adsorbed contaminant dose and that cooking has no effect on the contaminants. In this study, the non-carcinogenic health risk associated with the consumption of fish and vegetable species by the local inhabitants was assessed based on the target hazard quotients (THQs) and calculations were made using the standard assumption for an integrate USEPA risk analysis. Higher THQ values mean a higher probability of experiencing long-term non-carcinogenic health effects. Following equation was used for the calculation of THQ (Abbasi et al. 2013; Ahmed et al. 2015a, b):
where EFr is the exposure frequency (365 days/year), ED is the exposure duration (70 years) equivalent to the average lifetime of human (USEPA 1991); RfD is the oral reference dose (mg/kg/day), BW is the average body weight (adult, 60 kg) and AT is the averaging time for non-carcinogens (365 days/year × number of exposure years, assuming 70 years). The oral reference doses were 1.5, 0.02, 0.04, 0.0003, 0.003 and 0.004 mg/kg/day for Cr, Ni, Cu, As, Cd and Pb, respectively (Nadal et al. 2008; USEPA 2010). If the THQ is less than 1, the exposed population is unlikely to experience obvious adverse effects. If the THQ is equal to or higher than 1, there is a potential health risk (Wang et al. 2005), where interventions and protective measurements should be taken.
2.4.3 Carcinogenic risk
For carcinogens, risk was estimated as the incremental probability of an individual to develop cancer over a lifetime exposure to that potential carcinogen (i.e., incremental or excess individual lifetime cancer risk USEPA 1989). Below written equation (USEPA 1989, 2010; Ahmed et al. 2015a, b) was used for estimating the target carcinogenic risk:
CSFo oral carcinogenic slope factor of As and Pb calculated from the integrated risk information system (USEPA 2010) database was 1.5 and 8.5 × 10−3 (mg/kg/day)−1.
2.5 Statistical analysis
The data were statistically analyzed using the statistical package, SPSS 16.0 (SPSS, USA). The means and standard deviations of the metal concentrations in vegetables and fish samples were calculated. Multivariate post hoc Tukey tests were employed to examine the statistical significance in the differences of mean concentrations of heavy metals among fish species. Principal component analysis (PCA) and cluster analysis (CA) were used to obtain the detailed information of the dataset and gain insight into the distribution of heavy metals by detecting similarities or dissimilarities in samples. The PCA was performed using Varimax normalized rotation of the dataset using Ward’s Method (Abbasi et al. 2013; Islam et al. 2015b).
3 Results and discussion
3.1 Metal concentrations in vegetables
The concentrations of heavy metals [mg/kg dw (dry weight)] in vegetable species are listed in Table 2. The concentrations of metals varied among species of vegetables. The mean concentration of Cr ranged from 0.06 (M. acuminate) to 1.10 mg/kg (L. siceraria) (Table 2). Among An earlier study by Karim et al. (2008) conducted in Feni district, Bangladesh, and they collected 15 vegetable samples of eddoe, taro, potato, carambola, plantain, green papaya, callaloo and bottle-ground. They reported a considerable concentration of Cr in Bangladeshi vegetables (mean 27.14 mg/kg, range 23.31–33.84 mg/kg) where the main sources of heavy metals in those vegetables were due to the chemical changes in fertilizers and pesticides used in the agricultural lands. A recent study by Rahman et al. (2013) showed that mean concentrations of Cr from Bangladesh were 1.12 mg/kg (range 0.35–4.50 mg/kg) for leafy and 0.64 mg/kg (range 0.18–1.90 mg/kg) for non-leafy vegetables. Rahman et al. (2013) collected a total of 87 home-garden vegetables, namely arum leaf, pumpkin leaf, coriander leaf, radish leaf, gourd leaf, radish, spinach, red amaranth, arum stem, Indian spinach, arum tuber, bean, papaya, green chili and eggplant from Noakhali district in Bangladesh. In their study, the main sources of heavy metals in vegetables due to the use of metals contaminated groundwater as irrigation for vegetables production. In Bangladesh, the main sources of Cr in agricultural soils where the farmers use untreated or poorly treated wastewater from industries (Bhuiyan et al. 2011) and application of chemical fertilizers and pesticides for vegetable production (Islam et al. 2009).
Average concentration of Ni ranged from 0.08 (M. acuminate) to 0.93 mg/kg (L. siceraria) (Table 2). The average concentration of Ni in all vegetable species was 0.46 mg/kg, lower than the permissible limit (10 mg/kg), indicated that vegetables were not contaminated by Ni. The highest mean concentration of Cu was observed in A. cepa (3.00 mg/kg) followed by S. tuberosum (2.90 mg/kg) (Table 2). From Samta village in Bangladesh, Alam et al. (2003) reported Cu concentrations of 8.5 and 15.5 mg/kg in leafy and non-leafy vegetables (bottle-ground leaf, taro leaf, drumstick leaf, brinjal, ash gourd, snake gourd, potato, stem amaranth, ladies finger, green papaya, plantain, taro (Lati), eddoe, elephant foot and ghotkol) which was higher than the present study. Alam et al. (2003) reported that the sources of heavy metals in samples were derived from agricultural use of contaminated irrigation water, chemical fertilizers and various pesticides. However, Cu concentration in vegetables of the present study was comparable to the study in West Bengal, India (Roychowdhury et al. 2003), where the mean Cu concentration was 1.59 mg/kg (range 0.03–4.21 mg/kg).
The mean concentrations of As in vegetable species ranged from 0.13 mg/kg (S. lycopersicum, S. oleracea) to 0.52 mg/kg (P. vulgaris) (Table 2). The mean concentration of As in studied vegetables were higher than the FAO/WHO permissible limits (0.1 mg/kg), indicating As might pose risk from vegetable consumption. Study from Bangladesh by Rmalli et al. (2005) reported that As concentrations in vegetables ranged from 0.01 to 0.50 mg/kg, with a mean of 0.06 mg/kg. The range of As levels in homegrown vegetables from Samta village in Bangladesh was 0.02–0.49 mg/kg (Alam et al. 2003). The range of As was 0.07–3.99 mg/kg in vegetables from Chandpur and Jamalpur districts (Das et al. 2004) and <0.04–1.93 mg/kg in vegetables from Sathkhira, Rajshahi and Comilla districts, Bangladesh (Williams et al. 2006). The range of As levels in this study was comparable with the results from other studies (Alam et al. 2003; Rmalli et al. 2005) where slightly lower than the study conducted by Das et al. (2004). In vegetables, mean concentrations of Cd ranged from 0.01 (C. annuum) to 0.16 mg/kg (S. tuberosum) (Table 2). The concentration of Cd in vegetables from Samta village varied between 0.01 and 0.22 mg/kg (Alam et al. 2003). Cadmium concentrations in S. tuberosum, A. Cepa and D. Carota were higher than the FAO/WHO permissible limits (0.05 mg/kg) (Table 2), indicating these three species are contaminated by Cd. The highest mean concentration of Pb was observed in S. tuberosum (1.10 mg/kg) followed by L. siceraria (1.00 mg/kg) (Table 2). In the present study, the concentration of Pb in vegetables were in line with the study in Samta, Bangladesh (Alam et al. 2003), where the range of Pb in vegetables was 0.14–1.70 mg/kg, which indicated that the vegetables in the present study also are contaminated by heavy metals. Slightly higher concentration of Cr, Ni, Cd and Pb was observed in L. siceraria and S. tuberosum which could be due to the hyper-accumulative behavior and ion transmembrane transport in the endoderm cytoplasma membrane and water transport in the xylem vessel (Kumar et al. 2009; Li et al. 2012).
Elevated concentrations of As, Cd and Pb were observed in vegetables species (S. tuberosum, A. Cepa and D. Carota) were higher than the FAO/WHO permissible limits, indicating these three metals might pose risk from the consumption of these vegetable species. The FAO/WHO guideline values are decided based on contaminants, toxins and many substances regarding their occurrence in foods and their significance for human and animal health. The comparison results of the present study with the previous studies in Bangladesh indicated that the environment of Bangladesh still is polluted with heavy metals.
3.2 Metal concentrations in fish species
Although researchers have presented the elemental concentrations in various tissues, such as liver, kidneys, gills, gonads and muscles of fish (Ahmed et al. 2014), in the present study only fish muscles (edible portion) were considered for the determination of heavy metals since Bangladeshi people do not habitually consume the other parts of fish. The concentrations of Cr, Ni, Cu, As, Cd and Pb in eleven different fish species are listed in Table 3. As a whole, the mean concentrations of heavy metals in fish species showed the descending order of Ni > Cu > As > Pb > Cr > Cd. The mean concentration of Cr in fish species was 0.84 mg/kg, where the mean concentration ranged from 0.38 (mg/kg) (C. soborna) to 1.66 (mg/kg) (A. testudineus) (Table 3). The mean concentration of Ni was 5.20 (mg/kg), where the highest value was observed in A. testudineus (9.37 mg/kg). The Ni concentration in all examined fish species was higher than the maximum tolerable concentration (MTC) in fish (0.80 mg/kg), indicated that these species were contaminated by Ni.
Copper is an essential part of several enzymes and necessary for the synthesis of hemoglobin. Most aquatic organisms have evolved mechanisms to regulate concentrations of Cu in their tissues in the presence of variable concentrations in the ambient water, sediment and foods. Copper was detected in all examined fish species, and significant differences (p < 0.05) were observed for Cu content in A. testudineus and H. fossilis with other species. The mean concentration of Cu was observed 3.59 (mg/kg) with the range from 2.02 to 7.37 (mg/kg) (Table 3). Slightly higher concentration of Cr, Ni and Cu was observed in fish species (A. testudineus and H. fossilis) which might be due to the bottom dwelling habitat, and therefore, sediments could be the major sources of these metals in two fish species (Gupta et al. 2009; Tao et al. 2012; Islam et al. 2015c).
In the environment, As is widespread due to both anthropogenic and natural sources. The highest concentration of As was observed in H. fossilis (5.95 mg/kg) followed by A. testudineus (4.37 mg/kg). Arsenic concentration in these two species (A. testudineus and H. fossilis) showed significant differences (p < 0.05) with other fish species (Table 3) which might be due to the higher capability to enrich As from the water environment. Furthermore, these two omnivorous species are enriching metals especially As from the water column through ingestion (Islam et al. 2015c). The USEPA has set As in tissue residues of 1.3 mg/kg fw in freshwater fish as the criterion for human health protection (Burger and Gochfeld 2005). Therefore, two species of fish (A. testudineus and H. fossilis) are of concern for health risk due to As, whereas the other species were lower than MTC in fish. In fish samples, the mean concentrations of Cd ranged from 0.07 mg/kg (C. striata) to 0.83 mg/kg (H. fossilis) (Table 3). The average concentrations of Cd in fish species (except C. striata) were higher than MAC (0.10 mg/kg) (Table 3), indicated that these species might pose health hazards. The mean concentration of Pb ranged from 0.55 mg/kg (C. soborna) to 3.87 mg/kg (A. testudineus). Lead in all examined fish species was higher than the safe limit of 0.5 mg/kg set by WHO (2004), indicating the studied fish species were contaminated by Pb and might pose risk. From the literature survey, it was noticed that the species of A. testudineus and H. fossilis are bottom dwelling, and therefore, sediments could be the major sources of metal accumulation in these species (Tao et al. 2012). Among the fish species, the concentrations of heavy metals showed different patterns which could be the fact that the accumulation capacity of heavy metals in different species depends on their filtration rate, ingestion rate, gut fluid quality and detoxification strategies (e.g., storage in nontoxic form or elimination) (Wang and Rainbow 2008). The variation in metal concentration in the studied fish species revealed their different behavior and response to different metal elements in the environment (Tao et al. 2012).
3.3 Multivariate analysis
Multivariate principal component analysis (PCA) of heavy metals in the vegetables and fish species explains the cumulative variance of first three axes 77.1 % (vegetables) and 69.0 % (fish). In the PCA, first three principal component analyses were computed and the variance explained by them was 42.0, 20.0 and 15.1 % for vegetables and 27.1, 22.2 and 19.7 % for fish, respectively (Fig. 2). Overall, PCA revealed three major groups of the metals for both vegetables and fish. The first group revealed similar loadings of Cr, Ni, Cu and Pb in vegetables and Cr and Ni for fish were included in the first principal component (PCA1) explaining the greatest variance (42.0 % and 27.1 %), indicated that these were contributed by anthropogenic activities. The second group only included Cd for vegetables and As and Cd for fish which were mostly contributed by the industrial emissions in the vicinity of the sampling sites (Manzoor et al. 2006; Shah and Shaheen 2007). Third group comprised of As for vegetables and Cu and Pb for fish which were predominantly contributed by lithogenic sources (Iqbal and Shah 2011). The depositions of atmospheric particulates released by automobile emissions were believed to contribute these metals in the study areas from where the vegetable and fish samples were collected (Manzoor et al. 2006; Iqbal and Shah 2011; Pandey et al. 2012). PCA revealed that the apportionment of same kind of heavy metals in fish and vegetables was not similar which might be due to the emission behavior of heavy metals from the source to the environment. Furthermore, cluster analysis (CA) with dendrogram using Ward’s Method was adopted to divide the vegetable and fish species into several groups as shown in Fig. 3. Largely, the vegetable species were grouped into three major clusters. First cluster was composed of S. oleracea, S. lycopersicum, C. annuum, C. sativus, C. annuum, S. melongena, B. oleracea and M. acuminata, while second cluster consisted of P. vulgaris and L. siceraria. Third cluster comprised of S. tuberosum, D. carota, A. cepa and M. charantia. The vegetables in these clusters bear a resemblance based on their relative distribution of heavy metals, and hence, their nutritional value was comparable to each other. Moreover, on the basis of heavy metal concentrations in some vegetables and fish species showed strong significant correlations by forming primary groups/clusters with each other (Fig. 3).
3.4 Estimated daily intake (EDI)
This study estimates the dietary exposure of heavy metals through consumption of vegetables and fish in the daily diet of the adult’s people who live in the study area in Bangladesh. The estimated daily intakes (EDIs) of heavy metals were evaluated according to the average concentration of each heavy metal in each food and the respective consumption rate (Santos et al. 2004). The EDI of studied metals from consumption of fish and vegetables are shown in Tables 4 and 5. Total daily intakes of studied metals (except Cu) through the consumption of vegetables were higher than the maximum tolerable intake (MTDI) (Table 4), indicated that these vegetables might pose risk to the consumers in the study area, Bangladesh. On the other hand, total EDIs of studied metals through consumption of fish were lower than the MTDI (Table 5). The EDIs of heavy metals from fish were lower than MTDI which was due to the lower consumption rate of fish by Bangladeshi adults. Though the EDI of the studied metals was lower than the MTDI, but periodic surveillance will be necessary to set up regulatory norms for dietary intake of those fish species. However, the total EDI from vegetables consumption was higher than that of fish for the adults which could be attributed to the higher consumption rate of vegetables. The EDI revealed that vegetables are more responsible to create health risk than fish in the vicinity of Bogra district in Bangladesh.
3.5 Non-carcinogenic and carcinogenic risk
Target hazard quotients (THQs) of six heavy metals due to consumption of fish and vegetables are listed in Tables 6 and 7. The THQ value for individual metal (except As) in vegetable and fish species was less than unity, which is considered as safe for human consumption. Nevertheless, total values of THQ for Cu, As and Pb for vegetables and As and Pb for fish were greater than 1.0 (Tables 6, 7); consequently, the consumption of these vegetables and fish species was considered to be unsafe, and their consumption was not recommended. Therefore, the consumers are at high risk due to the exposure of Cu, As and Pb from vegetables and fish which were associated with non-carcinogenic risk. Given all metals in consideration, total THQ (sum of individual metal THQ) for the consumption of vegetable species (1.53 to 3.58) (Table 6) and fish species (1.57 to 6.03) (Table 7) and therefore potential health risk from vegetables and fish are of some concern. In this study, the single metal (except As) from single species of fish or vegetable did not pose any non-carcinogenic risk, whereas sum of individual metal THQ or individual species THQ showed non-carcinogenic risk (THQ > 1.0). The result showed the highest THQ of As (due to lower RfD value compared to its concentration) in fish sample which exceed the safe limit of 1.0, indicating that As might pose a potential health risk to the fish consumers in the study area. If people consume several species of fish and vegetables in their daily diet, then they exposed a considerable risk.
The target carcinogenic risk (TR) derived from the intake of As and Pb was calculated since these elements may promote both non-carcinogenic and carcinogenic effects depending on the exposure dose. Inorganic As is classified as a known carcinogen (USEPA group A) and Pb as probable carcinogen based on animal studies (USEPA group B2). The TR values of As and Pb for adults due to exposure to these elements from consumption of fish and vegetables are presented in Tables 6 and 7. In vegetables, TR values for As were ranged from 0.28 to 1.11 and for Pb 0.001 to 0.026 (Table 6). In fish species, TR values for As were ranged from 0.32 to 1.25 and for Pb 0.001 to 0.009 (Table 7). In general, the excess cancer risk lower than 10−6 is considered to be negligible, cancer risk above 10−4 is considered unacceptable, and cancer risk lying between 10−6 and 10−4 is generally considered an acceptable range (USEPA 1989, 2010). The carcinogenic risk of As and Pb was in the unacceptable range (>10−4) to the acceptable range (10−6 to 10−4). Considering the food-specific exposure, it was found that the TR values of As and Pb from consuming fish and vegetables were several times higher than the acceptable risk limit (10−4), indicating that the inhabitants consuming these vegetable and fish species are exposed to As and Pb with a lifetime cancer risk. Based on the results of the present study, the potential health risk for the inhabitants due to metal exposure through consumption of fish and vegetables should not be overlooked. In addition, there are also some other sources of metal exposures, such as consumption of other foodstuffs (e.g., rice, wheat, pulses, ground water, egg and meat) and dust inhalation, which are not included in this study. It is thus suggested that constant monitoring of both toxic and essential elements in all food commodities is needed in order to evaluate if any potential health risk to the consumers does exist.
4 Conclusions
In this study, concentrations of six heavy metals (Cr, Ni, Cu, As, Cd and Pb) in mostly consumed fish and vegetable species in Bangladesh were assessed. The concentrations of heavy metals in fish and vegetable species varied, and the estimated levels of heavy metals in vegetable species (L. siceraria and S. tuberosum) and fish species (A. testudineus and H. fossilis) were higher than the maximum tolerable concentration (MTC). The estimated daily intakes (EDIs) of Cr, Ni, As, Cd and Pb from vegetables were higher than the maximum tolerable daily intake (MTDI), suggesting a considerable risk. Single-element target hazard quotient (THQ) (except As) would not pose any potential risk, whereas sum of individual metal THQ or individual species THQ showed non-carcinogenic risk (THQ > 1.0). Also, the estimation showed that the target carcinogenic risk (TRs) of As and Pb was higher than the acceptable risk limit (10−4). From the health point of view, this study showed that the consumers of fish and vegetable species are exposed chronically to elemental pollution with carcinogenic and non-carcinogenic consequences. To figure out the fact regarding As poisoning in human body through fish and vegetable consumption, intensive investigation on the As speciation is needed, which is our future interest.
References
Abbasi MA, Iqbal J, Khan MA, Shah MH (2013) Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from lesser Himalayas, Pakistan. Ecotoxicol Environ Saf 92:237–244
Ahmad MK, Islam S, Rahman S, Haque MR, Islam MM (2010) Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int J Environ Res 4:321–332
Ahmed MK, Parvin E, Islam MM, Akter MS, Khan MS, Al-Mamun MH (2014) Lead and cadmium induced histopathological changes in gill, kidney and liver tissues of freshwater climbing perch Anabas testudineus (Bloch, 1792). Chem Ecol 30:532–540
Ahmed MK, Shaheen N, Islam MS, Al-Mamun MH, Islam S, Mohiduzzaman M, Bhattacharjee L (2015a) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292
Ahmed MK, Shaheen N, Islam MS, Al-Mamun MH, Islam S, Banu CP (2015b) Trace elements in two staple cereals (rice and wheat) and associated health risk implications in Bangladesh. Environ Monit Assess 187:326–336
Alam MG, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308:83–96
Alhashemi AH, Sekhavatjou MS, Kiabi BH, Karbassi AR (2012) Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland, Iran. Microchem J 104:1–6
Bean H, Schuler C, Leggett RE, Levin RM (2010) Antioxidant levels of common fruits, vegetables and juices versus protective activity against in vitro ischemia/reperfusion. Int Urol Nephrol 42:409–415
Bhuiyan MAH, Suruvi NI, Dampare SB, Islam MA, Quraishi SB, Ganyaglo S, Suzuki S (2011) Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ Monit Assess 175:633–649
Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99:403–412
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30:383–387
Fang Y, Sun X, Yang W, Maa N, Xin Z, Fu J, Liu X, Liu M, Mariga AM, Zhu X, Hu Q (2014) Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem 147:147–151
FAO (2006) Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. Food and agriculture organization of the United Nations regional office for Asia and the Pacific, Bangkok, Thailand
FAO/WHO (2002) Codex Alimentarius- General standards for contaminants and toxins in food. Schedule 1 maximum and guideline levels for contaminants and toxins in food. Joint FAO/WHO food standards programme, Codex Committee, Rotterdam. Reference CX/FAC 02/16
FAO/WHO (2011) Joint FAO/WHO Food Standards programme Codex committee on contaminants in foods. Food CF/5 INF/1, pp 1–89
FAO/WHO (2004) Safety evaluation of certain food additives and contaminants. WHO food additives series no. 52. World Health Organization, Geneva
Forti E, Salovaara S, Cetin Y, Bulgheroni A, Tessadri R, Jennings P, Pfaller W, Prieto P (2011) In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol in Vitro 25:454–461
Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157:449–458
HIES (household income and expenditure survey) (2011) Preliminary report on household income and expenditure survey-2010. Bangladesh Bureau of Statistics, Statistics division, Ministry of planning, Dhaka
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J Hazard Mater 192:887–898
Islam MM, Halim MA, Safiullah S, Hoque SAMW, Islam MS (2009) Heavy metal (Pb, Cd, Zn, Cu, Cr, Fe and Mn) content in textile sludge in Gazipur, Bangladesh. Res J Environ Sci 3:311–315
Islam MS, Ahmed MK, Al-Mamun MH, Hoque MF (2014a) Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ Earth Sci 73:1837–1848
Islam MS, Ahmed MK, Al-Mamun MH (2014b) Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Hum Ecol Risk Assess Int J 21:986–1006
Islam MS, Ahmed MK, Al-Mamun MH (2015a) Metal speciation in soil and health risk due to vegetables consumption in Bangladesh. Environ Monit Assess 187:288–302
Islam MS, Ahmed MK, Raknuzzaman M, Al-Mamun MH, Islam MK (2015b) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Ind 48:282–291
Islam MS, Ahmed MK, Raknuzzaman M, Al-Mamun MH, Masunaga S (2015c) Chemical speciation of trace metals in sediment and their bioaccumulation in fish of three urban rivers around Dhaka City, Bangladesh. Arch Environ Contam Toxicol 68:92–106
JECFA (2003) Summary and conclusions of the 61st meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). JECFA/61/SC. Rome
JECFA (2005) Codex general standard for contaminants and toxins in food and feeds. 64th meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), JECFA/64/CAC/RCP 49-2001
Karim RA, Hossain SM, Miah MMH, Nehar K, Mubin MSH (2008) Arsenic and heavy metal concentrations in surface soils and vegetables of Feni district in Bangladesh. Environ Monit Assess 145:417–425
Kumar NJI, Soni H, Kumar RN, Bhatt I (2009) Hyper accumulation and mobility of heavy metals in vegetable crops in India. J Agric Environ 10:29–38
Li QS, Chen Y, Fu H, Cui Z, Shi L, Wang L, Liu Z (2012) Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J Hazard Mater 227–228:148–154
Mansour SA, Belal MH, Abou-Arab AAK, Gad MF (2009) Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 75:601–609
Manzoor S, Shah MH, Shaheen N, Khalique A, Jaffar M (2006) Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. J Hazard Mater 137:31–37
Marti-Cid R, Llobet JM, Castell V, Domingo JL (2008) Dietary intake of arsenic, cadmium, mercury, and lead by the population of Catalonia, Spain. Biol Trace Elem Res 125:120–132
Muchuweti M, Birkett JW, Chinyanga E, Zvauya R, Scrimshaw MD, Lester JN (2006) Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implications for human health. Agric Ecosyst Environ 112:41–48
Nadal M, Ferré-Huget N, Martí-Cid R, Schuhmacher M, Domingo JL (2008) Exposure to metals through the consumption of fish and seafood by population living near the Ebro River in Catalonia, Spain: health risks. Hum Ecol Risk Assess Int J 14:780–795
Pandey R, Shubhashish K, Pandey J (2012) Dietary intake of pollutant aerosols via vegetables influenced by atmospheric deposition and wastewater irrigation. Ecotoxicol Environ Saf 76:200–208
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Swanson PD, Checkoway H (2003) Parkinson’s disease risks associated with dietary iron, manganese and other nutrient intakes. Neurology 60:1761–1766
Rahman MM, Asaduzzaman M, Naidu R (2013) Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J Hazard Mater 262:1056–1063
RDA (RecommendedDietaryAllowance) (1989) RecommendedDietaryAllowance, 10th edn. NationalAcademicPress, Washington
Rmalli SWA, Haris PI, Harrington CF, Ayub M (2005) A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci Total Environ 337:23–30
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal. India. Sci Total Environ 108(1–3):15–35
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ Monit Assess 185:3867–3878
Santos EE, Lauri DC, Silveira PCL (2004) Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Total Environ 327:69–79
Shah MH, Shaheen N (2007) Annual TSP and trace metal distribution in urban atmosphere of Islamabad in comparison with mega-cities of the world. Hum Ecol Risk Assess Int J 13:884–899
Shaheen N, Ahmed MK, Islam MS, Al-Mamun MH, Tukun AB, Islam S, Rahim ATMA (2015) Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’ foodstuffs (meat, milk and egg) in Bangladesh. Environ Sci Pollut Res. doi:10.1007/s11356-015-6013-2
Tao Y, Yuan Z, Xiaona H, Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol Environ Saf 81:55–64
Terra BF, Araujo FG, Calza CF, Lopes RT, Teixeira TP (2008) Heavy metal in tissues of three fish species from different trophic levels in a tropical Brazilian river. Water Air Soil Pollut 187:275–284
USEPA (1989) Risk assessment guidance for superfund. Human health evaluation manual part A, interim final, vol. I. Washington DC, United States Environmental Protection Agency; EPA/540/1-89/002
USEPA (2010) Risk-Based Concentration Table. http://www.epa.gov/reg3hwmd/risk/human/index.htm
US-EPA (1991) Technical support document for water quality-based toxics control (EPA/505/2-90-001). Washington, DC
Wang WX, Rainbow PS (2008) Comparative approaches to understand metal bioaccumulation in aquatic animals. Comp Biochem Physiol Part C 148:315–323
Wang X, Sato T, Xing B, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
WHO (1996) Guidelines for drinking-water quality, vol 2, 22nd edn. World Health Organization, Geneva
WHO (2004) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in ground waters. Environ Sci Technol 40(16):4903–4908
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407:1551–1561
Acknowledgments
The authors thank the authority of Patuakhali Science and Technology University (PSTU), Bangladesh, and Yokohama National University, Japan, for providing laboratory facilities to analyze vegetable and fish samples using conventional technique. The authors also delighted to express their gratefulness and sincerest thanks to Professor Dr. Md. Shams-Ud-Din (Vice Chancellor, PSTU), for his valuable suggestions and cooperation to carry out this research. Furthermore, we are thankful for the kind help from the members of the department of Soil Science Patuakhali Science and Technology University (PSTU), Bangladesh, during the field sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M.S., Ahmed, M.K., Habibullah-Al-Mamun, M. et al. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environ Syst Decis 36, 253–265 (2016). https://doi.org/10.1007/s10669-016-9592-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-016-9592-7