Abstract
Toxic metals and freshwater fish’s metalloid contamination are significant environmental concerns for overall public health. However, the bioaccumulation and sources of metal(loids) in freshwater fishes from Bangladesh still remain unknown. Thus, the As, Pb, Cd, and Cr concentrations in various freshwater fish species from the Rupsha River basin were measured, including Tenualosa ilisha, Gudusia chapra, Otolithoides pama, Setipinna phasa, Mystus vittatus, Glossogobius giuris, and Pseudeutropius atherinoides. An atomic absorption spectrophotometer was used to determine metal concentrations. The mean concentrations of metal(loids) in the fish muscle (mg/kg) were found to be As (1.53) > Pb (1.25) > Cr (0.51) > Cd (0.39) in summer and As (1.72) > Pb (1.51) > Cr (0.65) > Cd (0.49) in winter. The analyzed fish species had considerably different metal(loid) concentrations with seasonal variation, and the distribution of the metals (loids) was consistent with the normal distribution. The demersal species, M. vittatus, displayed the highest bio-accumulative value over the summer. However, in both seasons, none of the species were bio-accumulative. According to multivariate statistical findings, the research area’s potential sources of metal(loid) were anthropogenic activities linked to geogenic processes. Estimated daily intake, target hazard quotient (THQ), and carcinogenic risk (CR) were used to assess the influence of the risk on human health. The consumers’ THQs values were < 1, indicating that there were no non-carcinogenic concerns for local consumers. Both categories of customers had CRs that fell below the permissible range of 1E − 6 to 1E − 4, meaning they were not at any increased risk of developing cancer. The children’s group was more vulnerable to both carcinogenic and non-carcinogenic hazards. Therefore, the entry of metal(loids) must be regulated, and appropriate laws must be used by policymakers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, metal pollution in the aquatic ecosystem has already become a worldwide concern due to its serious threat to biological health and human beings (Hossain et al. 2018; Makedonski et al. 2017; Islam et al. 2022). In open water bodies, metal contamination has a serious impact on the residents (Vu et al. 2017; Ali et al. 2022a; Kubra et al. 2022). Another well-known problem with a striking trend is the health danger associated with eating aquatic foods contaminated with metals, particularly in developing like Bangladesh (Ali et al. 2016; Ahmed et al. 2019a). They can be bio-concentrated and bio-magnified through the trophic food chain due to their toxicity and inability to degrade, even though they appear insignificant in the environment (Ahmed et al. 2019b; Hossain et al. 2018; Ali et al. 2022b). Urbanization and industrialization have a significant negative effect on the aquatic environment due to the direct discharge of waste and untreated materials (Ahmed et al. 2021; Rahman et al. 2021; Islam et al. 2021a; 2022).
Aquatic foods are deemed potential sources of protein and essential amino acids for humans (Fuentes et al. 2009; Mohiuddin et al. 2022). Furthermore, because fish contain little fat and calories, they are considered the primary source of nutrients, vitamins, and energy (Miri et al. 2017; Fakhri et al. 2018). However, the situation becomes dangerous when people consume contaminated fish and other aquatic organisms over the safe limit (Ahmed et al. 2019a). The metal content’s density is five times higher than water in a particular aquatic environment (Ali et al. 2020a). Such concentration levels can damage valuable human organs like the liver, central nervous system, kidney, reproductive system, and intestinal tissue (Baki et al. 2018). On the other hand, aquatic organisms like fish from different habitats directly absorb contaminants from the environment, which leads to high fish mortality, abnormalities, and the extinction of a fish community (Khayatzadeh & Abbasi 2010; Ahmed et al. 2020; Rakib et al. 2021).
Humans can be exposed to toxic metals due to consuming contaminated fish through the food web. As most of the metal elements are resistant to microbial degradation, the concentration of those metals increases in an aquatic environment (Ahmed et al. 2015; Rani et al. 2021; Islam et al. 2021b). Regarding this problem, fish species and aquatic organisms are viewed as biomarkers or biomonitors, accumulating the metal contents in various organs (Baki et al. 2018). Determining the metal concentration in fish is crucial for the fish and for evaluating the threats to human health (Kalyoncu et al. 2012; Islam et al. 2015). Because some metal(loids), such as As, Cd, Pb, and Cr, are extremely poisonous, determining the concentration level is critical (Zhou et al. 2016; Kubra et al. 2022). Other metals like Cu, Mn, Fe, and Zn are required for the development of the human body and are not damaging to health until they reach a critical level (Roohani et al. 2013).
In general, long-term anthropogenic influences and natural consequences can result in bioaccumulation and biomagnification. The uptake, elimination, and accumulation rates are all variables (Islam et al. 2022; Bayen et al. 2005). According to a recent study, metal accumulation in fish is influenced by a variety of elemental chemical characteristics as well as ecological requirements, physiological conditions, individual size and age, life cycle, life history, and feeding preferences (Nyeste et al. 2019). Moreover, a fish species’ diet might change significantly throughout the lifespan, and as a result, the pattern of accumulated metals can differ among the various age groups of the same species (Subotić et al. 2013). Typically, fish tissues can collect metals through normal absorption. Fish absorb metals in various ways, including eating feed particles, ingesting particulates that are highly suspended in water, transitioning ion exchange into lipophilic tissues like gills, and adsorption on tissue and skin surfaces (Ahmed et al. 2016; Ali et al. 2020b). The bioaccumulation of metals in different fish species has been the subject of numerous studies, including those by Vu et al. (2017), Traina et al. (2019), Ali et al. (2022a, b), Varol et al. (2022) and others. Through a variety of routes, including ingestion, cutaneous contact, and inhalation, metals can have an effect on people. However, dietary intake is the primary route for exposure to metals (Zhuang et al. 2009). Planning human health risk indices will give the respected authorities specific information because metal can be passed from contaminated fish species to the human body.
One of Bangladesh’s commercial river systems, the Rupsha River, receives a variety of pollutants from industrial areas, manufacturing plants, chicken farms, and paint manufacturers (Kubra et al. 2022). Additionally, certain feeding stations, recreation areas, and brickfields discharge chemical waste directly into rivers (Islam et al. 2022). Various industrial operations and an increase in water demand for agriculture caused a significant impact on the river. Moreover, overexploitation and the discharge of unprocessed textile wastewater into rivers pose severe threats to river ecosystems (Singh and Kumar 2017). In addition, the Rupsha River is a potential source of economic and biological aspects. For instance, the poor local community depends on catching fish for daily consumption and selling them to the market to earn money. These include T. ilisha, O. pama, G. chapra, S. phasa, and so on. Around 3000 tons of fish caught from the river basin annually contribute to a significant fish supply to the national markets (Kubra et al. 2022). However, this commercially important river basin is gradually impacted by the attribution of different toxic metals. Although Samad et al. (2015) and Sabbir et al. (2018) conducted relevant studies, there has been no comprehensive research regarding bioaccumulation and human health risk from consuming some particular fish species. The current study will monitor the estimation of prospective human health risks due to the consumption of different commercial fish species. The human health setting as a result of fish consumption was flawless.
Therefore, the study aims to (1) determine some toxic metal(loid) concentrations in fish species commonly found in the Rupsha River basin, which are typically toxic both for fish and human health development; (2) evaluate bioaccumulation of the metal(loid)s in fish muscles, (3) assess the adverse human health impact due to intake of the contaminated fish specimens, and (4) identify the probable sources of the metal(loid)s in the fish species of the studied river. This is the first report to detect the probabilistic sources of heavy(loid)s in the fish species of the Rupsha River, located in the southwestern part of Bangladesh, and assess their health risk implications. This investigation will also help to highlight the current status of metal(loid) content in the studied area, which can pave the way to providing sufficient action platforms for the government authorities to control metal pollution.
Materials and methods
Study area
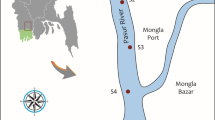
In the Rupsha River of Bangladesh, ten (10) different locations were used for the current investigation (Fig. 1). Upstream and downstream of the river, where the level of contamination is rising daily, were chosen as the study locations. Southwest Bangladesh is home to the Rupsha River, a distributary of the Ganges. On the other hand, the Bhairab and Madhumati rivers meet and merge to form the Pashur River. The river’s length is affected by tidal action as it joins the Bay of Bengal close to the Mongla channel, which flows next to Khulna via the Pashur River. At Chalna, it takes the name Pashur River before emptying into the Bay of Bengal (Saran et al. 2018). The average width is 486 m, with maximum and minimum widths of 650 and 322 m, respectively (Saran et al. 2018). The banks of this flowing, perennial river are home to several fisheries, shipyards, docks, and enterprises. Several common business practices have been used near the Rupsha River, which the Bangladeshi Department of Environment has recognized as Bangladesh’s most polluted area (DoE 2001). The glass and plastic industries, chemical complexes, steel mills, paper mill complexes, rayon mill complexes, cement factories, paint and dye manufacturing plants, numerous soap and detergent factories, many light industrial units, and waste from the brickyards and agricultural runoff, all directly discharge toxic effluent that has not been treated. From these industrial activities, metal(loids) as a point source may release to the aquatic environment at the study area which acts as anthropogenic source of heavy metals. Additionally, sewage and municipal wastes—unwanted byproducts mixed with Fe2O3·2H2O from the shipbreaking yard activities—are promptly released. Around 4500 m3/ha of the effluent from these enterprises enters the Bhairab-Rupsha River system, which flows into the Sundarbans (DoE 2001; Samad et al. 2015). Other diffuse sources of metal(loids) in the study river may arise due to the connecting of river channels with the Bhairab River and other unknown sources that contribute elevated levels of heavy metals in the riverine ecosystem. The sample sites were selected, located near pollution sources like factories and industrial units. The distance of the sampling sites was not unique as the pollution sources were not found at a unique distance.
Collection and processing of fish species
Ten primarily commercial fish species were studied in the current study, including T. ilisha (Ilish- Hilsa shad), G. chapra (Chaplia), O. pama (Poa), S. phasa (Phasa), M. vittatus (Tengra), G. giuris (Baila), P. atherinoides (Batashi), Heteropneustes fossilis (Singh), Trichogaster fasciata (Khailsha), and Cirrhinus reba (Tatkeni). Samples were collected in summer (Jun-Aug) and winter (Dec-Feb) seasons. Following orders, samples were transported to the lab for metal(loid) examination while being maintained in an airtight insulating ice box. Table 1 gives more information on the fish species that were gathered and are shown in Fig. 2. Scientific name, date of sampling, number of sampled specimens, and their habitat preferences of the fish species in the study area are shown in Table S1. Freshwater was used to rinse the fish samples in the lab to get rid of any surface adhesion, and a stainless steel blender cup was used to separate the edible muscles from the rest of the fish. The three subsamples of muscles were combined to create a composite sample for each species. After being freeze-dried for 48 h, the samples were ready for acid microwave digestion (Cabañero et al. 2007).
Microwave digestion of fish samples and metal(loid) analysis
Fish samples were deviated using a microwave accelerated reaction technique (MARS-5, Australia). A small amount of powder (1 g) was placed in a microwave-safe container with 20% ultrapure HNO3 and allowed to sit for 24 h to allow for digestion. The sample was then mixed with 6 mL of 98% ultra-pure HNO3, and the vessel was then switched out for the turntable motor assembly. In this procedure, three identical heating systems were employed: ramping time (15 min to heat up from room temperature to 180 °C), holding time (to heat the temperature from 0 to 180 °C and to hold the temperature for 10 min), and cooling time (cool down from 180 to 60 °C or less and time required for 15 min). Following digestion, the entire mixture was kept in a fume hood until it reached a temperature of 60 °C. The final solution volume was diluted with deionized water to 10 mL. An electric balance took the sample’s weight (AND EK 300 H, capacity 300 g, d = 0.01 g). Following the same scheme, a sample blank was also prepared to avoid contamination from the chemical or reagent used in the sample preparation. An atomic absorption spectrometer (AAS, GTA 120-AA240Z, Varian, Australia) was used to determine the presence of metals such as Pb, Cr, and Cd equipped with a graphite furnace, whereas As was detected using the hydride vapor generation technique in the AAS (SpectrAA 220, Varian, Australia). The limit of detection (LOD) for Cr, As, Cd, and Pb by the instrument was 0.00029, 0.001, 0.0002, and 0.0001 (mg/kg) and the limit of quantification (LOD) was 0.00099, 0.002, 0.0005, and 0.0005 (mg/kg), respectively.
Quality control in the analysis
Sigma-Aldrich of Switzerland provided a stock standard solution of 1000 ppm for each metal(loid) in order to create the calibration standards for the instruments. Throughout the study, deionized ultrapure water (conductivity 0.05 µS/cm) was used for sample preparation and standard dilution. All of the glassware and containers used in the experiment were carefully cleaned with a 20% nitric acid solution over the course of an entire night before being rinsed many times with deionized ultrapure water. Using the fish protein-certified reference material DORM-4 for trace elements, the analytical process was examined. In addition, the investigation included quality control using TORT-2 from the National Institute of Standards and Technology and the National Research Council of Canada. The results of certified and measured values were As (6.80 ± 0.09, 6.77 ± 0.13), Cr (1.87 ± 0.10, 1.85 ± 0.02), Cd (0.306 ± 0.005, 0.298 ± 0.01), and Pb (0.42 ± 0.07, 0.38 ± 0.18) which showed recovery rate 91.35–99.56% (Table S2). The certified and measured values indicated the good precision of the instrument analysis.
BAF
The bioaccumulation factor (BAF) evaluates the degree of metal contents in the fish species over time (Vu et al. 2017). It can be calculated considering the ratio of the metal concentration in the fish body and the concentration of the same metal in the living environment of the fish as follows (Wang et al. 2017):
where \({Cn}_{fish}\) is the metal concentration (mg/kg) in the edible fish tissues, and \({Cn}_{water}\) is the respective metal concentration (mg/L) in water. Based on the value ranges, BAF can be classified as follows, BAF < 1000: no accumulation probability; 1000 < BAF < 5000: bioaccumulative organism; BAF > 5000: extremely accumulative organism (Ali et al. 2020b, a). Metal(loid) statistical analysis such as the testing for normality of the datasets was performed by Kolmogorov and Smirnov (K-S) test in this work.
Human health risk assessment
EDI
The calculation of estimated daily intake (EDI) is primarily necessary to estimate the human health risk derived from the consumption of metal-contaminated aquatic foodstuffs (Ali et al. 2022b). In this case, EDI can be assessed based on the metal concentration level in aquatic food items and the consumption of the foods regularly (Bruno Lemos et al. 2012). The estimate of EDI can be done after following the USEPA-provided equation (USEPA 2000; Griboff et al. 2017):
where CN is the metal concentration (mg/kg-dry weight) in the fish sample, IGr is the ingestion rate according to the FAOSTAT database (period 20,122): 55.5 g/day for adults and 52.5 g/day for children (Ali et al. 2022b), and BWt is the bodyweight of the local consumers (adults: 70 kg and children: 15 kg) (USEPA 2008a).
Non-carcinogenic risk assessment
By calculating the possible risk of exposure to metal contaminants as a result of eating the contaminated foods, the total hazard quotient (THQ) can be used to determine the non-carcinogenic risk (USEPA 2011). THQ can be computed based on the ratio of the oral reference dose (RfD) to the estimated daily intake (EDI) for this aspect. The RfDs for the selected metal(loid)s As, Cd, Cr, and Pb in the current investigation are 0.0003, 0.001, 0.003, and 0.002, respectively (USEPA 2008b). The equation is followed by the estimation of THQ (Heshmati et al. 2018; Ali et al. 2022b).
where Ep is exposure frequency (365 days/year) (Ahmed et al. 2015), and Ed is the time period of exposure to the metal contents (65 years) (USEPA 2008a, b); At is the average time for non-carcinogenic elements (Ed \(\times\) Ep) (Ahmed et al. 2019a, b). The local consumers do not experience any non-carcinogenic impacts when the THQ values are less than unity (Abtahi et al. 2017).
HI evaluation
As humans consume various contaminated food items, the evaluation of hazard index (HI) is essential. Exposure to two or multiple contaminants may result in additives as well as interactive effects on human health (Saha et al. 2016). HI for the various pollutants can be assessed by the following equation (Ali et al. 2022a, b; Zhao et al. 2018):
where THQ denotes the risk value of the multiple metal contents available in the samples. Values higher than unity render significant non-carcinogenic impacts on human health (Yi et al. 2011).
CR assessment
The carcinogenic risk (CR) reveals the incremental possibility of cancer occurring in an individual for the exposure to the carcinogenic elements over a lifetime (Zhong et al. 2018). The acceptable range of carcinogenic effects ranges from 1E − 6 to 1E − 4 (USEPA 2000; Yin et al. 2015). Values greater than 1E − 4 refer to the high possibility of the carcinogenic effect occurring (Hu et al. 2017; Ali et al. 2022a, b). To assess this, the carcinogenic slope factor was considered for the particular metal contents, and the assessment equation is as follows (Gu et al. 2015; USEPA 2010):
where CSF is the oral carcinogenic slope factor which is identical for the particular metal content viz. As: 1.5 mg/kg/day, Cd: 6.35 mg/kg/day, and Pb: 0.0085 mg/kg/day (USEPA 2010). A value greater than 1E-5 denotes that the incremental possibility of cancer occurring would be more than 1 over 100,000 (Traina et al. 2019).
Statistical analysis
Microsoft Excel 2013 was used to investigate each and every mathematical result. Origin 2021 carried out multivariate analyses including principal component analysis and Pearson’s correlation matrices. The correlation matrix establishes if there is a positive or negative correlation between the metal contents (Wang et al. 2012; Merciai et al. 2014). Any association between the metal amounts indicates a lack of control when only one element is taken into account (Ali et al. 2022a; Kükrer et al. 2014). At p < 0.05, metal concentration variability in the fish samples was deemed significant. Kolmogorov–Smirnov and Shapiro–Wilk tests were carried out to determine a specific distribution of the contents in order to prevent problematic data distribution (Ali et al. 2022b). The sampling map was drawn using ArcGIS 10, and the normality tests were run using SPSS 23. Additionally, a two-way hierarchical heatmap employing similar groups of the elements connected to the gathered fish specimens at a distance between two nearest members was carried out using Origin 2021. The plotting of elements with identical characteristics in one cluster and differing contents in another cluster resulted in the contamination of fish sample statues (Sundaray et al. 2011).
Result and discussion
Level of metal(loid)s in the fish species
Metal distribution in the aquatic food items generally determines the environmental contamination status and human risk hazard (Fakhriet al. 2018). Accordingly, toxic metals found in the aquatic habitats, food chains, and the trophic level and the lifetime exposure to the metal contents signify a sign of bioaccumulation in different aquatic food materials (Jayapal et al. 2017). In the present study, the concentration of metal(loid)s considering summer and winter seasons in different commercial fish species is represented in Fig. 2. The As concentration in fish samples was ranged from 0.10 to 2.94 mg/kg with the mean value of 1.53 mg/kg, followed by Pb (range: 0.10 to 3.07 mg/kg, mean: 1.25 mg/kg), Cr (range: 0.15 to 0.77 mg/kg, mean: 0.51 mg/kg), and Cd (range: 0.06 to 0.95 mg/kg, mean: 0.39 mg/kg) in summer, and the concentration of As was ranged from 0.37 to 3.15 mg/kg with the mean value of 1.72, followed by Pb (range: 0.25 to 3.52 mg/kg, mean: 1.51 mg/kg), Cr (range: 0.24 to 1.01 mg/kg, mean: 0.65 mg/kg), and Cd (range: 0.07 to 1.06 mg/kg, mean: 0.49 mg/kg) in winter.
Arsenic (As)
As is typically present in our diets in a variety of chemical forms, but it might be difficult to quantify the total As content in seafood, which must contain at least 10% inorganic As (Ali et al. 2022b). Hence, humans can be exposed to inorganic As for a long time; the inorganic form of As is more lethal than the organic form (Baki et al. 2018). Moreover, exposure to inorganic As is detrimental to human health, causing dysfunction and failure of the lung, the digestive tract of the circulatory system, and the nervous system (Sarkar et al. 2017; Mandal and Suzuki 2002). In addition, the liver and skin can be extremely affected, and nausea, diarrhea, hypertension, diabetes, and cancer can occur due to intake of the instantly high As concentration (Cheng et al. 2010; Ali et al. 2016; Mateen et al. 2017; Centeno and Gray 2005). Intake of As mostly depends on the consumption of aqua products directly (Suñer et al. 1999). In our study area, the mean concentration (mg/kg) of As was observed in different fish species as follows: M. vittatus (3.04) > P. atherinoides (1.99) > Heteropneustes fossilis (1.81) > Cirrhinus reba (1.743) > Trichogaster fasciata (1.741) > O. pama (1.66) > G. giuris (1.60) > S. phasa (1.41) > G. chapra (1.04) > T. ilisha (0.24) (Table 1). In our study area, the As concentration in the specimens was higher than WHO (0.01 mg/kg) and FAO (1 mg/kg) values except for T. ilisha. However, all the concentrations were less than the MOFL (2014) standards. In comparison with some national studies, the concentration of As in our samples was higher than that of the Kirtankhola River (Ali et al. 2022b), Halda River (Ahmed et al. 2020), Meghna River (Ahmed et al. 2019a), Buriganga River (Ahmed et al. 2016), and Turag River (Rashid et al. 2017).
While findings from the Karnaphuli River (Ahmed et al. 2019b) and the Bangshi River (Rahman et al. 2012) were three and two times higher than our findings, for international studies, As concentration in the present work was almost two times higher than all other reports like Baiyangdian Lake, China (Zerizghi et al. 2020); Thamirabarani River, India (Arisekar et al. 2020); Ganga River, India (Maurya et al. 2019); Igbokoda River, Nigeria (Arojojoye et al. 2018); Swat River, Pakistan (Liu et al. 2020); and Perak River, Malaysia (Salam et al. 2020) (Table 1).
Chromium (Cr)
The availability of Cr in the diet significantly affects glucose and lipid metabolism (Saha et al. 2016). Moreover, Cr deficiency in public diets can retard the progress and glucose, protein, and lipid metabolism (Calabrese et al. 1985). However, excess ingestion of Cr may cause liver and kidney dysfunction along with pulmonary disorder (Forti et al. 2011; Alipour et al. 2015). In our study, Cirrhinus reba exhibited the highest Cr concentration (0.88 mg/kg) and followed by Heteropneustes fossilis (0.82 mg/kg), Trichogaster fasciata (80 mg/kg), P. atherinoides (0.69 mg/kg), Mystus vittatus (0.56 mg/kg), G. chapra (0.54 mg/kg),O. pama (0.50 mg/kg), G. giuris (0.43 mg/kg), and S. phasa (0.35 mg/kg) (Table 1). The mean concentration of Cr was far less than the international guidelines such as WHO (1985), Nauen (1983), and EU (2001). The mean concentration of Cr (0.58 mg/kg) in our work was higher than the Meghna River (Ahmed et al. 2019a) while less than the Karnaphuli River, Buriganga Bangshi River, and Turag River. In international studies, the mean concentration of Cr was slightly higher than Baiyangdian Lake, China; Thamirabarani River, India; Igbokoda River, Nigeria; Swat River, Pakistan; and Perak River, Malaysia. Nevertheless, it was higher than the Ganga River, India, which was insignificant.
Cadmium (Cd)
Even at a modest concentration of 1 mg/kg in meals, cadmium causes persistent poisoning (Ali et al. 2020a; Rahman et al. 2021). Consequently, the aquatic environment’s constituents are relatively lethal (Rahman et al. 2012). According to studies, consuming a lot of Cd in your diet may increase your risk of developing postmenopausal breast cancer and vascular conditions linked to dermal lesions (Itoh et al. 2014). In light of this, the Codex Committee on Food Additives (Ikem and Egilla 2008) and European Community legislation (EC 2001) set the allowable limit of Cd at 0.05 mg/kg in fish. The following fish species were found to have the highest Cd concentrations: P. atherinoides (1.0 mg/kg) > Heteropneustes fossilis (0.96 mg/kg) > Cirrhinus reba (0.69 mg/kg) > M. vittatus (0.56 mg/kg) > Trichogaster fasciata (0.42 mg/kg) > G. giuris (0.34 mg/kg) > O. pama (0.16 mg/kg) > S. phasa (0.11 mg/kg) > G. chapra (0.09 mg/kg) > T. ilisha (0.07 mg/kg). We observed that the mean concentration of Cd already surpassed the national and international guidelines from WHO (1985), Nauen (1983), EU (2001), and MOFL (2014) due to the presence of a high concentration of P. atherinoides.
Moreover, the mean value of Cd was also higher than other national studies regarding the Halda River, Karnaphuli River, Buriganga River, Bangshi River, and Turag River. The record from the Meghna River was the only one that remained at a higher extent than our found mean value. Likewise, our mean value was 47-, 5-, 5-, and 12-fold higher than some international findings such as Baiyangdian Lake, China; Thamirabarani River, India; Igbokoda River, Nigeria; Swat River, Pakistan; and Perak River, Malaysia, respectively. Only the Ganga River in India remained largely consistent with our mean finding.
Lead (Pb)
Lead is one of the essential metals that have a detrimental impact on both human and aquatic species (Ahmed et al. 2020; Ali et al. 2022b). Due to intake to a great extent, Pb enhances renal function, disrupts nervous system dysfunction, retards the skeletal hematopoietic system, and causes mental retardation, leading to death afterward (Zhong et al. 2018; Rahman et al. 2012). The European Community (EC 2001) and Food Standards Australia and New Zealand (FSANZ 2008) proposed upper limits for Pb impurities in fish species of 0.2 and 0.5 mg/kg, respectively. The concentration of Pb (mg/kg) was observed in different fish specimens, with the descending order as follows: Heteropneustes fossilis (3.27) > M. vittatus (3.14) > Cirrhinus reba (1.87) > G. giuris (1.33) > Trichogaster fasciata (1.27) > P. atherinoides (1.14) > O. pama (1.02) > G. chapra (0.34) > S. phasa (0.26) > T. ilisha (0.18). The mean concentration of Pb exceeded EU and MOFL guidelines, whereas it remained below the WHO and FAO suggested safety values. From national studies, the record from the Turag River was in line with our mean Pb finding, while the Halda River, Karnaphuli River, Meghna River, Buriganga River, and Bangshi River were 22, 10, three, 2.50, and three times higher than our mean Pb value, respectively. For international studies, Pb values from the Ganga River, India, and Perak River, Malaysia, were slightly higher than our obtained mean value. Our mean findings were 8, 3, 9, and 42 times higher than Baiyangdian Lake, China; Thamirabarani River, India; Igbokoda River, Nigeria; and Swat River, Pakistan, respectively.
Notably, young fish species uptake a higher rate of metal concentration than adults, as loading decreases during the maturation process (Rahman et al. 2012). For instance, Ndimele et al. (2017) reported a negative correlation between Pb concentration and age, meaning that juvenile species uptake more metal concentration compared with older groups. Higher Pb concentrations in young fish could be attributed to their high metabolic rate and underdeveloped hazardous metal neutralizing system (Jia et al. 2017). Again, compared to pelagic fish, demersal fish specimens have higher metal concentrations (Traina et al. 2019). The predatory fishes that make up the benthic fish species have highly concentrated and aggregated metals in their body tissue due to their extreme adaptability and close proximity to the sediment (Vieira et al. 2011). The diet consists primarily of aquatic plants and phytoplanktons, which produce lower metal concentrations in pelagic species than in demersal species (Hajeb et al. 2009). In other words, according to recent investigations, metal accumulation in demersal fish tissues is observed to a comparatively great extent (Velusamy et al. 2014). In our study, M. vittatus, P. atherinoides, and Heteropneustes fossilis show relatively higher metal accumulation than other pelagic ones (Table 1). This is because these species have close contact with the river basin’s metal-enriched sediment, impacting their feeding habits. Anandkumar et al. (2018) report that another factor is that predatory species eat metal and that the metal interacts with organisms that live on the bottom.
Consequently, the surpassed values from the suggested national and international organizations (WHO, EU, FAO, and MOFL) were explicitly observed in demersal species. Our findings were mainly like Velusamy et al. (2014) and Roméo et al. (1999), where Pb, Cd, Cu, and Cr were detected to a greater extent in the demersal specimens than in pelagic ones. Thus, detecting metal concentration in demersal fish species has become an environmental pollution indicator in a river basin (Buah-Kwofie et al. 2018). However, species variability brings about a particular action in accumulating the metal contents as different species have specific feeding habits and metabolic activities (Zhong et al. 2018; Chi et al. 2007).
BAF
Bioaccumulation factors in different fish species are presented in the Table 2. It is well known that one species has individual accumulation capability in response to various particulates (USEPA 2000). The calculation of BAF results in a fraction of the number in an efficient manner accumulated in different fish body organs like skin, gill, tissue, and viscera (Vu et al. 2017). The maximum BAF was recorded for As, followed by Cd, Pb, and Cr in the summer and winter seasons in the current study. In terms of fish specimens, the mean BAF was observed in the following descending order in summer and winter: M. vittatus (Summer: 348.06, winter: 375.81) > Heteropneustes fossilis (summer: 314.99, winter: 254.95) > P. atherinoides (summer: 288.75, winter: 227.17) > G. giuris (summer: 189.08, winder: 177.78) > Trichogaster fasciata (summer: 176.78, winter: 143.77) > Cirrhinus reba (summer: 163.70, winter: 157.56) > S. phasa (summer: 131.68, winter: 101.95) > O. pama (summer: 131.20, winter: 106.20) > G. chapra (summer: 93.05, winter: 81.58) > T. ilisha (summer: 23.96, winter: 34.30). From the hierarchy, we can see that the range of BAFs was from 4.71 to 758.91. As shown in Table 2, none of the species did surpass the corresponding threshold of bioaccumulative status (< 1000). Hence, all the specimens in the study area showed non-bioaccumulative conditions.
Human health risk assessment
EDI
Estimated daily intake (EDI) calculation was conducted to identify the substantial non-carcinogenic risk effect (THQ) along with the carcinogenic health impact (CR) of the consumption of contaminated aquatic foods (Liu et al. 2018). The EDI calculation follows the oral reference dose (Rfd) concerning certain metal content that delineates the response to the toxic metal element and avoids the harmful impact on public health (USEPA 2014; Baki et al. 2018). The EDIs for adults and children for the investigated fish species consumption are represented in Table 3. We observed that the demersal species exhibited relatively higher EDIs than pelagic ones. The EDIs were kept in the following descending order: As > Pb > Cr > Cd and compared with the recommended daily allowance proposed by WHO (2000). Lower EDIs than respective RDAs for specific metal elements indicate a negligible risk to human health from ingestion (Ahmed et al. 2019a). However, it would not be wise to consider it a permanent means to conclude as “acceptable range” and “unacceptable range” when it is lower than RDA/RfD (Baki et al. 2018).
Non-carcinogenic risk assessment
In Table 3, the calculated target hazard quotient (THQ) resulting from consuming the investigated fish species is shown. The estimation of THQs for both adults and children was noted in the declining order of, As > Cd > Pb > Cr. In adults, the range of THQ was from 7.64E − 07 to 7.97E − 03, whereas it was from 1.70E − 05 to 3.55E − 02 in children. Regarding the mean value of THQ, children possessed almost four times as much as adults. However, all THQs were lower than the threshold limit (< 1). As a result, lifetime consumption of such species would have no negative impact on human health (Yi et al. 2011). However, it is necessary to evaluate the hazard index as it refers to the alarming condition of the non-carcinogenic effect on public health (Liu et al. 2018). The estimated HI (total target hazard quotient) was 4.92E − 03 and 2.20E − 02 for adults and children, respectively. We can see that HIs were also less than the unit level (< 1), which revealed that the local consumers were safe from the non-carcinogenic impact. Our records were similar to those conducted by Khalil et al. (2020), where both THQ and HI were in a safe condition (< 1). Although THQ and HI are useful tools for assessing risk, they are not direct measurements because humans are susceptible to multiple effects of combined contaminants (Li et al. 2013).
Carcinogenic risk assessment
Only As, Pb, and Cd were considered to be potential oral carcinogenic slope variables when evaluating the CR (Ahmed et al. 2019a). The descending order of CRs in the current investigation was Cd > As > Pb for adults and children. The range of CRs was from 1.19E − 09 to 4.96E − 06 and from 5.31E − 09 to 2.21E − 05 in adults and children, respectively (Table 3). Among all the species, P. atherinoides exhibited the highest CR value in the children’s group. Therefore, children are relatively more susceptible to carcinogenic risk effects than adults, especially for the consumption of certain species. However, the cumulative CRs did not surpass the threshold range of 1E − 6 to 1E − 4. Thus, our CR observation would not pose any carcinogenic threat to the local consumers. Our CR result resembled Arisekar et al. (2020), who reported that CR values were acceptable near the Thamirabarani River, India. The increasing concentration of metal materials in the current study location can be a typical concern for carcinogenic risk issues due to spontaneous climate change, rapid industrialization, and economic activity.
Statistical result
The result of ANOVA showed that the metal distribution in the particular fish species was significantly different (p < 0.05) in both the summer and winter seasons. The Kolmogorov–Smirnov and Shapiro–Wilk tests showed that metal distribution followed the normal-distributed pattern. The analyzed correlation matrix with a p-value is depicted in Fig. 4. The conducted correlation matrix also describes the source of origin and the metal contents’ distribution pattern (Ahmed et al. 2021; Suresh et al. 2011). Our findings revealed a strong positive significant correlation (p 0.05) in metal(loid) pairs such as As–Pb (r = 0.757), Cr–Pb (r = 0.746), and Cd–Pb (r = 0.709). There is no negative correlation among the metal contents. Figure 3 demonstrates relationships among the metal contents based on the correlation coefficient. This also denoted that the most significant correlation was observed in As–Pb metal pair. Such relations indicated that the sources and the characteristics of the metal elements for the investigated species might be similar (Ali et al. 2022b). Also, the metal correlation revealed that metal accumulation was closely associated with the targeted fish tissues (Ahmed et al. 2019a). Moreover, a similar level of metal concentration can be attributed to the river basin from relevant sources associated with particular features and mutual dependence through transportation (Jiang et al. 2014). However, to better understand the basis of the origin of metal attribution, it is necessary to explore PCA (Borůvka et al. 2005).
The explored PCA represented compressed factor sets of variances of all considered datasets that exhibited the qualitative analysis (Singh et al. 2005; Ma et al. 2016). Figure 4 describes the corresponded PCA with three factors based on the Eigenvalues. The expressed cumulative variance was 95.08%, and three corresponding factors (PC 1, PC 2, and PC 3) appeared. The representative variances of the three factors were 73.8, 14.6, and 6.7%, respectively. PC1 was dominated by Cd and Pb due to their high loadings of 25.76 and 26.08%, respectively, whereas As and Cr dominated PC2 and PC3 with 65.66 and 56.47% for their loadings, respectively. The result indicated an internal relationship between metal elements and sources of origins, which might be attributed to the study area by untreated industrial activities and geogenic sources (Mohiuddin et al. 2011). In other words, human activities, like industrial waste and chemicals used in agriculture, are a big part of metal attribution and a small part of geogenic influence (Shikazono et al. 2012; Muhammad et al. 2011).
Hierarchical cluster analysis (HCA) was employed to identify the relationship between metal concentrations and the observable variables (Hossain et al. 2018). In the current study, a two-way HCA heatmap was conducted following the Ward linkage method associated with Euclidean distance, depicted in Fig. 5. The horizontal portion denoted identified metal(loid) content where fish species were presented in the vertical segment. For metal(loid) content, As and Pb constituted cluster 1, and Cr and Cd were confined to cluster 2. On the other hand, S. phasa, G. chapra, and T. ilisha made up cluster 1; Cirrhinus reba, P. atherinoides, Trichogaster fasciata, G. giuris, and O. pama made up cluster 2; and Heteropneustes fossilis and Mystus vittatus. Our HCA observation was in line with our explored PCA, which recommended a similar source of metal attribution in the current investigated area. Moreover, the findings of HCA denote the more or less similar accumulative characteristics of the metal contents in the targeted specimens (Suseno et al. 2010).
Source identification
Along with natural sources, it is widely accepted that anthropogenic actions such as As and Pb-containing agricultural manure and chemical use and application, as well as wood treatments containing copper arsenate, can be potential active reasons for As and Pb attribution to a river basin (Fu et al. 2014). The maximum concentration of As revealed that the study area was significantly impacted by employing agricultural chemicals and different wood treatments containing this particular metal element. Moreover, the concentration of Cr was increased in the study area due to the direct disposal of untreated wastes from petroleum production, upstream agricultural waste runoff, paint and inks, textile chemicals, and so on (Ahmed et al. 2021; Rahman et al. 2012). Common approaches for attribution of Cd to the river system included ostensible anthropogenic activities such as perfume and plastic manufacturing and processing, electroplating, and Cd-containing pesticides (Wang et al. 2015). However, natural inflow from the upstream as well as natural enduring corrosion from unknown sources cannot be denied for Cd intrusion in the study area. After figuring out where the metals are coming from, the metals will be controlled so that the environment of the river basin can improve. The deteriorating water quality in terms of metal concentrations results from human activities and natural sources (Wei et al. 2015). Arsenic, Cr, and Cd are used in various applications, including electroplating, nickel–cadmium batteries, residual cadmium alloys, foils, lubricants, and fertilizers (Kubra et al. 2022). Long-term use of fungicides, and algaecides, reduces the amount of heavy metals that are released into rivers during the processing of agricultural inputs (Ahmed et al. 2019a). High Cd levels are seen in discharge wastes from chemical plants and the battery sector (An et al. 2010). Pb has a long history of use in the steel industry as an anti-corrosive element (Abdollahi et al. 2013). According to Kumar et al. (2021), boat and steamer engine exhaust and oil leaks significantly impact the amount of Pb that enters aquatic bodies.
Conclusions
The Rupsa River in Bangladesh, which is significant commercially and is regularly contaminated by metal(loid)s, was the subject of the current investigation. Toxic metal(loid)s such as As, Cr, Cd, and Pb were investigated in 10 diverse fish species collected from the Rupsha River of Bangladesh in the summer and winter. Demersal fish, M. vittatus, were found to have the highest levels of metal content both in the summer and the winter. Fish species showed higher levels of metal(loid)s throughout the winter than during the summer. However, none of the examined specimens were metal-containing bio-accumulative species.
Most cases, especially As and Pb concentrations, surpassed some suggested national and international organizations. The statistical results revealed that metal concentration in the fishes differed significantly, and that metal concentration distribution among the specimens was normally distributed. According to multivariate analysis, As–Pb, Cr–Pb, and Cd–Pb all showed a strong positive significant connection. Meanwhile, the investigated PCA indicated that both geogenic and anthropogenic processes were the origin of the metal content. Our explored two-way HCA supported the PCA results regarding the relationship among the metal contents in the particular species. Because their THQ levels were below the threshold limit (1), the evaluation of THQ in both adults and children revealed that neither consumer would experience the non-carcinogenic effect. The cumulative CRs remained within the acceptable threshold (1E − 6 to 1E − 4) range, implying that the carcinogenic effect on consumers was negligible. Children, however, were more vulnerable than adults to both the carcinogenic and non-carcinogenic effects. The trend of the current increasing rate should be monitored by establishing some regulatory action by local policymakers.
Data availability
Data are available upon request on the corresponding author.
Code availability
Not applicable.
References
Abdollahi S, Raoufi Z, Faghiri I, Savari A, Nikpour Y, Mansouri A (2013) Contamination levels and spatial distributions of heavy metals and PAHs in surface sediment of Imam Khomeini Port, Persian Gulf, Iran. Marine Pollut Bullet 71(1–2):336–345
Abtahi M, Fakhri Y, Oliveri Conti G, Keramati H, Zandsalimi Y, Bahmani Z, Ghasemi SM (2017) Metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: a systematic review. Toxin Rev 36(4):331–341
Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, Hossain MM (2015) Human health risk assessment of metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ Sci Pollut Res 22(20):15880–15890
Ahmed MK, Baki MA, Kundu GK, Islam MS, Islam MM, Hossain MM (2016) Human health risks from metals in fish of Buriganga river, Bangladesh. Springerplus 5(1):1–12
Ahmed AS, Rahman M, Sultana S, Babu SOF, Sarker MSI (2019) Bioaccumulation and metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar Pollut Bull 145:436–447
Ahmed AS, Sultana S, Habib A, Ullah H, Musa N, Hossain MB, Sarker MSI (2019) Bioaccumulation of metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS ONE 14(10):e0219336
Ahmed ASS, Hossain MB, Semme SA, Babu SMOF, Hossain K, Moniruzzaman M (2020) Accumulation of trace elements in selected fish and shellfish species from the largest natural carp fish breeding basin in Asia: a probabilistic human health risk implication. Environ Sci Pollut Res 27(30):37852–37865
Ahmed ASS, Hossain MB, Babu SMOF, Rahman MM, Sarker MSI (2021) Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh. Environ Nanotechnol Monitor Manag 15:100416. https://doi.org/10.1016/j.enmm.2020.100416
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of metals in water and sediment of Karnaphuli River, Bangladesh. Environ Nanotechnol Monit Manage 5:27–35
Ali MM, Ali ML, Proshad R, Islam S, Rahman Z, Kormoker T (2020) Assessment of trace elements in the demersal fishes of a coastal river in Bangladesh: a public health concern. Thalassas: An Int J Marine Sci 36(2):641–655
Ali MM, Ali ML, Proshad R, Islam S, Rahman Z, Tusher TR, Al MA (2020) Metal concentrations in commercially valuable fishes with health hazard inference from Karnaphuli river, Bangladesh. Hum Ecol Risk Assess Int J 26(10):2646–2662
Ali MM, Rahman S, Rahman S, Islam MS, Rakib MRJ, Hossen S, Hanif MA, Rahman MZ, Kormoker T, Idris AM, Phoungthong K (2022) Distribution of metals in water and sediment of an urban river in a developing country: a probabilistic risk assessment. Int J Sedim Res 37(2):173–187
Ali MM, Ali ML, Rakib MR, Islam MS, Bhuyan MS, Senapathi V, Chung SY, Roy PD, Sekar S, MdTowfiqulIslam AR, Rahman MZ (2022) Seasonal behavior and accumulation of some toxic metals in commercial fishes from Kirtankhola tidal river of Bangladesh – a health risk taxation. Chemosphere 301:134660
Alipour H, Pourkhabbaz A, Hassanpour M (2015) Estimation of potential health risks for some metallic elements by consumption of fish. Water Qual Expo Health 7(2):179–185
An Q, Wu Y, Wang J, Li Z (2010) Assessment of dissolved heavy metal in the Yangtze River estuary and its adjacent sea, China. Environ Monit Assess 164(1):173–187
Anandkumar A, Nagarajan R, Prabakaran K, Bing CH, Rajaram R (2018) Human health risk assessment and bioaccumulation of trace metals in fish species collected from the Miri coast, Sarawak, Borneo. Mar Pollut Bull 133:655–663
Arisekar U, Shakila RJ, Shalini R, Jeyasekaran G (2020) Human health risk assessment of metals in aquatic sediments and freshwater fish caught from Thamirabarani River, the Western Ghats of South Tamil Nadu. Mar Pollut Bull 159:111496
Arojojoye OA, Oyagbemi AA, Afolabi JM (2018) Toxicological assessment of metal bioaccumulation and oxidative stress biomarkers in Clariasgariepinus from Igbokoda River of South Western Nigeria. Bull Environ Contam Toxicol 100(6):765–771
Baki MA, Hossain MM, Akter J, Quraishi SB, Shojib MFH, Ullah AA, Khan MF (2018) Concentration of metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol Environ Saf 159:153–163
Bayen S, Wurl O, Karuppiah S, Sivasothi N, Lee HK, Obbard JP (2005) Persistent organic pollutants in mangrove food webs in Singapore. Chemosphere 61(3):303–313
Borůvka L, Vacek O, Jehlička J (2005) Principal component analysis as a tool to indicate the origin of potentially toxic elements in soils. Geoderma 128(3–4):289–300
Bruno Lemos B, Letícia Ramos N, Fernando B (2012) Determination of Essential (Ca, Fe, I, K, Mo) and Toxic Elements (Hg, Pb) in Brazilian rice grains and estimation of reference daily intake. Food Nutrit Sci 3(1):129–134. https://doi.org/10.4236/fns.2012.31019
Buah-Kwofie A, Humphries MS, Pillay L (2018) Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso Wetland Park, South Africa. Sci Total Environ 621:273–281
Cabañero AI, Madrid Y, Cámara C (2007) Mercury–selenium species ratio in representative fish samples and their bioaccessibility by an in vitro digestion method. Biol Trace Elem Res 119:195–211
Calabrese EJ, Canada AT, Sacco C (1985) Trace elements and public health. Annu Rev Public Health 6(1):131–146
Centeno J, Gray M (2005) Arsenic in drinking water and health effects. In Metal Contaminants in New Zealand: Sources, Treatments, and Effects on Ecology and Human Health (pp 415–440). University of the Sunshine Coast, Queensland
Cheng TJ, Ke DS, Guo HR (2010) The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Res 44(19):5770–5776
Chi QQ, Zhu GW, Langdon A (2007) Bioaccumulation of metals in fishes from Taihu Lake, China. J Environ Sci 19(12):1500–1504
Commission of the European Communities (2001) Commission Regulation (EC) No. 221/2002 of 6 February 2002 amending regulation (EC) NO.466/2002 setting maximum levels for certain contaminants in foodstuffs. Off J European Communities 6:2002
Department of Environment (DoE) (2001) Water quality data of rivers Buriganga, Meghna, Balu, Shitalakhya and Jamuna. Department of Environment, Dhaka, Bangladesh
EU Commission (2001) EU Commission Regulation as regards metals. No. 466. Directive 2001/22/EC
Fakhri Y, Saha N, Miri A, Baghaei M, Roomiani L, Ghaderpoori M, Pouya RH (2018) Metal concentrations in fillet and gill of parrotfish (Scarusghobban) from the Persian Gulf and implications for human health. Food Chem Toxicol 118:348–354
Forti E, Salovaara S, Cetin Y, Bulgheroni A, Tessadri R, Jennings P, Prieto P (2011) In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol in Vitro 25(2):454–461
FSANZ (2008) Food Standards Australia and New Zealand. Contaminants and Natural Toxicants, Australia and New Zealand
Fu J, Zhao C, Luo Y, Liu C, Kyzas GZ, Luo Y, Zhu H (2014) Metals in surface sediments of the Jialu River, China: their relations to environmental factors. J Hazard Mater 270:102–109
Fuentes A, Fernández-Segovia I, Escriche I, Serra JA (2009) Comparison of physico-chemical parameters and composition of mussels (MytilusgalloprovincialisLmk.) from different Spanish origins. Food Chem 112(2):295–302
Griboff J, Wunderlin DA, Monferran MV (2017) Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J 130:236–244
Gu YG, Lin Q, Wang XH, Du FY, Yu ZL, Huang HH (2015) Metal concentrations in wild fishes captured from the South China Sea and associated health risks. Mar Pollut Bull 96(1–2):508–512
Hajeb P, Jinap S, Ismail A, Fatimah AB, Jamilah B, Rahim MA (2009) Assessment of mercury level in commonly consumed marine fishes in Malaysia. Food Control 20(1):79–84
Heshmati A, Ghadimi S, Khaneghah AM, Barba FJ, Lorenzo JM, Nazemi F, Fakhri Y (2018) Risk assessment of benzene in food samples of Iran’s market. Food Chem Toxicol 114:278–284
Hossain MB, Ahmed ASS, Sarker MSI (2018) Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environ Sci Pollut Res 25(31):31727–31736
Hu B, Jia X, Hu J, Xu D, Xia F, Li Y (2017) Assessment of metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int J Environ Res Public Health 14(9):1042
Ikem A, Egilla J (2008) Trace element content of fish feed and bluegill sunfish (Lepomismacrochirus) from aquaculture and wild source in Missouri. Food Chem 110(2):301–309
Islam MS, Ahmed MK, Al-Mamun MH (2015) Determination of metals in fish and vegetables and health implications in Bangladesh. Hum Ecol Risk Assess Int J 21:986–1006
Islam MS, Ahmed MK, Idris AM, Phoungthong K, Habib MA, Mustafa RA (2021a) Geochemical speciation and bioaccumulation of trace elements in different tissues of pumpkin in the abandoned soils: health hazard perspective in a developing country. Toxin Rev. https://doi.org/10.1080/15569543.2021.1977325
Islam MS, Idris AM, Islam ARMT, Ali MM, Rakib MRJ (2021b) Hydrological distribution of physicochemical parameters and metals in surface water and their ecotoxicological implications in the Bay of Bengal coast of Bangladesh. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15353-9
Islam MS, Phoungthong K, Ali MM, Ibrahim KA, Idris AM, Phoungthong K (2022) Assessing risk to human health for potentially toxic elements in farmed and wild giant tiger prawn (Paeneas monodon) in the coastal area of Bangladesh. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2106136
Itoh H, Iwasaki M, Sawada N, Takachi R, Kasuga Y, Yokoyama S, Tsugane S (2014) Dietary cadmium intake and breast cancer risk in Japanese women: a case–control study. Int J Hyg Environ Health 217(1):70–77
Jayapal S, Ramaiyan SK, Gopal D, Kothalia R, Manambarakat V, Ramalingam K (2017) Blue barred parrot fish (ScarusghobbanForsskal, 1775) culture in sea cages at Rameshwaram Island, Southeast coast of India
Jia Y, Wang L, Qu Z, Wang C, Yang Z (2017) Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes. Environ Sci Pollut Res 24:9379–9386
Jiang X, Teng A, Xu W, Liu X (2014) Distribution and pollution assessment of metals in surface sediments in the Yellow Sea. Mar Pollut Bull 83(1):366–375
Kalyoncu L, Kalyoncu H, Arslan G (2012) Determination of metals and metals levels in five fish species from Işıklı Dam Lake and Karacaören Dam Lake (Turkey). Environ Monit Assess 184(4):2231–2235
Khalil A, Jamil A, Khan T (2020) Assessment of metal contamination and human health risk with oxidative stress in fish (Cyprinus carpio) from Shahpur Dam, Fateh Jang, Pakistan. Arabian J Geosci 13(18):1–10
Khayatzadeh J, Abbasi E (2010) The effects of metals on aquatic animals. In The 1st International Applied Geological Congress, Department of Geology, Islamic Azad University–Mashad Branch, Iran (Vol 1, pp 26–28)
Kubra K, Mondol AH, Ali MM, Palash MAU, Islam MS, Ahmed ASS, Masuda MA, Islam ARMT, Bhuyan MS, Rahman MZ, Rahman MM (2022) Pollution level of trace metals (As, Pb, Cr and Cd) in the sediment of Rupsha River, Bangladesh: assessment of ecological and human health risks. Front Environ Sci 10:778544
Kükrer S, Şeker S, Abacı ZT, Kutlu B (2014) Ecological risk assessment of metals in surface sediments of northern littoral zone of Lake Çıldır, Ardahan, Turkey. Environ Monit Assess 186(6):3847–3857
Kumar S, Islam ARMT, Hasanuzzaman M, Roquia S, Khan R, Islam MS (2021) Preliminary assessment of heavy metals in surface water and sediment in Nakuvadra-Rakiraki River, Fiji using indexical and chemometric approaches. J Environ Manage 298:113517. https://doi.org/10.1016/j.jenvman.2021.113517
Li J, Huang ZY, Hu Y, Yang H (2013) Potential risk assessment of metals by consuming shellfish collected from Xiamen, China. Environ Sci Pollut Res 20(5):2937–2947
Liu Q, Liao Y, Shou L (2018) Concentration and potential health risk of metals in seafoods collected from Sanmen Bay and its adjacent areas, China. Mar Pollut Bull 131:356–364
Liu M, Xu Y, Nawab J, Rahman Z, Khan S, Idress M, Li G (2020) Contamination features, geo-accumulation, enrichments and human health risks of toxic metal (loids) from fish consumption collected along Swat river Pakistan. Environ Technol Innov 17:100554
Ma X, Zuo H, Tian M, Zhang L, Meng J, Zhou X, Liu Y (2016) Assessment of metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144:264–272
Makedonski L, Peycheva K, Stancheva M (2017) Determination of metals in selected black sea fish species. Food Control 72:313–318
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58(1):201–235
Mateen FJ, Grau-Perez M, Pollak JS, Moon KA, Howard BV, Umans JG, Navas-Acien A (2017) Chronic arsenic exposure and risk of carotid artery disease: The Strong Heart Study. Environ Res 157:127–134
Maurya PK, Malik DS, Yadav KK, Kumar A, Kumar S, Kamyab H (2019) Bioaccumulation and potential sources of metal contamination in fish species in River Ganga basin: possible human health risks evaluation. Toxicol Rep 6:472–481
Merciai R, Guasch H, Kumar A, Sabater S, García-Berthou E (2014) Trace metal concentration and fish size: variation among fish species in a Mediterranean river. Ecotoxicol Environ Saf 107:154–161
Miri M, Akbari E, Amrane A, Jafari SJ, Eslami H, Hoseinzadeh E, Taghavi M (2017) Health risk assessment of metal intake due to fish consumption in the Sistan region, Iran. Environ Monit Assess 189(11):1–10
MOFL (2014) Bangladesh Gazette, Bangladesh Ministry of Fisheries and Livestock, SRO no. 233/Ayen
Mohiuddin KM, Ogawa YZHM, Zakir HM, Otomo K, Shikazono N (2011) Metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Technol 8(4):723–736
Mohiuddin M, Hossain MB, Ali MM, Hossain MK, Habib A, Semme SA, Rakib RJ, Rahman A, Yu J, Al-Sadoon MK, Gulnaz A, Arai T (2022) Human health risk assessment for exposure to metals in finfish and shellfish from a tropical estuary. J King Saud Univ-Sci 34(4):102035
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98(2):334–343
Nauen CE (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular (FAO). no. 764
Ndimele PE, Pedro MO, Agboola JI, Chukwuka KS, Ekwu AO (2017) Heavy metal accumulation in organs of Oreochromis niloticus (Linnaeus, 1758) from industrial effluent-polluted aquatic ecosystem in Lagos, Nigeria. Environ Monit Assess 189:1–15
Nyeste K, Dobrocsi P, Czeglédi I, Czédli H, Harangi S, Baranyai E, ... Antal L (2019) Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): juveniles are useful bioindicators of recent pollution. Ecol Indic 101:1–10
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem 134(4):1847–1854
Rahman MS, Ahmed AS, Rahman MM, Babu SOF, Sultana S, Sarker SI, Rahman M (2021) Temporal assessment of metal concentration and surface water quality representing the public health evaluation from the Meghna River estuary, Bangladesh. Appl Water Sci 11(7):1–16
Rakib MRJ, Jolly YN, Begum BA, Choudhury TR, Fatema KJ, Islam MS, Ali MM, Idris AM (2021) Assessment of trace element toxicity in surface water of a fish breeding river in Bangladesh: a novel approach for ecological and health risk evaluation. Toxin Rev 41(2):420–436
Rani S, Ahmed MK, Xiongzhi X, Keliang C, Islam MS, Al-Mamun MH (2021) Occurrence, spatial distribution and ecological risk assessment of trace elements in surface sediments of rivers and coastal areas of the east coast of Bangladesh, North-East Bay of Bengal. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.149782
Rashid A, Siddika A, Rahman MTU (2017) Assessment the presence of Metal (HM) concentration in river water and fish species and associated human health hazard due to the fish consumption of Turag River in Bangladesh 15th Int. In Conf. on Environmental Engineering, Held as part of the Canadian Society for Civil Engineering Annual Conf. and General Meeting 2017
Roméo M, Siau Y, Sidoumou Z, Gnassia-Barelli M (1999) Metal distribution in different fish species from the Mauritania coast. Sci Total Environ 232(3):169–175
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci 18(2):144
Sabbir W, Rahman MZ, Hasan MM, Khan MN, Ray S (2018) Assessment of metals in river water, sediment and fish mussel in Rupsha River under Khulna district, Bangladesh. Int J Expt Agric 8(1):1–5
Saha N, Mollah MZI, Alam MF, Rahman MS (2016) Seasonal investigation of metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 70:110–118
Salam MA, Paul SC, Zain RAMM, Bhowmik S, Nath MR, Siddiqua SA, Amin MFM (2020) Trace metals contamination potential and health risk assessment of commonly consumed fish of Perak River, Malaysia. Plos One 15(10):e0241320
Samad MA, Mahmud Y, Adhikary RK, Rahman SBM, Haq MS, Rashid H (2015) Chemical profile and metal concentration in water and freshwater species of Rupsha River, Bangladesh. Am J Environ Prot 3(6):180–186
Saran SH, Rahman A, Yunus A (2018) Comparative analysis on flow and salinity of Rupsha-Passur River system of Bangladesh. J Modern Sci Technol 6(2):19–31
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2017) Differential modulation of cellular antioxidant status in zebrafish liver and kidney exposed to low dose arsenic trioxide. Ecotoxicol Environ Saf 135:173–182
Shikazono N, Tatewaki K, Mohiuddin KM, Nakano T, Zakir HM (2012) Sources, spatial variation, and speciation of metals in sediments of the Tamagawa River in Central Japan. Environ Geochem Health 34(1):13–26
Singh UK, Kumar B (2017) Pathways of metals contamination and associated human health risk in Ajay River basin, India. Chemosphere 174:183–199
Singh KP, Malik A, Sinha S, Singh VK, Murthy RC (2005) Estimation of source of metal contamination in sediments of Gomti River (India) using principal component analysis. Water Air Soil Pollut 166(1):321–341
Subotić S, Višnjić Jeftić Ž, Spasić S, Hegediš A, Krpo-Ćetković J, Lenhardt M (2013) Distribution and accumulation of elements (As, Cu, Fe, Hg, Mn, and Zn) in tissues of fish species from different trophic levels in the Danube River at the confluence with the Sava River (Serbia). Environ Sci Pollut Res 20:5309–5317
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186(2–3):1837–1846
Suñer MA, Devesa V, Munoz O, López F, Montoro R, Arias AM, Blasco J (1999) Total and inorganic arsenic in the fauna of the Guadalquivir estuary: environmental and human health implications. Sci Total Environ 242(1–3):261–270
Suresh G, Ramasamy V, Meenakshisundaram V, Venkatachalapathy R, Ponnusamy V (2011) Influence of mineralogical and metal composition on natural radionuclide concentrations in the river sediments. Appl Radiat Isot 69(10):1466–1474
Suseno H, Hudiyono S, Wisnubroto DS (2010) Effects of concentration, body size and food type on the bioaccumulation of Hg in farmed tilapia Oreochromis mossambicus. Aust J Basic Appl Sci 4(5):792–799
Traina A, Bono G, Bonsignore M, Falco F, Giuga M, Quinci EM, Sprovieri M (2019) Metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): potential human health risk estimation. Ecotoxicol Environ Saf 168:466–478
USEPA (2000) Guideline for assessing chemical contaminant data for use in fish advisories, Vol. I: Fish Sampling and Analysis, Third edition. Office of Water. U.S. Environmental Protection Agency, Washington, DC (Document No. EPA 823-B-00–007. November 2000)
USEPA (2008) Environmental Protection Agency. Integrated Risk Information System. CRC.
USEPA (2008) Integrated risk information system. United States Environmental Protection Agency, Washington, DC, USA. https://www.epa.gov/iris. Accessed date: 25 September 2021
USEPA (2010) Integrated Risk Information System (IRIS). United States Environmental Protection Agency, Washington, DC, USA. www.epa.gov/ncea/iris/index.html. Accessed date: 15 September 2021
USEPA (2011) USEPA regional screening level (RSL) summery table: November 2011. http://www.epa.gov/regshwmd/risk/human/Index.htm. Accessed 18 Jul 2022
USEPA (2014) U.S. Environmental Protection Agency. Region 4 human health risk assessment supplemental guidance. U.S. Environmental Protection Agency, Washington DC
Varol M, Kaçar E, Sünbül MR, Islam ARMT (2022) Levels of metals and elements in tissues of fish species in the Kızılırmak River (Turkey) and assessment of health risks and nutritional benefits. Env Res 214:113791. https://doi.org/10.1016/j.envres.2022.113791
Velusamy A, Kumar PS, Ram A, Chinnadurai S (2014) Bioaccumulation of metals in commercially important marine fishes from Mumbai Harbor, India. Marine Pollut Bullet 81(1):218–224
Vieira C, Morais S, Ramos S, Delerue-Matos C, Oliveira MBPP (2011) Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra-and inter-specific variability and human health risks for consumption. Food Chem Toxicol 49(4):923–932
Vu CT, Lin C, Yeh G, Villanueva MC (2017) Bioaccumulation and potential sources of metal contamination in fish species in Taiwan: assessment and possible human health implications. Environ Sci Pollut Res 24(23):19422–19434
Wang C, Liu S, Zhao Q, Deng L, Dong S (2012) Spatial variation and contamination assessment of metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicol Environ Saf 82:32–39
Wang Y, Yang L, Kong L, Liu E, Wang L, Zhu J (2015) Spatial distribution, ecological risk assessment and source identification for metals in surface sediments from Dongping Lake, Shandong, East China. CATENA 125:200–205
Wang Q, Chen M, Shan G, Chen P, Cui S, Yi S, Zhu L (2017) Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci Total Environ 598:814–820
Wei X, Gao B, Wang P, Zhou H, Lu J (2015) Pollution characteristics and health risk assessment of heavy metals in street dusts from different functional areas in Beijing, China. Ecotoxicol Environ Safe 112:186–192. https://doi.org/10.1016/j.ecoenv.2014.11.005
WHO (1985) Guideline for drinking water quality, 2nd edition. Recommendation. World Health Organization General. 1:30–113
WHO (2000) WHO technical report series. Evaluation of certain food additives and contaminants. Fifty-third report of the joint FAO/WHO expert committee on food additives (JECFA). World Health Organization Geneva, Switzerland. http://www.Who.Int/foodsafety/publications/jecfa-reports/en/. Accessed date: 07 October 2021
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of metals in sediment and human health risk assessment of metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159(10):2575–2585
Yin S, Feng C, Li Y, Yin L, Shen Z (2015) Metal pollution in the surface water of the Yangtze Estuary: a 5-year follow-up study. Chemosphere 138:718–725
Zerizghi T, Yang Y, Wang W, Zhou Y, Zhang J, Yi Y (2020) Ecological risk assessment of metal concentrations in sediment and fish of a shallow lake: a case study of Baiyangdian Lake, North China. Environ Monit Assess 192(2):1–16
Zhao XM, Yao LA, Ma QL, Zhou GJ, Wang L, Fang QL, Xu ZC (2018) Distribution and ecological risk assessment of cadmium in water and sediment in Longjiang River, China: implication on water quality management after pollution accident. Chemosphere 194:107–116
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of metals in freshwater fish in the central and eastern North China. Ecotoxicol Environ Saf 157:343–349
Zhou H, Yang WT, Zhou X, Liu L, Gu JF, Wang WL, Liao BH (2016) Accumulation of metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 13(3):289
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407(5):1551–1561
Acknowledgements
This manuscript is part of a master’s thesis of Khadijatul Kubra from the Department of Aquaculture, Faculty of Fisheries, Patuakhali Science and Technology University (PSTU), Bangladesh. We express our gratitude to PSTU academic members Dr. Muhammad Abdur Razzak, the Department of Aquaculture, and others. The Khulna, Bangladesh-based Quality Control (QC) Laboratory’s management and personnel deserve the authors’ sincere gratitude and appreciation for providing the lab space. Furthermore, the authors thank the Goiano Federal Institute and the National Council for Scientific and Technological Development (CNPq/Brazil). Malafaia G. holds a productivity scholarship from CNPq (Proc. No. 308854/2021-7).
Author information
Authors and Affiliations
Contributions
Mir Mohammad Ali: designed, writing — original draft and proofreading during the manuscript drafting stage. Md. Abu Bakar Siddique: Instrumental setup, Methodology, Formal analysis, Investigation, Data curation, Validation, Writing—original draft preparation, Writing—reviewing and editing. Khadijatul Kubra: Writing – original draft and proofreading during the manuscript drafting stage. Abu Reza Md. Towfiqul Islam: Designed, Planned, Conceptualization, Writing – original draft and proofreading during the manuscript drafting stage. Edris Alam: Formal analysis, proofreading during the manuscript drafting stage. Anwar Hossain Mondol: Formal analysis, proofreading during the manuscript drafting stage. Ehsanul Karim: software, mapping, proofreading during the manuscript drafting stage. Guilherme Malafaia: statistical analysis, formal analysis, interpretation, manuscript editing, literature review, proofreading. Shahrina Akhtar: manuscript editing, literature review, proofreading. A.S. Shafiuddin Ahmed: statistical analysis, formal analysis, interpretation, software, mapping, proofreading during the manuscript drafting stage. Md Saiful Islam: formal analysis, validation, manuscript editing, literature review, software, mapping, proofreading during the manuscript drafting stage. Md. Mostafizur Rahman: formal analysis, validation, manuscript editing, literature review, software, mapping, proofreading during the manuscript drafting stage. Md. Zillur Rahman: formal analysis, validation, proofreading during the manuscript drafting stage.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, M.M., Kubra, K., Alam, E. et al. Bioaccumulation and sources of metal(loid)s in fish species from a subtropical river in Bangladesh: a public health concern. Environ Sci Pollut Res 31, 2343–2359 (2024). https://doi.org/10.1007/s11356-023-31324-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31324-8