Abstract
Emerging contaminants (ECs) originated from different agricultural, biological, chemical, and pharmaceutical sectors have been detected in our water sources for many years. Several technologies are employed to minimise EC content in the aqueous phase, including solvent extraction processes, but there is not a solution commonly accepted yet. One of the studied alternatives is based on separation processes of emulsion liquid membrane (ELM) that benefit low solvent inventory and energy needs. However, a better understanding of the process and factors influencing the operating conditions and the emulsion stability of the extraction/stripping process is crucial to enhancing ELM’s performance. This article aims to describe the applications of this technique for the EC removal and to comprehensively review the ELM properties and characteristics, phase compositions, and process parameters.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diverse “emerging” contaminants (ECs) are demanding our attention (Richardson and Kimura 2017) because of their detrimental effects on ecological and/or human health. Typically, ECs are present at extremely low concentrations from nanogram per litre (ng L−1) up to microgram per litre (μg L−1), causing poor detection and assessments. The recent advancement of technology has encouraged many researchers to minimise the content of ECs in the environment as they are now able to detect a broad range of contaminants at extremely low concentrations by many means (Galindo-Miranda et al. 2019). There are several groups of emerging compounds reported, such as hormones and other endocrine-disrupting compounds, algal and cyanobacterial toxins, disinfection by-products, gasoline additives, organometallics, organophosphate flame retardants and plasticisers, pharmaceuticals and personal care products, brominated flame retardants, perfluorinated compounds, polar pesticides, and their degradation/transformation products and surfactants and their metabolites (Petrovic et al. 2008). These contaminants entered the aquatic and terrestrial environments via several sources, including anthropogenic and naturally occurring chemicals, pharmaceuticals and personal care products (PPCPs), engineered nanomaterials, illicit drugs, and antibiotic resistance genes. To the best of our wisdom, ECs are not circulated in drinking water supplies or, at least, not being adequately monitored yet. Lack of monitoring will cause harmful ecological and human health effects (Noguera-Oviedo and Aga 2016). Though residues of the chemicals were detected in natural waters, the effluent of wastewater treatment facilities was recognised as the key source of the introduction of pharmaceutical ECs. Recently, a number of pharmaceuticals that have been developed in the common priority list are associated with the water cycle based on intake, physicochemical properties, persistence, toxicity, incidence, and resistance to treatment (Boleda et al. 2011) as shown in Table 1.
Pharmaceutical waste is among the most studied group of emerging contaminants in the water ecosystem at a concentration range as low as ng·L−1 to μg·L−1(Mompelat et al. 2009; Nikolaou et al. 2007; Ternes et al. 2002; Zuccato and Castiglioni 2009). Approximately there are 3000 various substances used as pharmaceutical ingredients, including antibiotics, painkillers, and impotence drugs. This involves beyond 4000 molecules with various biological and physicochemical properties and different biochemical reaction forms. Most medications are administered orally, while some medications are metabolised, while others remain unchanged until they are excreted (Monteiro and Boxall 2010). The mixture of pharmaceuticals and their metabolites would then reach the urban water treatment plants and sewage treatment plants. Easily detected pharmaceutical compounds in the contaminated water include acetaminophen, carbamazepine, diclofenac, ibuprofen, and salicylic acid (Stackelberg et al. 2007). Surprisingly, out of 713 pharmaceuticals checked (of which 142 are transformation products), Lyons (2014) had identified 631 pharmaceutical types (of which 127 are transformation products) surpass their detection limit. This worrying situation calls for a better method for the removal of these contaminants from the aqueous phase.
EC removal from wastewater and drinking water is tricky as there is no all-inclusive method available. In general, removals of these pollutants in the wastewater treatment processes can be considered acceptable. However, many reported failing to entirely eliminate pharmaceutical pollutants in water by using traditional treatment systems in municipal and industrial wastewater treatment plants (WWTPs) (Balabanič et al. 2012; Chaouchi and Hamdaoui 2014b). The collected chemicals were easily released into the groundwater to a certain degree due to poor handling in WWTPs (Jarrett 2017). Several methods such as biodegradation, ozonation, electrooxidation, photocatalysis, and Fenton process have been introduced to remove ECs (Kyzas et al. 2015). The processes listed in Fig. 1 give an overview of available removal techniques that were reported to eliminate a significant portion of pharmaceutical content in the wastewater. These conventional methods are classified as bio-electrochemical (Jiang et al. 2016; Wu et al. 2017; Yang et al. 2015; Zhang et al. 2016, 2017, 2018), chemical (Angeles et al. 2020), and physical treatment. The eco-friendly biological method suffers from a limited capacity for upscale, difficulty in technical integration, as well as concerns on microbial activities. Meanwhile, polymeric membrane and activated carbon are subclasses of physical treatment. The first is typically associated with fouling and high energy requirement (Helmecke et al. 2020), while the latter has inconsistent results and requires additional post-treatment. In general, biological and physical treatment is less efficient and could be costly. Additionally, chemical treatments such as ozonation, electrochemical, TiO2 photocatalysis, H2O2/UV oxidation, and solar photoelectron-Fenton oxidation were used to remove ECs (Skoumal et al. 2006), wherein some cases resulted relatively better than biological and physical methods.
Unfortunately, they acquire some drawbacks such as high investment and maintenance costs, secondary pollutant formation, and complex operating procedures (Grassi et al. 2012; Treacy 2019). The main disadvantages of the chemical method are environmentally unfriendly in nature and might require post-treatment. It may also produce by-products as reported by Rivera-Utrilla et al. (2013). Unfortunately, these methods may not fully eradicate pharmaceutical compounds, as stated by Chaouchi and Hamdaoui (2014b), due to many reasons, mainly the complexity of the process (Krzeminski et al. 2019). To a certain degree, the collected chemicals were simply released without adequate treatment in the WWTPs (Jarrett 2017). In addition, various polluting compounds in trace amounts have been found in WWTPs, for which traditional treatment technologies have not been particularly developed (Gros et al. 2010). Another chemical method investigated for removing pharmaceuticals contaminated water is solvent extraction (Kyzas et al. 2015) which offers an efficient and quick separation (Hosseinzadeh et al. 2014). Consequently, the assimilation of conventional wastewater treatment with advanced technologies, especially liquid membranes, has grown to be of enormous interest to overcome the limitations mentioned. Liquid membrane separation was investigated and proven to be a feasible method to separate pharmaceutical ECs, specifically acetaminophen, from polluted water.
This article will specifically discuss the utilisation of ELM for the mentioned motive. ELM invented by Li (1968) has shown excellent potential for the application extraction of ECs. ELM is currently being applied as an optional strategy to separation, comprising three stages: emulsification, extraction, and demulsification. It offers many desirable features such as a large interfacial area to volume ratio for mass transfer, simultaneous extraction and stripping, cost-effectiveness, low energy consumption, low solvent concentration performance, and low solvent quantity requirements. In addition, ELM is also estimated to be around 40% cheaper than traditional extraction methods (Kislik 2010). These advantages sparked the interest for ELM to be studied thoroughly for industrial applications, for instance, separation of numerous kinds of metal ions (Ahmad et al. 2012; Kunthakudee et al. 2016; Murugan et al. 2009; Zhao et al. 2010), organic compounds (Lee 2011; Ng et al. 2010; Sunsandee et al. 2013; Sunsandee et al. 2017), and inorganic compounds (Lichang et al. 2016). Unfortunately, poor emulsion stability remains as a hindrance in the industrial application of ELM. It is usually governed by membrane breakage in ELM systems which involve the rupture of the emulsion, and leakage of internal phase and extracted solute to the external phase causes the decrease in volume of the stripping phase. This causes the driving force for mass transfer, reduces the concentration gradient, and increases the external feed concentration, thereby lowering the extraction efficiency. Typically, it is caused by the emulsion formulation and condition of emulsification. Thus, this article aims to illuminate the all-inclusive review on ELM’s properties and characteristics, phase compositions, and process parameters correspondingly and describe the applications of this technique for EC removal. This study will be significant in the appropriate selection of organic phase compositions and elucidating some of the effects of some ELM process parameters which impact on the extraction efficiency (%).
Liquid membrane (LM)
Membranes are commercially used for various purposes such as water purification, gas applications, chemical, biotechnology, and biomedical applications where it consists of the semipermeable layer. A semipermeable polymeric membrane layer, which can be heterogeneous, homogeneous, asymmetric, symmetric, solid, or liquid, splits up two phases and constricts the transport of different chemical species in a specific manner (Porter 1990). As for liquid membrane, the semipermeable layer is named the membrane phase, where it serves as a selective immiscible liquid layer that segregates the feed and stripping phase.
Liquid membranes have been extensively studied due to their immense potential to replace traditional techniques available for solvent separation. Similar to other developing technologies, the liquid membrane has various other names such as “liquid protraction”, “carrier-mediated extraction”, “facilitated transport”, and “two-stage” (Boyadzhiev and Lazarova 1995). A liquid membrane system consists of processes where liquid-liquid extraction along with membrane separation was incorporated simultaneously. Three types of liquid membranes are commonly reported, bulk liquid membrane (BLM), supported (R. Kumar et al. 2021; Rajendaren et al. 2021) or immobilised liquid membrane (SLM or ILM), and emulsion liquid membrane (ELM) (Al-Obaidi et al. 2020), which differ in terms of the configuration as well as its transport mechanism (as shown in Fig. 2). On top of that, these liquid membranes differ in terms of their design, formulation as well as method of contact with the external feed phase(Parhi 2013). However, all three configurations of the liquid membrane are similar in the way that it is assisted by the extracting reagent to transport the solute. The extracting agent can either be stagnant or mobile between the feed and the internal phase to precisely remove the targeted solute (Kislik 2010).
Transport mechanism of liquid membrane
Passive or active transport can achieve the transfer phenomena of a substance via the membrane phase in liquid membranes. They are classified according to the solubility of the solute in the membrane phase as well as the presence and mechanism of the extracting agent in the membrane phase.
Passive transport
The solute permeates easily via this method due to its solubility in the membrane phase. The process relies on the solute concentration gradient, where this selective transport took place from a high concentration region to the lower one (Kislik 2010). When the concentration equilibrium is reached, there is no net transportation of solute. No reaction is involved in this mechanism, and the solute remains in the same form throughout the phases. The transported solution must be transformed into a state in which the receiving aqueous droplets cannot diffuse back, to maintain a substantial concentration gradient over a long period of time. This is to minimise the solute in the external feed phase as it is solely dependent on the concentration gradient. The illustration of this process is shown in Fig. 3. While this approach is straightforward and affordable, its selectivity and efficiency are not always favourable. Additionally, as the separation occurs, the neutralising or anchoring component in the inner aqueous phase is depleted earlier in the droplets near the globule’s surface. As a result, the solute must diffuse inward and outward before being released and neutralised. This causes the process to become diffusion-limited and slows down with time (Patnaik 1995). In order to circumvent this constraint, a carrier is introduced into the membrane phase to boost speed and selectivity, which leads to the facilitated transport mechanism.
Facilitated transport
During active transport or facilitated transport, the membrane phase contains a carrier that has been dissolved in an organic solvent. The transfer of a substrate through the membrane phase is accomplished in accordance with the double layer model (Eljaddi et al. 2017) via the following steps: (1) The substrate (S) is diffused in the stagnant layer of the source phase. (2) At the first interface between the source and membrane phase, the carrier (C) and substrate form a connection. (3) Diffusion of the entity (carrier-substrate (C-S)) from its initial location in the membrane phase to the second membrane interface. (4) The dissociation of the entity and the regeneration of the carrier. (5) In the receiving phase, there is a diffusion of the substrate. The carrier works as a catalyst by increasing the rate of transfer, increasing the solubility of the chemical region within the membrane. The material transfer process comes to a halt when the concentration differential between the feed phase and receiving phase reaches zero. It is referred to as simple facilitated diffusion if it only involves one species to be transported. However, if the source phase contains two species capable of associating with the carrier, this is referred to as coupled facilitated transport. There are two types of coupled facilitated transport, which are typically referred to as type I (co-transport) and type II (counter transport).
For type I, a solute must be soluble in all phases (feed, membrane, and stripping). A modification is made by introducing a stripping agent on the contrary side of the membrane phase to increase the solute’s mass transfer. The stripping agents will react towards the transferred solute in the membrane phase and yield an insoluble compound, as illustrated in Fig. 4. The insoluble compound will be restricted from diffusing through the membrane phase, hence accumulating in the stripping phase. In the internal stripping phase, the solute concentration is also preserved by the reaction at zero. In type I, the reaction only involves the solute and stripping agent without the presence of a carrier. To simplify, this type of transport can be expressed in the following equilibrium reactions as shown in Eq. 1 whereby the carrier is neutral, and the external feed phase contains a pair of associated and dissociated ions (Aa+ aX−, cation and anion), which are reversibly connected by carrier B.
Type II transport mechanism mainly relies on the assistance of the carrier to transport the solute across the membrane phase, whereby the carrier can be acidic or basic. This mechanism was named “carrier-mediated” transport (Teng et al. 2014). The targeted solute is typically insoluble in the membrane phase, thus requiring assistance to move from the feed to the stripping phase. The transportation process involves a chemical reaction to form a solute-carrier complex and dissociation of this complex to strip the targeted solute in the internal phase, shown in Fig. 5. The two chemical reactions mentioned taking place at the external-membrane and the membrane-internal interface. The solute turned into a solute-carrier complex via a reversible reaction before being transported into the internal stripping phase. The reaction product that is soluble in the membrane phase will diffuse to the membrane-internal interface through the membrane layer. Following that is another chemical reaction that will cause the dissociation of the solute-carrier complex. The targeted solute will be stripped and released into the internal phase from this second chemical reaction. The carrier remains unchanged and will circulate back to the membrane-external interface to continue the same loop. For example, in the case of an acidic carrier where the process is carried out by a cation exchange proton, the cations migrate in the opposite direction of protons from high pH to low pH value; the balance in the external feed-membrane interface is expressed as shown in Eq. 2. When the carrier in the membrane phase exchanges an anion (basic) with the substrate at the external feed-membrane phase, a neutral entity (C−B+) is formed as shown in Eq. 3. The process is governed by the association of the substrate and carrier, the gradient of the anion X concentration, and the electrical neutrality of the source and receiving phases.
The transport mechanism that has been widely explored in the liquid membrane is the type II transport mechanism. The metal ions were removed in water with protons as counter-transport ions. The coupled transport is similar to facilitated transport as a carrier agent is integrated into the membrane. The difference was spotted as the carrier agent couples the flow of two species which enables one of the species to move against its concentration gradient (San Román et al. 2010). However, this is bound to happen only on the terms that the concentration gradient of the second coupled species is sufficiently large. In the cases of ionic species, the transport mechanism occurs to maintain the electroneutrality of the solution.
The concentration gradient of the solute-carrier complex at the external and internal interface serves as the main driving force in the whole process. According to Wan et al. (1997), the concentration gradient could be maximised through reaction with stripping agent since the solute is insoluble in the membrane phase. Besides, the pH difference between the internal and external phases has a significant influence (Kargari et al. 2006). This mechanism enables the carrier molecules to carry the solvent as much as possible, thus reducing the amount of carrier required during the membrane process.
Emulsion liquid membrane
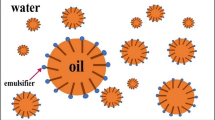
For the application of EC removal, researchers choose ELM over the other type of liquid membrane. Even though SLM offers a simpler configuration and process, it is a three-phase system with a liquid membrane phase immobilised in the membrane support between two aqueous feed and stripping phases. The higher usage of materials leads to high costs with approximately the same extraction efficiency as to using ELM. Thus, since ECs have a low concentration in wastewater, ELM is more preferable since it is more economical. Besides the conventional solvent extraction processes, ELM has been a promising alternative technique with a plus factor of low solvent inventory and energy requirements (Chakraborty et al. 2010). Additionally, in extraction and stripping operations of ELM, the solute simultaneously preconcentrates the solute in a single unit. However, numerous problems constantly arise when the ELM is involved in the separation process (Teng et al. 2014). The emulsion stability always has been the main concern in the ELM. Factors that need to be controlled to overcome this problem involve ionic strengths, pH, temperature, or any factors that disrupt the membrane stability during the separation. Desiring for a very stable emulsion causes breaking it down to be a difficult task. As a result, recovering the receiving phase and replenishing the carrier are highly unlikely. A proper understanding of the process and factors influencing the system needs to be understood to resolve these issues. In general, an ELM can be envisioned as a “bubble within a bubble” occurrence (A. Kumar et al. 2019). As depicted, the innermost bubble is the internal phase, and the outer bubble is the membrane phase containing the carriers. The phase outside the bubble is known as the external feed phase. A huge number of these bubbles are available in an ELM set-up, as shown in Fig. 6.
Role of various constituents during ELM formulation
The development of the ELM system can be considered a simple process, although it is hard to maintain long-term performance. For an efficient separation, the emulsion must possess high viscosity, neutral buoyancy for the emulsion to suspend in the external phase, and sufficient surfactant concentration for emulsion stability (Hiroshi 1990). Therefore, the ELM formulation, which includes carrier, diluents, and stripping agents, is important. The effectiveness of an ELM process typically depends on the choice of components since it will indirectly affect the extraction efficiency. Besides, the suitable ELM formulation will produce a stable emulsion, hence promising high efficiency.
The membrane phase consists of an organic solvent containing carrier, surfactant, and diluent (Manikandan et al. 2014; Othman et al. 2014). The membrane phase serves as a selective barrier, and it provides a large surface area for solute transport during the extraction process, as depicted in Fig. 7. Carrier in the membrane phase is an important component in ELM formulation where it is responsible for forming a complex and serves as a shuttle carrying solute (Aziz et al. 2008; Perera and Stevens 2009). Carrier can be classified into three groups (Zihao and Jufu 1992): acidic, basic (Ritchey and Ashbrook 1984), and solvating. The selection of a suitable carrier is vital for the ELM system to achieve maximum extraction efficiency. The thermodynamic and kinetics are the main concerns in selecting the best carrier. The carrier should thermodynamically favour the solute ion from the external feed phase and forming a carrier-solute complex. At the same time, the kinetics of the solute extraction reaction must be fast. For rapid solute extraction from the external phase, carriers with greater stability of the solute-carrier complex are preferable. The solubility of the carrier is another vital aspect to be looked at. The carrier should have high solubility in the membrane phase but insoluble in the internal and external phases.
Many studies prove that its concentration is also important to achieve high extraction efficiency besides the carrier type. Research carried out by Kumbasar (2009) revealed that a high carrier concentration enhanced the extraction capability of an ELM system. However, excessively high concentration triggers emulsion breakage due to interfacial characteristics of solute-extractant complex (Valenzuela et al. 2005). According to Sengupta et al. (2007), increasing carrier concentration resulted in a larger emulsion size due to an increase of interfacial tension. Meanwhile, high emulsion viscosity due to high carrier concentration is not beneficial as the emulsion diameter is enlarged (Chaouchi & Hamdaoui, 2014; Chiha et al. 2006), where this condition will significantly affect the extraction rate.
On the other hand, surfactant stabilises the emulsion by suppressing the interfacial tension between two immiscible phases, and its presence also influences the transport rate of solute (Chakraborty et al. 2010; Perera and Stevens 2009). Surfactant is known to have two parts which is hydrophilic polar head group and the other one is a hydrophobic non-polar tail group (Bjorkegren and Fassihi 2011). The hydrophilic polar head group attracts the aqueous phase (internal and external phases) where it is used to avoid surfactant expulsion, whereas the hydrophobic non-polar tail highly attracts the organic phase. This condition causes an expanding free energy system and modification of organic solvent liquid structure, which means less work is required for surfactant molecules to surface. In general, surfactants are categorised into four major classes, such as anionic, cationic, zwitterionic, and non-ionic surfactants (Chakraborty et al. 2010).
Surfactant and extractant are both solubilised in diluents forming membrane phase. In the ELM system, diluent serves as media for carrier and surfactant, where it acts as a barrier separating two aqueous phases. The general requirements of diluent are as follows:
-
i.
Compatible with carrier and surfactant (Perera and Stevens 2009).
-
ii.
Low solubility in the internal and external phase.
-
iii.
Moderate viscosity due to its effect on membrane stability.
-
iv.
Low dielectric constant. Dielectric constant represents diluent polarity where diluents with dielectric content less than 15 are non-polar (Marcus 2004).
-
v.
Low toxicity to prevent pollution.
-
vi.
Economic.
Besides that, an aliphatic diluent is preferred due to its minimal solubility in water compared to an aromatic diluent. In addition, aliphatic diluent has better stability (Othman et al. 2006).
A stripping agent is required as an accumulation point of the solute once it dissociated at the internal-membrane interface from the solute-carrier complex. A considerable guide by Ho and Kamalesh (1992) in choosing appropriate stripping agents with their associated carrier has been prepared where acidic stripping agent is commonly used with the acidic carrier while both acidic and basic stripping agents can be used with a basic carrier. There are two possible mechanisms of solute transport assisted by a carrier, co-transport and counter transport, depending on the stripping agent used. The difference in pH between the external and internal phases creates a driving force. The difference in pH between the two phases should be kept as low as possible to avoid emulsion swelling (Malik et al. 2012). pH adjustment can be conducted by various chemicals, depending on the carrier type.
Extraction of ECs using ELM
Attention paid to ELM as a promising method for removing pollutants present in wastewater, especially ECs, is growing. Unfortunately, ELM’s main drawback is the emulsion stability, while understanding the complexity of the parameters influencing the stability is not an easy task. Thus, the membrane phase composition must be selected very carefully since the selection affects both the extraction efficiency and ELM stability.
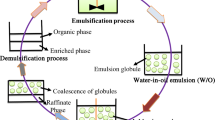
Generally, the ELM process consists of four main steps, which are emulsification, permeation or extraction process, settling, and demulsification (breaking of the emulsion) as shown in Fig. 8. The internal phase and membrane phase are emulsified in the first stage to create an emulsion. The produced emulsion is then disseminated into the external feed phase, which contains the extracted solute. Removal of low-concentrated solute molecules is accomplished by mass transfer in which the prepared emulsion is dispersed into the external feed phase containing the required solute concentration. During the extraction process, these solute molecules pass through the membrane phase, which consists of 0.1–3-mm emulsion globules, and subsequently react with internal phase reagent, which is accessible in the form of fine internal phase droplets (A. Kumar et al. 2019). Following the extraction step, the solute-loaded primary emulsion is separated from the external feed phase by gravitational settling. The membrane phase is then recovered using the demulsification process, where the primary emulsion containing the enhanced solute is de-emulsified to recover the membrane phase constituents and the enriched solute. Finally, the membrane phase ingredients are recovered for reuse in the formulation of the ELM. ELM performance was determined by either calculating extraction efficiency (%) or recovery (%) using Eqs. 4 and 5.
where Cinitial and Cfinal are the initial and final concentration of solute in the external phase, while Cint is the concentration of solute in the stripping phase after extraction. Tr is the treat ratio.
Table 2 elucidates briefly existing literature studies on the removal of ECs using ELM. Typically, Span 80 and kerosene are the most often employed surfactant and diluent, respectively. However, the proper composition of the membrane phase is largely determined by the type of solute to be treated. For example, Shirasangi et al. (2020) conducted a screening experiment in order to select the suitable carrier (TOA/TBP) and the stripping agent (NaOH/Na2CO3/NaCl). Extraction efficiency using TOA with NaOH, Na2CO3, and NaCl were 85.02%, 73.76%, and 25.64%, respectively. Similarly, extraction efficiency using TBP with NaOH, Na2CO3, and NaCl were 69.39%, 60.44%, and 10.17%, respectively. It is discovered that stripping efficiency improves proportionately with stripping agent basicity and that basicity decreases in the following trend of NaCl < Na2CO3 < NaOH, and due to mild methylparaben acidity, it forms a more rapid complex with TOA than TBP. Alreda Akkar and Muslim Mohammed (2021) studied on the separation of organic acid pollutants (acetic acid, benzoic acid, phenol) using kerosene as a membrane phase, Span 80 as a surfactant, and NaOH as a stripping agent in the inner phase of W/O emulsions. According to the effective diffusivities, an extraction efficiency of 98% was achieved, and the mobility of these compounds in the membrane phase is as follows: benzoic acid > acetic acid > phenol.
In the case of penicillin G, Span 80 and non-ionic polyamine PARABAR 9551 are used as a surfactant, and Amberlite LA2 is used as a carrier, Na2CO3 as a stripping agent, and kerosene as a diluent (Lee 2000), while for the extraction of acetaminophen, Chaouchi and Hamdaoui (2014) used hexane, Aliquat 336 potassium chloride, KCl, and Span 80 as diluent, carrier, stripping agent, and surfactant, accordingly. Besides that, Chaouchi and Hamdaoui (2015b) also studied the extraction of the endocrine-disrupting compound propylparaben (PP). The study showed promising method results by using trioctylphosphine oxide (TOPO), hexane, Na2CO3, and Span 80 as the carrier, diluent, stripping agent, and surfactant, respectively. For the extraction of acetic acid, Lee (2015) and Lee and Hyun (2010) use the same diluent and stripping agent. However, Lee (2015) uses C9232 as surfactant and TBP and TOPO as carrier, while Lee and Hyun (2010)as a surfactant and TOA as a carrier extraction efficiency of 91.7 % andSeveral parameters significantly affect the extraction performance in the ELM process, including carrier concentration, stripping agent concentration, emulsification time, agitation speed, and pH value (Ahmad et al. 2012; Chaouchi and Hamdaoui 2014b, 2016; Othman et al. 2016).
Effect of carrier concentration
Carrier concentration is important as it acts as a shuttle to transport solute, which directly affects the extraction efficiency. The carrier concentration has two main roles which are transporting solute molecules from the external feed phase to the internal phase via complex formation and boosting ELM stability and efficiency by increasing the membrane phase viscosity. Several studies proved that both types of carrier and carrier concentration affect the ELM performance (Kumar et al. 2019). Increasing carrier concentration enhances the formation of solute-carrier complex. However, unnecessary high carrier concentration will only reduce the stripping reaction rate since solutes remain in the membrane phase. A high amount of carrier present in the ELM could also bring the increment in mass transport resistance where the accumulation of complex occurred during the process (Goyal et al. 2011). In addition, a massive amount of carrier could in turn encourage emulsion swelling, which diminishes the effectiveness of the process and eventually causes the membrane layer to become thinner, making it prone to breakdown (Chiha et al. 2006). As reported by Mohammed et al. (2020a, 2020b), the transportation and stripping efficiency of tetracycline increased to 96.3% as carrier concentration increased to 4% (v/v). However, the ELM instability began to rise with increases in the carrier concentration to 6, 8, and 10% (v/v), consequently affecting the extraction and stripping percentage as the concentration, the interfacial tensions, and the accumulation of the carrier molecules in the membrane phase increase. Subsequently, the viscosity increases and leads to bigger emulsion droplet size formation (swelling). A similar result was found by Seifollahi and Rahbar-Kelishami (2017); the ability of diclofenac extraction increased with increasing concentration due to the increment of diclofenac ions-TBAB complex species, thus increasing the extraction efficiency. However, incorporation of a carrier higher than 0.04 M reduces the percentage of diclofenac removal. It may also be due to the formation of more complexes, which lead to an increase in organic phase resistance. Thus the extent of the ion transfers decreases. Economically, a lower extractant concentration is always preferable, as it is the most expensive component of the membrane phase. This result is also in line with Jusoh et al. (2019), which studied the effect of Amberlite LA2 concentration on the extraction of succinic. The optimum concentration obtained for the extraction of succinic acid is 0.7M, with an extraction efficiency of 71%. Succinic acid migrates towards the external contact when the concentration of Amberlite LA2 increases. The combination formed at the external interface of Amberlite LA2 and succinic acid then reacts reversibly to permeate across the membrane phase. Thus, this will enhance the flux of succinic acid across the membrane due to the concentration gradient of the complexes. On the other hand, a further increase of carrier concentration leads to a decrease of 55% extraction efficiency. This is because a less viscous emulsion was formed due to the high concentration of carrier. In addition, it could also result in a mass transport resistance increment owing to the accumulation of complex in the membrane phase.
Effect of stripping agent concentration
The stripping agent concentration determines stripping efficiency and extraction efficiency. Thus, it is important to employ optimum concentration to achieve maximum extraction efficiency. Stripping agent concentration should not be above the optimum level as it may cause membrane swelling and breakage, which will lower the extraction efficiency of the membrane. Excessive stripping agents may hydrolyse surfactant and decrease emulsion stability. A study conducted by Zereshki et al. (2018) observed that increasing the concentration of NaOH as a stripping agent from 0.001 to 0.04 M increases extraction efficiency, but increasing the concentration to higher concentrations has the opposite effect. Further increase than 0.04M in concentration gradually resulted in emulsion swelling, which leads to low extraction efficiency. Muthusaravanan et al. (2019) discovered that when the concentration of NaOH increased, there was also an increase in the extraction efficiency of norfloxacin. Due to the ionic potential of the stripping agent, the stripping agent concentration exerted stress on the norfloxacin molecules present in the aqueous phase. When the NaOH concentration was increased, the ionic potential increased, resulting in high extraction efficiency. Similar results were obtained by Garavand et al. (2018) where excess NaOH can potentially reduce the stability of the emulsion by reacting with Span 80 and influencing the surfactant characteristics. Additionally, it results in a thick emulsion with bigger droplet size. Jusoh et al. (2020) vary the concentration of stripping agent from 0.5 to 2 M for the extraction of polyphenols. It was observed that the extraction increased with NaOH concentration up to 1 M and further increase of concentration leads to decrease in extraction efficiency. Low concentrations of NaOH are insufficient to remove polyphenols from complexes, resulting in polyphenol-TBP complexes accumulating in the liquid membrane phase, which limits complex formation, whereas high concentrations of NaOH cause excess NaOH to accumulate in the stripping phase, which prevents polyphenols from being transported from the internal interface. Furthermore, excessive NaOH is unfavourable since it might cause surfactant hydrolysis, decreasing stability. The ELM process’s effectiveness and economy are indicated by a suitable treatment ratio (volume ratio of emulsion to external phase). Othman et al. (2011) found that a small emulsion volume is enough to treat a high volume of wastewater. Minimal emulsion volume is often desired to make it economical.
Effect of emulsification time
On the other hand, sufficient emulsification time must be employed to ensure a stable and uniform emulsion size. Prolonging emulsification time produces smaller and stable emulsion as well as enhances the intensity of the solution. With adequate emulsification time, more significant numbers of fine droplets are produced. Smaller emulsion requires more time to coalesce, hence better stability. However, an inappropriate long time of emulsification is counterproductive. Emulsion prepared at unnecessary long emulsification time is exposed to higher shear, which can cause emulsion breakage (Chiha et al. 2006). In addition, intense emulsification causes the surfactant to escape out of the water-oil interface, thus reducing its concentration. This condition will raise the interfacial stress between the interfaces and produce bigger emulsion droplets that are unstable. Jusoh et al. (2016) demonstrate the effect of emulsification time (3 to 20 min) on the water in oil emulsion stability. It is found that emulsification time of 5 min produces stable emulsion compared to others due to sufficient time for smaller internal droplets to form, thus leading to longer time that is needed to coalesce. This shows that the organic membrane and internal aqueous solution were not homogenised adequately due to the short emulsifying period, while on the contrary, prolonged emulsification time will decrease the emulsion stability due to high shear exposure, thus leading to emulsion breakage. Therefore, it is important to find adequate emulsification time to produce smaller inner droplets, which leads to better emulsion stability and extraction efficiency. Similar results were obtained by Mohammed et al. (2020b), where an emulsification time of 7 min was considered to be the best time with the highest ciprofloxacin extraction efficiency of about 99%. The study was carried out on different emulsification times ranging from 5 to 15 min. Formation of large droplet size of 10.41 m, which allows the coalescence phenomena to occur readily, was found at 5 min due to insufficient emulsification time. However, prolonging the emulsification time to 10 and 15 min causes membrane breaking due to severe internal shearing, resulting in a very high number of emulsion globules per unit volume, facilitating their diffusion into the external feed phase. This is in line with Rosly et al. (2020) where longer emulsifying time raises shear stress and interfacial tension due to high homogenisation pressure. In contrast, insufficient emulsifying time may inhibit blended surfactant interfacial activity from adsorbing at W/O interface. In this study, 3 min of emulsification time is chosen as the optimum duration for forming a stable emulsion with a minimum average droplet size distribution of 8.1 m. A sufficient amount of time is necessary to guarantee that the blended surfactant fully migrates to and adsorbs at the water-oil interface, lowering the interfacial tension and promoting tiny droplet breakup.
Effect of agitation speed
Another parameter affecting extraction efficiency is the agitation speed during the extraction process. Multiple researchers found that ELM extraction increase with an increase in stirring speed (Ahmad et al. 2011; Kumbasar 2008). A high rate of stirring reduces globules size, providing a larger mass transfer area, which is preferable to enhance its extraction performance. However, further increase of the speed resulted in unstable droplets, causing membrane leakage due to the membrane wall’s thinning. Manzak and Tutkun (2011) studied the effect of agitation speed (250–400 rpm) and elucidated those speeds above 300 rpm will decrease the extraction efficiency due to leakage of lactic acid caused by shearing of emulsion. A similar result was obtained by Zereshki et al. (2018) for the removal of cationic dye where the agitation speed within the range of 130–630 rpm was studied. The size of emulsion globules reduces as the stirring speed is increased from 130 to 430 rpm, resulting in an increase in the mass transfer area. Higher than 430 rpm of stirring speed results in emulsion instability and breakup, resulting in ineffective extraction efficiency due to excessive stress. The effect of agitation speed on the extraction efficiency was also studied by Abbassian and Kargari (2016a, 2016b), whereby two different effects are observed by increasing the agitation speed. When agitation speed increases, the impellers’ applied shear forces on the emulsion globules rise, resulting in the formation of smaller globules. Secondly, increasing the agitation speed increases the rate of emulsion globule rupture. The increase in mass transfer area caused by the formation of smaller globules at higher agitation speeds is insufficient to compensate for internal phase reagent release via emulsion breakup. As a result, once a threshold speed of agitation is reached, the overall extraction rate reduces significantly, as does the extraction efficiency. Mohammed et al. (2020a, 2020b) investigating ciprofloxacin extraction from an aqueous solution revealed a significant influence of the emulsion preparation step on the extraction efficiency. The emulsion breakage decreases with increasing the homogeniser speed from 3000 to 12,700 rpm, while the minimum breakage percentage of 0.063% was observed. At the same time, the extraction efficiency peaked at 98.8% while maintaining minimal breakage (Mohammed et al. 2020a). The internal droplets are smaller in size at higher rotation speeds, with the diameter decreasing from 18.5 μm at 3000 rpm to 4.31 μm at 12,700 rpm. This affects the surface area and increases the rate of solute transfer. However, as the homogeniser speed increases (up to 19,700 rpm), the stability decreases, and the extraction efficiency is reduced to 87.5%. On the other hand, low homogeniser speed (below 12,700) causes instability and higher chances of breakage due to the larger droplet size, and the coalescence phenomenon occurs in a short time. Besides speed, it was also proved that inadequate emulsification time (5 min) would produce big droplet size (10.41 μm) that will promote coalescence, subsequently heighten the breakage, and lower the extraction efficiency. This is in line with Akkar and Mohammed (2021) where increasing the agitation level increased the interfacial area and the coefficient of mass transfer. Nevertheless, emulsion droplets are prone to rupture at a certain level of agitation, resulting in acid value enrichment and extraction. Agitation speeds of 200, 300, and 400 rpm were conducted for extraction efficiency, and it can be seen that 300 rpm was the most suitable agitation speed for phenol extraction.
Effect of pH value
The pH of the external feed phase affects the efficiency, particularly in carrier-mediated transport mechanisms. This is because the reaction to form a solute-carrier complex is a pH-dependent process to produce solute in ionic form. Also, the equilibrium constant of the solute-carrier extraction reaction is sensitive towards pH value of the external phase since the pH value affected the acceleration of the destabilisation of the droplets. Sujatha and Rajasimman (2021) screen out parameters that have influences on the ELM stability, and arsenic extraction and the pH of the external feed solution were the last in the order. Thus, based on the preliminary screening and inference, the value of non-significant parameters is fixed, which includes the pH value. Fewer focuses have been offered in the extraction of ECs in terms of breakage and extraction efficiency. However, studies show that in some application, the extraction efficiency and emulsion breakage was affected by the pH value as depicted in Table 3. The pH value of the external feed phase used varies depending on the type of solute being extracted. Some solute has higher extraction efficiency in acidic condition, while some is better in basic condition. Interestingly, Razo-Lazcano et al. (2018) demonstrated the influence of acid concentration on the droplet sizes. As the pH decreases, the superficial charge of the droplet increases. Thus, the droplet size is larger at low HCl concentrations because the generated superficial charge is not enough to provoke a repulsion capable of preventing the coalescence between the droplets. Davoodi-Nasab et al. (2018) investigate the effect of the initial pH of the external feed phase on the gadolinium extraction by varying the pH values in the range of 0.1 to 2. It was discovered that increasing the acidity of the external feed phase resulted in a decrease in extraction efficiency. This is because the rate at which gadolinium complexes with D2EHPA, which works as a cation exchanger, is directly proportional to the acidity of the external feed phase. However, lowering the pH from 2 to 0.1 resulted in a less stable emulsion. This is due to the hydrolysis of Span 80 since it is chemically unstable in moderately acidic and basic conditions, thus undergoing hydrolysis. This causes the osmotic swelling ratio to increase and gadolinium extraction to decrease. A similar study was found by Jusoh et al. (2020), where the effect of pH from 3 to 10 on polyphenol extraction was studied, and it was found that the optimum pH value is at pH 5. The acidic environment aids in the preservation of polyphenols, which are then ready for extraction into the organic phase. An increase in pH value increases the extraction from 42 to 65 % and reduced to 33% with pH 10. Very acidic conditions are unfavourable since they tend to degrade the surfactant’s stabiliser properties. Polyphenols in sterilisation condensate dissociate to their anionic form at higher pH values (7–10), resulting in a considerable reduction in their extraction by a neutral carrier. While on the contrary, for the extraction of ciprofloxacin conducted by Mohammed et al. (2020a, 2020b), it was discovered that the suitable external feed phase pH value is at 8 with an extraction efficiency of 98.85%. This could be owing to greater H+ concentrations at lower pH causing instability, as well as a decrease in extraction efficiency due to the neutral extractant (TBP) being unable to form a significant complex with the ciprofloxacin molecule at this pH range. However, when the external phase pH is raised to a more neutral level, the extraction efficiency increases to more than 71%, and the breakage percentage decreases. This can be explained by protons being released as a result of an anion exchange reaction, as well as an increase in pH causing the production of additional species. Different trends of extraction efficiency at various external feed phase pH values can be observed from Table 3. In terms of phenol extraction, it was concluded as the change of the external pH caused by the emulsion breakage introduces the highly alkaline internal phase reagent into the external phase. Thus, it decreases the acidity of the external phase. It can be explained as the dissociation reversal of phenol reaction when there are changes in pH value as it is decreased (increase in H+ concentration) which leads to the remaining of phenol in an oil-soluble state. In addition, the breakage introduces the phenolate ions into the external phase, which lowers the extraction efficiency.
Emulsion stability
Due to the weak stability of ELM, several industrial applications of separation processes based on ELM are limited for practical use. An emulsion’s stability is described as the emulsion’s resistance towards high shear stress and droplet coalescence. Stirring of the emulsion during the extraction process could jeopardise the emulsion stability. In order to achieve high extraction efficiency, the emulsion must be stable enough during this process. The stability of the emulsion might range from a few minutes to years. The stability of the ELM depends on the stability of the interfacial film, which is dependent on a number of factors, including the emulsion droplet size, the method of emulsion preparation, the surfactant adsorption-desorption kinetics, the mixing intensity, the constituent concentrations, the stripping phase concentration, the external feed phase pH, the phase volumes, and the surfactants, extractants, stripping agents, and diluents (Kumbasar 2009; Othman et al. 2010). Therefore, the selection of ELM components and parameters is important as it affects the ELM stability and extraction efficiency. The interfacial rheological features of ELM, such as the interfacial tension gradient, interfacial viscosity, and elasticity, also have a significant effect on ELM stability (Alsabagh et al. 2016). The other critical characteristic that has a significant effect on ELM stability is the droplet size (Ahmad et al. 2012). Both the preparation methods and the emulsion composition played an important role in producing stable emulsion associated with the droplet size of the emulsion. Emulsion droplet size is usually represented by Sauter mean diameter (d32), where according to Canselier et al. (2002), it is considered to be effective to represent the average surface diameter of the emulsion as shown in Eq. 6:
where ni and di are the number and diameter of droplets belonging to the ith class, respectively, while V and A are the dispersed phase’s total volume and area.
Smaller droplets result in increased breakage resistance and extraction efficiency, whereas larger droplets result in decreased ELM stability and extraction efficiency (%) (Hachemaoui et al. 2010; Patnaik 1995). Several studies were summarised on the stability of ELM for EC extraction as tabulated in Table 4. It can be elucidated that low droplet leads to low membrane breakage and high extraction efficiency. ELM’s stability is mostly attributable to the surfactant molecules forming a protective interfacial film or barrier with viscoelastic properties that function as a barrier to aggregation and coalescence of emulsion droplets (Mosayebi and Abedini 2013). Kusumastuti et al. (2017) had discovered that an emulsion with droplets ranging in size from 0.3 to 10 μm (ideally 0.8–3 μm) results in excellent extraction rates and great ELM stability. This is in line with the study conducted by Mohammed et al. (2020a, 2020b), where at optimum parameter, a droplet size of 4.31 μm, membrane breakage 0.063 %, and extraction efficiency of 98.8% were achieved. Extraction of organic acids was conducted by Alreda Akkar and Muslim Mohammed (2021) where the droplet size obtained is 15 μm for acetic acid having a frequency of 68%, 25μm for benzoic acid having a frequency of 27%, and 115 μm for phenol having a frequency of 28% which proves large droplet size decreases the extraction efficiency. Meanwhile, excessive amount of stable emulsion is undesirable since it causes difficulties with its settling and de-emulsification during the third stage of the ELM process (Ahmad et al. 2011).
Despite the promising features of ELM, its major drawback is emulsion’s instability, which has impeded the widespread applications of ELM on a larger scale. Unstable emulsion tends to rupture or break, hence diminishing the efficiency of the system (Park et al. 2004). According to Kumar et al. (2012), two thermodynamically unstable interfaces result in emulsion instability. Three main phenomena could cause instability of emulsion, which are coalescence, emulsion swelling, and membrane breakage, as shown in Fig. 9(Chakraborty et al. 2010). Extensive studies are conducted to minimise the occurrence of emulsion instability to achieve better extraction efficiency.
Coalescence
Coalescence occurred because of the aggregation of two or more emulsion droplets, resulting in the creation of a larger one (Ahmad et al. 2015). The approach of two or more droplets indicates the beginning of the coalescence, leading to emulsion destruction. Coalescence occurs in many steps where it is initiated by droplet contact (Borwankar et al. 1992). The pressure increases in the contact area, flattening both surfaces of the droplets, which causes the continuous phase film between the drop starts and drain out. Then, film rupture occurred, and the droplets are completely coalescing (Kawasaki et al. 2009).
Swelling
Swelling involves the transportation of the external aqueous phase into the emulsion, where it causes the volume of the internal phase to expand. Swelling is destructive from a process point of view because of three main factors (Kulkarni and Mahajani 2002; Malik et al. 2012; Park et al. 2004; Wan and Zhang 2002): (1) the thickness of the membrane is reduced, thus causing breakage, (2) the driving force for solute extraction is reduced, (3) alteration of the viscosity of ELM, which eventually influences the dispersion of emulsion in the external feed phase. Swelling can be divided into two types: osmotic and entrainment (Ho and Kamalesh 1992; Park 2006). Osmotic swelling is owing to the difference in osmotic pressure between the external and internal phases. In contrast, entrainment swelling is caused by re-dispersion and frequent coalescence of emulsion globules throughout the extraction process (Van et al. 1987; Wan and Zhang 2002).
Emulsion swelling is induced by osmotic pressure via two possible mechanisms (Wen and Papadopoulos 2000). Firstly, the surfactant molecules in the external phase tend to form a complex with water molecules. The hydrophilic part of the surfactant is hydrated at the membrane-external interface, which is later dehydrated when it reaches the membrane-internal interface (Yan and Pal 2001). The water will be released into the aqueous internal phase, hence causing the emulsion to swell. The second mechanism is via the act of reversed micelles that transport water molecules. In this case, the surfactant aggregates and stabilises water molecules in the oil membrane phase. The hydrophilic heads of surfactants retain water within the micelles, while surfactants’ hydrophobic tails face the liquid membrane’s non-polar portion. The micelles form at the membrane-external interface and move the water molecules into the stripping process, similar to the first mechanism.
Membrane breakage
Membrane breakage causes the expulsion of the internal phase to the external phase. This condition causes the detriment of stripping phase agent and entrapped solute; hence, lower extraction efficiency can be anticipated. The release of the internal phase does not only affect the separation efficiency, but the stripping phase agent will contaminate the external phase (Mat et al. 2006). Besides, the membrane breakage will also ruin down the transfer process by decreasing the mass transfer area (Martin and Davies 1977). It is said that around 0.1% membrane breakage is acceptable for a practical process (Draxler and Marr 1986).
Membrane breakage is quantified based on the change of H+ ion concentration in the external phase, which can be easily measured by a pH meter. Estimation of membrane breakage ε (%) can be made according to the following equation:
where Vi is the initial volume of the internal phase while Vs is the volume of the internal phase leaked into the external phase, which can be calculated by mass balance as shown in the equation below.
where VExt is the initial volume of the external phase and pHo and pH are the initial pH of the external phase and pH of the external phase being in contact with emulsion after stirring, respectively. \( {C}_{OH^{-}}^i \) is the initial concentration of OH– in the internal phase (Ahmad et al. 2018).
Methods are proposed to overcome the problem of ELM stability which are selecting the appropriate membrane phase compositions, increasing the emulsifier concentration and viscosity using Newtonian additives, increasing the viscosity of membrane phase, and adding a modifier to convert the emulsion’s Newtonian behaviour to a non-Newtonian behaviour (A. Kumar et al. 2019). Most of the researchers focus on selecting the appropriate membrane phase compositions to overcome the instability problem. Membrane phase compositions were chosen based on factors such as utilisation of new type of surfactant, agitation time, size of emulsion droplet, concentration of emulsifying agent, and pH of internal droplets (Barad et al. 2010). Nevertheless, increasing the emulsifier concentration also has been explored to improve ELM stability. Non-ionic surfactants with HLB (hydrophilic-lipophilic balance) values less than 5 have been the most commonly employed emulsifiers in ELM technology such as Span 80 (Abbassian and Kargari 2016b). Increasing the emulsifier concentration strengthens the interfacial film and boosts resistance to coalescence. However, establishing adsorptive and mechanical barriers at the interfaces nonetheless reduces solute transfer rates due to reduced internal motion of small droplets within big emulsion globules (Dâas and Hamdaoui 2010). Binks et al. (2007) discovered that the resistance to creaming and coalescence is both increased as is the emulsion viscosity and completely stable emulsions can be achieved at low overall emulsifier concentration.
The stability also can be improved by increasing the membrane phase viscosity which can be conducted by the addition of nanoparticles, the usage of viscosity-enhancing agent, or reducing the transportation of water molecules from the external feed phase to stripping phase (Al-Obaidi et al. 2020; Jusoh et al. 2016; Weidemann et al. 2018). Nonetheless, it significantly reduces the diffusion coefficient as well as the mass transfer rate of the desired species. Both of the mentioned methods did reduce the ELM stability, but they reduce the permeability of the membrane which can lead to long transfer path, low diffusivity, and low selectivity (Kumar et al. 2019). Mohammed et al. (2020a, 2020b) improvised the current ELM design and reducing the risk of emulsion instability paves a new pathway for the process. The work introduced nano-fluid (Pickering) emulsion liquid to eliminate toxic contaminants from aqueous solutions or recognised in short as NFELM. The membrane phase consists of n-heptane (diluent), oleic acid–modified Fe2O3 nanoparticles (stabilising agent), and hydrochloric acid–homogenised tributyl phosphate (TBP, extractant) (HCl) as the internal aqueous phase to develop the nano-fluid emulsion. Within 12 min of mixing time, over 97% was efficiently extracted by the NFELM method with stripping efficiency at 96.5% without significant emulsion breakage. High efficiency and acceptably low emulsion instability result from tinier internal droplets at an increased emulsification speed. This improves the surface area of the droplet and speeds up the rate of mass transfer. Elongating the emulsification time to 10 and 15 min increases breakage and decreases extraction efficiency to 83% and 69%, respectively, while at 15 min, the stripping efficiency decreased to 59.5 %. This is due to high internal shearing, which generates a large number of droplets of NFE per unit volume, thus speeding up their transport to the external phase. However, a high number of NFE droplets encourage the coalescence phenomenon and thus create a large-sized emulsion droplet that increases membrane breakage.
The addition of a modifier to convert the emulsion’s Newtonian behaviour to a non-Newtonian behaviour also was attempted in order to increase the membrane stability. Few studies use low amount of viscoelastic polymers such as polybutadiene (PBD), polyisobutylene (PIB), styrene butadiene rubber (SBR), and polystyrene (PS) as stabilisers (Abbassian and Kargari 2016b; Kargari and Abbassian 2015; Lee 2000; Park et al. 2006). This addition can improve the stability without compromising features like permeability and diffusivity for the ELM. Generally, the membrane phase and the dissolved polymers have great ELM resilience against shear stress without reducing much the rates and the permeability of the membrane. The dissolved polymers also reduce the viscosity of the membrane phase next to the zero shear rate, which further enhances the strength and stability of the membrane by significantly minimising emulsion swelling. Abbassian and Kargari (2016a, 2016b) studied the ELM stability with an appropriate formulation of ELM using styrene butadiene rubber (SBR) as stabiliser. A stable ELM system was obtained by the addition of 3 wt.% of SBR to the membrane phase. There is an increase on the stability of the ELM from 5 to 35 min with the addition of SBR, and it is able to achieve extraction efficiency of 90.1%. Besides that, the addition of blended surfactants is also an alternative to improve membrane stability. Rosly et al. (2020) studied ELM process using a bi-functional diluent with blended non-ionic surfactant for phenol removal. A little amount of Tween 80 binds synergistically with the molecules of Span 80 to form a stable elastic network that reduces droplet coalescence and avoids emulsion breakdown, hence improving emulsion stability. Interactions between surfactants may alter the structural organisation, physicochemical properties, and functional properties of the surfactant molecules, hence influencing the stability and functionality of the emulsion. The optimum formulation for the blended non-ionic surfactant between Span 80 and Tween 80 was achieved at HLB 5, with an extraction efficiency of 83.4%.
Demulsification process
In general, demulsification is a process to destabilise the emulsion to recover and allows reusability of the membrane phase in ELM (Ooi et al. 2015). Besides reducing secondary pollution, demulsification could also lower the operational expenditure of the process (Lu-ting 2006). Demulsification separates the aqueous internal phase and membrane phase via chemical, physical (Teng et al. 2014), or combination methods. The physical method includes heating, freeze and thaw, microwave radiation, ultrasonication, membrane separation, and centrifugation. Meanwhile, the chemicals used to demulsify the emulsion in ELM systems can be found in Table 5.
An emulsion is known as a thermodynamically unstable system and will eventually separate into water and oil phases. However, optimised surfactant concentration and the ratio between phases allow the emulsion produced to be stable for a long duration. The collapse of ELM occurs through a series of steps (shown in Fig. 10a) which are flocculation and coalescence (Lu et al. 1997). Flocculation refers to the formation of floc by the internal phase scattered droplets. The readily large clusters will further coalesce into a larger unit and lead to the decrease of drop numbers. With gravity, the large internal drops will easily sink in the interface of the membrane and internal phase and coagulate with the water phase; thus, demulsification process occurs. Destabilisation of an emulsion is also a result of creaming and sedimentation. In the end, the emulsion droplets will coalesce, becoming large enough to separate into two phases. Before coalescing, the droplets interact with each other, resulting in the drainage of the continuous phase film between the droplets. This phenomenon causes the droplet’s film to break and coalesce (Kamp et al. 2017). On the other hand, a different mechanism was proposed to separate the phases in the emulsion. Sun et al. (1998) reported on the possibility of emulsion breakage by a hydrophilic porous glass membrane. As demonstrated in Fig. 10b, the emulsion droplets pass through the membrane by transmembrane pressure, deformed, squeezed, collided, and broken. In this method, attention must be paid to the wettability property of the membrane and pore size.
Thermal treatment is a relatively simpler method compared to the others. It can be carried out by direct heating, or the temperature of the emulsion can be elevated by microwave radiation. The first is dependent on the temperature and heating time, while electrolyte concentration, power supply, and exposure time have a significant influence on the latter. For instance, Jiao et al. (2013)completely demulsify the emulsion at a temperature higher than 75°C after 30 min of heating. Chan and Chen (2002) reported that demulsification is complete at 420 W and 12 s while maintaining the electrolyte concentration at 0.5M. Microwave is a clean, cheap, and convenient heating method, and this method is able to reduce the reaction times to yield higher separation efficiency (Fortuny et al. 2007). According to Kusumastuti et al. (2020), the microwave could save energy up to 97% and 99% compared to that of ultrasonic probe and centrifuge, respectively. However, this method requires the presence of a high concentration of electrolytes, for instance, NaCl, KCl, and NaNO3, to achieve high demulsification efficiency (Fortuny et al. 2007). On the other hand, the electric field method was used by Othman et al. (2010). It was claimed that this method promotes irreversible rupturing of stable emulsion (Othman et al. 2010), and the droplets coalesce to a greater extent when the external field surpasses a certain critical value (Lu et al. 1997). Unfortunately, this method was ineffective towards emulsions with high water content (Abismaïl et al. 1999) and required very high electric field strength (Chan and Chen 2002). The freeze-thaw method was successfully used by Lin et al. (2007) where the study proposed a collision mechanism to destabilise the emulsion. Lin et al. (2008) hypothesised that there are four steps involved to separate the phases in the emulsion. Firstly, that the continuous oil phase freezes rapidly at first on cooling and forms a solid cage to fix droplets. The cage broke as the droplet phase freezes gradually and producing fine gaps where some liquid droplet phase permeates and then forms a large network bridging droplet. During thawing, the network fuses and coalesces.
Li (1978) introduced the method of centrifugation for W/O demulsification. To break the emulsion completely, centrifugation was coupled with another high shear device by pumping the half-broken emulsion into the unit (Teng et al. 2014). In fact, the addition of some liquid was found to be necessary to reduce the total emulsion’s viscosity to allow complete emulsion breaking. However, the dilution of the extracted solute in the internal phase is frequently associated with the additional aqueous media in the ELM system. Juang and Lin (2004) used an ultrasonic probe at high power to sonicate the emulsion. The work inferred that the process of emulsion destruction by using ultrasonication is a function of exposure time. In their study, minimal exposure of 5 min is required to allow the destruction of the emulsion. The author claimed that the destruction of W/O emulsion is impossible at a power below 33W. This method is claimed to be chemical-free and clean, and the breaking of an emulsion can be done real quick (Susunu et al. 2008). Anarakdim et al. (2020) successfully demulsified the membrane phase by heating up to 80°C for 2h with water content less than 4%. Yet, this method may possess another problem which increases the energy and cost requirement of the system. On the contrary, Moyo and Tandlich (2015) reveal that thermal treatment alone is insufficient to completely break the emulsion unless a chemical (or known as demulsifier) is added. Apparently, such a combination allows complete separation of the phases, but the membrane phase change in colour, due to the presence of the foreign chemical in the system. In this regard, utilisation of chemicals in the demulsification of ELM is not preferred, and the removal of the demulsifier is another completely new issue to be addressed before the membrane phase can be reused. According to Bjorkegren and Fassihi (2011) this method modifies the characteristics of the membrane phase component and limits its reusability. Furthermore, it requires an additional process to separate the emulsion so that the solute can be retrieved and the membrane phase can be reused, which is not economically feasible (Jilska and Geoff 2008).
Nevertheless, destabilisation of emulsion in ELM system by chemical treatment is frequently reported (Chaouchi & Hamdaoui, 2014; Devulapalli and Jones 1999), in which alcohols are typically used. Evidently, these works reported complete phase separation. The selection of a suitable demulsifier and its concentration, HLB value, and reaction time are among the crucial factors to be duly considered (Abdulredha et al. 2020; Al-Sabagh et al. 2013). Typically, a demulsifier has higher surface activity than that of emulsifying agents to destabilise the emulsion (Abullah et al. 2016). The chemicals used in demulsification have the function to induce droplet coalescence, and the bigger droplets formed can be separated easily. Similar to the physical treatment, the addition of a demulsifier into the system induces flocculation and coalescence before phase separation is achieved (Hassanshahi et al. 2020).
General criticism on ELM for EC removal
Having stated all the parameters involved in ELM for EC removal over the decades, the following conclusions can be obtained by taking into consideration the application by all these parameters. Firstly, emulsion instability hinders the system from being implemented in industrial applications. The emulsion’s stability is characterised as resistance to liquid membrane rupture at high shear stress, swelling, and coalescence. The emulsion can be destabilised by sedimentation, osmotic swelling, creaming, coalescence, and droplets’ flocculation. In addition, emulsion instability hinders the system from being implemented in industrial application. The emulsion’s stability is characterised as resistance to liquid membrane rupture at high shear stress, swelling, and coalescence. Since stability is the major problem for ELM system, more focus should be given towards the method to overcome the problem and improve on emulsion stability without losing the main function of ELM.
The emulsion stability can be significantly improved with surfactant blend and introduce nanoparticles into the liquid membrane formulation as studied by Shirasangi et al. (2020). An extraction of 93.54% of methylparaben was achieved using emulsion consisting of 2% w/v TOA, 2% w/v Span 80, and 0.1 N NaOH. Furthermore, 100% extraction was obtained using 2% w/v blended surfactant at a contact time of 10 min: Span 80 and Span 20 (HLB 5.7) with MWCNT122. Multiple cycle experiments have shown that the emulsions can be used up to four cycles without de-emulsification in the batch process. Meanwhile, the combination of mixed surfactant and MWCNT provides greater emulsion stability and opens up a broader scope for the industrial application of the technique. Besides that, blending non-ionic surfactant also may be an alternative to tackle this problem as conducted by Rosly et al. (2020), whereby the interactions between surfactants may alter the structural organisation, physicochemical properties, and functional properties of the surfactant molecules, thus enhancing the stability and extraction efficiency. A stable W/O emulsion was obtained at 7:3 palm oil to kerosene ratio, blended surfactant at HLB 5, 3% w/v of blended surfactant concentration, 0.1M of sodium hydroxide as stripping agent, and 3:1 of organic to internal ratio with an efficiency of 83.4%. Addition of polymers also may increase the emulsion stability as studied by Abbassian and Kargari (2016a, 2016b) with an appropriate formulation of emulsion, and the emulsion stability without polymer can be obtained at 5–10 min, whereas with polymers at 35 min.
Generally, petroleum-based chemicals have been widely used as a diluent in the liquid membrane formulation. Apparently, this type of diluent is not environmentally friendly. Recently the whole scientific community has been pivotally debating on the concern of the environment, economy, and energy regarding the particular concern over the sustainability, environmentally benign, and equity as green technology. Therefore, researchers have been carefully choosing an alternative renewable diluent as a better replacement for this diluent. Jusoh et al. (2020) selected vegetable-based palm cooking oil as a diluent to recuperate succinic acid from the fermentation broth. This diluent is easily accessible and may contain organic surface-active agents, which might enhance the stability of emulsion usage of chemical diluent. Next, the reusability of the emulsion would therefore reduce the cost of operation of the system. Although the process of demulsification requires another set of processes and equipment, its contribution towards improving the economic value of the overall process must never be overlooked. With the growing number of studies reporting the possibility of separating oil and water in ELM systems, this technology can be used on a larger scale provided that the ideal formulation is identified to allow demulsification. In this regard, a thorough study that paves the way for the cradle-to-cradle approach via this technology is highly recommended.
In spite of that, one of the benefits of ELM is the low maintenance cost of the system. However, it might consume a large space to be implemented on an industrial scale (Jilska and Geoff 2008). Thus, one of the upgraded versions of ELM that has been progressively employed is supported liquid membrane. This hybrid technology between two types of the membrane provides high performance and better stability and reduces the space constraint. It is now time to articulate a successful laboratory-scale ELM systematically to make it applicable on an industrial scale. Of course, there are challenges in doing so, both from the perspective of economic and operational. For example, (i) the essential materials in establishing the suitability for additional and advanced mass production; (ii) fully understanding the inhibition and diffusion mechanism; and (iii) technical necessities (effective costing for fabrication, data gathering, and wireless networks) urge to be reflected, although this is not in the dimension of this review.
Conclusion
Herein, the most recent works on removing ECs present in domestic or industrial wastewater have been reviewed. ELM technology is one of the most efficient methods of separation that were operated without pressure or voltage because the separation is based on a concentration difference. The extraction approach based on ELM has been proposed as a potential method for removing various contaminants and valuable products from industrial wastewater and effluent streams. The results provided in this review paper (Table 2) demonstrate that significant research efforts are being made to determine the optimal membrane phase composition for a variety of contaminants. Appropriate selection of membrane phase ingredients is based on a single critical parameter, namely extraction efficiency. The most frequently used surfactant, stripping agent, and diluent for EC removal/extraction are Span 80, sodium hydroxide, and hexane/kerosene, respectively. At the same time, the most relevant carriers are Aliquat 336 and tributyl phosphate. A considerable gap in experimental works in implementing the technology in industrial size might become a challenge. Nevertheless, separation and analysis evidently prove that ELM can be one of the feasible technologies to remove ECs. ELM’s advantage in removing the ECs even in low concentration without fouling problems such as polymeric membrane separation might have been an upper hand for this technology to be implemented widely. With the emerging innovative and green ELM, the technology could be cheaper and greener while maintaining its effectiveness. The ELM process technology will undoubtedly contribute to the establishment of new possibilities for future research and development and the broader membrane community.
References
Abbassian K, Kargari A (2016a) Modification of membrane formulation for stabilization of emulsion liquid membrane for extraction of phenol from aqueous solutions. Journal of Environmental Chemical Engineering 4(4, Part A):3926–3933. https://doi.org/10.1016/j.jece.2016.08.030
Abbassian K, Kargari A (2016b) Effect of polymer addition to membrane phase to improve the stability of emulsion liquid membrane for phenol pertraction. Desalination and Water Treatment 57(7):2942–2951. https://doi.org/10.1080/19443994.2014.983981
Abdulredha MM, Siti Aslina H, Luqman CA (2020) Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arabian Journal of Chemistry 13(1):3403–3428. https://doi.org/10.1016/j.arabjc.2018.11.014
Abismaïl B, Canselier JP, Wilhelm AM, Delmas H, Gourdon, C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrasonics Sonochemistry 6:75–83
Abullah MMS, Al-Lohedan HA, Attah AM (2016) Synthesis and application of amphiphilic ionic liquid based on acrylate copolymers as demulsifier and oil spill dispersant. Journal of Molecular Liquids 219:54–62. https://doi.org/10.1016/j.molliq.2016.03.011
Ahmad AL, Kusumastuti A, Derek CJC, Ooi BS (2011) Emulsion liquid membrane for heavy metal removal: an overview on emulsion stabilization and destabilization. Chemical Engineering Journal 171(3):870–882
Ahmad, A. L., Kusumastuti, A., Derek, C. J. C., & Ooi, B. S. (2012). Emulsion liquid membrane for cadmium removal: studies on emulsion diameter and stability. Desalination, 287(0), 30–34.
Ahmad AL, Shah BMMH, Ooi BS, Kusumastuti A (2015) Cadmium removal using vegetable oil based emulsion liquid membrane (ELM): membrane breakage. Jurnal Teknologi
Ahmad AL, Zaulkiflee ND, Kusumastuti A, Buddin MMHS (2018) Removal of acetaminophen from aqueous solution by emulsion liquid membrane: emulsion stability study. Industrial & Engineering Chemistry Research 58(2):713–719
Akkar SAA, Mohammed SAM (2021) Design of intelligent network to predicate phenol removal from waste water by emulsion liquid membrane. Materials Science Forum 1021:115–128. https://doi.org/10.4028/www.scientific.net/MSF.1021.115
Al-Obaidi Q, Alabdulmuhsin M, Tolstik A, Trautman JG, Al-Dahhan M (2020) Removal of hydrocarbons of 4-nitrophenol by emulsion liquid membrane (ELM) using magnetic Fe2O3 nanoparticles and ionic liquid. Journal of Water Process Engineering 101729:101729. https://doi.org/10.1016/j.jwpe.2020.101729
Alreda Akkar SA, Muslim Mohammed SA (2021) The feasibility of emulsion liquid membrane for the extraction of organic acids from wastewater. IOP Conference Series: Materials Science and Engineering 1076(1):012021. https://doi.org/10.1088/1757-899X/1076/1/012021
Alsabagh AM, Hassan ME, Desouky SEM, Nasser NM, Elsharaky EA, Abdelhamid MM (2016) Demulsification of W/O emulsion at petroleum field and reservoir conditions using some demulsifiers based on polyethylene and propylene oxides. Egyptian Journal of Petroleum 25(4):585–595. https://doi.org/10.1016/j.ejpe.2016.05.008
Al-Sabagh AM, Nasser NM, Abd El-Hamid TM (2013) Investigation of kinetic and rheological properties for the demulsification process. Egyptian Journal of Petroleum 22(1):117–127. https://doi.org/10.1016/j.ejpe.2012.11.013
Anarakdim K, Matos M, Cambiella A, Senhadji-Kebiche O, Gutiérrez G (2020) Effect of temperature on the heat treatment to recover green solvent from emulsion liquid membranes used in the extraction of Cr(VI). Chemical Engineering and Processing - Process Intensification 158108178. https://doi.org/10.1016/j.cep.2020.108178
Angeles LF, Mullen RA, Huang IJ, Wilson C, Khunjar W, Sirotkin HI, McElroy AE, Aga DS (2020) Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems. Environmental Science: Water Research & Technology 6(1):62–77. https://doi.org/10.1039/C9EW00559E
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresource Technology 99(6):1578–1583
Balabanič D, Hermosilla D, Merayo N, Klemenčič AK, Blanco Á (2012) Comparison of different wastewater treatments for removal of selected endocrine-disruptors from paper mill wastewaters. Journal of Environmental Science and Health, Part A 47(10):1350–1363. https://doi.org/10.1080/10934529.2012.672301
Balasubramanian A, Venkatesan S (2012) Removal of phenolic compounds from aqueous solutions by emulsion liquid membrane containing ionic liquid [BMIM]+[PF6]− in tributyl phosphate. Desalination 289:27–34
Barad JM, Chakraborty M, Bart H-J (2010) Stability and performance study of water-in-oil-in-water emulsion: extraction of aromatic amines. Industrial & Engineering Chemistry Research 49(12):5808–5815. https://doi.org/10.1021/ie901698u
Binks BP, Desforges A, Duff DG (2007) Synergistic stabilization of emulsions by a mixture of surface-active nanoparticles and surfactant. Langmuir 23(3):1098–1106. https://doi.org/10.1021/la062510y
Bjorkegren S, Fassihi R (2011) A study of the heavy metal extraction process using emulsion liquid membranes. In Department of Chemical Engineering: Vol. PhD. Chalmers University of Technology
Boleda MR, Galceran MT, Ventura F (2011) Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environmental Pollution 159(6):1584–1591
Borwankar RP, Lobo LA, Wasan DT (1992) Emulsion stability- kinetics of flocculation and coalescence. Colloids and Surfaces 69:135–146
Boyadzhiev L, Lazarova Z (1995) Chapter 7 Liquid membranes (liquid pertraction). In R. D. Noble & S. A. Stern (Eds.), Membrane Science and Technology (Vol. 2, pp. 283–352). Elsevier. http://www.sciencedirect.com/science/article/pii/S0927519306800094
Canselier JP, Delmas H, Wilhelm AM, Abismaïl B (2002) Ultrasound Emulsification—An Overview. Journal of Dispersion Science and Technology 23(1–3):333–349. https://doi.org/10.1080/01932690208984209
Chakraborty M, Bhattacharya C, Datta S (2010) Emulsion liquid membranes: definitions and classification, theories, module design, applications, new directions and perspectives. In: Kislik VS (ed) Liquid Membranes Principles and Applications in Chemical Separations and Wastewater Treatment (1st ed., pp. 141–199). Elsevier Science
Chan C-C, Chen Y-C (2002) Demulsification of W/O emulsions by microwave radiation. Separation Science and Technology 37(15):3407–3420. https://doi.org/10.1081/SS-120014434
Chanukya BS, Navin KR (2013) Extraction of alcohol from wine and color extracts using liquid emulsion membrane. Separation and Purification Technology 105:41–47
Chanukya, B.S. Kumar, Manish Rastogi, & K., N. (2013). Optimization of lactic acid pertraction using liquid emulsion membranes by response surface methodology. Separation and Purification Technology, 111, 1–8, 1.
Chaouchi S, Hamdaoui O (2014a) Acetaminophen extraction by emulsion liquid membrane using Aliquat 336 as extractant. Separation and Purification Technology 129:32–40. https://doi.org/10.1016/j.seppur.2014.03.021
Chaouchi S, Hamdaoui O (2014b) Extraction of priority pollutant 4-nitrophenol from water by emulsion liquid membrane: emulsion stability, effect of operational conditions and membrane reuse. Journal of Dispersion Science and Technology 35(9):1278–1288
Chaouchi, S., & Hamdaoui, O. (2015a). Removal of 4-nitrophenol from water by emulsion liquid membrane. Desalination and Water Treatment, 2015(0), 1–5.
Chaouchi S, Hamdaoui O (2015b) Extraction of endocrine disrupting compound propylparaben from water by emulsion liquid membrane using trioctylphosphine oxide as carrier. Journal of Industrial and Engineering Chemistry 22:296–305. https://doi.org/10.1016/j.jiec.2014.07.023
Chaouchi S, Hamdaoui O (2016) Removal of 4-nitrophenol from water by emulsion liquid membrane. Desalination and Water Treatment 57(12):5253–5257. https://doi.org/10.1080/19443994.2015.1021104
Chiha M, Samar MH, Hamdaoui O (2006) Extraction of chromium (VI) from sulphuric acid aqueous solutions by a liquid surfactant membrane (LSM). Desalination 194(1–3):69–80
Cristina MIM, Daniela R (2003) Removal of uranyl ions uo22+ from dilute solutions by ELM technique. I. Transport Mechanism of Uranyl Ions with Ammonium Quaternary Salts as Carrier. Analele Universitatii Bucuresti : Chimie
Dâas A, Hamdaoui O (2010) Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. Journal of Hazardous Materials 178:973–981
Davoodi-Nasab P, Rahbar-Kelishami A, Safdari J, Abolghasemi H (2018) Evaluation of the emulsion liquid membrane performance on the removal of gadolinium from acidic solutions. Journal of Molecular Liquids 262:97–103. https://doi.org/10.1016/j.molliq.2018.04.062
Devulapalli R, Jones F (1999) Separation of aniline from aqueous solutions using emulsion liquid membranes. Journal of Hazardous Materials 70(3):157–170
Draxler J, Marr R (1986) Emulsion liquid membranes part I: phenomenon and industrial application. Chemical Engineering and Processing: Process Intensification 20(6):319–329
Eljaddi T, Laurent L, Miloudi H (2017) Review on mechanism of facilitated transport on liquid membranes. Journal of Membrane Science and Research 3(3). https://doi.org/10.22079/jmsr.2017.50137.1110
Fortuny M, Oliveira CBZ, Melo RLFV, Nele M, Coutinho RCC, Santos AF (2007) Effect of salinity, temperature, water content, and pH on the microwave demulsification of crude oil emulsions. Energy & Fuels 21(3):1358–1364
Galindo-Miranda JM, Guízar-González C, Becerril-Bravo EJ, Moeller-Chávez G, León-Becerril E, Vallejo-Rodríguez R (2019) Occurrence of emerging contaminants in environmental surface waters and their analytical methodology – a review. Water Supply 19(7):1871–1884. https://doi.org/10.2166/ws.2019.087
Garavand F, Razavi SH, Cacciotti I (2018) Synchronized extraction and purification of L-lactic acid from fermentation broth by emulsion liquid membrane technique. Journal of Dispersion Science and Technology 39(9):1291–1299. https://doi.org/10.1080/01932691.2017.1396225
Goyal RK, Jayakumar NS, Hashim MA (2011) Chromium removal by emulsion liquid membrane using [BMIM]+[NTf2]− as stabilizer and TOMAC as extractant. Desalination 278(1–3) 50–56. https://doi.org/10.1016/j.desal.2011.05.001
Grassi M, Kaykıoğlu G, Belgiorno V, Lofrano G (2012) Removal of emerging contaminants from water and wastewater by adsorption process. Emerging Compounds Removal from Wastewater: Natural and Solar Based Treatments, 15–38
Gros M, Petrović M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environment International 36(1):15–26
Gupta S, Khandale PB, Chakraborty M (n.d.) Application of emulsion liquid membrane for the extraction of diclofenac and relationship with the s. 2020, Volume 41(3):10
Hachemaoui A, Belhamel K, Bart H-J (2010) Emulsion liquid membrane extraction of Ni(II) and Co(II) from acidic chloride solutions using bis-(2-ethylhexyl) phosphoric acid as extractant. Journal of Coordination Chemistry 63(13):2337–2348
Hassanshahi N, Hu G, Li J (2020) Application of ionic liquids for chemical demulsification: a review. Molecules 25(21):4915. https://doi.org/10.3390/molecules25214915
Helmecke M, Fries E, Schulte C (2020) Regulating water reuse for agricultural irrigation: risks related to organic micro-contaminants. Environmental Sciences Europe 32(1):4. https://doi.org/10.1186/s12302-019-0283-0
Hiroshi T (1990) Chemical applications. CRC Press, Japan
Ho WS, Kamalesh KS (1992) Membrane handbook. Chapman & Hall
Holmberg K, Jönsson B, Kronberg B, Lindman B (2002) Surfactants and polymers in aqueous solution. John Wiley & Sons, Ltd. https://doi.org/10.1002/0470856424
Hosseinzadeh M, Alizadeh M, Ranjbar M, Pazouki M (2014) Improvement of the solvent extraction of rhenium from molybdenite roasting dust leaching solution using counter-current extraction by a mixer-settler extractor. International Journal of Engineering Transactions A: Basic 27(4):651–658
Jarrett A (2017) Pharmaceutical Disposal and Water Quality, PennState Extension.
Jiang C, Liu L, Crittenden JC (2016) An electrochemical process that uses an Fe0/TiO2 cathode to degrade typical dyes and antibiotics and a bio-anode that produces electricity. Frontiers of Environmental Science & Engineering 10(4):15. https://doi.org/10.1007/s11783-016-0860-z
Jiao H, Juntong WP, Xu ZC (2013) Extraction performance of bisphenol A from aqueous solutions by emulsion liquid membrane using response surface methodology. Desalination 313:36–43
Jilska MP, Geoff WS (2008) Use of emulsion liquid membrane systems in chemical and biotechnological separations. In: Pabby AK, Rizvi SSH, Sastre AM (eds) Handbook of Membrane Separations (Vol. 1–0, pp. 709–740). CRC Press. https://doi.org/10.1201/9781420009484.ch25
Juang R-S, Lin K-H (2004) Ultrasound-assisted production of W/O emulsions in liquid surfactant membrane processes. Colloids and Surfaces A: Physicochemical and Engineering Aspects 238(1):43–49
Jusoh N, Othman N, Nasruddin NA (2016) Emulsion liquid membrane technology in organic acid purification. Malaysian Journal of Analytical Science 20(2):436–443. https://doi.org/10.17576/mjas-2016-2002-28
Jusoh N, Noah NFM, Othman N (2019) Extraction and recovery optimization of succinic acid using green emulsion liquid membrane containing palm oil as the diluent. Environmental Progress & Sustainable Energy 38(3):e13065. https://doi.org/10.1002/ep.13065
Jusoh N, Rosly MB, Othman N, Rahman HA, Noah NFM, Sulaiman RNR (2020) Selective extraction and recovery of polyphenols from palm oil mill sterilization condensate using emulsion liquid membrane process. Environmental Science and Pollution Research 27(18):23246–23257. https://doi.org/10.1007/s11356-020-07972-5
Kamp J, Villwock J, Kraume M (2017) Drop coalescence in technical liquid/liquid applications: a review on experimental techniques and modeling approaches. Reviews in Chemical Engineering 33(1):1–47. https://doi.org/10.1515/revce-2015-0071
Kargari A, Abbassian K (2015) Study of phenol removal from aqueous solutions by a double emulsion (W/O/W) system stabilized with polymer. Separation Science and Technology 50(7):1083–1092. https://doi.org/10.1080/01496395.2014.982765
Kargari A, Kaghazchi T, Sohrabi M, Soleimani M (2006) Application of experimental design to emulsion liquid membrane pertraction of gold (III) ions from aqueous solutions. Iranian Journal of Chemical Engineering 3(1(Winter)):77–91
Kawasaki J, Kosuge H, Egashira R, Asawa T (2009) Mechanical entrainment in W/O/W emulsion liquid membrane. Separation Science and Technology 44(1):151–168
Kedari CS, Pandit SS, Parikh KJ, Tripathi SC (2010) Removal of 241Am from aqueous nitrate solutions by liquid surfactant membrane containing 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester as ion carrier. Chemosphere 80(4):433–437. https://doi.org/10.1016/j.chemosphere.2010.04.056
Kislik VS (2010) Chapter 1—introduction, general description, definitions, and classification. Overview. In: Kislik VS (ed) Liquid Membranes (pp. 1–15). Elsevier. https://doi.org/10.1016/B978-0-444-53218-3.00001-5
Kohli HP, Gupta S, Chakraborty M (2018) Extraction of ethylparaben by emulsion liquid membrane: statistical analysis of operating parameters. Colloids and Surfaces A: Physicochemical and Engineering Aspects 539:371–381. https://doi.org/10.1016/j.colsurfa.2017.12.002
Krzeminski P, Tomei MC, Karaolia P, Langenhoff A, Almeida CMR, Felis E, Gritten F, Andersen HR, Fernandes T, Manaia CM, Rizzo L, Fatta-Kassinos D (2019) Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: a review. Science of The Total Environment 648:1052–1081. https://doi.org/10.1016/j.scitotenv.2018.08.130
Kulkarni PS, Mahajani VV (2002) Application of liquid emulsion membrane (LEM) process for enrichment of molybdenum from aqueous solutions. Journal of Membrane Science 201(1–2):123–135
Kumar A, Thakur A, Panesar PS (2019) A review on emulsion liquid membrane (ELM) for the treatment of various industrial effluent streams. Reviews in Environmental Science and Bio/Technology 18(1):153–182. https://doi.org/10.1007/s11157-019-09492-2
Kumar R, Ansari SA, Kandwal P, Mohapatra PK (2021) Selective permeation of 90Y from a mixture of 90Y/90Sr through diglycolamide impregnated supported liquid membranes. Applied Radiation and Isotopes 170:109604. https://doi.org/10.1016/j.apradiso.2021.109604
Kumar R, Murugesan SK, Nanjaian M (2012) Multiple emulsion: A review. International Journal of Recent Advances in Pharmaceutical Research 2(1):9–19
Kumbasar RA (2008) Selective separation of chromium (VI) from acidic solutions containing various metal ions through emulsion liquid membrane using trioctylamine as extractant. Separation and Purification Technology 64(1):56–62
Kumbasar RA (2009) Selective extraction and concentration of cobalt from acidic leach solution containing cobalt and nickel through emulsion liquid membrane using PC-88A as extractant. Separation and Purification Technology 64:273–279
Kunthakudee N, Sunsandee N, Pancharoen U, Ramakul P (2016) Separation of yttrium from rare earth using hollow fiber-supported liquid membrane: factorial design analysis. Desalination and Water Treatment 57(9):3985–3994. https://doi.org/10.1080/19443994.2014.989275
Kusumastuti A, Syamwil R, Anis S (2017) Emulsion liquid membrane for textile dye removal: Stability study.:020026. https://doi.org/10.1063/1.4976890
Kusumastuti A, Anis S, Ahmad AL, Ooi BS, Shah Buddin MMH (2020) Emulsion liquid membrane for heavy metals removal: emulsion breaking study. Jurnal Teknologi 82(5). https://doi.org/10.11113/jt.v82.14539
Kyzas GZ, Fu J, Lazaridis NK, Bikiaris DN, Matis KA (2015) New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. Journal of Molecular Liquids 209:87–93
Lee SC (2000) Continuous extraction of penicillin G by emulsion liquid membranes with optimal surfactant compositions. Chemical Engineering Journal 79(1):61–67
Lee SC (2011) Extraction of succinic acid from simulated media by emulsion liquid membranes. Journal of Membrane Science 381(1):237–243
Lee S (2015) Removal of acetic acid from simulated hemicellulosic hydrolysates by emulsion liquid membrane with organophosphorus extractants. Bioresource Technology 192:340–345
Lee SC, Hyun K-S (2010) Development of an emulsion liquid membrane system for separation of acetic acid from succinic acid. Journal of Membrane Science 350(1):333–339
Li NN (1978) Separating hydrocarbons with liquid membranes, ExxonMobil Research and Engineering Co, United States (patent no. US3410794A)
Li NN (1968) Separating Hydrocarbons with Liquid Membranes, United States (patent no. 3410794)
Lichang Z, Qinlin C, Chao K, Xin M, Zunliang Y (2016) Rare earth extraction from wet process phosphoric acid by emulsion liquid membrane. Journal of Rare Earths 34(7):717–723
Lin C, He G, Li X, Peng L, Dong C, Gu S, Xiao G (2007) Freeze/thaw induced demulsification of water-in-oil emulsions with loosely packed droplets. Separation and Purification Technology 56(2):175–183. https://doi.org/10.1016/j.seppur.2007.01.035
Lin C, He G, Dong C, Liu H, Xiao G, Liu Y (2008) Effect of oil phase transition on freeze/thaw-induced demulsification of water-in-oil emulsions. Langmuir 24(10):5291–5298. https://doi.org/10.1021/la704079s
Lu G, Lu Q, Li P (1997) Break-down of liquid membrane emulsion under high electric field. Journal of Membrane Science 128(1):1–6
Lu-ting P (2006) Extraction of amino-J acid from waste-water by emulsion liquid membrane. The Chinese Journal of Process Engineering 6(5):738–741
Lyons G (2014) Pharmaceuticals in the Environment: A growing threat to our tap water and wildlife, CHEM Trust Report
Majeed, N., & Adnan, M. (2016). Demulsification of remaining waste (water in oil emulsions) after removal of phenol in emulsion liquid membrane process. https://doi.org/10.13140/RG.2.2.33651.68644
Malik MA, Hashim MA, Nabi F (2012) Extraction of metal ions by ELM separation technology. Journal of Dispersion Science and Technology 33(3):346–356
Manikandan GN, Bogeshwaran K, Jamuna P, Sandhya S (2014) A review on emulsion liquid membranes on heavy metal separation. International Journal of ChemTech Research 6(9):4328–4332
Manzak A, Tutkun O (2005) Extraction of citric acid through an emulsion liquid membrane containing Aliquat 336 as carrier. Separation Science and Technology 39(10):2497–2512
Manzak A, Tutkun O (2011) The extraction of lactic acid by emulsion type of liquid membranes using Alamine 336 in Escaid 100. The Canadian Journal of Chemical Engineering 89(6):1458–1463. https://doi.org/10.1002/cjce.20501
Marcus Y (2004) Principles of solubility and solutions. Marcel Dekker
Martin TP, Davies GA (1977) The extraction of copper from dilute aqueous solutions using a liquid membrane process. Hydrometallurgy 2:315–334
Othman N, Mat HB, Goto M (2006) Separation of silver from photographic wastes by emulsion liquid membrane system. Journal of Membrane Science. 282:171-177.
Masry BA, Aly MI, Daoud JA (2021) Selective permeation of Ag+ ions from pyrosulfite solution through Nano-Emulsion Liquid Membrane (NELM) containing CYANEX 925 as carrier. Colloids Surf A Physicochem Eng Asp:610125713. https://doi.org/10.1016/j.colsurfa.2020.125713
Mohammed AA, Atiya MA, Hussein MA (2020a) Studies on membrane stability and extraction of ciprofloxacin from aqueous solution using Pickering emulsion liquid membrane stabilized by magnetic nano-Fe2O3. Colloids and Surfaces A: Physicochemical and Engineering Aspects 585:124044. https://doi.org/10.1016/j.colsurfa.2019.124044
Mohammed AA, Atiya MA, Hussein MA (2020b) Removal of antibiotic tetracycline using nano-fluid emulsion liquid membrane: breakage, extraction and stripping studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects 595:124680. https://doi.org/10.1016/j.colsurfa.2020.124680
Mompelat S, Le BB, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environment International 35:803–814
Monteiro SC, Boxall ABA (2010) Occurrence and fate of human pharmaceuticals in the environment. Reviews of Environmental Contamination and Toxicology:53–154
Mosayebi A, Abedini R (2013) Using demulsifiers for phase breaking of water/oil emulsion. Petroleum & Coal 55(1):26–30
Moyo F, Tandlich R (2015) Optimisation of the emulsion liquid membrane composition and demulsification for rhodium extraction. Waste Treatment and Recovery 1(1). https://doi.org/10.1515/lwr-2015-0002
Murugan A, Palanivelu K, Manickam V (2009) Removal of Cu (II) using emulsion liquid membrane. International Journal of ChemTech Research, 1
Muthusaravanan S, Priyadharshini SV, Sivarajasekar N, Subashini R, Sivamani S, Dharaskar S, Dhakal N (2019) Optimization and extraction of pharmaceutical micro-pollutant—norfloxacin using emulsion liquid membranes. Desalination and Water Treatment 156:238–244. https://doi.org/10.5004/dwt.2019.23833
Ng YS, Jayakumar NS, Hashim MA (2010) Performance evaluation of organic emulsion liquid membrane on phenol removal. Journal of Hazardous Materials 184(1–3):255–260
Nikolaou A, Meric S, Fatta D (2007) Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal Bional Chem 387(4):1225–1234
Noguera-Oviedo K, Aga DS (2016) Lessons learned from more than two decades of research on emerging contaminants in the environment. J Hazard Mater 316:242–251
Ooi Z-Y, Harruddin N, Othman N (2015) Recovery of Kraft lignin from pulping wastewater via emulsion liquid membrane process. Biotechnology Progress, In Press 31:1305–1314
Othman N, Mat HB, Goto M (2006) Separation of silver from photographic wastes by emulsion liquid membrane system. J Membr Sci 282:171–177
Othman N, Mili N, Zailani SN, Mohammad NAB (2010) Extraction of Remazol Brilliant Orange 3R from textile wastewater using tetrabutyl ammonium bromide. Jurnal Teknologi 53:29–39
Othman N, Zailani S, Mili N (2011) Recovery of synthetic dye from simulated wastewater using emulsion liquid membrane process containing tri-dodecyl amine as a mobile carrier. Journal of Hazardous Materials 198:103–112
Othman N, Zing-Yi O, Harruddin N, Norimie R, Jusoh N, Zailani SN (2014) Carrier assisted emulsion liquid membrane process for recovery of basic dye from wastewater using continuous extractor. Jurnal Teknologi 67(2):69–74
Othman N, Noah NFM, Poh KW, Yi OZ (2016) High performance of chromium recovery from aqueous waste solution using mixture of palm-oil in emulsion liquid membrane. Procedia Engineering 148:765–773
Parhi PK (2013) Supported liquid membrane principle and its practices: a short review. Journal of Chemistry 2013:1–11
Park Y (2006) Development and optimization of novel emulsion liquid membranes stabilized by non-Newtonian conversion in Taylor-Couette flow for extraction of selected organic and metallic content. In School of Civil and Environmental Engineering: Vol. PhD. Georgia Institute of Technology
Park Y, Forney LJ, Kima JH, Skelland AHP (2004) Optimum emulsion liquid membranes stabilized by non-Newtonian conversion in Taylor–Couette flow. Chemical Engineering Science 59(2004):5725–5734
Park Y, Skelland AHP, Forney LJ, Kim J-H (2006) Removal of phenol and substituted phenols by newly developed emulsion liquid membrane process. Water Research 40(9):1763–1772. https://doi.org/10.1016/j.watres.2006.03.005
Patel M, Kumar R, Kishor K, Mlsna T, Pittman CU, Mohan D (2019) Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chemical Reviews 119(6):3510–3673. https://doi.org/10.1021/acs.chemrev.8b00299
Patnaik PR (1995) Liquid emulsion membranes: principles, problems and applications in fermentation processes. Biotechnology Advances 13(2):175–208. https://doi.org/10.1016/0734-9750(95)00001-7
Peng W, Jiao H, Shi H, Xu C (2012) The application of emulsion liquid membrane process and heat-induced demulsification for removal of pyridine from aqueous solutions. Desalination 286:372–378
Perera JM, Stevens G (2009) Use of emulsion liquid membrane systems in chemical and biotechnological separations. Handbook of Membrane Separations : Chemical, Pharmaceutical, Food and Biotechnology Applications
Petrovic M, Radjenovic J, Postigo C, Kuster M, Farre M, Lopez de Alda M, Barcelo D (2008) Emerging contaminants in wastewaters: sources and occurrence: emerging contaminants from industrial and municipal waste-occurrence, analysis and effects. Handbook of Environmental Chemistry, Vol. 5. Part S1: Water Pollution, 1–36
Porter MC (1990) Handbook of industrial membrane technology. Noyes Publications
Rajendaren V, Saufi SM, Zahari MAKM, Mohammad AW (2021) Membrane support formulation and carrier selection in supported liquid membrane for extraction of zwitterionic form of glutamic acid. 11th Malaysian Technical Universities Conference on Engineering & Technology, 41, 116–121. https://doi.org/10.1016/j.matpr.2020.11.1016
Razo-Lazcano TA, del Pilar González-Muñoz M, Stambouli M, Pareau D, Hernández-Perales L, Avila-Rodriguez M (2018) Chlorpheniramine recovery from aqueous solutions by emulsion liquid membranes using soy lecithin as carrier. Special Issue on Formula VIII 536:68–73. https://doi.org/10.1016/j.colsurfa.2017.07.050
Richardson SD, Kimura SY (2017) Emerging environmental contaminants: challenges facing our next generation and potential engineering solutions. Environmental Technology & Innovation 8:40–56
Ritchey GM, Ashbrook AW (1984) Solvent extraction principles and applications to process metallurgy, Prt I. Elsevier Science Publisher
Rivera-Utrilla J, Sanchez-Polo M, Ferro-Garcia MA, Prados-Joya G, Ocampo-Perez R (2013) Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93(7):1268–1287
Rosly MB, Jusoh N, Othman N, Rahman HA, Sulaiman RNR, Noah NFM (2020) Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chemical Engineering and Processing - Process Intensification 148:107790. https://doi.org/10.1016/j.cep.2019.107790
San Román MF, Bringas E, Ibañez R, Ortiz I (2010) Liquid membrane technology: fundamentals and review of its applications. Journal of Chemical Technology & Biotechnology 85(1):2–10. https://doi.org/10.1002/jctb.2252
Schöller C, Chaudhuri JB, Pyle DL (1993) Emulsion liquid membrane extraction of lactic acid from aqueous solutions and fermentation broth. Biotechnology and Bioengineering 42(1):50–58
Seifollahi Z, Rahbar-Kelishami A (2017) Diclofenac extraction from aqueous solution by an emulsion liquid membrane: parameter study and optimization using the response surface methodology. Journal of Molecular Liquids 231:1–10. https://doi.org/10.1016/j.molliq.2017.01.081
Sengupta B, Bhakhar MS, Sengupta R (2007) Extraction of copper from ammoniacal solutions into emulsion liquid membranes using LIX 84 I®. Hydrometallurgy 89(3–4):311–318
Shirasangi R, Kohli HP, Gupta S, Chakraborty M (2020) Separation of methylparaben by emulsion liquid membrane: optimization, characterization, stability and multiple cycles studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects 597:124761. https://doi.org/10.1016/j.colsurfa.2020.124761
Skoumal M, Cabot P-L, Centellas F, Arias C, Rodríguez RM, Garrido JA, Brillas E (2006) Mineralization of paracetamol by ozonation catalyzed with Fe2+, Cu2+ and UVA light. Applied Catalysis B: Environmental 66(3–4):228–240
Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL (2007) Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Science of the Total Environment 377:255–272
Sujatha S, Rajasimman M (2021) Development of a green emulsion liquid membrane using waste cooking oil as diluent for the extraction of arsenic from aqueous solution – screening, optimization, kinetics and thermodynamics studies. Journal of Water Process Engineering 41:102055. https://doi.org/10.1016/j.jwpe.2021.102055
Sun D, Duan X, Li W, Zhou D (1998) Demulsification of water-in-oil emulsion by using porous glass membrane. Journal of Membrane Science 146(1):65–72. https://doi.org/10.1016/S0376-7388(98)00096-9
Sunsandee N, Leepipatpiboon N, Ramakul P (2013) Selective enantioseparation of levocetirizine via a hollow fiber supported liquid membrane and mass transfer prediction. Korean Journal of Chemical Engineering 30(6):1312–1320. https://doi.org/10.1007/s11814-013-0044-5
Sunsandee N, Kunthakudee N, Chutvirasakul B, Phatanasri S, Ramakul P (2017) Enantioseparation of (S)-amlodipine from pharmaceutical wastewater by hollow-fiber supported liquid membrane: Central composite design and optimization. Https://Www.Crossref.Org/WebDeposit/, 72, 207–215. https://doi.org/10.5004/dwt.2017.20191
Susunu N, Kikumoto S, Tokuyama H (2008) Quantitative approach to ultrasonic emulsion separation. Ultrasonics Sonochemistry 16:145–149
Teng TT, Soniya M, Muthuraman G, Talebi A (2014) Role of emulsion liquid membrane (ELM) in separation processes. In: Aziz HA, Mojiri A (eds) Wastewater Engineering: Advanced Wastewater Treatment Systems (pp. 149–157). IJSR Publications
Teramoto M, Yamashiro T, Inoue A, Yamamoto A, Matsuyama H, Miyake Y (1991) Extraction of amino acids by emulsion liquid membranes containing di (2-ethylhexyl)phosphoric acid as a carrier biotechnology; coupled, facilitated transport; diffusion. Journal of Membrane Science 58(1):11–32
Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Haist-Gulde B, Preuss G, Wilme U, Zulei-Seibert N (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36(17):3855–3863
Treacy J (2019) Drinking water treatment and challenges in developing countries. In: Potgieter N, Hoffman ANT (eds) The relevance of hygiene to health in developing countries. IntechOpen. https://doi.org/10.5772/intechopen.80780
Valdez-Carrillo M, Abrell L, Ramírez-Hernández J, Reyes-López JA, Carreón-Diazconti C (2020) Pharmaceuticals as emerging contaminants in the aquatic environment of Latin America: a review. Environmental Science and Pollution Research 27(36):44863–44891. https://doi.org/10.1007/s11356-020-10842-9
Valenzuela F, Fonseca C, Basualto C, Correa O, Tapia C, Sapag J (2005) Removal of copper ions from a waste mine water by a liquid emulsion membrane method. Minerals Engineering 18(1):33–40
Van N-X, Huang S-A, Shi Y-J (1987) Removal of acetic acid from wastewater with liquid surfactant membranes: an external boundary layer and membrane diffusion controlled model. Separation Science and Technology 22(2–3):801–818
Wan YH, Zhang XJ (2002) Swelling determination of W/O/W emulsion liquid membranes. Journal of Membrane Science 196(2):185–201
Wan YH, Wang XD, Zhang XJ (1997) Treatment of high concentration phenolic waste water by liquid membrane with N503 as mobile carrier. Journal of Membrane Science 135(2):263–270
Weidemann E, Niinipuu M, Fick J, Jansson S (2018) Using carbonized low-cost materials for removal of chemicals of environmental concern from water. Environmental Science and Pollution Research 25(16):15793–15801. https://doi.org/10.1007/s11356-018-1781-0
Wen L, Papadopoulos KD (2000) Visualization of water transport in W1/O/W2 emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 174(1–2):159–167
Wu D, Sun F, Zhou Y (2017) Degradation of chloramphenicol with novel metal foam electrodes in bioelectrochemical systems. Electrochimica Acta 240:136–145. https://doi.org/10.1016/j.electacta.2017.04.059
Xie ZN, Chen QL (2014) Demulsification of emulsion liquid membrane extracting rare earth ions in leaching liquid of phosphate rock. Advanced Materials Research 926–930:64–67. https://doi.org/10.4028/www.scientific.net/AMR.926-930.64
Yan J, Pal R (2001) Osmotic swelling behavior of globules of W/O/W emulsion liquid membranes. Journal of Membrane Science 190(1):79–91
Yang W, Han H, Zhou M, Yang J (2015) Simultaneous electricity generation and tetracycline removal in continuous flow electrosorption driven by microbial fuel cells. RSC Advances 5(61):49513–49520. https://doi.org/10.1039/C5RA05545H
Zereshki S, Daraei P, Shokri A (2018) Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. Journal of Hazardous Materials 356:1–8. https://doi.org/10.1016/j.jhazmat.2018.05.037
Zhang S, Song H-L, Yang X-L, Yang K-Y, Wang X-Y (2016) Effect of electrical stimulation on the fate of sulfamethoxazole and tetracycline with their corresponding resistance genes in three-dimensional biofilm-electrode reactors. Chemosphere 164:113–119. https://doi.org/10.1016/j.chemosphere.2016.08.076
Zhang S, Song H-L, Yang X-L, Huang S, Dai Z-Q, Li H, Zhang Y-Y (2017) Dynamics of antibiotic resistance genes in microbial fuel cell-coupled constructed wetlands treating antibiotic-polluted water. Chemosphere 178:548–555. https://doi.org/10.1016/j.chemosphere.2017.03.088
Zhang S, Song H-L, Yang X-L, Li H, Wang Y-W (2018) A system composed of a biofilm electrode reactor and a microbial fuel cell-constructed wetland exhibited efficient sulfamethoxazole removal but induced sul genes. Bioresource Technology 256:224–231. https://doi.org/10.1016/j.biortech.2018.02.023
Zhao L, Fei D, Dang Y, Zhou X, Xiao J (2010) Studies on the extraction of chromium(III) by emulsion liquid membrane. Journal of Hazardous Materials 178(1):130–135
Zihao W, Jufu F (1992) Viscosity effects on the swelling of liquid surfactant membrane. CIESC Journal 43:148–153
Zuccato E, Castiglioni S (2009) Illicit drugs in the environment. Phil. Trans. R. Soc. A 367:3965–3978
Acknowledgements
This work was financially supported by Ministry of Higher Education Malaysia grant under the LRGS scheme (203/PJKIMIA/67215002) and FRGS scheme (FRGS/1/2017/TK02/UITM/03/11). Financial assistance under the USM Fellowship programme is also acknowledged. We thank the anonymous reviewers whose insightful comments and suggestions helped improve and clarify this manuscript.
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
Nur Dina Zaulkiflee: Collected the data, performed the analysis, wrote the paper.
Abdul Latif Ahmad: Supervising, checking, performed the analysis.
Nuur Fahanis Che Lah: Performed the analysis, wrote the paper.
Meor Muhammad Hafiz Shah Buddin: Performed the analysis, wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaulkiflee, N.D., Ahmad, A.L., Che Lah, N.F. et al. Removal of emerging contaminants by emulsion liquid membrane: perspective and challenges. Environ Sci Pollut Res 29, 12997–13023 (2022). https://doi.org/10.1007/s11356-021-16658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16658-5