Abstract
This study was conducted to identify the bioactive phytochemicals in Salvia officinalis essential oil, to determine the polyphenols in the aqueous extract (SOE), and to evaluate their protective role against cadmium (Cd)-induced oxidative damage and genotoxicity in rats. Six groups of female rats were treated orally for 2 weeks including the control group, CdCl2-treated group, SOE-treated groups at low or high dose (100 and 200 mg/kg b.w), and CdCl2 plus SOE-treated groups at the two doses. The GC-MS analysis identified 39 compounds; the main compounds were 9-octadecenamide, eucalyptol, palmitic acid, and oleic acid. However, the HPLC analysis showed 12 polyphenolic compounds and the majority were coumaric acid, chlorogenic acid, coffeic acid, catechin, vanillin, gallic acid, ellagic acid, and rutin. In the biological study, rats received CdCl2 displayed severe disturbances in liver and kidney indices alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (Alb), total protein (TP), total bilirubin (T. Bil), direct bilirubin (D. Bil), creatinine, uric acid, and urea, lipid profile, tumor necrosis factor-alpha (TNF-α), alpha-fetoprotein (AFP) and CEA), glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT), malondialdehyde (MDA), nitric oxide (NO), gene expressions, DNA fragmentation, and histological alterations in the liver and kidney tissue. SOE showed a potent antioxidant and mitigated these alterations in serum and tissue. Moreover, the high dose succeeded to normalize most of the tested parameters and histological features. It could be concluded that S. officinalis is a promising source for bioactive compounds with therapeutic benefits against environmental toxicants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is known to induce direct health hazards to humans in different forms. The concentration of Cd in the environment increases because of industrial activities, soil disruption, and volcanic activity (Godt et al. 2006). Cd induces severe damage to different organs such as the lung, kidney, liver, testes, placenta, and bones (Pari and Murugavel 2007). After ingestion, Cd transports to the bloodstream through the albumin and erythrocytes and accumulates in the kidneys (Satarug 2018), gut, and liver (Tinkov et al. 2018a). The excretion of Cd from the body is very slow, and it occurs mainly through the kidneys via urine, milk during lactation, and saliva. The International Agency for Research on Cancer (IARC) classified Cd as a carcinogenic agent (WHO 1992). The risk of Cd in humans includes hepatic and renal dysfunction, testicular damage, pulmonary edema, adrenal hematopoietic system damage, and osteomalacia (Tinkov et al. 2018b). Thiol groups (-SH) of the amino acid cysteine found the protein is the critical target of Cd. The inactivation of enzyme sulfhydryl groups induces several deficits in the function of nuclei, mitochondria, and endoplasmic reticulum (Genchi et al. 2020). Cd toxicity is primarily attributed to its role in blocking the chain of mitochondrial electron transport through impairing of electron flow via the complex III (e.g., cytochrome c oxidoreductase, cytochrome bc1 complex, and ubiquinone). Moreover, Cd suppresses the uncoupler-stimulated respiration, inhibits ADP, and increases the permeability of ions in the inner mitochondrial membrane by making an opening of the mitochondrial permeability transition pore (Belyaeva et al. 2008). Furthermore, Cd inhibits the production of lactate dehydrogenase, ATPase, SOD, and GPx activities and enhances the peroxidation of lipids and the generation of ROS (Cannino et al. 2009). Additionally, it is involved in Fenton reactions; although it does not have any catalytic effect in Fenton reactions, it can increase the release of free redox-active metals through the production of ROS and the indirect displacing of the endogenous Fenton metals such as Fe2+ from the proteins (Cuypers et al. 2010). Cd-induced ROS production led to the accumulation of these free radicals which in turn influence the membrane of mitochondria resulting in sequences of events such as apoptosis (Chatterjee et al. 2008). It was also reported that Cd induces its carcinogenicity through different mechanisms including the production of ROS, oxidative damage, induction of inflammatory processes, attenuation of apoptosis, epigenetics, DNA damage, decreased repair capacity of DNA, modification in the gene expression, aberrant DNA methylation, and cell proliferation (Buha et al. 2018; Pizzino et al. 2014; Zhou et al. 2013).
Salvia genus is represented by about 900 species distributed all over the world (Fu et al. 2013), and it is a very important genus in the Lamiaceae family. Different species of Salvia are used as spices or flavoring agents in food besides their economic importance in cosmetics and perfumery (Abu-Darwish et al. 2013; Senatore et al. 2004, 2006). Different Salvia species are used in folk medicine for the remediation of about sixty various ailments such as aches, hemorrhage, cold, tuberculosis, bronchitis, menstrual disorders, and epilepsy (Kamatou et al. 2008; Topcu 2006). Additionally, several species of Salvia showed antioxidant, antitumor, anti-inflammatory, antimicrobial, antifungal, anticholinesterase, estrogenic, and antiplasmodial properties besides their effective role in treating psoriasis and eczema (Fu et al. 2013; Moghaddam et al. 1998). Therefore, different species of Salvia were subjected for comprehensive studies for the isolation and characterization of different phytochemicals and pharmacognostic of their bioactive secondary metabolomics (Al-Qudah et al. 2020; Hasan et al. 2016; Lehbili et al. 2018) and to evaluate their pharmacological activities (Güzela et al. 2019; Khare et al. 2019; Marcinek and Krejpcio 2017). However, the bioactive compounds in a plant are different due to the variety and other environmental factors (Russo et al. 2013). Therefore, this study was conducted to identify the bioactive secondary metabolomics of Salvia officinalis grown in Egypt and to evaluate their protective role against Cd-induced oxidative stress, genotoxicity, and pathological alterations in the liver and kidney in rats.
Materials and methods
Chemicals and kits
The following chemicals and kits were used in the current study: cadmium chloride (CdCl2) (sigma, St. Louis, Mo, USA); transaminase (ALT, AST) kits (Spectrum Diagnostics Co., Cairo, Egypt); alkaline phosphatase (ALP), creatinine, uric acid, urea, total bilirubin (TB), direct bilirubin (DB), nitric oxide (NO), lipid peroxidase (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), triglycerides (TG), cholesterol (Cho), and high- and low-density lipoprotein (HDL, LDL) kits (Eagle Diagnostics, Dallas, TX, USA); and alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and tumor necrosis factor-alpha (TNF-a) kits (Orgenium, Helsinki, Finland). All other reagents and chemicals used were of the highest purity commercially available.
Plant materials, extract, and essential oil preparation
The dried plants (aerial parts) of S. officinalis were purchased from an herbalist in El-Arish City, North Sinai, Egypt. The plant was authenticated by a plant taxonomist at the Department of Botany, Faculty of Science, Al-Arish University, and a voucher specimen was kept in the Department Herbarium. Twenty-five g of dry powder was steeped in 250 ml of sterilized boiling water for 20 min with incessant moving. The crude mixture was filtrated and kept in sterile dark bottles at 4 °C until used. The essential oil (EO) was extracted from the plant material by the Clevenger distiller apparatus according to Mohamed and Mustafa (2019). In brief, the sample was covered by distilled water and the temperature was adjusted at 66 °C. The condenser was attached and the extraction time was 3 h. The obtained mixture was separated and oil was collected, treated with sodium sulfate anhydrous to remove the water, and then stored at 4 °C in a refrigerator until used
Gas chromatography-mass spectrometry (GC-MS) analysis
The identification of bioactive constituents of EO was carried out using a GC-MS system (Agilent Technologies) equipped with the gas chromatograph (7890B) and mass spectrometer detector (5977A) as described in our previous work (Abdel-Wahhab et al. 2020a). The bioactive constituents were identified by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
Polyphenols determination by HPLC
Polyphenols were detected using an Agilent HPLC 1260 series according to our previous work (Gheraibia et al. 2020). In brief, Agilent 1260 series HPLC was used for the analysis of polyphenols. C18 column (4.6 mm × 250 mm i.d., 5 μm) was used for the separation and the mobile phase consisted of water (A) and 0.02% trifluoroacetic acid in acetonitrile (B) and the flow rate was 1 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0–5 min (80% A); 5–8 min (40% A); 8–12 min (50% A); and 12–16 min (80% A). The multi-wavelength detector was monitored at 280 nm, the injection volume was 10 μl for each sample solution, and the column temperature was maintained at 35 °C.
Animals
Forty-two adult female Albino rats (180–200 g) were obtained from the Faculty of Science, Al-Arish University, North Sinai, Egypt. The animals were housed in solid-bottom cages with free access to food and water in an air-conditioned room (20–24 °C and 12-h/12-h dark/light cycle) in the Faculty of Science, Al-Arish University. All animals were kept for 1 week as an adaptation period before the start of the experiment. All animals received humane care in compliance with the guidelines of the Animal Care and Use Committee of the Faculty of Science, Al-Arish University, North Sinai, Egypt, and the National Institutes of Health (NIH publication 86-23 revised 1985).
Experimental protocol
After the adaptation period, animals were separated into 6 groups (7 rats/group) and treated orally using a stomach tube for 14 days as follows: groups 1, untreated control received distilled water; group 2, rats received CdCl2 (2 mg/kg b.w); groups 3 and 4 received S. officinalis extract (SOE) at low (LD, 150 mg/kg b.w) or high (HD, 300 mg/kg b.w) dose; and groups 5 and 6 received CdCl2 plus SOE (LD) or SOE (HD). Body weight was recorded daily throughout the experimental period. After 24 h of the last treatment (day 15), blood samples were taken from all animals within different treatment groups from the retro-orbital venous plexus under isoflurane anesthesia (1–4 %). The blood samples were left to cool at room temperature then centrifuged for 15 min at 3000 rpm and 4 °C. Sera were stored at − 20 °C until use for the determination of the biochemical parameters using a spectrophotometer according to the kit instructions.
After the collection of blood samples, all animals were euthanized and liver and kidney samples of all animals were dissected. Samples of each organ were fixed in natural formalin, hydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. Sections (5-μm thick) were cut and stained with hematoxylin and eosin (H&E) for histological examination (Bancroft et al. 1996). Other samples were collected, weighed, and homogenized in phosphate buffer (pH 7.4), centrifuged at 1700 rpm and 4 °C for 10 min. The supernatants were used for MDA, GPx, CAT, GSH, and SOD determination according to Lin et al. (1998) and Abdel-Wahhab et al. (2021). Another sample from the liver of each animal was collected and stored at − 80 °C for cytogenetic analysis.
Cytogenetic analysis
Gene expression assay
One hundred milligrams of the frozen liver samples was used for total RNA extraction by Trizol reagent (Invitrogen) and the complementary deoxyribonucleic acid (cDNA) was synthesized as described by Abdel-Wahhab et al. (2020b). Real-time PCR was used to evaluate the quantitative expression of mRNA for GPx, SOD, CAT, Bax, Bcl-2, and glyceraldehyde3-phosphate dehydrogenase (GAPDH) as the control. The selected primers were designed from published GenBank sequences. Sequences of GPx, SOD, CAT, Bax, Bcl-2, and GAPDH primers and annealing temperature used for real-time PCR are shown in Table 1. Melting curve analysis was conducted following each real-time PCR. Gene expression data were normalized to GAPDH and analyzed using the 2−ΔΔCt method (Livak and Schmittgen 2001; Abdel-Wahhab et al. 2020b).
DNA fragmentation assay
The hepatic DNA content was detected calorimetrically according to Burton (1956) and modified by Perandones et al. (1993). The percentage of DNA fragmentation was calculated according to the following formula:
Moreover, DNA fragmentation was also detected by agarose gel electrophoresis following the method of Kuo et al. (2005), and the DNA bands were observed and photographed under a UV trans-illuminator.
Statistical analysis
Data were stated as mean ± SE and were analyzed statistically by one-way ANOVA followed by Duncan-test as a post hoc using SPSS for Windows (Version 21; SPSS Inc., Chicago, IL, USA). The statistical significances for DNA fragmentation were analyzed by using a t test. All statements of significance were based on a probability of P ≤ 0.05.
Results
The GC-MS analysis of the essential oil of S. officinalis aqueous showed the presence of 39 compounds (Table 2). The main compounds were 9-octadecenamide, (Z)-(55.80%), eucalyptol (4.81), palmitic acid, TMS derivative (3.96%), oleic acid, (Z)-, TMS derivative (3.11%), 1,6-bis(2-propyl-1-yloxy)hexane (2.39%), myristic acid, TMS derivative (2.23%), eicosane-1,2-diol, isopropylidene derivative (2.1%), dodecane, 2,6,10-trimethyl (2.05%), stearic acid, TBDMS derivative (2.02%), hexane, 3,3-dimethyl (1.5%), tetracosane (1.47%), docosane-1,2-diol, isopropylidene derivative (1.41%), nonadecane, 2-methyl (1.35%), hexadecane, 2,6,11,15-tetramethyl (1.2%), decane, 2,3,5,8-tetramethyl (1.1%), and 2-methyltetracosane (1.05%) besides other detectable compounds. These compounds are belonging to different classes such as fatty amides, monoterpenoid, fatty acids, isopropylidene derivative, and alkanes. However, the HPLC analysis of SEO identified 12 polyphenols (Table 3) and the paramount compounds based on their concentrations order were coumaric acid, chlorogenic acid, coffeic acid, catechin, vanillin, gallic acid, ellagic acid, and rutin.
The in vivo results revealed that animals administrated SOE (LD( or SOE (HD) gained weight, whereas a significant reduction in body weight was found in the rats that received CdCl2 along the experimental period compared with the negative control group. Animals treated with CdCl2 plus SOE (LD) or SOE (HD) showed a significant improvement in body weight compared to CdCl2 alone (Fig. 1). It is worthy to mention that the sharp decrease in body weight of animals in the CdCl2-alone group was started on day 3 of the treatment and continued until the end of the treatment period
The biochemical results presented in Table 4 revealed that animals that received CdCl2 exhibited a significant elevation in ALT, AST, TB, DB, creatinine, urea, and uric acid and a significant reduction in Alb and TP compared with the control groups. Treatment with SOE alone at both doses did not significantly affect the biochemical parameters except TP which increased significantly and NO which was decreased than the negative control group. Co-administration of CdCl2 plus SOE alleviated the elevation of these biochemical parameters resulted from CdCl2 in a dose-dependent manner (Table 4).
The effect of different treatments on lipid profile (Table 5) showed that CdCl2 increased in cholesterol, triglycerides, and LDL-Ch and decreased HDL-Ch significantly compared with the control. A significant augmentation in HDL-Ch was noticed in the rats who received SOE (LD), while the animals who received SOE (HD) showed a considerable increase in HDL-Ch and LDL-Ch and a significant decrease in cholesterol and triglycerides compared with the untreated control rats. The combined treatment with CdCl2 plus SOE (LD) or SOE (HD) showed a significant decrease in cholesterol, triglycerides, and LDL-Ch and a significant increase in HDL-Ch compared with those received CdCl2 alone.
The effect of different treatments on liver and kidney GSH, GPx, GST, SOD, and MDA (Table 6) revealed a significant increase in the antioxidant markers in rats who received SOE at both doses. However, the hepatic MDA did not significantly affect by both doses but renal MDA level was significantly decreased in animals that received the high dose of SOE. Animals that received CdCl2 plus SOE (LD or HD) exhibited a notable increase in SOD, CAT, and GPx and a significant decrease in MDA in the hepatic and renal tissue compared with those in CdCl2-alone group.
TNF-α, AFP, and CEA increased significantly in animals treated by CdCl2 alone as compared with the negative control (Table 7). Animals who received SOE alone (LD or HD) exhibited a significant reduction in TNF-α and AFP; however, CEA showed a significant increase. Meanwhile, animals that received CdCl2 plus the extract displayed significant improvement in these parameters towards the control levels and the HD could normalize TNF-α and AFP.
To confirm the disturbances in the activity of the antioxidant enzyme, the profiles of corresponding gene expression were examined in the hepatic tissue by RT-qPCR. As illustrated in Fig. (2), the relative mRNA expression levels of GPx1 (2A), SOD (2B), and CAT (2C) were markedly down-regulated by 30, 30, and 20 %, respectively, in the CdCl2-intoxicated animals when compared with those in the control group. The levels of expression transcript of the target genes were highly significantly up-regulated in SOE-alone-treated rats compared with the control animals and were additionally significantly up-regulated in the groups that received CdCl2 plus SOE (LD or HD) compared with the CdCl2-treated group. Despite this increase, the mRNA expression levels in CdCl2 plus SOE–treated rats did not reach those of the untreated control animals. Additionally, the alterations in the activities of these antioxidant enzymes and the relative expression of their genes are commonly in a positive correlation.
The qPCR assay was further utilized for mRNA expression of Bax and Bcl-2 (the apoptotic genes) in hepatic tissue. Our results showed that exposure to CdCl2 pronouncedly altered mRNA expression of both Bax and Bcl-2, where the expression of Bax was up-regulated (Fig. 3A), and the expression of Bcl-2 was down-regulated (Fig. 3B) compared with the control group. However, these alterations were pronouncedly attenuated by SOE even at the low dose. Additionally, the effect of different treatments on DNA fragmentation (Table 8) showed a significant increase in DNA fragmentation percentage in animals that received CdCl2. However, DNA fragmentation percentage in animals administrated with SEO was comparable to the untreated control group. Co-administration of CdCl2 and SOE significantly reduced the percentage of DNA fragmentation compared with the group that received CdCl2 alone and the inhibition percent reached 37.97 and 43.04% in the groups that received SOE (LD) and SOE (HD), respectively. The agarose gel electrophoresis of the DNA (Fig. 4) confirmed the colorimetric assays of DNA fragmentation and endorsed the change in the gene transcript levels. The liver samples of CdCl2-intoxicated rats showed a smear (a hallmark of necrosis) DNA fragmentation with no ladder formation which indicated a random DNA degradation when compared with the control or SOE-treated groups. Treatment of CdCl2 plus SOE (LD) or SOE (HD) markedly suppressed the fragmentation of DNA, where DNA was still localized at the starting point. Moreover, the DNA electrophoretic patterns in animals treated with SOE were comparable to the control groups.
Agarose gel electrophoresis of extracted DNA from liver of rats; results of the DNA fragmentation assay confirm that treatment with SOE attenuates CdCl2-induced DNA damage in the liver of rats. Lane M, DNA ladder; lanes 1–2, untreated control group; lanes 3–4, CdCl2-treated group; lanes 5–6, SOE (LD)-treated group; lanes 7–8 SOE (HD) and lanes 9–10, CdCl2 plus SOE (LD)–treated group; and lanes 11–12, CdCL2 plus SOE (HD)–treated group
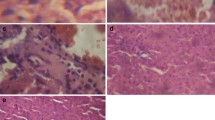
The pathological examination of the untreated control liver sections showed normal histology with normal central vein and normal hepatic lobule (Fig. 5A). The liver sections of animals that received SOE (LD) of SOE (HD) (Fig. 5B and C) showed normal central vein surrounded by the hepatocyte cords, normal vesicular nuclei, and eosinophilic cytoplasm. The liver of animals that received CdCl2 showed necrosis and shrunken hepatocytes with chromatin condensation, markedly dilated congested central vein, and fibrous tissues (Fig. 5D). The liver sections of animals that received SOE (LD or HD) plus CdCl2 showed normal liver architecture (Figs. 5E, F).
Photomicrographs of liver sections of A control showing normal histology with normal central vein and normal hepatic lobule; B and C rats treated with the low dose or high dose of SOE alone, respectively, showing normal liver tissue formed of central vein surrounded by cords of hepatocytes (arrows) with normal vesicular nuclei and eosinophilic cytoplasm; D the liver section of rats treated with CdCl2 showing necrosis and shrunken hepatocytes (arrows) with chromatin condensation (N), markedly dilated congested central vein (cong) and fibrous tissues. E and F Liver of rats treated with low or higher dose of SOE plus CdCl2 showing normal liver tissue formed of central vein surrounded by cords of hepatocytes with normal vesicular nuclei and eosinophilic cytoplasm (H&E stain)
The examination of the untreated control kidney showed normal glomerular and tubular structure and distal and proximal convoluted tubules (Fig. 6A). The kidney sections of the rats treated with SOE (LD) or SOE (HD) showed normal renal tissue with a normal tubular and glomerular picture of both glomerular and tubular (Figs. 6B, 5C). The kidney sections of rats in the CdCl2-alone group showed disruption of the normal renal architecture accompanied by a shrinkage of glomerular tufts, interstitial hemorrhages, and few distorted tubules with vacuolated cytoplasm and pyknotic nuclei (Fig 6D). The kidney sections of SOE (LD) plus CdCl2–treated rats showed normal glomerular architecture with normal tubular histology (Fig. 6E). However, the kidney of CdCl2 plus SOE (HD)–treated rats showed a normal histological picture of both glomerular and tubular tissue with interstitial hemorrhage (Fig. 6F).
Photomicrographs of kidney sections of A control group showing normal glomerular (G) and tubular structure, distal and proximal convoluted tubules (T); B kidney section of rats treated with CdCl2 showing disruption of the normal renal architecture accompanied with a shrinkage of glomerular tufts, interstitial hemorrhages and few distorted tubules with vacuolated cytoplasm (T) and pyknotic nuclei; C kidney section of rats treated with the low dose of SOE showing normal renal tissue with normal tubular (T) and glomerular picture (G); D kidney section of rats treated with the high dose of SOE showing the normal histological picture of both glomerular (G) and tubular (T) sections; E kidney section of rats treated with the low dose of SOE plus CdCl2 showing normal glomerular architecture (G) with normal tubular histology (T); F kidney section of rats treated with the high dose of SOE plus CdCl2 showing normal histological picture of both glomerular (G) and tubular (T) tissue with interstitial hemorrhage (H&E stain)
Discussion
The results of GC-MS identified 39 compounds and the major compounds were 9-octadecenamide, (Z)- which is belonging to amide compounds, and eucalyptol which is belonging to monoterpenoid. In this concern, Baj et al. (2013) isolated 37 compounds from the essential oil from sage leaves grown in Poland, most of them belonging to monoterpenoids. Moreover, Mohamed and Mustafa (2019) in Sudan identified 42 compounds and the main compound was α-terpineol followed by camphor, α-pinene, camphene, and β-cymen. Additionally, Abu-Darwish et al. (2013) isolated 25 compounds. The major compounds were oxygen-containing monoterpenes including 1,8-cineole and camphor. The HPLC analysis of the water extract of S. officinalis revealed that this plant is rich in polyphenols, and the majorities were coumaric, chlorogenic, coffeic, gallic, and ellagic acids, catechin, vanillin, and rutin. It was reported that the major phytochemicals in S. officinalis are alkaloids, fatty acids, carbohydrate, glycosidic derivatives such as flavonoid glycosides, saponins, and cardiac glycosides as well as phenolic compounds such as tannins and coumarins, steroids, polyacetylenes, terpenes, and terpenoids such as sesquiterpenoids, terpenoids (mono-, di-, and tri-) and some waxes (Badiee et al. 2012; El Hadri et al. 2010). Moreover, these phytochemicals were mainly isolated from the essential oil, aqueous, and methanolic extracts (Ghorbani and Esmaeilizadeh 2017). The differences in the chemical composition between the current results and the previous data may be due to several environmental factors such as water availability, climate, and altitude (Russo et al. 2013).
Cd as an environmental pollutant can enter the food chain via different routes and induces severe adverse health effects to the vital organs in humans (Bernhoft 2013). International organizations such as WHO and ATSDR have grouped Cd as the most hazardous chemicals (Andjelkovic et al. 2019) which harm the liver and kidney (Andjelkovic et al. 2019; Rani et al. 2014; Sanjeev et al. 2019). Additionally, S. officinalis is well-documented in traditional medicine around the globe for its beneficial effects. Its bioactive ingredients have been extensively studied and reviewed by various extraction techniques (Jakovljević et al. 2019). Nowadays, many research studies have been conducted to find new biological effects for this plant. In this study, we evaluated the role and the mechanism(s) of action of SOE against Cd intoxication in a rat model. The selected doses of Cd and the extract were literature-based (El-Kady et al. 2009; Arabi et al. 2014, respectively).
Our results showed that Cd alone administration induced a loss of body weight and a significant elevation in liver and kidney indices, NO, cholesterol, triglycerides, LDL, serum cytokines, hepatic and renal MDA, mRNA expression of Bax accompanied by a significant decrease in HDL, hepatic and renal antioxidant enzymes, and their mRNA gene expression. The decrease in body weight and the elevation of ALT and AST in intoxicated rats reported herein is similar to that reported previously and indicated that the reduction of body weight is mainly owing to the detrimental effect on liver function (Padilla et al. 2010). The serum ALT and AST are involved in the catabolism of the amino acids and the production of bile, so these enzymes act as critical biomarkers of liver function. The increase in these transaminases in the serum indicated the leakage of these enzymes into the bloodstream due to the severe damage of the membrane of the hepatocyte (Hall and Cash 2012; Kang et al. 2013). Moreover, the decrease in Alb and TP indicated the increase in excretion of high molecular mass protein (Genchi et al. 2020). The elevation of creatinine, uric acid, and urea reported in our study agreed with Borges et al. (2008). Although the increase of urea is considered the first marker in kidney dysfunction, creatinine is the most trustable marker and it rises if the kidneys suffer any damaging insult. In this concern, Hussein et al. (2014) reported that the pathological changes in the renal tissue include significant increases in serum urea and creatinine in rats exposed to Cd. The disturbances in lipid profile in Cd-treated rats suggested that this element generates oxidative stress which disturbs the balance between antioxidant and pro-oxidant resulting in the damage of cell function and unfavorable biological reactions leading to dyslipidemia (Olisekodiaka et al. 2012). Similar outcomes were reported by several authors and indicated an increase in cholesterol, triglycerides, and LDL levels in rats exposed to Cd (Badisa et al. 2007; Genchi et al. 2020; Murugavel and Pari 2007).
Several reports indicated that the hepatotoxicity of Cd attributed mainly ROS generation, protein, lipid peroxidation, and inflammation since the main mode of action of this metal is the generation of ROS and the diminish of the antioxidant defense system (SOD, CAT, GSH, and GPx) (Andjelkovic et al. 2019; Rahimzadeh et al. 2017). The generation of ROS including hydroxyl, hydrogen peroxide, and superoxide radicals modulates different components in the cell components mainly protein, lipids, and carbohydrates leading to the discrepancy of the metabolic dysfunction and cell integrity (Kaur et al. 2020). Moreover, MDA is a well-known player of lipid peroxide, whose malignant activities lead to injury to parenchymal cells (Andjelkovic et al. 2019) and its forms interfere with many biomolecules such as DNA, acetaldehyde, and the advanced glycation end products that comprise cell integrity (Li et al. 2015). Taken together, the elevation of liver and kidney indices and the oxidative markers (NO and MDA) along with the diminution of the hepatic and renal antioxidant enzymes suggesting that Cd exposure promotes the early oxidative damage leading to the development of consequential pathological conditions owing to its prolonged retention in different tissues (Abdel-Aziem et al. 2011; Renugadevi and Prabu 2010; Winiarska-Mieczan 2018).
The elevation of serum cytokine levels in rats treated with Cd in our study is in agreement with previous reports (Bonaventura et al. 2017; Markiewicz-Górka et al. 2019). These results suggested that exposure to Cd stimulates the cytokines production leading to cellular immune response disorders and all of these disturbances are consequences of the oxidative damage of Cd (Bonaventura et al. 2017; Turley et al. 2019). These results also confirmed that Cd affects the macrophages M1-type which is accountable for the inflammatory response via the releasing of the pro-inflammatory cytokines (Saqib et al. 2018). Furthermore, TNF-α is a cytokine produced by activated macrophages in response to pathogens and other harmful stimuli and is a necessary factor for local and systemic inflammation (Kany et al. 2019). In addition, TNF-α amplifies and prolongs the inflammatory reactions by triggering other cells to release cytokines such as IL-1β and media such as NO and ROS, all of which promote further inflammation and tissue damage (Elkhadragy and Abdel-Moneim 2017; Alghasham et al. 2013).
Additionally, the disturbances in mRNA expression of the pro-apoptotic gene (Bax), anti-apoptotic gene (Bcl-2), and the antioxidant enzymes (CAT, GPx, and SOD) along with the elevation of DNA fragmentation confirmed the hypothesis that the mechanism of Cd induces its toxicity via oxidative damage as suggested previously (Abdeen et al. 2019; Abdel-Aziem et al. 2011; Zhu et al. 2020; Mężyńska et al. 2018; Genchi et al. 2020). The pathological changes in the liver and kidney reported herein were also in accordance with the previous reports who indicated that Cd exposure induces severe histological alterations in these organs and others and all of these changes resulted from the oxidative damage of this element (El-Kady et al. 2009; Satarug 2018; Zhu et al. 2020).
In current results, administration of SOE alleviated and/or prevent the hazards of Cd. Animals who received SOE alone did not significantly affect the biochemical parameters, gene expression, or histology of the liver and kidney. The protective role of SOE is primarily due to the antioxidant and radical savaging properties of the bioactive constitutes in the extract. The highest content of these bioactive compounds and polyphenols gave this extract a great advantage in the therapy of several diseases resulted from several environmental toxins which have oxidative damage to living organisms. Natural antioxidants are well known to protect cells against the overproduction of ROS, consequently, counteract oxidative stress–mediated cells and tissue damage.
Previous reports indicated that SOE has a potent antioxidant activity and increases the resistance of the liver against oxidative damage (Horvẚthovẚ et al. 2016; Kolac et al. 2017; Poulios et al. 2020). It protects against oxidative and DNA damage through the elevation of glutathione peroxidase activity (Kozics et al. 2013). In our study, we found that SO essential oil is rich in 9-octadecenamide and other fatty acids which were reported to have strong antioxidant activity (Aktumsek et al. 2013; Olaoluwa et al. 2018; Nengroo and Rauf 2019; Karimi et al. 2015). Moreover, the extract is rich in polyphenols mainly coumaric, chlorogenic, catechin, ellagic, and gallic acids, vanillin, naringenin, and rutin which are well-known antioxidants. Godarzi et al. (2020) reported that coumaric acid protects the kidneys against ischemia-reperfusion (I/R) injury through its antioxidants and anti-inflammatory effects and exhibited hepatoprotective efficiency via the inhibition of lipid peroxidation, the generation of intracellular ROS, and the up-regulation of the detoxifying enzymes (Shen et al. 2019). Chlorogenic and coffeic acids also were reported to possess antioxidants and prevent ROS-induced DNA damage (Tomac et al. 2020).
Additionally, it was reported that catechins exert antioxidant activity via different direct and indirect mechanisms including chelating metal ions, scavenging ROS, enhancing the antioxidant enzymes, suppressing the pro-oxidant enzymes, and inducing the enzymes of phase II detoxification and the antioxidant enzymes (Ping-Hsiao et al. 2007; Bernatoniene and Kopustinskiene 2018). Vanillin also was reported to exhibit stronger antioxidant effects than Trolox and modulates the intracellular antioxidant activity such as SOD, CAT, and GSH-Px (Zhao et al. 2017). The high concentration of ellagic and gallic acids reported herein in SOE supports the previous findings of Jasicka-Misiak et al. (2018) and Zhang et al. (2014) who reported that SOE possesses a potent antioxidant activity because of its high content of ellagic and gallic acids. In the same concern, naringenin and rutin exert a potential antioxidant effect through the control of the effectors’ mechanisms of ROS generation (Nishimura et al. 2013). Taken together, antioxidant and radical scavenging activities of SOE are due to the high content of bioactive compounds and polyphenols which were able to counteract the oxidative damage of Cd and protect DNA, protein, and lipid damage in rats. Moreover, the high dose was more effective against Cd-induced oxidative damage and genotoxicity than the low dose due to the higher content of these bioactive constitutes.
Conclusion
The current results showed that S. officinalis grown in Egypt is rich in bioactive compounds including polyphenols. The GC-MS identified 39 compounds, most of them belonging to polyunsaturated fatty acids; moreover, the HPLC identified 12 polyphenols. The results also showed that Cd induced severe biochemical and cytokines alterations, oxidative damage leading to genotoxicity, DNA damage, and histological abnormalities in the liver and kidney tissues. S. officials extract could prevent these effects in a dose-dependent manner. This effect may be due to the synergistic antioxidant and radical savaging effects of the bioactive compounds.
References
Abdeen A, Abou-Zaid OA, Abdel-Maksoud HA, Aboubakr M, Abdelkader A, Abdelnaby A, Abo-Ahmed AI, El-Mleeh A, Mostafa O, Abdel-Daim M, Aleya L (2019) Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ Sci Pollut Res Int 26(24):25167–25177
Abdel-Aziem SH, El-Nekeety AA, Barakat IA, Mohamed IM, Abdel-Wahhab MA (2011) Aquilegia vulgaris extract protects against the oxidative stress and the mutagenic effects of cadmium. Exp Toxicol Pathol 63(4):337–344
Abdel-Wahhab MA, El-Nekeety AA, Salman AS, Hathout AS, Abdel-Aziem SH, Sabry BA, Hassan NS, Abdel-Aziz MS, Aly SA, Jaswir I (2020a) Bioactive compounds from Aspergillus niger extract enhance the antioxidant activity and prevent the genotoxicity in AFB1-treated rats. Toxicon 181:57–68
Abdel-Wahhab MA, El-Nekeety AA, Hathout AS, Salman AS, Abdel-Aziem SH, Hassan NS, Abdel-Aziz MS (2020b) Secondary metabolites from Bacillus sp. MERNA97 extract attenuates the oxidative stress, genotoxicity and cytotoxicity of aflatoxin B1 in rats. Food Chem Toxicol 141:111399. https://doi.org/10.1016/j.fct.2020.111399
Abdel-Wahhab MA, Hassan MA, El-Nekeety AA, Abdel-Aziem SH, Hassan NS, Jaswir I, Salleh HM (2021) Zinc loaded whey protein nanoparticles mitigate the oxidative stress and modulate antioxidative gene expression in testicular tissues in rats. J Drug Deliv Sci Technol 61:102322. https://doi.org/10.1016/j.jddst.2021.102322
Aboshanab MHA, El-Nabarawi MA, Teaima MH, El-Nekeety AA, Abdel-Aziem SH, Hassan NS, Abdel-Wahhab MA (2020) Fabrication, characterization and biological evaluation of silymarin nanoparticles against carbon tetrachloride-induced oxidative stress and genotoxicity in rats. Int J Pharm 587:119639. https://doi.org/10.1016/j.ijpharm.2020.119639
Abu-Darwish MS, Cabral C, Ferreira IV, Gonçalves MJ, Cavaleiro C, Cruz MT, Al-bdour TH, Salgueiro L (2013) Essential oil of common sage (Salvia officinalis L.) from Jordan: assessment of safety in mammalian cells and is antifungal and anti-inflammatory potential. Biomed Res Int 2013: Article ID 538940. https://doi.org/10.1155/2013/538940
Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A (2013) Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem 141:91–97
Alghasham A, Salem TA, Meki AR (2013) Effect of cadmium polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: protective effect of curcumin. Food Chem Toxicol 59(22):160–164
Al-Qudah MA, Tashtoush HI, Khlaifat EF, Ibrahim SO, Saleh AS, Al-Jaber HI, Abu Zarga MH, Abu Orabi ST (2020) Chemical constituents of the aerial parts of Salvia judaica Boiss from Jordan. Nat Prod Res 34(20):2981–2985
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16(2):274. https://doi.org/10.3390/ijerph16020274
Arabi S, Arshami J, Haghparast A (2014) Effects of Salvia officinalis L. extract on biochemical blood parameters in male rats. J Adv Med Biomed Res 2014 22(94):34–43
Badiee P, Nasirzadeh AR, Motaffaf M (2012) Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J Pharm Technol Drug Res 1:7. https://doi.org/10.7243/2050-120X-1-7
Badisa VL, Latinwo LM, Odewumi CO, Ikediobi CO, Badisa RB, Ayuk-Takem LT, Nwoga J, West J (2007) Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ Toxicol 22:141–144
Baj T, Ludwiczuk A, Sieniawska E, Woèniak KS, Widelski J, Zieba K, G£owniak K (2013) GC-MS analysis of essential oils from salvia officinalis l.: comparison of extraction methods of the volatile components. Acta Pol Pharm 70(1):35–40
Bancroft D, Stevens A, Turmer R (1996) Theory and practice of histological technique, 4th edn. Churchill LivingStone, Edinburgh, London and New York, pp 273–292
Belyaeva EA, Dymkowska D, Wieckowski MR, Wotczak L (2008) Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol 231:34–42
Bernatoniene J, Kopustinskiene DM (2018) The role of catechins in cellular responses to oxidative stress. Molecules 23(4):965. https://doi.org/10.3390/molecules23040965
Bernhoft RA (2013) Cadmium toxicity and treatment. Sci World J 394652:1–7
Bonaventura P, Lamboux A, Albarède F, Miossec P (2017) Regulatory effects of zinc on cadmium-induced cytotoxicity in chronic inflammation. PLoS One 12(7):e0180879. https://doi.org/10.1371/journal.pone.0180879
Borges LP, Brandao R, Godoi B, Nogueira CW, Zenidoi G (2008) Oral administration of diphenyl diselenide protects against cadmium-induced liver damage in rats. Chem Biol Interact 171:15–25
Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 19:1501. https://doi.org/10.3390/ijms19051501
Burton K (1956) A study of the conditions and mechanisms of the diphenylaminereaction for the estimation of deoxyribonucleic acid. Biochem J 62:315–323
Cannino G, Feruggia E, Luparello C, Rinaldi AM (2009) Cadmium and mitochondria. Mitochondrion 9:377–384
Chatterjee S, Kundu S, Bhattacharyya A (2008) Mechanism of cadmium induced apoptosis in the immunocyte. Toxicol Lett 177:83–89
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois T, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940
El Hadri A, del Río MAG, Sanz J, Coloma AG, Idaomar M, Ozonas BR, González JB, Reus MI (2010) Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An R Acad Nac Farm 76:343–356
El-Kady AA, Sharaf HA, Abbès S, Ben Salah-Abbès J, Naguib KM, Oueslati R, Abdel-Wahhab MA (2009) Adsorption of Cd2+ ions on an Egyptian montmorillonite and toxicological effects in rats. Appl Clay Sci 44(1-2):59–66
Elkhadragy MF, Abdel-Moneim AE (2017) Protective effect of Fragaria ananassa methanolic extract on cadmium chloride (CdCl2)- induced hepatotoxicity in rats. Toxicol Mech Methods 27(5):335–345
Fu Z, Wang H, Hu X, Sun Z, Han C (2013) The pharmacological properties of Salvia essential oils. J Appl Pharm Sci 07:122–127
Gandhi M, Aggarwal M, Puri S, Singla SK (2013) Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male Wistar rat. Int Braz J Urol 39:108–117
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17:3782. https://doi.org/10.3390/ijerph17113782
Gheraibia S, Belattar B, Abdel-Wahhab MA (2020) HPLC analysis, antioxidant and cytotoxic activity of different extracts of Costus speciosus against HePG-2 cell lines. South Afr J Bot 131:222–228
Ghorbani A, Esmaeilizadeh M (2017) Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med 7:433–440
Godarzi SM, Gorji AV, Gholizadeh B, Mard SA, Mansouri E (2020) Antioxidant effect of p-coumaric acid on interleukin 1- and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrologia 40(3):311–319
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol (London, England) 1:22
Güzela S, Özayb Y, Kumas M, Uzun C, Özkorkmaz G, Yıldırım Z, Ülger M, Güler G, Çelik A, Çamlıca Y, Kahraman A (2019) Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher & Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed Pharmacother 111:1260–1276
Hall P, Cash J (2012) What is the real function of the liver ‘function’ tests? Ulster Med J 81(1):30–36
Hasan MR, Al-Jaber HI, Al-Qudah MA, Abu Zarga MA (2016) New sesterterpenoids and other constituents from Salvia dominica growing wild in Jordan. Phytochem Lett 16:12–17
Hassan MA, El-Nekeety AA, Abdel-Aziem SH, Hassan NS, Abdel-Wahhab MA (2019) Zinc citrate incorporation with whey protein nanoparticles alleviate the oxidative stress complication and modulate gene expression in the liver of rats. Food Chem Toxicol 125:439–445
Horvẚthovẚ E, Srancíkova A, Regendová-Sedláčková E, Melušová M, Meluš V, Netriová J, Krajčovičová Z, Slameňová D, Pastorek M, Kozics K (2016) Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 31:51–59
Hussein SA, Omnia M, Fayed AM (2014) Protective effects of alpha-lipoic acid and melatonin against cadmium-induced oxidative stress in erythrocytes of rats. J Pharmacol Toxicol 9:1–24
Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H, Banjari I (2019) Review Bioactive Profile of Various Salvia officinalis L. Preparations Plants 8:55. https://doi.org/10.3390/plants8030055
Jasicka-Misiak I, Poliwoda A, Petecka M, Buslovych O, Shlyapnikov V, Wieczorek P (2018) Antioxidant Phenolic Compounds in Salvia officinalis L. and Salvia sclarea L. Ecol Chem Eng 25:133–142
Kamatou GP, Makunga NP, Ramogola WPN, Viljoen AM (2008) South African salvia species: a review of biological activities and phytochemistry. J Ethnopharmacol 119(3):664–672
Kang MY, Cho SH, Lim YH, Seo JC, Hong YC (2013) Effects of environmental cadmium exposure on liver function in adults. Occup Environ Med 70(4):268–273
Kany S, Vollrath JT, Relja B (2019) Cytokines in Inflammatory Disease. Int J Mol Sci 20(23):6008. https://doi.org/10.3390/ijms20236008
Karimi E, Jaafar HZE, Ghasemxadeh A, Ebrahimi M (2015) Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol Res 48:9. https://doi.org/10.1186/0717-6287-48-9
Kaur G, Shivanandappa TB, Kumar M, Kushwa AS (2020) Fumaric acid protect the cadmium-induced hepatotoxicity in rats: owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn Schmiedeberg's Arch Pharmacol 393:1911–1920
Khare R, Upmanyu N, Jha M (2019) Exploring the potential effect of Methanolic extract of Salvia officinalis against UV exposed skin aging: In vivo and In vitro model. Curr Aging Sci:12. https://doi.org/10.2174/1874609812666190808140549
Kolac UK, Ustuner MC, Tekin N, Ustuner D, Colak E, Entok E (2017) The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J Med Food 20(12):1193–1200
Kozics K, Klusova V, Rančíková A, Mučaji P, Slameňová D, Hunáková L, wicz KB, Horváthová E (2013) Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem Toxicol 141:2198–2206
Kuo JH, Jan MS, Jeng J, Chiu HW (2005) Induction of apoptosis in macrophages by air oxidation of dioleoylphosphatidylglycerol. J Control Release 108:442–452
Lehbili M, AlabdulMagid A, Kabouche A, Voutquenne-Nazabadioko L, Abedini A, Morjani H, Gangloff SC, Kabouche Z (2018) Antibacterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri. Nat Prod Res 32:2683–2691
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124
Limaye PV, Raghuram N, Sivakami S (2003) Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin induced diabetic rats. Mol Cell Biochem 243:147–152
Lin CC, Hsu YF, Lin TC, Hsu FL, Hsu HY (1998) Antioxidant and hepatoprotective activity of Punicalagin and Punicalin on carbon tetrachloride induced liver damage in rats. J Pharm Pharmacol 50:789–794
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25(4):402–408
Marcinek K, Krejpcio Z (2017) Chia seeds (Salvia hispanica): health promoting properties and therapeutic applications- a review. Rocz Panstw Zakl Hig 68(2):123–129
Markiewicz-Górka I, Pawlas K, Jaremków A, Januszewska L, Pawłowski P, Pawlas N (2019) Alleviating effect of α-lipoic acid and magnesium on cadmium-induced inflammatory processes, oxidative stress and bone metabolism disorders in Wistar rats. Int J Environ Res Public Health 16(22):4483. https://doi.org/10.3390/ijerph16224483
Mężyńska M, Brzóska MM, Rogalska J, Piłat-Marcinkiewicz B (2018) Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: a study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients 11(1):21. https://doi.org/10.3390/nu11010021
Moghaddam FM, Amiri R, Alam M, Hossain MB, Van der Helm D (1998) Structure and absolute stereochemistry of Salvimirzacolide, a new sesterterpene from Salvia mirzayanii. J Nat Prod 61:279–281
Mohamed AY, Mustafa AA (2019) Gas Chromatography-Mass Spectrometry (GC-MS) analysis of essential oil Salvia officinalis in Sudan. 10.20944/preprints201902.0218.v1.
Murugavel P, Pari L (2007) Diallyl tetrasulfide protects cadmium-induced alterations in lipids and plasma lipoproteins in rats. Nutr Res 27:356–361
Nengroo ZR, Rauf A (2019) Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind Crop Prod 140:111596. https://doi.org/10.1016/j.indcrop.2019.111596
Nishimura FDY, Almeida AC, Ratti BA, Ueda-Nakamura T, Nakamura CV, Ximenes VF (2013) Silva SDO (2013) Antioxidant effects of quercetin and naringenin are associated with impaired neutrophil microbicidal activity. Evid Based Complement Alternat Med 2013:1–7. https://doi.org/10.1155/2013/795916
Olaoluwa O, Moronkola D, Taiwo O, Iganboh P (2018) Volatile oil composition, antioxidant and antimicrobial properties of Boerhavia erecta L. and Euphorbia hirta L. Trends Phytochem Res 2:171–178
Olisekodiaka MJ, Igbeneghu CA, Onuegbu AJ, Oduru R, Lawal AO (2012) Lipid, lipoproteins, total antioxidant status and organ changes in rats administered high doses of cadmium chloride. Med Princ Pract 21:156–159
Padilla MA, Elobeid M, Ruden DM, Allison DB (2010) An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int J Environ Res Public Health 7(9):3332–3347
Pari L, Murugavel P (2007) Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology 234:44–50
Perandones CE, Illera VA, Peckham D, Stunz LL, Ashman RF (1993) Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol 151:3521–3529
Ping-Hsiao S, Chi-Tai Y, Gow-Chin Y (2007) Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem 55:9427–9435
Pizzino G, Bitto A, Interdonato M, Galfo F, Irrera N, Mecchio A, Pallio G, Ramistella V, De Luca F, Minutoli L, Squadrito F, Altavilla D (2014) Oxidative stress and DNA repair and detoxification gene expression in adolescents exposed to heavy metals living in the Milazzo-Valle del Mela area (Sicily, Italy). Redox Biol 2:686–693
Poulios E, Giaginis C, Vasios GK (2020) Current state of the art on the antioxidant activity of sage (Salvia spp.) and its bioactive components. Planta Med 86(4):224–238
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA (2017) Cadmium toxicity and treatment: an update. Caspian J Intern Med 8(3):135–145
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24(4):378–399
Renugadevi J, Prabu SM (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62(5):471–481
Russo A, Formisano C, Rigano D, Senatore F, Delfine S, Cardile V, Rosselli S, Bruno M (2013) Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem Toxicol 55:42–47
Sanjeev S, Bidanchi RM, Murthy MK, Gurusubramanian G, Roy VK (2019) Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res Int 26(20):20631–20653
Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R (2018) Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 9(25):17937–17950
Satarug S (2018) Dietary Cadmium intake and its effects on kidneys. Toxics 6:15. https://doi.org/10.3390/toxics6010015
Senatore F, Apostolides AN, Piozz F (2004) Chemical composition of the essential oil of Salvia multicaulis Vahl. var. simplicifolia Boiss. J Chromatogr A 1052:237–240
Senatore F, Arnold NA, Piozzi F, Formisano C (2006) Chemical composition of the essential oil of Salvia microstegia Boiss. et Balansa growing wild in Lebanon. J Chromatogr A 1108:276–278
Shen Y, Song X, Li L, Sun J, Jaiswal Y, Huang J, Liu C, Yang W, Williams L (2019) Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed Pharmacother 111:579–587
Tinkov AA, Gritsenko VA, Skalnaya MG, Cherkasov SV, Aaseth J, Skalny AV (2018a) Gut as a target for cadmium toxicity. Environ Pollut 235:429–434
Tinkov AA, Filippini T, Ajsuvakovae OP, Skalnaya MG, Aasethf J, Bjørklundh G, Gatiatulinai ER, Popova EV, Nemereshinai ON, Huangk PT, Vinceti M, Skalny AV (2018b) Cadmium and atherosclerosis: a review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ Res 162:240–260
Tomac I, Šeruga M, Labuda J (2020) Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem 325:126787. https://doi.org/10.1016/j.foodchem.2020.126787
Topcu G (2006) Bioactive triterpenoids from Salvia species. J Nat Prod 69:482–487
Turley AE, Zagorski JW, Kennedy RC, Freeborn RA, Bursley JK, Edwards JR, Rockwell CE (2019) Chronic low-level cadmium exposure in rats affects cytokine production by activated T cells. Toxicol Res 8(2):227–237
WHO World Health Organization (1992) Environmental health criteria 135. In Cadmium-environmental aspects; World Health Organization: Geneva, Switzerland
Winiarska-Mieczan A (2018) Protective effect of tea against lead and cadmium-induced oxidative stress-a review. Biometals 31(6):909–926
Zhang L, Wang Y, Xu M, Wu DM, Chen JH (2014) Quantification of gallic acid and ellagic acid from the seed of Cornus officinalis by UHPLC method and their antioxidant activity. Chem Eng Commun 201:4. https://doi.org/10.1080/00986445.2013.780165
Zhao D, Sun J, Sun B, Zhao M, Zheng F, Huang M, Li H (2017) Intracellular antioxidant effect of vanillin, 4-methylguaiacol and 4-ethylguaiacol: three components in Chinese Baijiu. RSC Adv 7(73):46395–46405
Zhou Z, Wang C, Liu H, Huang Q, Wang M, Le Y (2013) Cadmium induced cell apoptosis, DNA damage, decreased DNA repair capacity, and genomic instability during malignant transformation of human bronchial epithelial cells. Int Med Sci 10:1485–1496
Zhu MK, Li HY, Bai LH, Wang LS, Zou XT (2020) Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult Sci 99(6):3215–3228
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the National Research Centre, Dokki, Cairo, Egypt, project # 12050305.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. Authors HM Rashwan, HE Mohammed, AA El-Nekeety, and ZK Hamza carried out the experimental work and the biochemical analysis. Author SH Abdel-Azeim carried out the genetic analysis. Author NS Hassan carried out the histological part. Author MA Abdel-Wahhab wrote the protocol, managed the project, managed the analyses of the study, performed the statistical analysis, and wrote the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol of the current study was approved by the ethics Animal Care and Use Committee of the National research Center, Dokki, Cairo, Egypt (approval # 12050305/2019).
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashwan, .M., Mohammed, H.E., El-Nekeety, A.A. et al. Bioactive phytochemicals from Salvia officinalis attenuate cadmium-induced oxidative damage and genotoxicity in rats. Environ Sci Pollut Res 28, 68498–68512 (2021). https://doi.org/10.1007/s11356-021-15407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15407-y