Abstract
Rosa persica is a member of the Rosaceae family that has a wide range of pharmacological properties. In this study, the antioxidant and therapeutic potential of this plant was investigated on cadmium (Cd)-induced hepatotoxicity. Rosa persica extract (RPE) was prepared by a maceration method in hydroalcoholic solvent, and its antioxidant properties were determined. Then, 36 mice were divided to six groups and treated for 2 weeks as follows: control, Cd (3 mg/kg), RPE (50 mg/kg), and groups 4–6 received Cd (3 mg/kg) and 12.5, 25, and 50 mg/kg of RPE respectively. The total polyphenol, flavonoids contents, and total antioxidant capacity in RPE were measured 263.4 ± 7.2 mg rutin equivalent/g extract, 72.3 ± 2.3 mg quercetin equivalent/g extract, and 8.46 ± 0.27 μmol ferrous sulfate/g extract, respectively. The in vivo results showed that Cd elicited remarkable hepatic injury that was manifested by the significant increase in serum hepatic enzymes. In addition, Cd significantly increased the levels of lipid peroxidation (LPO) and tumor necrosis factor-alpha (TNF-α) and decreased total thiol molecules (TTM) and total antioxidant capacity (TAC) in hepatic tissue. However, RPE decreased serum hepatic enzyme levels and improved oxidative hepatic damage by lowering the LPO and TNF-α levels and raising TAC and TTM in in Cd-treated groups. Although the RPE increased the metallothionein (MT) protein content, there was no change in MT gene expression. The present study showed that the RPE due to having antioxidant properties might partially prevent hepatic oxidative damage by the improvement of oxidant/antioxidant balance in animals exposed to Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most important toxic metals, due to its accumulation in the different organs such as the testis, kidney, and liver (Amamou et al. 2015). The human exposure to Cd mostly results from smoking and industrial processes such as smelting and refining of metals, battery and plastic industries, and food contamination (Dastan et al. 2019; Ige et al. 2012; Heshmati et al. 2017). Cd causes a wide range of different health disorders, including cardiovascular disease (Li et al. 2019), testicular atrophy (Yang et al. 2018), osteoporosis (Chen et al. 2017), and hepatorenal dysfunction (Dastan et al. 2019; El-Boshy et al. 2015). In addition, Cd has been categorized as carcinogenic to animals and humans (Hartwig 2013).
The liver plays a critical role in metabolism and xenobiotic detoxification (Gu and Manautou 2012). Cd is accumulated mostly in the hepatic tissue and can cause severe injury in this organ (Arroyo et al. 2012). Different mechanisms have been suggested to explain the Cd-induced liver toxicity such as mitochondrial dysfunction, disruption of autophagic flux, and especially inflammatory and oxidative stress pathways (Zou et al. 2019; Okoye et al. 2019; Liu et al. 2015),
Oxidative stress is the outcome of an imbalance between free radicals and intracellular antioxidants, which can lead to tissue injury (Cuypers et al. 2010). The previous studies suggested that Cd induces oxidative stress through depletion of endogenous antioxidants and interference with mitochondrial respiratory chain (El-Boshy et al. 2015; Wang et al. 2004). This toxic metal has a high affinity for variety of functional groups especially sulfhydryl groups. Therefore, Cd can inhibit several enzymes and subsequently disrupt some metabolic processes in hepatocytes (Begic et al. 2017; Rani et al. 2014).
Metallothioneins (MTs) are low-molecular-weight cysteine-rich proteins and are expressing in several tissues especially the liver and kidney. These proteins play a key role in the homeostatic control of trace elements and the detoxification of toxic metals such as Cd. MTs protect the human body against oxidative damages through scavenging intracellular free radicals (Abdeen et al. 2019a; Li et al. 2015).
In recent years, the use of traditional medicine has become remarkably common in world (Bhosale and Banerjee 2020). Rosaceae family has been used for cosmetic, therapeutic, and aromatherapy purposes since long time ago. In traditional medicine, Rosaceae family has been used to treat depression, intestinal ulcer, asthma, cough, diarrhea, fever, insomnia, vascular contraction, headache, colds, bacterial infections, and previous reports revealed antioxidant, anti-inflammatory, analgesic, antispasmodic, and anticonvulsants properties (Boskabady et al. 2011; Mohebitabar et al. 2017; Sadraei et al. 2016).
Rosa persica (RP) is a member of the Rosaceae family that found not only in Iran but also in Afghanistan and central Asia. Despite the hydroalcoholic extract of Rosa persica contained polyphenolic antioxidant ingredients with potent free radical scavenging activity (Jassbi et al. 2003), there is little evidence of its medicinal properties. Therefore, the current study was designed to assess whether treatment with this herbal extract exerts any beneficial effects in improving of hepatotoxicity induced by Cd in mice.
Materials and methods
Chemicals
2,4,6-tripyridyl-s-triazine (TPTZ), bovine serum albumin (BSA), 5,5’dithiobis-2-nitro benzoic acid (DTNB), and 2-thiobarbituric acid (TBA) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Cadmium chloride monohydrate (CdCl2 ∙ H2O), ethanol, ascorbic acid powder, rutin and folin reagents, aluminum chloride reagent, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) powder were purchased from Merck (Darmstadt, Germany).
Plant materials: collection and extraction procedure
The RP plant was collected in late spring, from Hamadan province of Iran. The obtained plant was identified by herbarium unit, school of pharmacy, Hamadan University of Medical Sciences (HUMS), Hamadan, Iran with the code number (NO: 223). Rosa persica extract (RPE) was prepared by maceration method in hydroalcohol solvent. Briefly, 100 g of plant aerial parts was powdered using electric mills and added to 1200 ml ethanol (80%) for 72 h in dark condition. Then, the extract was filtrated and evaporated to become dry in a rotary evaporator (Heidolph, Germany) under vacuum at 40 °C. The resulting extract was kept at 4 °C (Moradkhani et al. 2014). Yield percentage was calculated by using following formula:
weight of the extract obtained (A) and total weight of the sample loaded (B)
Phytochemical analysis
Determination of total flavonoid content
Total flavonoid content was measured using the method of Ordonez et al. (2006). To prepare the stock solution of the plant extract, 100 mg of the dried extract was weighed and dissolved in 100 ml of ethanol (90% w/v). After making a serial dilution in the range of 0.5–0.03 mg/ml, 0.5 ml of each dilution was mixed with 0.5 ml of 2% AlCl3-ethanol solution and incubated for 1 h. The flavonoid content was assayed by calculating the rise in yellow color due to production of complex flavonoid aluminum. The absorbance was detected in duplicates in a 96-well plate with a microplate reader (Biotec, Tecan US, Inc.) set at 415 nm against different concentration of quercetin as the standard: Y = 1.5055x-1.592, r2 = 0.99.
Determination of total polyphenol content
The total polyphenol content of extract was determined by Folin–Ciocalteu colorimetric method with some modifications (Rezvani-Kamran et al. 2017). Briefly, 100 mg of the dried extract was dissolved in 100 ml of ethanol (90% w/v) and a range of two-fold serial dilution was prepared (0.5–0.03 mg/ml). One milliliter of each dilution was mixed with 2 ml of Na2CO3 (2%) and 0.1 ml of 50% Folin–Ciocalteu reagent. Then, the mixture was vortexed and incubated at 40 °C for 30 min. The absorbance was detected in duplicates in a 96-well plate with a microplate reader set at 765 nm against different concentration of rutin, as the standard curve: Y = 0.783x-0.3937, r2 = 0.9947.
Determination of total antioxidant activity
The total antioxidant power of extract was measured by determining its ability to reduce Fe+3 to Fe+2 using ferric-reducing antioxidant power (FRAP) method (Benzie and Strain 1996). In the first stage, 100 mg of the dried extract was dissolved in 100 ml of ethanol (90% w/v) and a range of serial dilution was prepared (0.5–0.03 mg/ml). Then, the FRAP reagent was prepared by mixing 1 volume of 20 mM FeCl3, 10 volumes of 300 mM acetate buffer (pH 3.6), and also 1 volume of 10 mM TPTZ in 40 mM HCL. It should be noted that all the solutions were freshly prepared before their uses. Antioxidant power value was reported in terms of μmol Fe+2/g of sample using ferric chloride standard curve: Y = 0.0006x + 0.0007, r2 = 0.999.
DPPH radical scavenging assay
Free radical scavenging activity was determined by DPPH reagent, as described earlier with some modifications (Abdeen et al. 2019b). Briefly, DPPH was dissolved in 25 ml ethanol to obtain a solution of 0.04% w/v. An aliquot of 80 μl of DPPH was mixed with 200 μl extract samples with different logarithmic concentrations (1–10,000 μg/ml) into 96-well plate and incubated for 30 min at room temperature. Optical density was detected at 517 nm using microplate reader. Ascorbic acid was used as standard, and the scavenging ability of the extract on DPPH was calculated using the equation:
absorbance of control (A); absorbance of the sample (B)
Animal experiments
Animals
Male albino mice (22–27 g) were obtained from animal house of HUMS. Animals were maintained in conventional conditions at a temperature of 23 ± 2 °C, with a relative humidity of 45–55% and a 12-h/12-h light/dark cycle. They were supplied with standard laboratory diet and water ad libitum, and left to acclimatize for 1 week before the experimental procedures. It should be noted that the study protocol was approved by HUMS Ethics Committee with the ethical number, IR.UMSHA.REC.1394.510, in accordance with the guideline of the Research Ethics Committee of the Ministry of Health and Medical Education, Iran (adopted on April 17, 2006), based on the Helsinki Protocol (Helsinki, Finland, 1975).
Preliminary studies
The possible acute toxicity of RPE was evaluated on mice at doses of 5, 50, 500, 1000, and 2000 mg/kg. After 24 h, the hepatic function was studied by evaluating the enzymatic activity of alanine transaminase (ALT) and histological changes. No biochemical and pathological changes were observed in the dose range of 5–500 mg/kg. At doses 1000 and 2000 mg/kg, the activity of the ALT enzyme increased, which was in line with the pathological changes. Therefore, the maximum safe dose of 50 mg/kg was selected (in sub-acute exposure) as 1/10 of the maximum acute dose (500 mg/kg). Subsequently, doses of 12.5, 25, and 50 mg/kg were selected as therapeutic doses. It should be noted that the toxic dose of Cd was determined 3 mg/kg based on a pilot study.
Experimental protocol and groups
The animals were randomly divided to six groups (n = 6, each). The mice were treated for two consecutive weeks by intraperitoneal (i.p) injection as follows: group 1 received normal saline solution. Group 2 received 3 mg/kg/day of Cd. Group 3 received 50 mg/kg/day of RPE, and groups 4, 5, and 6 were administrated Cd (3 mg/kg/day) following treatment with 12.5, 25, and 50 mg/kg/day of RPE, respectively. It should be noted that the RPE was mixed in normal saline solution and injection volume considered 0.2 ml for each animal. At the end of study course, the animals were weighted and then anaesthetized with mixtures of ketamine and xylazine (5:1) (Harchegani et al. 2017). Blood samples were collected by cardiac puncture, and serum separated for biochemical experiments.
After laparotomy surgery, the liver was removed and washed in normal saline. About 500 mg of hepatic tissue was separated for determination of biochemical biomarkers and another part of tissue was fixed in 10% formaldehyde solution for histopathological analysis.
Liver function test
The enzymatic activities of alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) in serum were determined using commercial kits (Pars Azmoon, Tehran, Iran).
Preparation of hepatic tissue homogenate
The sample homogenates were prepared in a ratio of 0.1 g of hepatic tissue to 1 ml of KCl (1.15%) and centrifuged at 3000 g for 10 min at 4 °C. Then, the supernatants were separated for the biochemical analysis.
Measurement of lipid peroxidation
The hepatic lipid peroxidation (LPO) was assayed using the thiobarbituric acid reactive substances (TBARS) method with some modifications (Ohkawa et al. 1979). Briefly, a reaction mixture was prepared containing TBA (0.2%) in H2SO4 (0.05 M). One hundred micriliters of liver homogenate supernatant and 500 μl reaction mixture was mixed and heated for 30 min in boiling water bath. The samples were detected by microplate reader set at 532 nm against malonedialdehyde (MDA) as the standard.
Measurement of total antioxidant capacity
Total antioxidant capacity (TAC) in liver homogenate supernatant was measured according to reduction of Fe3+ to Fe2+ as described by Benzie and Strain 1996. In this experiment, the complex between Fe2+ and TPTZ, as an indicator, gives a blue color with an absorbance maximum at 593 nm.
Measurement of total thiol molecules
Total thiol molecules (TTM) were measured using DTNB as the reagent and its absorbance was read against a blank at 412 nm (Hu 1994). Two hundred microliters of Tris-EDTA buffer solution (0.25 M Tris base, 20 mM EDTA, pH 8.2) was mixed with liver homogenate supernatant (10 μl), and its absorbance was determined at 412 nm. Then, 10 μl of DTNB solution (10 mmol/l in absolute methanol) was added and incubated at 37 °C for 15 min. The absorbance of the samples (A2) and DTNB blank (B) was read again at 412 nm. The level of thiol molecules was calculated by reduced glutathione as standard.
Measurement of TNFα
Quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kit was used for liver TNFα assay, according to the manufacturer’s instructions (Shanghai crystal Day Biotech Co., LTD, China). This technique is based on direct sandwich procedure, in which two monoclonal antibodies are against separate antigenic elements on the TNFα molecule. The TNFα in liver homogenate supernatant reacts with peroxidase-conjugated anti-TNFα antibodies and anti-TNFα antibodies bound to microtitration well. The optical density of the produced color was detected using microplate reader set at 450 nm against standard curve.
Measurement of metallothionein protein
The level of metallothionein (MT) protein in liver homogenate supernatant was assayed by ELISA kit (Shanghai crystal Day Biotech Co., LTD, China), according to direct sandwich procedure. The optical density of the produced color was detected using microplate reader set at 450 nm against standard curve.
Protein assay
At the end of the experiment, protein content was determined in each tissue homogenate supernatant based on Bradford method using BSA as a standard.
RT-PCR assay
Hepatic total RNA was extracted by TRIzol reagent. In the next stage, cDNA was synthesized using revert aid first strand cDNA synthesis kit (Cinna Gen Co, Iran). The primers were designed for RT-PCR process as follow: MT primers F: CTCCGTAGCTCCAGCTTCAC and R: AGGAGCAGCAGCTCTTCTTG resulting a 137 bp product, β- actin (as housekeeping gene) primers F: GGCCAACCGTGAAAAGATGA and R: CAGCCTGGATGGCTACGTACA resulting a 77 bp product. The RT-PCR process was performed by adding cDNA to PCR master mix and the products were analyzed using SensoQuest Thermal Cycler. The thermal cycling programs after initial denaturation at 95 °C for 5 min were as follows: 95 °C for 10 s, (MT: 54 °C, β-actin: 54 °C) for 10 s and 72 °C for 15 s that repeated for 35 cycles and followed by a final extension at 72 °C for 2 min. Finally, a volume 20 μl of PCR products were separated on 2% agarose gel and stained with ethidium bromide. The intensity of the MT and β-actin bands was quantified by densitometry. The MT level was normalized to that of β-actin.
Histopathological analysis
After fixation of hepatic tissue with 10% formaldehyde solution, the paraffin-embedded block was prepared and cut into 4–6 μm thick sections by a rotary microtome. Finally, the samples were dyed by hematoxylin and eosin (H&E) and pictured by the camera under a microscope for histopathological examination (Cardiff et al. 2014).
Statistical analysis
All data expressed as the mean ± standard error (SEM). The data were analyzed by SPSS, version 16.0 (SPSS, Inc., Chicago, IL, USA) using analysis of variance (ANOVA) and Tukey’s post hoc test were used if variables were normally distributed. P < 0.05 was considered statistically significant.
Results
Phytochemical analysis
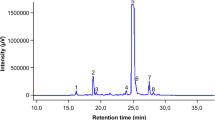
In the current study, the yield percentage of extraction was determined 55.87% of dried plant material. In DPPH experiment, the average of IC50 was calculated 8.28 μg/ml (in the range of 6.6–10.3 μg/ml) for vitamin C and 16.33 μg/ml (in the range of 11.9–22.2 μg/ml) for RPE (Fig. 1). Also, the total polyphenol, flavonoids contents, and total antioxidant capacity in RPE were measured 263.4 ± 7.2 mg rutin equivalent/g extract, 72.3 ± 2.3 mg quercetin equivalent/g extract, and 8.46 ± 0.27 μmol ferrous sulfate/g extract, respectively.
Animal’s weight
The body weights of different groups over the two weeks study period were monitored. There was no change in body weights in Cd and RPE-treated Cd groups (data not shown).
Liver function status
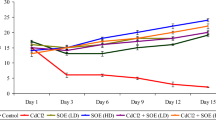
In the Cd group, a significant increase was observed in serum levels of AST, ALT, and ALP as compared to the control group (P < 0.001). RPE was able to reduce the increased levels of AST and ALP at the employed doses of 25 and 50 mg/kg (P < 0.05), and ALT level in dose of 50 mg/kg (P < 0.01) (Fig. 2).
Effect of RPE on hepatic serum enzymes in Cd-exposed mice. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ≠≠≠P < 0.001 vs. control group; *P < 0.05 and **P < 0.01 vs. Cd group. ALT, alanine aminotransferase (a); AST, aspartate aminotransferase (b); ALP, alkaline phosphatase (c); Cd, cadmium (equal 3 mg/kg); RPE, Rosa persica extract
Biomarkers of oxidative stress
As shown in Fig. 3, Cd significantly increased LPO level (P < 0.001) and remarkable decreased TAC and TTM contents of liver tissue (P < 0.001) in comparison with control. Administration of RPE decreased LPO level in dose of 50 mg/kg (P < 0.05) as compared to the Cd group. In addition, RPE could improve TAC level in dose of 50 mg/kg (P < 0.01), and TTM contents in doses of 25 and 50 mg/kg (P < 0.01 and P < 0.05, respectively), in animals exposed to Cd.
Effect of RPE on hepatic oxidative stress biomarkers in Cd-exposed mice. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ≠≠≠P < 0.001 vs. control group; *P < 0.05 and **P < 0.01 vs. Cd group. LPO, lipid peroxidation (a); TAC, total antioxidant capacity (b); TTM, total thiol molecules (c). Cd, cadmium (equal 3 mg/kg); RPE, Rosa persica extract
TNFα assay
In Cd-treated mice, a significant increase in liver TNFα was observed after the treatment period when compared to the untreated mice (P < 0.001). Treatment with different doses of RPE, 25 and 50 mg/kg, caused a significant decrease in liver TNFα level (P < 0.05) (Fig. 4).
Effect of RPE on hepatic TNF-α level in Cd-exposed mice. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ≠≠≠P < 0.001 vs. control group; *P < 0.05 vs. Cd group. TNF-α, tumor necrosis factor alpha; Cd, cadmium (equal 3 mg/kg); RPE, Rosa persica extract
Metallothionein assay
As shown in Fig. 5a, hepatic MT protein content was significantly higher in mice treated with Cd than the control group (P < 0.001). In addition, mice that received both Cd and RPE (50 mg/kg) showed higher MT protein content than those receiving Cd alone (P < 0.01). Despite these changes, no significant change in MT gene expression was observed in the different groups (Fig. 5b).
Effect of RPE on MT protein contents (a) and MT mRNA levels (b) in the hepatic tissue of mice treated with Cd. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ≠≠≠P < 0.001 vs. control group; **P < 0.01 vs. Cd group. MT, metallothionein; Cd, cadmium (equal 3 mg/kg); RPE, Rosa persica extract
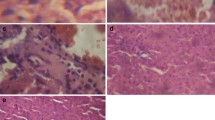
Histopathological changes
As shown in Fig. 6 and Table 1, pathological changes such as hyperplasia of Kupffer cells, necrosis, inflammation, disruption of hepatocytes sinusoidal dilatation, and infiltration of mononuclear cells were observed in hepatic tissue of the Cd group. RPE could prevent some of these changes especially inflammation and necrosis.
Discussion
Antioxidants protect various tissues against the destructive effects of free radicals (Pathak and Thakur 2019). Among these, natural antioxidants are mostly secondary metabolites including flavonoid and phenolic acid compounds, which are formed by plant cells for their sustenance under adverse environments (Ghasemzadeh and Ghasemzadeh 2011).
Our findings showed that the RPE contained high levels of phenolic constituents. In agreement with our findings, Koczka et al. (2018) reported high amounts of polyphenol compounds in some Rosa species. It seems that the high amounts of these compounds may be associated to the different climate conditions because long light exposure and rainfall scarcity in the natural habitat of this plant may be involved in the activation of phenol biosynthesis (Miled et al. 2017). In addition, plant response to abiotic stresses involves the biosynthesis of some metabolites of the phenylpropanoid pathway, and phenolic constituents may be intermediates of this pathway for plant defense (Ksouri et al. 2008; Miled et al. 2017).
In this study, the antioxidant potential of RPE was determined by two different biochemical assays: the total antioxidant capacity and scavenging activity on DPPH radicals. Our findings showed that RPE moderately scavenges DPPH radical as compared to ascorbic acid (vitamin C). However, the RPE exhibited a remarkable total antioxidant capacity, which makes this plant to have more antioxidant activity than other similar plants such as Rosa pimpinellifolia (Mavi et al. 2004). This evidence may be due to the phenolic compounds contained in RPE.
Serum liver enzymes such as aminotransferase are well-known as sensitive biomarkers for early acute hepatic injury (Goorden et al. 2013). In the present study, the administration of Cd exerts liver failure as confirmed by the increase in serum hepatic enzymes. The elevated levels of serum ALT and AST indicate the loss of functional integrity of hepatocellular membrane (Contreras-Zentella and Hernández-Muñoz 2016). Additionally, a raised level of ALP may indicate the biliary dysfunction and/or cholestasis (Bhakuni et al. 2017).
Following administration of RPE, a significant improve was observed in the levels of serum liver enzymes in Cd-exposed animals. It seems the phenolic and flavonoid compounds in RPE could stabilize the hepatocyte membrane and protect these cells against destructive effects of Cd, which may decrease the leakage of the hepatic enzymes into the blood serum. In support of our findings, it has been reported that the phenolic and flavonoid content of some Rosaceae species are able to inhibit hepatotoxicity induced by some chemical agents (Liu et al. 2011; Tao et al. 2016; Zhang et al. 2013).
Oxidative stress and inflammatory reactions play a key role in Cd-induced hepatic damages as described by Amamou et al. (2015) and Liu et al. (2015). In this study, the level of LPO was significantly increased in the Cd group alongside the TNFα changes. The previous studies showed that Cd induced oxidative reactions in different tissues throughout different pathways. For instance, Cd increases the formation of superoxide anion (O2−) and thereby can convert Fe3+ to Fe2+ to create hydroxyl radicals (OH) by the Fenton reaction, which in turn promotes oxidative damage especially lipid peroxidation (Watjen and Beyersmann 2004). In addition, Cd can play its destructive effects through reducing the activity of cellular enzymatic antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (Jihen el et al. 2009; Sanjeev et al. 2019). On the other hand, TNFα, as a pre-inflammatory factor, may be attributed to the down regulation of antioxidative enzymes and subsequently ROS production. This cellular process has been described to be involved in both cell survival and cell death, and the key source of ROS that contributes to TNF-α-induced cell death is the mitochondrion (Kim et al. 2010). Gottlieb et al. reported that TNF-α induces an early decrease in mitochondrial membrane potential (Δψm) and subsequent ROS generation, which promotes a late decrease in Δψm, and that Bcl-XL expression, as a antiapoptotic molecule, prevents the early decrease in Δψm and ROS formation after TNF-α stimulation (Gottlieb et al. 2000). Following administration of RPE, a significant decrease was observed in the TNF-α and LPO levels. It seems that the phenolic compounds of RPE are responsible for the antioxidant properties. It causes chain breaking that can act against free radicals formed during lipid peroxidation which might inhibit continual hydrogen abstraction and consequently preventing chain propagation step. In addition, decreased TNF-α level in treatment groups may be associated with reduces the generation of inflammatory molecules stimulated by Cd. In accordance with our results, Tursun et al. (2016) showed that Rosa rugosa flower extract contains remarkable inhibitory activity on some of the inflammatory cytokines, such as IL-1β and IL-6, are known to play vital roles in the induction and perpetuation of inflammation process in macrophages.
In the current study, depletion of hepatocellular thiol resource and its antioxidant capacity was observed following Cd exposure. These findings are consistent with previous study by Amamou et al. (2015) that GSH level decreased in hepatic tissue exposed to Cd (Amamou et al. 2015). Previously, it has been described that Cd can be bound to cysteine in glutathione and attenuates the cell antioxidant defense (Sandbichler and Höckner 2016). In treatment groups, a remarkable improve was observed in the TTM contents alongside the changes of the TAC in hepatic tissue that may be related to flavonoid contents of RPE. Myhrstad et al. (2002) showed that flavonoids have key role in the regulation of the intracellular GSH levels. This effect may be exerted in part through gamma-glutamylcysteine synthetase gene regulation that could be effective in increase intracellular total thiol, and improve the antioxidant capacity of the hepatic tissue (Myhrstad et al. 2002).
Metallothionein, a low-molecular-weight cysteine-rich protein, is induced by different stressor such as heavy metals (Chen et al. 2014). In this study, no significant changes in MT gene expression were observed in the Cd-treated groups compared to the control group. Therefore, despite the increased concentration of MT protein in the treatment group with the maximum dose of extract, it cannot be said that the RPE has an inducible effect on MT expression. Our findings are in agreement with Vicente-Sanchez et al. (2008), who show that MT gene expression does not necessarily indicate tissue MT protein level. The discrepancy between the findings of the amount of MT protein with its gene expression may be due to the decreased rate of MT protein degradation and/or increased translation efficiency for MT synthesis, which should be considered in future studies.
The increase in MT protein vs. decrease in hepatic TTM reserves is another important finding in this study. As the results show, a small part of the TTM contents is MT protein. On the other hand, Wong and Klaassen (1981) have shown that increased cellular MT levels occur when the levels of cellular antioxidant thiols such as glutathione are significantly reduced. This evidence may explain the discrepancy between the total thiols levels and the MT level following Cd exposure. In addition, the studies have shown independent effects of some of the plant flavonoids on increasing MT protein levels (Vicente-Sanchez et al. 2008; Weng et al. 2011). Based on our phytochemical findings, RPE contains flavonoids, and therefore, it is likely that the increased MT by RPE is due, at least in part, to flavonoids and other bioactive constituents.
Conclusion
The current study demonstrated that RPE is effective herbal medicine in partial protecting against Cd-induced liver damage, as evidenced by improved hepatic tissue oxidative/antioxidant balance. Additionally, elevated MT protein level and reducing hepatic TNF-α may play an important role in improving Cd-induced hepatotoxicity. This therapeutic potential could be related to the flavonoid and phenolic contents of the RPE, and consequently its antioxidant properties.
References
Abdeen A, Abou-Zaid OA, Abdel-Maksoud HA, Aboubakr M, Abdelkader A, Abdelnaby A, Abo-Ahmed AI, El-Mleeh A, Mostafa O, Abdel-Daim M, Aleya L (2019a) Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ Sci Pollut Res Int 26(24):25167–25177
Abdeen A, Abdelkader A, Abdo M, Wareth G, Aboubakr M, Aleya L, Abdel-Daim M (2019b) Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ Sci Pollut Res Int 26(1):240–249
Amamou F, Nemmiche S, Kaouthar Meziane R, Didi A, Yazit SM, Chabane-Sari D (2015) Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem Toxicol 78:177–184
Arroyo V, Flores K, Ortiz L, Gómez-Quiroz L, Gutiérrez-Ruiz M (2012) Liver and cadmium toxicity. J Drug Metab Toxicol S5:1–7
Begic A, Djuric A, Ninkovic M, Stevanovic I, Djurdjevic D, Pavlovic M, Jelic K, Pantelic A, Zebic G, Dejanovic B (2017) Disulfiram moderately restores impaired hepatic redox status of rats subchronically exposed to cadmium. J Enzyme Inhib Med Chem 32(1):478–489
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Bhakuni GS, Bedi O, Bariwal J, Kumar P (2017) Hepatoprotective activity of morin and its semi-synthetic derivatives against alcohol induced hepatotoxicity in rats. Indian J Physiol Pharmacol 61(2):175–190
Bhosale VV, Banerjee D (2020) Scientific validation of herbal medicine. In: Sen S, Chakraborty R (eds) Herbal medicine in India. Springer, Singapore
Boskabady MH, Shafei MN, Saberi Z, Amini S (2011) Pharmacological effects of rosa damascena. Iran J Basic Med Sci 14(4):295–307
Cardiff RD, Miller CH, Munn RJ (2014, 2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc (6):pdb-rot073411
Chen L, Ma L, Bai Q, Zhu X, Zhang J, Wei Q, Li D, Gao C, Li J, Zhang Z, Liu C, He Z, Zeng X, Zhang A, Qu W, Zhuang Z, Chen W, Xiao Y (2014) Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J Biol Chem 289(32):22413–22426
Chen X, Ren S, Zhu G, Wang Z, Wen X (2017) Emodin suppresses cadmium-induced osteoporosis by inhibiting osteoclast formation. Environ Toxicol Pharmacol 54:162–168
Contreras-Zentella ML, Hernández-Muñoz R (2016) Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxidative Med Cell Longev 2016:1–12
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T (2010) Cadmium stress: an oxidative challenge. Biometals 23(5):927–940
Dastan D, Karimi S, Larki-Harchegani A, Nili-Ahmadabadi A (2019) Protective effects of Allium hirtifolium Boiss extract on cadmium-induced renal failure in rats. Environ Sci Pollut Res Int 26:18886–18892
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29:104–110
Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: role and biochemical activity in plants and human. J Med Plant Res 5(31):6697–6703
Goorden SM, Buffart TE, Bakker A, Buijs MM (2013) Liver disorders in adults: ALT and AST. Ned Tijdschr Geneeskd 157(43):A6443
Gottlieb E, Vander Heiden MG, Thompson CB (2000) Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 20(15):5680–5689
Gu X, Manautou JE (2012) Molecular mechanisms underlying chemical liver injury. Expert Rev Mol Med 14:e4–e4
Harchegani AL, Hemmati AA, Nili-Ahmadabadi A, Darabi B, Shabib S (2017) Cromolyn sodium attenuates paraquat-induced lung injury by modulation of proinflammatory cytokines. Drug Res 67(05):283–288
Hartwig A (2013) Cadmium and cancer, cadmium: from toxicity to essentiality. Springer, pp 491-507
Heshmati A, Karami-Momtaz J, Nili-Ahmadabadi A, Ghadimi S (2017) Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere 173:207–215
Hu ML (1994) Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 233:380–385
Ige SF, Olaleye SB, Akhigbe RE, Akanbi TA, Oyekunle OA, Udoh UA (2012) Testicular toxicity and sperm quality following cadmium exposure in rats: ameliorative potentials of Allium cepa. J Hum Reprod Sci 5(1):37–42
Jassbi AR, Zamanizadejarib S, Tahara S (2003) Polyphenolic antioxidant constituents of Rosa persica. J Chem Soc Pak 4(25):323–327
Jihen el H, Imed M, Fatima H, Abdelhamid K (2009) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress. Ecotoxicol Environ Saf 72(5):1559–1564
Kim J, Lee S, Park J, Yoo Y (2010) TNF-α-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-XL. Cell Death Differ 17(9):1420–1434
Koczka N, Stefanovits-Bányai É, Ombódi A (2018) Total polyphenol content and antioxidant capacity of rosehips of some rosa species. Medicines 5(3):1–10
Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C (2008) Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol 331(11):865–873
Li Y, Yang H, Liu N, Luo J, Wang Q, Wang L (2015) Cadmium accumulation and metallothionein biosynthesis in cadmium-treated freshwater mussel Anodonta woodiana. PLoS One 10(2):e0117037
Li H, Fagerberg B, Sallsten G, Borné Y, Hedblad B, Engström G, Barregard L, Andersson EM (2019) Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Cohort Study. Environ Health 18(1):56
Liu Y-T, Lu B-N, Peng J-Y (2011) Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem 125(2):719–725
Liu L, Tao R, Huang J, He X, Qu L, Jin Y, Zhang S, Fu Z (2015) Hepatic oxidative stress and inflammatory responses with cadmium exposure in male mice. Environ Toxicol Pharmacol 39(1):229–236
Mavi A, Terzi Z, Ozgen U, Yildirim A, Coskun M (2004) Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), Malva neglecta (Malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), Urtica dioica (Urticaceae). Biol Pharm Bull 27(5):702–705
Miled HB, Barka ZB, Hallegue D, Lahbib K, Ladjimi M, Tlili M, Sakly M, Rhouma KB, Ksouri R, Tebourbi O (2017) Hepatoprotective activity of Rhus oxyacantha root cortex extract against DDT-induced liver injury in rats. Biomed Pharmacother 90:203–215
Mohebitabar S, Shirazi M, Bioos S, Rahimi R, Malekshahi F, Nejatbakhsh F (2017) Therapeutic efficacy of rose oil: a comprehensive review of clinical evidence. Avicenna J Phytomed 7(3):206–213
Moradkhani S, Kobarfard F, Ayatollahi SA (2014) Phytochemical investigations on chemical constituents of Achillea tenuifolia Lam. Iran J Pharm Res 13(3):1049–1054
Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO (2002) Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med 32(5):386–393
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Okoye CN, MacDonald-Jay N, Kamunde C (2019) Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquat Toxicol 214:105264
Ordonez AAL, Gomez JD, Vattuone MA, lsla MI (2006): Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 97(3), 452-458
Pathak S, Thakur M (2019): Plants as natural antioxidants-a review. International Journal of Management, Law & Science Studies 3(7)
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24(4):378–399
Rezvani-Kamran A, Salehi I, Shahidi S, Zarei M, Moradkhani S, Komaki A (2017) Effects of the hydroalcoholic extract of Rosa damascena on learning and memory in male rats consuming a high-fat diet. Pharm Biol 55(1):2065–2073
Sadraei H, Asghari G, Jalali F (2016) Assessment of hydroalcoholic and hexane extracts of Rosa persica Mich. flower on rat ileum spasm. Res Pharm Sci 11(2):160–167
Sandbichler A, Höckner M (2016) Cadmium protection strategies—a hidden trade-off? Int J Mol Sci 17(1):1–22
Sanjeev S, Bidanchi RM, Murthy MK, Gurusubramanian G, Roy VK (2019) Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res Int 26(20):20631–20653
Tao X, Sun X, Xu L, Yin L, Han X, Qi Y, Xu Y, Zhao Y, Wang C, Peng J (2016) Total flavonoids from Rosa laevigata Michx fruit ameliorates hepatic ischemia/reperfusion injury through inhibition of oxidative stress and inflammation in rats. Nutrients 8(7):418
Tursun X, Zhao Y, Alat Z, Xin X, Tursun A, Abdulla R, AkberAisa H (2016) Anti-inflammatory effect of Rosa rugosa flower extract in lipopolysaccharide-stimulated RAW264.7 macrophages. Biomol Ther 24(2):184–190
Vicente-Sanchez C, Egido J, Sanchez-Gonzalez PD, Perez-Barriocanal F, Lopez-Novoa JM, Morales AI (2008) Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol 46(6):2279–2287
Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36(11):1434–1443
Watjen W, Beyersmann D (2004) Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17(1):65–78
Weng CJ, Chen MJ, Yeh CT, Yen GC (2011) Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. New Biotechnol 28(6):767–777
Wong KL, Klaassen CD (1981) Relationship between liver and kidney levels of glutathione and metallothionein in rats. Toxicology 19(1):39–47
Yang S-H, Yu L-H, Li L, Guo Y, Zhang Y, Long M, Li P, He J-B (2018) Protective mechanism of sulforaphane on cadmium-induced sertoli cell injury in mice testis via nrf2/are signaling pathway. Molecules 23(7):1774
Zhang S, Lu B, Han X, Xu L, Qi Y, Yin L, Xu Y, Zhao Y, Liu K, Peng J (2013) Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol 55:60–69
Zou H, Wang T, Yuan J, Sun J, Yuan Y, Gu J, Liu X, Bian J, Liu Z (2019) Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes. Toxicol Lett 321:32–43
Funding
The financial support for conducting the present study was provided by the Vice-chancellor of Research and Technology, Hamadan University of Medical Sciences, Hamadan, I.R. Iran (Grant No. 9412187265).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by HUMS Ethics Committee with the ethical number, IR.UMSHA.REC.1394.510, in accordance with the guideline of the Research Ethics Committee of the Ministry of Health and Medical Education, Iran (adopted on April 17, 2006), based on the Helsinki Protocol.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradkhani, S., Rezaei-Dehghanzadeh, T. & Nili-Ahmadabadi, A. Rosa persica hydroalcoholic extract improves cadmium-hepatotoxicity by modulating oxidative damage and tumor necrosis factor-alpha status. Environ Sci Pollut Res 27, 31259–31268 (2020). https://doi.org/10.1007/s11356-020-09450-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09450-4