Abstract
Increased levels of environmental pollutants are linked to almost all human disorders; the efficient method to manage the human health is through naturally available dietary molecule. Solanum torvum (ST) Swartz (Solanaceae) commonly called Turkey Berry is found in Africa, Asia, and South America. Its fruit, part of traditional Indian cuisine, is a widely consumed nutritious herb, acclaimed for its medicinal value. ST aqueous extract (STAe) (250, 500, and 1000 mg/kg b.w., 6 days; oral) against acute Cadmium (Cd) (6.3 mg/kg b.w., single dose; oral) toxicity was evaluated in rats. Protective effect was assessed using serum markers, tissue antioxidants, oxidant derivatives, glycoprotein, and histopathological studies. The activities of serum marker enzymes were increased (40–60 %); antioxidant enzymes such as SOD and CAT, GSH, and its metabolic enzyme activities were decreased (50–80 %) in the liver and kidney upon Cd intoxication. During STAe pre-treatment, at doses of 250 and 500 mg/kg b.w., the above changes were brought to near normal (25–63 %). Tissue 4-hydroxynonenal, 3-nitrotyrosine, and protein carbonyls were increased (8–15 fold) in Cd-alone-treated rats, whereas pre-supplementation of STAe significantly decreased their levels and inhibited the protein glycosylation effectively. The pharmacological effect of STAe was confirmed by histopathological observations. Based on previous literature and present investigation, we conclude that ST may serve as a potential functional food against environmental contaminant such as heavy metal-induced oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide experimental reports and scientific innovations reveal that several plant extracts and their phytoconstituents have been shown to retard or reduce oxidative stress. A major obstacle in the exploration of natural antioxidants is that they differ in their mode of action in vitro and in vivo (Singh and Sharma 2009). Studies on numerous plant products/extracts have shown that their radical scavenging or quenching efficacy was high in vitro (Capasso 2013) while the contrary has been observed in vivo studies. However, fewer have compiled antioxidant properties of plant extracts both in vitro and in vivo (Celep et al. 2013; Zhou et al. 2013; Rui et al. 2016).
One of the major oxidative stress inducers for the biological systems is environmental pollutants. Among the environmental pollutants, the contribution of heavy metals is significantly high. Heavy metals induce detrimental effects, which ultimately leads to irreparable cellular damage at a molecular level (Karak et al. 2015). Cadmium (Cd) is one such metal which induces cellular damage through the process of oxidative stress by chelating thiol proteins (Cuypers et al. 2010); it results in the interruption of cellular redox homeostasis leading to the liberation of nascent radicals, which in turn would damage biomolecules such as DNA, lipids, and proteins (Mortensen et al. 2011; Karak et al. 2015). Further, its low excretion rate and longer half-life result in its accumulation in various organs (Cuypers et al. 2010; Zhang et al. 2015). Prolonged systemic exposure to Cd mainly affects the liver and kidney, the central metabolic and excretory organs (Karak et al. 2015). The evaluation of protective status in these two vital organs against Cd-induced toxicity would directly reflect the therapeutic efficacy of compounds or plant extracts under investigation.
Though, Solanum torvum (ST) Swartz (Solanaceae) fruit has a long history of its use as a culinary agent and as an ingredient in folk and herbal medications in various cultures, there are only ∼50 scientific reports on its pharmacological and phytochemical research aspects. The earlier reported pharmacological studies include antimicrobial, antiulcerogenic, antiplatelet, antioxidant, antidiabetic, analgesic, anti-inflammatory, and cytoprotective effects (Mohan et al. 2010; Takahashi et al. 2010; Gandhi et al. 2011; Balachandran et al. 2012; 2015; Ramamurthy et al. 2012; Sivapriya et al. 2015). Our previous study confirms that the aqueous extract of ST fruit (ST aqueous extract (STAe)) has potent in vitro antioxidant property when compared to its methanol and ethanol extracts (Ramamurthy et al. 2012). Even though ST has a diverse range of phytochemicals, the lack of experimental evidence of its role at the molecular level has cloaked its therapeutic importance against metal toxicity.
Materials and methods
Fruit material collection and preparation
S. torvum fruits were collected from reputable farms, Panruti, Tamil Nadu, India, postal code 607805, during November and December 2010 and authenticated by Prof. P. Jayaraman, Director, Plant Anatomy Research Centre, National Institute of Herbal Science, Chennai, India (PARC/2009/993). The dried fruits of ST were made into fine powder. The extraction process was carried out using Soxhlet apparatus in solvents such as ethanol (10 % w/v), methanol, and water according to their ascending order of polarity. ST fruits aqueous extract was concentrated by lyophilization process and stored at 4 °C in a refrigerator and used for further studies.
In vitro antioxidant properties
Procedure mentioned in Ghosh et al. (2010) was adopted to evaluate the superoxide radical and nitric oxide radical scavenging and iron chelation properties of STAe. DNA nicking experiment was carried out using M13mp 18 plasmid DNA (Lee et al. 2002).
Hepato- and nephroprotective effect of STAe (in vivo antioxidant property)
Animals
The Wistar strain male albino rats were purchased from an authorized vendor. Animals were maintained and the animal experiments were conceded according to the guidelines of the Institutional Animal Ethical Committee, Pondicherry University, and Pondicherry, India (PU/AEC/10/28).
Experimental design
The animals were divided into seven groups of six animals. Food and water were provided ad libitum.
-
Group 1:
Control animals
-
Group 2:
Animals treated with cadmium (Cd) alone [(6.3 mg/kg b.w., single dose on the sixth day; after the initiation of experiment, orally) (Cd in the form of Cd nitrate)]
-
Group 3:
Animals supplemented with STAe [(250 mg/kg b.w. orally; pre-treated—5 days + 1 day) + Cd (as per group 2)]
-
Group 4:
Animals supplemented with STAe [(500 mg/kg b.w. orally; pre-treated—5 days + 1 day) + Cd (as per group 2)]
-
Group 5:
Animals supplemented with STAe [(1000 mg/kg b.w. orally; pre-treated—5 days + 1 day) + Cd (as per group 2)]
-
Group 6:
Animals supplemented with vitamin E [(250 mg/kg b.w. pre-treated—5 days + 1 day; orally) + Cd (as per group 2)]
-
Group 7:
Animals supplemented with vitamin E alone (250 mg/kg b.w. orally)
Sample preparation (serum and tissues)
After the experimental period, animals were sacrificed (seventh day; after the initiation of experiment) by cervical decapitation. Blood was collected in heparinized tubes, and the serum obtained was used for various biochemical assays. The liver and kidney tissues were excised from the sacrificed animals and washed in phosphate-buffered saline. The portions of tissues were fixed in 10 % formalin for histopathological studies, while the portions of tissue were frozen (for western blot analysis) and fresh tissue samples were used for various biochemical estimations.
Biochemical assays
Lactate dehydrogenase (LDH), alanine transaminase (SGPT), aspartate transaminase (SGOT), alkaline phosphatase (ALP), and γ-glutamyltransferase (GGT) were estimated by the commercially available kits from Agappe Diagnostics Ltd., Agappe Hills, Ernakulam district, Kerala, India 683562. Uric acid and creatinine were estimated by the commercially available kits from Beacon Diagnostics Ltd., Kabilpore, Navsari, India 396 424. The Lowry et al. (1951) method was adopted to estimate the total protein in different experimental conditions.
Lipid peroxidation and antioxidant analysis
The level of lipid peroxidation (LPO) in terms of thiobarbituric acid reactive substances (TBARS) was determined as described by Ohkawa et al. (1979). Glutathione (GSH) level was assayed by the method of Ellman (1959). SOD, CAT, GPx, and GST activities were measured as mentioned in Ramesh et al. (2012). Protein carbonyl level was estimated by dinitrophenylhydrazine (DNPH) method (Oliver et al. 1987).
Western blot
Oxidative modification of proteins was detected by protein immunoblot using anti-dinitrophenylhydrazine, anti 3-nitro tyrosine and anti 4-hydroxynonenal (HNE) antibodies. Protein (25 μg) was loaded in 12 % SDS-PAGE. The electrophoresis was carried out at 100 V for 90 min. For the analysis of protein carbonyls, 25 μg protein was incubated with 10 μl of 10 mM DNPH for 10 min at room temperature. Subsequently, 5 μL of neutralization solution was added to protein sample and vortexed. Protein derivative samples were run on 12 % SDS-PAGE. Electroblotting was carried out with 0.2-μm PVDF membrane, at 55 V for 50 min, and the membrane was blocked in 1 % BSA in TBST buffer. After blocking, the membrane was washed (four times for 15 min) and incubated with rabbit anti-DNPH antibody (Invitrogen Molecular probes, USA) at 4 °C for overnight. The excess and unbound antibody was washed (four times for 15 min) and then, the membrane was incubated with goat anti-rabbit IgG horseradish peroxidase conjugate (Calbiochem) for 2 h at room temperature. After removing the excess unbound antibody by washing (four times for 15 min), the bands in the membrane were visualized by enhanced chemiluminescence method. The protein nitration, [3-nitro tyrosine monoclonal antibody (Cayman Chemical Company, USA)] and anti-HNE (Alpha Diagnostics Intl.) immunoblotting experiments were carried out with the same experimental conditions except the derivatization step for protein carbonyls.
Glycoprotein staining
Glycoprotein staining was performed using periodic acid-Schiff’s reagent method. Protein sample (25 μg) was run on 12 % reduced SDS-PAGE. After the run, the gel was fixed in 40 % methanol and then washed with 3 % acetic acid (three times for 10 min). The gel was incubated with 1 % periodic acid for 15 min in dark at 4 °C and then washed with 3 % acetic acid (three times for 10 min). After the completion of washing, the gel was incubated in Schiff’s reagent at 4 °C in the dark for 15 min and then washed in 0.5 % sodium bicarbonate and the gel was viewed under Syngene gel document system and photographed.
HPTLC fingerprint analysis
HPTLC analysis was performed as explained in Ramamurthy et al. (2012).
Histopathological studies
Ten percent formalin-fixed tissues were dehydrated and cleared in alcohol and xylene and embedded in paraffin. Sections of 5-μm thickness were stained with hematoxylin and eosin (H and E), and histopathological alterations were captured (under ×10) with Olympus CX41 microscope (Olympus, USA).
Statistical analysis
Statistical Package for Social Sciences (SPSS, version 16) was utilized to analyze the data obtained in different experimental conditions. One-way ANOVA was performed followed by Tukey’s comparison test for comparison of results between the control and test groups. Differences were considered significant at P ≤ 0.05.
Results and discussion
In vitro radical quenching property
Oxyradicals are highly unstable and abundantly generated in stress conditions. Hence, the superoxide and nitric oxide quenching abilities of STAe were assessed at different concentrations (10, 50, 100, 250, and 500 μg/mL). The percent of superoxide and nitric oxide radical inhibition ranged from 15 to 87 %; however, superoxide radical was readily inhibited (∼87 %). STAe was found to be more efficient than well-known antioxidant, catechin, at 500-μg/mL concentration. The EC50 of superoxide and nitric oxide radicals were 86.5 and 200 μg/mL, respectively. The commendable inhibitory effect on oxyradicals may be due to the synergistic action of phytoconstituents present in STAe (Gandhi et al. 2011; Ramamurthy et al. 2012). The nitric oxide radical inhibition increased with increasing concentrations of the extract (15–78 %) with a correlation coefficient value (r 2) of 0.9 (Table 1). Bivalent transition metal ions catalyze the radical reactions, which in turn enhances the oxidative processes. Hence, metal ion chelation may be the indirect way of antioxidant mechanism. The inhibition of ferrozine-Fe2+ complex in the presence of STAe directly correlated with Fe2+ chelation. The absorbance of ferrozine-Fe2+ complex decreased linearly with increasing concentrations of STAe. The metal ion chelation efficacy of STAe ranges from 22 to 74 % Na2EDTA equivalents with EC50 value 60 μg (Table 1). The inhibitory concentration of plant extract showed the same efficacy of standard such as EDTA, EC50 (58 μg).
The oxyradical-mediated DNA strand breaks, and the assessment of radical quenching ability of antioxidants on agarose gel is considered as an efficient and reliable in vitro assay. Single-strand M13 DNA is in circular form, induction of single or multiple nicks by oxyradicals in the phosphodiester backbone of DNA will create linear form. Conversion of DNA from circular to linear form directly correlates with the radical concentration and frequency of radical hits to circular DNA. Hence, radical-mediated conversion of DNA from circular to linear form and its protection of DNA integrity in the presence of antioxidant are a reliable indicator for antioxidant potential. Present study well documented that DNA shearing was found in Fenton’s reaction mixture-treated DNA sample (Fig. 1). Lanes 3, 4, and 5 represent the DNA samples treated with Fenton’s reaction mixture, with increasing concentration of plant extract. The inhibition of single-strand breaks was increased in STAe-treated samples as evident from increased circular DNA integrity on agarose gel (lanes 3, 4, and 5). Further DNA nicking assay result is positively correlated with free radical quenching assay.
Our previous study, evaluated the free radical quenching nature of the different extracts of the ST fruit (Ramamurthy et al. 2012). Further, we found that the aqueous extract showed potential radical quenching effect when compared with methanol and ethanol extracts. In addition, the present in vitro assay showed the oxyradical quenching activity and metal chelation effect of STAe. We further extended our study to in vivo experiment to support the in vitro results of STAe. The in vivo antioxidant studies are accurate and reliable. However, it also depends on many factors such as bioavailability and dosage (Niki 2010).
We evaluated the protective effect of STAe against Cd-induced acute toxicity in Wistar male albino rat liver and kidney. To determine the toxic effect of STAe, three different doses such as low, medium, and high (250, 500, and 1000 mg/kg b.w.) were selected. The toxic effect of STAe was studied by oral supplementation for 20 days. The non-toxic nature of STAe was concluded based on hematological, biochemical, and histopathological analyses (data not shown).
The protective effect of STAe on hydroxyl radicals (Fenton’s reaction) induced DNA strand breaks (nicks). Lane 1 100-bp ladder; Lane 1—M13 DNA; Lane 2 M13 DNA + Fenton’s reaction mixture (Ferric chloride + H2O2 + ascorbic acid); Lane 3 100 μg STAe + DNA + Fenton’s reaction mixture; Lane 4 250 μg STAe + DNA + Fenton’s reaction mixture; Lane 5 500 μg STAe + DNA + Fenton’s reaction mixture; Lane 6 vitamin C (10 μg/mL) + DNA + Fenton’s reaction mixture; Lane 7 catechin (10 μg/mL) + DNA + Fenton’s reaction mixture. The M13 (ss) DNA (50 ng) was incubated with Fenton’s reagent for 15 min at room temperature, along with STAe (various concentrations), catechin, and vitamin C (we have used very low concentration of standard). Agarose gel electrophoresis was performed at 50 V for 30 min and bands were illuminated in syngene gel documentation system
In vivo antioxidant property
The hepatic and renal functional markers were analyzed in the serum of control and test animals (Table 2). The serum biomarkers such as ALP, ALT, LDH, AST, and GGT activities were significantly increased (P ≤ 0.001) in Cd-administered rats (group 2) when compared with control groups (group 1). Pre-supplementation of STAe at 250 and 500 mg/kg b.w. decreased (P ≤ 0.001) the above enzyme activities when compared to group 2 rats. The renal functional metabolites such as creatinine and urea levels were decreased significantly in Cd-administered animals (group 2), when compared to group 1 rats. Whereas, pre-supplementation (250 and 500 mg/kg b.w.) of STAe showed normal level as similar to group 1 animals. The protective effect of STAe in Cd-induced injury might be due to the presence of various antioxidant compounds present in the extract (Ramamurthy et al. 2012; Gandhi et al. 2011; Yadav and Khandelwal 2009). The phytoconstituents may act synergistically or antagonistically, based on the dose, duration, bioavailability, and interactions among them. In the present study, we observed an interesting result; at low and medium doses (250 and 500 mg/kg b.w.), supplementation protects the Cd-induced organ injury. However, pre-supplementation of STAe at 1000 mg/kg b.w. did not show any positive effect, at the same time it did not aggravate the Cd-induced organ injury. This finding infers that STAe is non-toxic at high concentration even in the presence of toxicant. Similarly, the protective effect of STAe is comparable with vitamin E effect. Vitamin E alone did not show any changes in marker enzymes and histopathological observations (data not shown) when compared to group 1 (control animal).
The TBARS level is believed to be the underlying marker for oxidative stress-induced injury. The TBARS in Cd-intoxicated rat liver and kidney were increased fourfolds (P ≤ 0.001) when compared with normal animals (group 1). STAe pre-supplementation inhibited membrane perturbations (Dewanjee et al. 2013) and brought down to 1.5-fold (P ≤ 0.001), which was similar to the standard antioxidant (vitamin E) used in the current investigation (Table 3).
The quantitative measurement of TBARS was also done by determining 4-HNE using anti-4-HNE antibodies through western blot analysis (Fig. 2a, b). The intensified bands were observed in (relatively high when compared to control animals) Cd-administered group of the liver and kidney (230 and 303 %, respectively). Pre-supplementation of STAe showed weak signals with low-intensity (112 and 102 %, respectively) bands similar to control group. The results clearly indicated abundant HNE adducts formations in group 2, whereas STAe pre-supplementation (250 and 500 mg/kg b.w.) inhibited HNE adducts through the prevention of Cd-induced radical generation.
a. Experimental design. b. Western blot analysis for 4-hydroxynonenal (4-HNE) adducts in liver and kidney of control and experimental animals. Figure 2a Liver homogenate. Figure 2b Kidney homogenate. Group 1 control, Group 2 Cd-alone-treated animals (6.3 mg/kg b.w), Group 3 STAe 250 mg/kg b.w. + Cd treatment (as in group 2 animals), Group 4 STAe 500 mg/kg b.w. + Cd treatment (as in group 2 animals), Group 5 STAe 1000 mg/kg b.w. + Cd treatment (as in group 2 animals), Group 6 vitamin E (250 mg/kg b.w.) + Cd treatment (as in group 2 animals). Bottom of the figure shows the densitometry values (fold change)
GSH and its metabolism were first-line defense mechanism against Cd-induced oxidative stress (Nair et al. 2013). GSH concentration was found to be lowered (P ≤ 0.001) in group 2 when compared to normal animals (group 1). GPx and GST activities were diminished at P ≤ 0.001 level in liver and kidney of Cd-administered animals (group 2) when compared to control animals (group 1). Pre-treatment with STAe nearly normalized the GSH level and activities of GPx and GST (groups 3 and 4). Upon increasing the dose of STAe (1000 mg/kg b.w.), no changes were observed in antioxidant effect (group 5 vs group 2). The decreased GSH level might be due to an increase in GGT activity found in serum (Table 3). Other possible mechanisms might be the increase of TBARS due to the decrease in the availability of NADPH, a co-factor for GSSG to GSH conversion, thus, ultimately leading to the disturbance of glutathione metabolism (Cuypers et al. 2010; Spiazzi et al. 2013).
Cd has strong affinity towards thiol (−SH) functional groups; hence, it forms thermodynamically stable Se-SH-Cd complex, which leads to a decrease in GPx activity ((Cuypers et al. 2010); Nazimabashir et al. 2015). However, the radical quenching and metal ion chelation efficacy of STAe protect glutathione and its metabolic enzymes from Cd-induced oxidative stress. Similar reports were found in different plant extracts against Cd-induced oxidative stress (Yadav and Khandelwal 2009; Wang et al. 2012).
Tissue antioxidant enzyme activities (SOD and CAT) were significantly (P ≤ 0.01) decreased in the liver and kidney of Cd-administered animals (group 2) when compared to normal animals (group1). The effect of STAe on the activities of CAT and SOD in liver and kidney were increased (Table 3) (at a dose of 250 and 500 mg/kg b.w.) when compared to Cd-administered animals (group 2). However, supplementation of STAe at 1000 mg/kg b.w. has not shown any positive effect on CAT and SOD activities against Cd-induced oxidative stress.
Oxidative modifications of proteins
The chemical nature of ROS/RNS is highly unstable and reactive. Hence, it is very difficult to directly measure the radicals in vivo; we evaluated the oxidative damage by analyzing the oxidized products of protein, lipids, and DNA which can be considered as better alternative biomarkers (Dalle-Donne et al. 2003). The oxidative modifications of proteins were assessed in the form of protein carbonyl-DNPH adduct by spectroscopic method in control and experimental groups (Dkhar and Sharma 2010) (Table 3). The protein carbonyl level increased 20 and eightfold (P ≤ 0.001) in Cd-administered rats when compared to control group in the liver and kidney, respectively. STAe pre-administration reduced carbonyl level to twofold (P ≤ 0.01) when compared to group 2 rats.
The oxidative derivatives of proteins are multiple products, which could not be measured by a single assay. However, the abundant ones are carbonyls and 3-nitrotyrosine (Souza et al. 2008). The protein carbonyls were analyzed by using western blot analysis employing anti-DNP antibody in liver and kidney samples of different experimental groups. The band intensity in western blot directly reflects protein carbonyl formations in normal (group 1) and experimental animals. The very high level of carbonyls with dark bands was observed in Cd-administered animals (Fig. 3a, b), whereas weak signals with reduced band intensity were observed in pre-supplementation of STAe at doses of 250 and 500 mg/kg b.w.; it is similar to vitamin E supplementation. Interestingly, 3-nitro tyrosine also showed similar results in control and Cd-intoxicated groups (Fig. 3c, d). The western blot analysis for carbonyls and nitration were well correlated and sustained with other results of Cd-induced stress. Again, a higher dose (used in the present study) did not show any positive results. The plant extract and its metabolite-mediated reduction of protein nitration have been observed earlier (Bixby et al. 2005).
Western blot analysis for oxidative modifications of proteins (carbonylation (a, b) and nitration (c, d) against Cd-induced oxidative stress and reducing effect of STAe in different experimental groups. Groups were treated as mentioned in materials and methods, experimental design as mentioned in Figure 2a. a, b Protein carbonyl formation in the liver and kidney. The carbonyls content was determined using anti-DNP antibody. The western blot analysis was performed as mentioned in material methods. c, d Protein nitration formation in the liver and kidney. The nitration of proteins was determined using anti-3-nitro tyrosine antibody. The western blot analysis was performed as mentioned in materials and methods. Bottom of the figure shows the densitometry values (fold change). (Note: We observed streak pattern of protein nitration and carbonylation in all the samples, which is common in free radical research; (Subbaiah et al. 2015; Valdez et al. 2000; Velsor et al. 2003))
Glycoproteins
The glycoprotein content and its degree of glycosylation have become prognostic biomarkers for many diseases like cancer, diabetes, and neurological disorders (Dalziel et al. 2014). The oxidative modifications of total glycoproteins could not be assessed by western blot analysis; hence, we performed reducing SDS-PAGE followed by periodic acid-Schiff staining for the determination of glycoprotein profile. Cd administration induced the hyper-glycosylation resulting high glycoprotein in group 2 animals (Fig. 4a, b), which might be due to free radical-induced aberrations in the glycosylation process. The degree of protein glycosylation was aberrantly increased in Cd-treated groups with red high intensified bands (as seen highly intensified bands in group 2 animals—Fig. 4a, b), but pale red bands were seen in STAe-supplemented groups. Band intensity is based on polysaccharide composition in proteins (Marklova and Albahri 2007). The decreased glycoprotein in STAe-treated animals might be due to the inhibition of glycosylation by STAe. Further, liver protein profile shows high degree of glycosylated protein than proteins in the kidney. Exposure to Cd showed that radical generation is high in the liver than in the kidney (Cuypers et al. 2010). An interesting observation in the present study is that STAe-treated groups showed light intensity bands; in addition, few bands disappeared, which infers that STAe acts as a glycosylation inhibitor.
Glycosylated protein in Cd-induced oxidative stress and pre-supplementation of STAe in control and experimental groups. a Liver homogenate. b Kidney homogenate: M marker. Groups were treated as mentioned in “Materials and methods” and “Experimental design” as mentioned in Figure 2a. Proteins were separated out in 12 % SDS-PAGE and staining was performed using PAS Schiff’s reagent, as explained in “Material and methods”. The significant changes in glycosylation is marked (arrow) in the figure
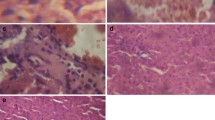
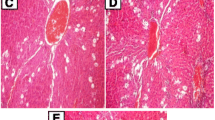
Histopathology
Control group showed the normal architecture of the liver and kidney, whereas the Cd-administered liver showed more pathological changes like the degeneration of hepatocytes and irregularity in cell size and shape; the condensed chromatin in many hepatocytes, micro-necrosis, and pyknotic nuclei with degenerated chromatin was also observed. Portal inflammation and cytoplasmic vacuolization were observed in Cd-administered groups (Fig. 5). Upon pre-supplementation of STAe, the above changes were decreased in groups 3 and 4, whereas there is no difference in group 5 animals. In control animals, the cortex and medulla of the kidneys showed a normal pattern (Fig. 6). Tubular necrosis and glomerular widening were observed in the kidneys of rats treated with Cd (Fig. 6). The presence of fatty vacuole, lymphocyte aggregation in glomeruli, and cloudy swelling confirmed Cd toxicity (Lee et al. 2015). When supplemented with STAe (at 250 and 500 mg/kg. b.w.), all degradative changes of Cd toxicity were reverted back to normal, as evident from the hepatic and renal histology. As observed in other biochemical assays, higher dose (1000 mg/kg b.w.) showed similar architecture of Cd alone-treated rats. STAe has diversified phytochemicals, which may have multiple mechanisms against oxidative stress. In present investigations, we found a different mode of actions of STAe for the inhibition of Cd-induced free radical-mediated tissue injury. Similarly, different constituents of the plant extract have varying degrees of activities, such as radical quenching ability, (Yadav and Khandelwal 2009; Wang et al. 2012) prevention of HNE adducts formation, protein carbonyls (Dkhar and Sharma 2010) and 3-nitrotyrosine (Bixby et al. 2005), and inhibition of glycosylation (Marklova and Albahri 2007) to elicit their pharmacological property. Hence, the diverse effect of STAe might be due to the synergistic effect of phytochemicals present in the extract.
Histological studies of the liver stained with H and E in control and experimental animals. Groups were treated as mentioned in “Materials and methods” and “Experimental design” as mentioned in Figure 2a. The various changes were observed in control and different experimental groups under ×10 magnifications (marked in figure). The pathological abnormalities of Cd were effectively reduced with STAe at 250 and 500 mg/kg b.w. At higher concentration of STAe (group 5), we did not observer any difference when compared to group 2 animals. However, STAe alone did not show any difference when compared to control rats (data not shown). Asterisk indicates cell death; small arrow indicates cytoplasmic vacuoles; long arrow indicates sinusoids, CV central vein (magnification ×10 and scale bar also shown)
Histological section of the kidney in control and experimental animals. Groups were treated as mentioned in “Materials and methods” and “Experimental design” as mentioned in Figure 2a. The pathological abnormalities were marked in Cd- and STAe-supplemented groups. Swelling with thickened blood vessel, fatty vacuole, fatty infiltrate, and lymphocyte aggregate and lymphocyte infiltration were seen in the kidney of Cd-treated rats. The pathological abnormalities of Cd were effectively reduced with STAe at 250 and 500 mg/kg b.w. At a concentration of 1000 mg/kg b.w. STAe (group 5), we did observe any difference when compared to Cd-treated animals. STAe alone did not show any difference when compared to control rats (data not shown). Long arrow indicates lymphocyte aggregation/infiltration and GL-glomeruli (magnification ×10)
HPTLC analysis
Finally, we evaluated the active principles responsible for pharmacological activity. In general, fruits are rich source for bioactive principles that have various therapeutic applications (Arthan et al. 2002). The STAe active principles were analyzed by high-performance thin layer chromatography (HPTLC). STAe contains the high quantity of gallic acid (1896 ± 179 μg/g of extract), moderate level of rutin (367 ± 38.4 μg/g of extract) and trace amount of ferulic acid (∼85 μg/g) where as quercetin, caffeic acid and catechin were undetectable. Thus, the antioxidant and hepato- and nephroprotective effect of STAe were mainly due to the gallic acid and trace extent, the different quantities of other flavanoids present.
Conclusion
The in vitro studies confirm STAe free-radical quenching property. To further validate ST, in vivo experiments against Cd-induced liver and kidney injuries were conducted, which proved its efficacy. ST fruit extract fetches its protective effect through increasing antioxidant enzyme activities and decreasing oxidative markers in STAe pre-treated animals. Finally, the protective effect of STAe was confirmed by the critical histological observations of the liver and kidney. All results were well correlated and elucidate the hepatoprotective and nephroprotective effect of STAe against Cd-induced toxicity which may be due to the synergistic effect of various active principles of its constituents.
References
Arthan D, Svasti J, Kittakoop P et al (2002) Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 59:459–463
Balachandran C, Duraipandiyan V, Dhabi NA et al (2012) Antimicrobial and antimycobacterial activities of methyl caffeine isolated from Solanum torvum Swartz. fruit. Indian J Microbiol 52:676–681
Balachandran C, Emi N, Arun Y et al (2015) In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem Biol Interact 242:81–90
Bixby M, Spieler L, Menini T et al (2005) Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: a comparative study with green tea and wines using a protein nitration model and mammalian cell cytotoxicity. Life Sci 77:345–358
Capasso A (2013) Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 18:609–700
Celep E, Aydın A, Kırmızıbekmez H et al (2013) Appraisal of in vitro and in vivo antioxidant activity potential of cornelian cherry leave. Food Chem Toxicol 62:448–455
Cuypers A, Plusquin M, Remans T et al (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940
Dalle-Donne I, Rossi R, Giustarini D et al (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Dalziel M, Crispin M, Scanlan CN et al (2014) Emerging principles for the therapeutic exploitation of glycosylation. Science 343:36–38
Dewanjee S, Gangopadhyay M, Sahu R et al (2013) Cadmium induced pathophysiology: prophylactic role of edible jute (Corchorus olitorius) leaves with special emphasis on oxidative stress and mitochondrial involvement. Food Chem Toxicol 60:188–198
Dkhar P, Sharma R (2010) Effect of dimethylsulphoxide and curcumin on protein carbonyls and reactive oxygen species of cerebral hemispheres of mice as a function of age. Int J Dev Neurosci 28:351–357
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Gandhi CR, Ignacimuthu S, Paulraj MG (2011) Solanum torvum Swartz. fruit containing phenolic compounds shows antidiabetic and antioxidant effect in streptozotocin induced diabetic rats. Food Chem Toxicol 49:2725–2733
Ghosh J, Das J, Manna P et al (2010) Protective effect of the fruits of Terminalia arjuna against cadmium-induced oxidant stress and hepatic cell injury via MAPK activation and mitochondria dependent pathway. Food Chem 123:1062–1075
Karak T, Paul RK, Das S, Das DK, Dutta AK, Boruah RK (2015) Fate of cadmium at the soil-solution interface: a thermodynamic study as influenced by varying pH at South 24 Parganas, West Bengal. India Environ Monit Assess 187:713. doi:10.1007/s10661-015-4923-6
Lee JC, Kim HR, Kim J et al (2002) Antioxidant property of an ethanol extract of the stem of Opuntiaficus-indica var. Saboten. J Agric Food Chem 50:6490–6496
Lee JY, Tokumoto M, Fujiwara Y, Satoh M (2015) Involvement of ubiquitin-coding genes in cadmium-induced protein ubiquitination in human proximal tubular cells. J Toxicol Sci 40:901–908
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Marklova E, Albahri Z (2007) Screening and diagnosis of congenital disorders of glycosylation. Clin Chim Acta 385:6–20
Mohan M, Kamble S, Gandhi P et al (2010) Protective effect of Solanum torvum on doxorubicin-induced nephrotoxicity in rats. Food Chem Toxicol 48:436–440
Mortensen ME, Wong LY, Osterloh JD (2011) Smoking status and urine cadmium above levels associated with subclinical renal effects in U.S. adults without chronic kidney disease. Int J Hyg Environ Health 214:305–310
Nair AR, DeGheselle O, Smeets K et al (2013) Cadmium induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14:6116–6143
Nazimabashir, Manoharan V, Miltonprabu S (2015) Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chem Biol Interact 242:179–193
Niki E (2010) Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biol Med 49:503–515
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituricacid reaction. Anal Biochem 95:351–358
Oliver CN, Anh BW, Moerman EJ et al (1987) Age-related changes in oxidized proteins. J Biol Chem 262:5488–5491
Ramamurthy CH, Kumar MS, Sujatha V et al (2012) Evaluation of antioxidant, radical scavenging activity and polyphenolics profile in Solanum torvum L. fruits. J Food Sci 77:907–913
Ramesh T, Kim SW, Sung JH et al (2012) Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol 47:77–84
Rui H, Chen C, Zhang X, Shen Z, Zhang F (2016) Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J Hazard Mater 301:304–313
Singh PP, Sharma P (2009) Antioxidant basket: do not mix apples and oranges. Indian J Clin Biochem 24:211–214
Sivapriya M, Gowda SS, Srinivas L (2015) Protective effect of sundakai (Solanum torvum) seed protein (SP) against oxidative membrane damage in human erythrocytes. J Membr Biol 248:1137–1144
Souza M, Peluffo G, Radi R (2008) Protein tyrosine nitration—functional alteration or just a biomarker? Free Radical Biol Med 45:357–366
Spiazzi CC, Manfredini V, Barcellos da Silva FE et al (2013) γ-Oryzanol protects against acute cadmium-induced oxidative damage in mice testes. Food Chem Toxicol 55:526–532
Subbaiah KCV, Valluru L, Rajendra W et al (2015) Newcastle disease virus (NDV) induces protein oxidation and nitration in brain and liver of chicken: ameliorative effect of vitamin E. The Int J Biochem Cell Biol 64:97–106
Takahashi K, Yoshioka Y, Kato E et al (2010) Methyl caffeate as an alpha-glucosidase inhibitor from Solanum torvum fruits and the activity of related compounds. Biosci Biotechnol Biochem 74:741–745
Valdez LB, Alvarez S, Arnaiz SL et al (2000) Reactions of peroxynitrite in the mitochondrial matrix. Free Radic Biol Med 29:349–356
Velsor LW, Ballinger CA, Patel J et al (2003) Influence of epithelial lining fluid lipids on NO2-induced membrane oxidation and nitration. Free Radic Biol Med 34:720–733
Wang W, Sun Y, Liu J et al (2012) Protective effect of theaflavins on cadmium-induced testicular toxicity in male rats. Food Chem Toxicol 50:3243–3250
Yadav N, Khandelwal S (2009) Therapeutic efficacy of picroliv in chronic cadmium toxicity. Food Chem Toxicol 47:871–879
Zhang S, Jin Y, Zeng Z, Liu Z, Fu Z (2015) Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem Res Toxicol 28:2000–2009
Zhou G, Chen Y, Liu S et al (2013) In vitro and in vivo hepatoprotective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J Ethnopharmacol 148:664–670
Acknowledgments
The authors acknowledge the Department of Science and Technology (DST), Government of India, New Delhi, India, for the financial support in the form of DST–FIST and DST Research Grant (NO.SR/FT/LS-63/2011). The first author (CH. Ram) acknowledges the CSIR, India, for the financial assistance in the form of CSIR–SRF no: 09/559/0084/2012/EMR–I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ramamurthy, C.H., Subastri, A., Suyavaran, A. et al. Solanum torvum Swartz. fruit attenuates cadmium-induced liver and kidney damage through modulation of oxidative stress and glycosylation. Environ Sci Pollut Res 23, 7919–7929 (2016). https://doi.org/10.1007/s11356-016-6044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6044-3