Abstract
Seed priming is a crucial method to induce tolerance capabilities in plants against various abiotic stresses. Seed priming is associated with an induction of number of physiological and biochemical changes in plants by the treatment of natural and/or synthetic compounds to the seeds before their germination. The aim of this study was to investigate whether salicylic acid (SA)-seed priming is involved in the regulation of hexavalent chromium [Cr(VI)] and UV-B toxicity in maize seedlings. For this, the accumulation of dry mass, Cr and SA, chlorophyll fluorescence, oxidative stress markers i.e. reactive oxygen species (ROS; O ·−2 , ·OH and H2O2) and antioxidants were determined. Treatment of Cr(VI) (50 and 250 µM) declined growth and chlorophyll fluorescence parameters- Fv/Fm, Fv/F0, Fm/F0 and qP which accompanied by an increase in NPQ and the accumulation of Cr, and a decline in level of SA. The UV-B also exerts similar effects on growth, chlorophyll fluorescence and level of SA, and damaging effects become intense when combined with Cr(VI). SA-seed priming reduced Cr(VI) and UV-B toxicity on growth which accompanied by a decline in the accumulation of Cr. Cr(VI) and UV-B enhanced generation of O ·−2 , ·OH and H2O2 which subsequently cause damage to lipids and proteins and thus, a decrease in membrane stability was noticed. Both stresses enhanced activities of superoxide dismutase and ascorbate peroxidase while activities of catalase and glutathione reductase were inhibited significantly. Furthermore, the results show that Cr(VI) and UV-B declined contents of total ascorbate and glutathione. This study suggests that Cr(VI) and UV-B might alter biosynthesis of SA as indicated by a decreased level of SA. However, SA-seed priming might act as a signal that reduces the accumulation of Cr and ROS and triggers up-regulation of antioxidants, which subsequently counteract Cr(VI) and UV-B toxicity and hence an improved growth was noticed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals are posing great threat to plant productivity as they are potential sources of abiotic stresses and Cr is one of them. The major sources of hexavalent chromium [Cr(VI)] are industrial inputs as it is frequently used in steel, alloys, cast iron, chrome plating, dyes and pigments, textile, leather tanning and wood preservation (Shanker et al. 2005). Cr production has been estimated to be more than hundred million tons globally and has significantly been increased since the 1950s (Adriano 1986; Shanker et al. 2005). The concentration of Cr in natural soil ranges from 10 to 50 mg/kg and can even reach up to 125 g/kg (Adriano 1986). In India, the concentration of Cr varies from 161.8 to 6227.8 mg/kg (average of 2652.3 mg/kg) near industrial sites which is far above than acceptable limit (Gowd et al. 2010). The Cr(VI) is reported to be highly toxic because it has potential to pass through the plasma membrane and can subsequently oxidize various biomolecules. The hazardous effects of Cr(VI) on seed germination, mitosis, growth, leaf area, pigments, photosynthesis, nitrogen and sulfur metabolism and stomatal functioning have been reported in the earlier studies (Ali et al. 2006; Schiavon et al. 2008; Gangwar and Singh 2011; Dotaniya et al. 2014). Production of ROS under Cr(VI) stress resulted in an oxidative stress, which leads damage to proteins, lipids and DNA (Gangwar and Singh 2011; Patnaik et al. 2013; Chen et al. 2014). Thus, Cr(VI) toxicity affects plants from molecular to the ecosystem level. Besides this, entry of Cr(VI) into the food chain poses great threat to the human beings. Therefore, studies on investigations of Cr(VI) toxicity responses and reduction of its load in the food chain are still a matter of research.
The wide spread applications of chlorofluorocarbons (CFCs) in refrigerating devices and their subsequent release into the atmosphere is causing depletion of the stratospheric ozone layer resulting into the enhanced solar UV-B radiation at the Earth’s surface (WMO 2007). Although due to the successful implementation of Montreal Protocol there has been a reduction in ozone depleting substances and solar UV-B as well, however, terrestrial ecosystems still appear to be sensitive to UV-B due to the variations in its irradiance (Ballaré et al. 2011). Photoautotrops are absolutely dependent on the solar radiation for their survival and thus cannot escape the damaging impact of UV-B (Sullivan and Teramura 1989; Mishra et al. 2009; Yu et al. 2013; Pandey and Pandey-Rai 2014). The deleterious effects of UV-B on plant growth and development, photosynthesis and biomass production have been observed under field and controlled conditions (Casati et al. 2008; Lavola et al. 2013; Liu et al. 2013; Nawkar et al. 2013; Choudhary and Agrawal 2014). Teramura and Sullivan (1991) observed 19–25 % reduction in yield of soybean due to the enhanced solar UV-B radiation. Furthermore, Pandey and Pandey-Rai (2014) also reported damage to photosystem II and ribulose-1,5-bisphosphate carboxylase/oxygenase (RUBISCO), and reductions in photosynthetic capacity, ribulose-1,5-bisphosphate (RuBP) regeneration and quantum yield by UV-B radiation. Hui et al. (2013) have reported ultrastructure changes in Bryum argenteum after exposure to the UV-B radiation. Therefore, in spite of enormous studies, research dealing with an impact of UV-B on photoautotrophs is still a matter of investigation because of its damaging nature at higher rate.
Salicylic acid (SA), a phenolic compound, is a potent signaling molecule in plants and is well established for its role in eliciting specific responses to the biotic stresses (O’Donnell et al. 2001; Kuźniak et al. 2013). Besides this, SA is also known to be involved in abiotic stress signaling including plant responses to heavy metals and UV-B (Metwally et al. 2003; Freeman et al. 2005; Bandurska and Ciéslak 2013; Choudhary and Agrawal 2014). SA may be synthesized in plants either directly by shikimate/chorismate (isochorismate) pathway or indirectly via phenylalanine (Bandurska and Ciéslak 2013). SA has been shown to accumulate in plants in response to various abiotic stresses and this correlates with the accumulation of the antioxidants such as reduced glutathione (GSH) and glutathione reductase (Freeman et al. 2005; Zhou et al. 2009; Cui et al. 2012). Furthermore, it has been suggested that SA is involved directly in signaling of antioxidant responses, however, the signaling mechanisms are still unclear and need further investigations.
In natural field conditions, multiple stresses [like Cr(VI) and UV-B] are likely to co-exist and simultaneously they may cause more severe damage to plants and other non-selective organisms. Besides this, the negative impact of one stress may get modified up to certain degree by other stressors. Keeping the above facts into consideration, this study has been undertaken to investigate: (1) effect of Cr(VI) and UV-B alone and together on the accumulation of dry mass, photosynthesis, oxidative stress and antioxidant system in maize seedlings, (2) effect of Cr(VI) and UV-B on SA level, and (3) probable involvement of SA seed priming in the regulation of Cr(VI) and UV-B toxicity in maize seedlings. Maize is the third most important crop in the world and is reported to be sensitive against increased UV-B radiation (Correia et al. 2000; Yin and Wang 2012). An Indian report also illustrates that maize is sensitive to UV-B as shown by alterations in chlorophyll fluorescence characteristics (Shine and Guruprasad 2012). Therefore, we have chosen maize as a model organism to investigate an impact of SA-seed priming in the regulation of Cr(VI) and UV-B toxicity.
Material and methods
Plant material and growth conditions
Seeds of maize (Zea mays L. cv. Nootan) were surface-sterilized, thoroughly washed and soaked for 24 h in sterilized distilled water. Pre-soaked seeds were sown in plastic trays filled with acid washed sterilized sand and incubated for 2 days in the darkness. Seedlings were grown in controlled conditions (light/dark regime of 16/8 h at 30/26 °C, relative humidity of 60–70 %, photosynthetic active radiation (PAR) of 350 µmol photons m−2 s−1) and watered regularly. Seven days after sowing, uniform seedlings were gently transferred in 0.2 strength Rorison nutrient medium (pH 6.8) containing mM: 0.4 Ca (NO3)2, 0.2 MgSO4 and 0.2 KH2PO4, and μM: 0.1 CuSO4.5H2O, 0.2 ZnSO4.7H2O, 9.2 H3BO3, 1.8 MnCl2.4H2O, 0.2 Na2MoO4.2H2O and 10 Fe-EDTA. The nutrient medium was changed regularly and aerated with sterile air to avoid root anoxia.
Cr(VI) and UV-B treatments
After acclimatization in the nutrient medium for 3 days, on 11th day, one set of healthy and uniform sized seedlings were transferred into fresh nutrient medium containing 0, 50 and 250 µM of Cr(VI) (as K2Cr2O7) and thereafter half of the seedlings from Cr(VI) treated set were irradiated with three successive exposures of UV-B (0.4 µmol photons m−2 s−1) for 4 h daily from 12th to 14th day. Similarly, another set of seedlings grown without Cr(VI) was also irradiated with UV-B. Prior to this, on the basis of screening experiments two concentrations of Cr(VI) (50 and 250 µM; environmentally relevant) and UV-B radiation (corresponds to 5.2 kJ m−2) were selected for this study. The used UV-B dose is biologically effective according to the biological spectral weighting function (Flint and Caldwell 2003). Samples were irradiated with fluorescent UV-B tube (TL 40 W/12 Philips, Holland) with its main output at 312 nm together with white light (50 µmol photons m−2 s−1, PAR). The radiation was filtered through 0.127 mm cellulose acetate (Johnston Industrial Plastics, Toronto, Canada) to remove all incident UV-C (<280 nm). The intensity of UV-B radiation at the surface of leaves was measured with a Power Meter (Spectra Physics, Model 407, A-2, USA). Seedlings grown without the treatment of either stress served as a control. On 15th day, seedlings from each set were harvested and parameters related with growth, the accumulation of Cr and salicylic acid (SA), chlorophyll fluorescence, oxidative stress and antioxidant defense system in maize seedlings were analyzed.
Experiments with SA-seed priming
To test an involvement of SA in the regulation of Cr(VI) and UV-B toxicity, experiments were carried out with SA-pretreated maize seeds. For this, pretreatment of maize seeds with SA was performed. Uniform sized seeds of maize were presoaked in 500 µM of SA (as sodium salt) for 24 h. After this, same protocol was followed for Cr(VI) and UV-B exposures.
Determination of growth
Growth was measured in terms of dry weight. The seedlings from treated and untreated samples were selected randomly; oven dried for 48 h at 70 °C and then dry mass was recorded by using single pan electronic balance (Contech-CA 223, India).
Determination of the accumulation of Cr and SA
For the measurement of the accumulation of Cr, control and treated seedlings were harvested randomly. Seedlings were washed thoroughly with double distilled water to remove adsorbed culture medium. Oven dried sample of each treatment (50 mg) was digested in a mixed acid (HNO3:HClO4; 85:15, v/v) at 80 °C until a transparent solution was obtained. After cooling, the digested samples were filtered using Whatman No. 42 filter paper and the filtrate was maintained up to 25 ml with double distilled water. Concentrations of Cr in filtrate of digested samples were estimated using an atomic absorption spectrometer (iCE3000 series).
SA and SA conjugates were extracted and determined as described by O’Donnell et al. (2001). The 500 mg tissue was homogenized and extracted with 3 ml 90 % methanol followed by 2 ml 100 % methanol. The combined extracts were then divided into two parts 1) dried down and resuspended in either 2.5 ml 5 % trichloroacetic acid (TCA) (for determination of free SA) or phosphate buffer (for determination of total SA). Conjugated forms of SA were hydrolyzed by boiling for 30 min in acidified phosphate buffer. Both fractions were then extracted twice with an equal volume of ethylacetate:cyclopentane:isopropanol (100:99:1), dried down, and resuspended in 20 % methanol. SA was identified and quantified by HPLC (Metrohm 820 IC). Mobile phase used was isocratic with 100 % methanol at the flow rate of 1 ml min−1 and monitoring was done at 254 nm. Identification and recovery of SA was determined by spiking a non-induced sample with a known amount of an authentic standard of SA. The level of SA was expressed in µg g−1 dry weight.
Measurement of chlorophyll fluorescence
For the determination of photosynthetic performance of maize seedlings, chlorophyll fluorescence measurements were taken in dark adapted leaves of control and treated seedlings using hand held leaf fluorometer (FluorPen FP 100, Photon System Instruments, Czech Republic). The following fluorescence parameters: maximum quantum efficiency of photosystem II (PSII) (Fv/Fm), activity of PS II (Fv/F0), electron transport rate through PS II (Fm/F0), photochemical quenching (qP) and non-photochemical quenching (NPQ) were measured. Measurements were taken in second leaf of nine different plants of each treatment during each experiment.
Determination of superoxide radical, hydrogen peroxide and hydroxyl radical
Superoxide radical (O ·−2 ) was measured according to the method of Elstner and Heupel (1976) by monitoring the nitrite formation from hydroxylamine in the presence of O ·−2 . The absorbance of the coloured aqueous phase was recorded at 530 nm. A standard curve prepared with NaNO2 was used to calculate the production rate of O ·−2 . For estimation of hydrogen peroxide (H2O2), the method of Velikova et al. (2000) was adopted. Hydrogen peroxide concentration was estimated based on the absorbance of reaction mixture at 390 nm using a standard curve of H2O2. Hydroxyl radical (·OH) was quantified by using dimethyl sulfoxide (DMSO) as a molecular probe, which is oxidized by ·OH generated under stress conditions into methane sulfinic acid (MSA), a stable compound (Babbs et al. 1989). The absorbance of samples was recorded at 425 nm and an extinction coefficient of 2088 M−1 cm−1 was used to calculate ·OH generation. The results are presented as nmol MSA g−1 fresh weight.
Determination of oxidative damages and membrane stability index
Lipid peroxidation was estimated as a malondialdehyde (MDA) content adopting the method of Hodges et al. (1999). Malondialdehyde content was calculated using an equation: MDA (nmol ml−1) = (A − B/157, 000) × 106 where A = [(A532 + TBA) − (A600 + TBA) − (A532 − TBA − A600 − TBA)] and B = [(A440 + TBA − A600 + TBA)0.0571]. Oxidative damage to proteins was estimated in terms of carbonyl groups according to the method of Levine et al. (1994) using dinitrophenylhydrazine. The absorbance of derivatized carbonyls was measured at 375 nm and content was calculated using an extinction coefficient of 22,000 M−1 cm−1. Membrane stability index (MSI) of the leaves of maize seedlings was measured according to the method of Sairam et al. (2002). The MSI was calculated using a formula: [1 − (C1/C2)] × 100.
Assay of antioxidant enzymes activities
Fresh leaves (1 g) were homogenized in 20 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1 % polyvinylpyrrolidone, with the addition of 1 mM ascorbate in case of ascorbate peroxidase (APX) assay. The homogenate was centrifuged at 20,000g for 10 min at 4 °C and supernatant was used as an enzyme extract.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of p-nitroblue tetrazolium chloride (NBT) according to the method of Giannopolitis and Reis (1977). One unit of SOD activity was expressed as the amount of enzyme required to cause 50 % inhibition of reduction of NBT as measured at 560 nm. Catalase (CAT, EC 1.11.1.6) activity was determined by following the method of Aebi (1984). The decomposition of H2O2 was followed for 3 min by an absorbance decrease at 240 nm (extinction coefficient 29.4 mM−1 cm−1) and one unit enzyme activity is represented as 1 µmol H2O2 decomposed min−1. Glutathione reductase (GR, EC 1.6.4.2) activity was estimated by measuring a decrease in absorbance due to oxidation of NADPH at 340 nm for 3 min (Schaedle and Bassham 1977). The reaction was initiated by the addition of NADPH. Corrections were made for the background absorbance at 340 nm, without NADPH and activity is expressed in units, representing 1 nmol of NADPH oxidized min−1. Ascorbate peroxidase (APX, EC 1.11.1.11) activity was determined by following a decrease in absorbance due to oxidation of ascorbate at 290 nm for 1 min (Nakano and Asada 1981). Ascorbate peroxidase activity was calculated by using an extinction coefficient of 2.8 mM−1 cm−1 and enzyme activity is expressed in units, representing 1 nmol of ascorbate oxidized min−1. Protein in each sample was estimated according to the method of Bradford (1976).
Determination of non-enzymatic antioxidants
Total ascorbate (ASC) content in treated and untreated seedlings was determined by the method of Gossett et al. (1994). This assay is based on reduction of ferric ion into ferrous ion with ascorbic acid in acid solution followed by formation of red chelate between ferrous ion and 2,2-bipyridyl. Standard curve was prepared with L-ascorbic acid and used for calculation of ASC.
Total glutathione (GSH + GSSG) content was determined by following the enzyme recycling method of Brehe and Burch (1976) with some modifications. This assay is based on sequential oxidation of GSH by 5,5-dithiobis-2 nitrobenzoic acid (DTNB) and reduction of oxidized glutathione (GSSG) in the presence of NADPH and GR (Type III from bakers’ yeast; Sigma, Chemical Company). The amount of GSH was calculated by using a standard curve prepared with reduced glutathione (GSH).
Statistical analysis
Results were statistically analyzed by analysis of variance (ANOVA). Duncan’s multiple range test was applied for mean separation for significant differences (P < 0.05) among treatments using SPSS software (Version 10, SPSS Inc., Chicago). Results presented are mean ± standard error of three independent experiments with three replicates in each experiment (n = 9).
Results
Growth and the accumulation of Cr and SA
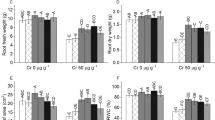
Growth was measured in terms of dry mass. Treatment of 50 and 250 µM Cr(VI) declined (P < 0.05) dry mass by 12 and 21 %, respectively compared to the control (Fig. 1). The UV-B also suppressed (P < 0.05) dry mass by 13 %. Combined treatment of Cr(VI) and UV-B further declined dry mass in maize seedlings as reduction reached up to 31 % under 250 µM Cr(VI) + UV-B combination. However, SA-priming of seeds significantly (P < 0.05) alleviated Cr(VI) and UV-B-induced toxicity in seedlings. For instance, SA-priming with seeds together with 50 and 250 µM Cr(VI), and UV-B significantly (P < 0.05) lowered reduction in dry mass and it was only 5, 12 and 6 %, respectively compared to the control (Fig. 1).
Effect of chromium (VI) and enhanced UV-B radiation alone and in combination on the accumulation of dry mass (growth) in non-SA primed and SA-primed maize seedlings. Means are ± standard error of three independent experiments with three replicates in each experiment (n = 9). Bars followed by different letters show significant (P < 0.05) difference from each other according to the Duncan’s multiple range test
Maize seedlings grown under 50 and 250 µM of Cr(VI) accumulated about 216 ± 9.1 and 1012 ± 26.3 µg Cr g−1 dry weight, respectively (Table 1). Under combined treatments of Cr(VI) and UV-B, the accumulation of Cr further increased and it was reached up to 1256 ± 52.7 µg Cr g−1 dry weight under 250 Cr(VI) + UV-B combination. However, in SA-primed seedlings, the accumulation of Cr decreased significantly (P < 0.05) compared to the Cr(VI) alone and Cr(VI) + UV-B combinations. As under 50 µM Cr(VI), 250 µM Cr(VI), 50 µM Cr(VI) + UV-B and 250 µM Cr(VI) + UV-B, the accumulation of Cr was only 142 ± 3.7, 643 ± 27.0, 158 ± 4.7 and 741 ± 26.7 µg Cr g−1 dry weight, respectively (Table 1).
Data related to SA content are presented in Table 1. Treatment of 50 and 250 µM Cr(VI) caused a decline (P < 0.05) in SA level by 16 and 41 %, respectively compared to the control. The UV-B alone also caused a reduction (P < 0.05) in SA level by 10 %. Combined treatments of Cr(VI) and UV-B caused greater decline in SA level as it was declined by 33 and 50 % under 50 µM Cr(VI) + UV-B and 250 µM Cr(VI) + UV-B, respectively compared to the control (Table 1).
Chlorophyll fluorescence
Cr(VI) and UV-B alone and in combination significantly (P < 0.05) decreased chlorophyll fluorescence characteristics except NPQ (Table 2). Upon exposure of 50 and 250 µM of Cr(VI), Fv/Fm, Fv/F0, Fm/F0 and qP decreased by 8 and 19 %, 14 and 24 %, 11 and 21 % and 10 and 20 %, respectively compared to the control. Similarly, UV-B exposure also declined Fv/Fm, Fv/F0, Fm/F0 and qP up to 9, 12, 11 and 12 %, respectively compared to the control. Combined treatments of Cr(VI) and UV-B further raised a decrease in above parameters (Table 2). In contrast to Fv/Fm, Fv/F0, Fm/F0 and qP, NPQ was raised by Cr(VI) and UV-B treatments. It was increased (P < 0.05) by 9, 16 and 7 % following 50 and 250 µM of Cr(VI) and UV-B treatments, respectively compared to the control (Table 2). Combined treatments of Cr(VI) and UV-B further raised values of NPQ. The maximum increase was reached up to 21 % following 250 µM Cr(VI) + UV-B combination (Table 2). Results related to SA-primed seedlings showed that Cr(VI) and UV-B induced reduction in Fv/Fm, Fv/F0, Fm/F0 and qP and a rise in NPQ were lower than Cr(VI) and UV-B alone treated seedlings (Table 2).
Reactive oxygen species
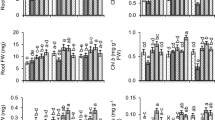
The level of superoxide radical (O ·−2 ) was enhanced (P < 0.05) with the increasing doses of Cr(VI) and a further rise in the content of O ·−2 was noticed when seedlings were exposed with combined doses of Cr(VI) and UV-B (Fig. 2a). Cr(VI) exposure at 50 and 250 µM caused 30 and 89 % rise in the level of O ·−2 over the control value, and the combined doses of Cr(VI) and UV-B (50 µM Cr(VI) + UV-B and 250 µM Cr(VI) + UV-B) further accelerated content of O ·−2 (85 and 149 %, respectively) compared to the control. The UV-B individually also caused significant (P < 0.05) rise (26 %) in the content of O ·−2 . The accumulation of H2O2 showed same pattern of an increment but concentration of H2O2 was substantially higher when compared to the O ·−2 (Fig. 2b). The UV-B exposure enhanced content of H2O2 by 34 % compared to the control. The amount of H2O2 was raised up to 40 and 98 % over the value of the control following treatment of 50 and 250 µM of Cr(VI) while together with UV-B it was increased by 88 and 122 %, respectively. Results pertaining to ·OH are presented in Fig. 2c. The level of ·OH was lower than H2O2, however, it showed similar increasing pattern as observed in the case of H2O2 and O ·−2 . Both the stresses individually accelerated formation of ·OH and their combined doses further enhanced the rate of ·OH generation (Fig. 2c). However, SA-priming of seeds lowered Cr(VI) and UV-B induced rise in O ·−2 , H2O2 and ·OH (Fig. 2a–c).
Effect of chromium (VI) and enhanced UV-B radiation alone and in combination on superoxide radical (a), hydrogen peroxide (b) and hydroxyl radical (c) contents in the leaves of non-SA primed and SA-primed maize seedlings. Means are ± standard error of three independent experiments with three replicates in each experiment (n = 9). Bars followed by different letters show significant (P < 0.05) difference from each other according to the Duncan’s multiple range test
Oxidative damage to lipids and proteins, and membrane injury
Lipid and protein peroxidation rates following Cr(VI) and UV-B exposure are shown in Fig. 3a, b, respectively. Exposure of Cr(VI) at 50 and 250 µM increased (P < 0.05) lipid and protein peroxidations by 14 and 33 % and 15 and 22 %, respectively compared to the control. The UV-B exposure also enhanced (P < 0.05) lipid and protein peroxidations by 17 and 14 %, respectively. The rate of peroxidations got further accelerated when both the stresses [(50 and 250 µM Cr(VI), and UV-B] were applied simultaneously. The maximum increase in lipid and protein peroxidations was noticed under 250 µM Cr(VI) + UV-B combinations as their amounts were increased up to 67 and 41 %, respectively compared to the control (Fig. 3a, b). The results pertaining to the membrane stability index (MSI) are depicted in Fig. 3c. The MSI significantly (P < 0.05) declined by Cr(VI) and UV-B treatments and their combined treatments further decreased MSI. Exposure of Cr(VI) at 50 and 250 µM and UV-B alone declined MSI by 8, 18 and 7 %, respectively (Fig. 3c). Maximum decline in MSI (by 26 %) was noticed when seedlings were exposed with combined doses of UV-B and 250 µM Cr(VI). On the contrary, in SA-primed seedlings a lowering in Cr(VI) and UV-B induced rise in lipids and protein oxidations was noticed (Fig. 3a, b).
Effect of chromium (VI) and enhanced UV-B radiation alone and in combination on lipid peroxidation (a), protein oxidation (b) membrane stability (c) in the leaves of non-SA primed and SA-primed maize seedlings. Means are ± standard error of three independent experiments with three replicates in each experiment (n = 9). Bars followed by different letters show significant (P < 0.05) difference from each other according to the Duncan’s multiple range test
Enzymatic antioxidants
The results pertaining to the activities of antioxidant enzymes in maize seedlings exposed to Cr(VI) and UV-B stresses are presented in Fig. 4. The activities of SOD and APX were increased (P < 0.05) following the treatments of Cr(VI) and UV-B, and both the stressors [50 and 250 µM of Cr(VI) and UV-B] simultaneously further stimulated their activities (Fig. 4a, b). Maximum enhancement in the activity was recorded in the case of SOD (217 %) and APX (72 %) over the values of their respective control. Conversely, CAT and GR activities were inhibited significantly (P < 0.05) in maize seedlings exposed to both stressors [50 and 250 µM of Cr(VI) and UV-B] alone and together (Fig. 4c, d). Exposure of Cr(VI) at 50 and 250 µM and UV-B inhibited CAT and GR activities by 20, 32 and 16 %, and 26, 35 and 7 %, respectively compared to the control (Fig. 4c, d). Moreover, combined treatments of Cr(VI) and UV-B enhanced inhibitions in CAT and GR activities. However, in SA-primed seedlings raised activities of SOD, APX, CAT and GR were noticed (Fig. 4).
Effect of chromium (VI) and enhanced UV-B radiation alone and in combination on superoxide dismutase (a), ascorbate peroxidase (b) catalase (c) and glutathione reductase (d) activities in the leaves of non-SA primed and SA-primed maize seedlings. Means are ± standard error of three independent experiments with three replicates in each experiment (n = 9). Bars followed by different letters show significant (P < 0.05) difference from each other according to the Duncan’s multiple range test
Non-enzymatic antioxidants
Data related to the total ascorbate and glutathione pools are presented in Fig. 5. Results reveal that 50 and 250 µM of Cr(VI) and UV-B declined (P < 0.05) total ascorbate and glutathione pools by 12, 45 and 13 % and 12, 48 and 15 %, respectively compared to the control. Combined treatments of Cr(VI) and UV-B intensified a decline in the ascorbate and glutathione pools as their amounts were decreased by 54 and 58 %, respectively under 250 µM Cr(VI) + UV-B combination. On the other hand, SA-priming of seeds alone had significantly (P < 0.05) increased total ascorbate and glutathione pools as their amounts were increased by 22 and 6 %, respectively (Fig. 5). Besides this, SA-priming of seeds significantly (P < 0.05) alleviated Cr(VI) and UV-B induced decline in ascorbate and glutathione pools (Fig. 5). As under 50 and 250 µM of Cr(VI), UV-B, 50 µM Cr(VI) + UV-B and 250 µM Cr(VI) + UV-B, a decline in ascorbate and glutathione pools was only 1, 12, 1, 10 and 18 % and 3, 16, 4, 14 and 22 %, respectively compared to the control (Fig. 5).
Effect of chromium (VI) and enhanced UV-B radiation alone and in combination on total ascorbate (a) and total glutathione (b) in the leaves of non-SA primed and SA-primed maize seedlings. Means are ± standard error of three independent experiments with three replicates in each experiment (n = 9). Bars followed by different letters show significant (P < 0.05) difference from each other according to the Duncan’s multiple range test
Discussion
The present study investigates an involvement of SA-priming of seeds in the regulation of Cr(VI) and UV-B toxicity in maize. This study confirms that SA-priming of seeds arrests Cr(VI) and UV-B-induced oxidative stress and toxicity in maize seedlings. The results reveal that Cr(VI) significantly (P < 0.05) declined growth of maize seedlings (Fig. 1). The Cr(VI)-induced a decrease in growth may be related to the altered biosynthesis of SA (as indicated by a declined level of SA), and photosynthesis (chlorophyll fluorescence) due to an increased generation of ROS which subsequently cause damage to lipids and proteins, and down-regulation of antioxidants-CAT, GR, ascorbate and glutathione (Figs. 1, 2, 3, 4, 5; Tables 1, 2). The Cr(VI) being a non-essential element has multifaceted negative effects on physiology and biochemistry of plants. Dotaniya et al. (2014) and Hou et al. (2014) have reported that Cr(VI) causes a decrease in seed germination, root elongation and mitosis and thus, reduces the accumulation of biomass in wheat, cabbage, lettuce, cucumber and maize. Furthermore, studies have revealed that Cr(VI) decreased photosynthetic pigments, rate of photosynthesis (Duman et al. 2014) and nitrogen and sulfur metabolism (Schiavon et al. 2008; Gangwar and Singh 2011). The Cr(VI) also increased the generation/accumulation of ROS (O ·−2 , H2O2 and ·OH) which subsequently cause peroxidations of phospholipids and proteins (Gangwar and Singh 2011; Chen et al. 2014; Duman et al. 2014) and modulation in the activities of antioxidant enzymes, and also in the status of non-enzymatic antioxidants (Gangwar et al. 2011; Duman et al. 2014). Rodriguez et al. (2012) have also reported that Cr(VI) induces toxicity at different photosynthetic levels in pea. However, SA-priming of seeds protects maize seedlings against Cr(VI) toxicity by reducing the accumulation of Cr and ROS, and triggering up-regulation of antioxidants. In SA-primed seedlings, a lowering in the accumulation of Cr may be assigned to the more than one mechanisms (1) Cr(VI) exclusion that lowers uptake, or active efflux from the roots, i.e. by the mechanisms leading to a lower cytoplasmic Cr content (Metwally et al. 2003), (2) protective role of SA on plasma membrane integrity (Singh et al. 2013), (3) haem oxygenase-1 mediated signaling cascade that results into a lowering of metal uptake (Cui et al. 2012) and (4) an increased cell wall thickness in root epidermis, thereby declining an absorption of Cr and accumulation (Singh et al. 2013). Similarly, El-Tayeb et al. (2006) have noticed that SA protects photosynthetic pigments and membrane damage in sunflower under copper stress. Bai et al. (2014) have found that SA alleviates Cd toxicity in perennial ryegrass by regulating oxidative stress and damage. Moreover, Liu et al. (2014) have observed that foliar application of SA alleviates salt stress in cotton seedlings.
The role of SA in the regulation of biotic stress is extensively studied (Catinot et al. 2008; Nakai et al. 2013; Alazem and Lin 2014). Besides this, studies have also demonstrated that SA plays an important role in the regulation of various abiotic stresses (Metwally et al. 2003; El-Tayeb et al. 2006; Cui et al. 2012; Liu et al. 2014). Based on the results of previous studies, a great agronomical potential of SA has been proposed in improving stress tolerance of agricultural crops. However, application of SA depends on the concentration of SA, the state of the plants and the mode of application (Miura and Tada 2014). In general, low concentration of SA alleviates abiotic stress toxicity, while higher concentrations cause oxidative stress, and thus reduce abiotic stress tolerance (Yang et al. 2004; Hayat et al. 2014).
Similarly, UV-B-induced adverse effects on physiology and biochemistry of plants have also been reported (Singh et al. 2012; Bandurska and Ciéslak 2013; Choudhary and Agrawal 2014). Recently, Choudhary and Agrawal (2014) have found that enhanced UV-B radiation declined growth and proposed that higher accumulation of SA was associated with higher UV-B sensitivity of pea. However, Bandurska and Ciéslak (2013) have observed that the accumulation of SA in barley seedlings renders UV-B protection. Our results are in accordance with Bandurska and Ciéslak (2013) and reveal that SA-priming of seeds protects maize seedlings against UV-B toxicity by increasing the level of antioxidants (ascorbate and glutathione) which successfully counteract ROS-mediated oxidative stress (Figs. 2, 3, 4, 5).
Photosynthetic efficiency of maize seedlings as determined by the chlorophyll fluorescence was severely affected by Cr(VI) and UV-B stresses (Table 2). The results reveal that Cr(VI) and UV-B stresses decreased Fv/Fm, Fv/F0 and Fm/F0 ratios and qP (Table 2). The Fv/Fm and qP are frequently used fluorescence parameters to assess the maximum photochemical efficiency of PS II and photochemical quenching, respectively in plants. Besides Fv/Fm, Fv/F0 and Fm/F0 are also being used to investigate photosynthetic efficiency of plants in the changing environmental conditions. The Fv/F0 indicates the activity of PS II while Fm/F0 indicates electron transport rate through PS II (Xing et al. 2010). Studies have shown that stress factors generally affect functional activity of PS II and thus decrease these ratios (Xing et al. 2010; Singh et al. 2013). Under Cr(VI) and UV-B stresses, a decline in Fv/Fm, Fv/F0 and Fm/F0 ratios and qP suggests about structural and functional alterations in photosynthetic process as indicated by decreased accumulation of biomass in maize seedlings. Cambrollé et al. (2011) have also reported that metal stress declined fluorescence characteristics such as Fv/Fm and ФPSII directly involving interference of metal with Ca ions of PS II. An increase in NPQ under Cr(VI) and UV-B treatments suggests about mechanisms that are involved in dissipating an excess excitation energy in order to protect maize seedlings from photodynamic damage under Cr(VI) and UV-B stresses (Table 2). Furthermore, an increase in NPQ may be explained on the basis of down-regulation of PS II function to avoid over-reduction of QA in order to match a decreased demand for electrons through NADPH consumption (Genty et al. 1990), hence it is involved in the photoprotection under stresses.
Cr(VI) alone and together with UV-B enhanced the generation/accumulation of ROS in a dose dependent manner (Fig. 2). ROS are unavoidable consequences of an oxygen metabolism and their generation/accumulation inevitably takes place where generation/consumption ratio of electron is imbalanced or high. In the present study, the generation/accumulation of ROS might have occurred due to an interaction of metal with carriers in the electron transport systems as reported in the earlier studies (Ali et al. 2006; Xing et al. 2010; Singh et al. 2013). It is also evident from data of chlorophyll fluorescence. An increase in ROS was coincided with an increase in lipids and proteins oxidations and thus a decrease in MSI was noticed (Figs. 2, 3). ROS can readily interact with the cellular biomolecules such as lipids and proteins and various key metabolic enzymes and produce damaging effects and thus result into a declined growth and altered photosynthetic performance in maize seedlings. However, in SA-primed seedlings a lowering in Cr(VI) and UV-B-mediated enhancement in ROS and oxidative damages was observed (Figs. 1, 2, 3).
The capacity of an organism to tolerate stress factor can be determined through its expressed antioxidant system which comprises of enzymatic and non-enzymatic antioxidants. The Cr(VI) alone and together with UV-B increased the activities of SOD and APX compared to the control (Fig. 4a, b). The results suggested that an increase in the activities of these enzymes might not be sufficient to protect maize seedlings from oxidative stress as indicated by decreased accumulation of biomass (Fig. 1). In contrast to the activities of SOD and APX, the activities of CAT and GR were inhibited by Cr(VI) and UV-B alone as well as in combination (Fig. 4c, d). These results imply that inhibitions in the activities of these enzymes might be related with the Cr(VI) and UV-B sensitivity of maize seedlings due to greater accumulation of ROS. Sharma et al. (2003) have concluded that CAT is an iron-porphyrin biomolecule and its decreased activity indicates that Cr(VI) is either interacting with iron in metabolic pool or affecting the availability of active form of iron. Furthermore, a decreased CAT activity might have resulted into a greater accumulation of H2O2 (Fig. 2b) which in turn causes severe damage to the cellular system directly or by generating highly reactive ·OH (Fig. 2c). A decrease in GR activity by heavy metal ions could result from the attack caused by a metal ion-induced ROS, which may be possible in case of non-redox metals (Buettner and Jurkiewiez 1996). Inhibition in GR activity as observed in the present study resulted into decreased pool of GSH that leads to severe oxidative damage to the cellular system because it failed to provide sufficient amount of GSH to eliminate ROS. However, significant improvement in the activities of enzymatic antioxidants in SA-primed seedlings suggested their role in mitigating ROS mediated damages to lipids and proteins as evidenced from better accumulation of dry mass (Figs. 1, 2, 3, 4).
In addition to the enzymatic antioxidants, plants also possess an array of efficient non-enzymatic antioxidants that include ascorbate, glutathione etc., which efficiently scavenge singlet oxygen (1O2) and ·OH for which no enzymatic antioxidants are known to be evolved. Ascorbate is known as a major primary antioxidant, reacts directly with 1O2, O ·−2 and ·OH (Buettner and Jurkiewiez 1996). Further, Gao and Zhang (2008) have shown an importance of ascorbate against oxidative stress in Arabidopsis ascorbate-deficient (vtc1) mutant under UV-B stress. Another important low molecular weight antioxidant- GSH, not only plays a role in the synthesis of phytochelatins and in removal of ROS but also acts as a substrate in the detoxification of lipid peroxidation and protein oxidation sub-products through the activity of glutathione-S-transferase (Kalinowska and Pawlik-Skowronska 2010). Further, Chen et al. (2010) have observed that exogenous addition of GSH alleviates Cd-induced reduction in growth of barley seedlings by depressing the accumulation of O ·−2 , H2O2 and MDA. In the present study, a decline in total ascorbate and glutathione under Cr(VI) and UV-B radiation could not support the cell to control lipid peroxidation and protein oxidation as indicated by the increased contents of MDA and RCG (Figs. 2, 3, 5). The significant improvement in total ascorbate and glutathione in SA-primed seedlings suggested that these non-enzymatic antioxidants up-regulated by SA and supported maize seedlings to regulate the oxidative stress under Cr(VI) and UV-B toxicity as indicated by better growth in SA-primed seedlings than non-SA primed ones (Figs. 1, 5).
Conclusion
The present study demonstrated that Cr(VI) and UV-B alone and together produced damaging effects in maize seedlings by altering photosynthesis and enhancing oxidative stress and damages to lipids and proteins. This Cr(VI) and UV-B toxicity coincides with a declined level of SA and thus, suggesting that SA is involved in the regulation of Cr(VI) and UV-B toxicity responses as indicated by inhibited CAT and GR activities and enhanced level of oxidative stress. However, SA-priming of seeds protects maize seedlings against Cr(VI) and UV-B toxicity by reducing the accumulation of Cr and ROS and triggering up-regulation of antioxidants and thus imparts Cr(VI) and UV-B tolerance to maize seedlings. The results of this study also point out that elevation of free SA levels in plants, either by exogenous feeding or genetically may enhance their tolerance against multiple abiotic stresses. Furthermore, the findings are also significant as it has been demonstrated that multiple stresses occur in natural fields where one stressor may modify the effects of other and SA may play a positive role in the regulating their toxicity in plants. The probable model for action of SA under Cr(VI) and UV-B stress in maize seedlings is given in Fig. 6.
Abbreviations
- F0 :
-
Minimal fluorescence
- Fv (Fm − F0):
-
Variable fluorescence in dark adapted leaves
- Fv/Fm :
-
Maximum photochemical efficiency of PS II
- Fv/F0 :
-
The activity of PS II
- Fm/F0 :
-
Electron transport rate through PS II
- qP:
-
Photochemical quenching
- NPQ:
-
Non-photochemical quenching
- ASC:
-
Ascorbate
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GSH:
-
Glutathione
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- ·OH:
-
Hydroxyl radical
- ROS:
-
Reactive oxygen species
- RCG:
-
Reactive carbonyl groups
- O ·−2 :
-
Superoxide radical
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
References
Adriano DC (1986) Elements in the terrestrial environment. Springer, New York, pp 105–123
Aebi II (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alazem M, Lin NS (2014) Roles of plant hormones in the regulation of host-virus interactions. Mol Plant Pathol. doi:10.1111/mpp.12204
Ali NA, Dewez D, Didur O, Popovic R (2006) Inhibition of photosystem II photochemistry by Cr is caused by the alteration of both D1 protein and oxygen evolving complex. Photosynth Res 89:81–87
Babbs CF, Pham Jo Ann, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90:1267–1270
Bai X, Dong Y, Kong J, Xu L, Liu S (2014) Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul. doi:10.1007/s10725-014-9971-3
Ballaré CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF (2011) Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci 10:226–241
Bandurska H, Ciéslak M (2013) The interactive effect of water deficit and UV-B radiation on salicylic acid accumulation in barley roots and leaves. Environ Exp Bot 94:9–18
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brehe JE, Burch HB (1976) Enzymatic assay for glutathione. Anal Biochem 74:189–197
Buettner GR, Jurkiewiez BA (1996) Chemistry and biochemistry of ascorbate. In: Cadenas E, Packer L (eds) Handbook of antioxidants. Marcel Dekker, New York, pp 91–115
Cambrollé J, Mateos-Naranjo E, Redondo-Gómez S, Luque T, Figueroa ME (2011) Growth, reproductive and photosynthetic responses to copper in the yellow-horned poppy, Glaucium flavum Crantz. Environ Exp Bot 71:57–64
Casati P, Campi M, Chu F, Suzuki N, Maltby D, Guan S, Burlingame AL, Walbot V (2008) Histone acetylation and chromatin remodeling are required for UV-B–dependent transcriptional activation of regulated genes in maize. Plant Cell 20:827–842
Catinot J, Buchala A, Abou-Mansour E, Métraux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett 582:473–478
Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Chen YL, Hong XQ, He H, Luo HW, Qian TT, Li RZ, Jiang H, Yu HQ (2014) Biosorption of Cr(VI) by Typha angustifolia: mechanism and responses to heavy metal stress. Bioresour Technol 160:89–92
Choudhary KK, Agrawal SB (2014) Ultraviolet-B induced changes in morphological, physiological and biochemical parameters of two cultivars of pea (Pisum sativum L.). Ecotoxicol Environ Saf 100:178–187
Correia CM, Coutinho JF, Björn LO, Torres-Pereira JMG (2000) Ultraviolet-B radiation and nitrogen effects on growth and yield of maize under Mediterranean field conditions. Eur J Agron 12:117–125
Cui W, Li L, Gao Z, Wu H, Xie Y, Shen W (2012) Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J Exp Bot 63:5521–5534
Dotaniya ML, Das H, Meena VD (2014) Assessment of chromium efficacy on germination, root elongation, and coleoptile growth of wheat (Triticum aestivum L.) at different growth periods. Environ Monit Assess 186:2957–2963
Duman F, Koca FD, Sahan S (2014) Antagonist effects of sodium chloride on the biological responses of an aquatic plant (Ceratophyllum demersum L.) exposed to hexavalent chromium. Water Air Soil Pollut. doi:10.1007/s11270-014-1865-5
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
El-Tayeb MA, El-Enany AE, Ahmed NL (2006) Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). Plant Growth Regul 50:191–199
Flint SD, Caldwell MM (2003) A biological spectral weighting function for ozone depletion research with higher plants. Physiol Plant 117:137–144
Freeman JL, Garcia D, Kim D, Hopf A, Salt DE (2005) Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol 137:1082–1091
Gangwar S, Singh VP (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: implication of oxidative stress. Sci Hortic 129:321–328
Gangwar S, Singh VP, Srivastava PK, Maurya JN (2011) Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol Plant 33:1385–1397
Gao Q, Zhang L (2008) Ultraviolet-B induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol 165:138–148
Genty B, Harbinson J, Briantais JM, Baker NR (1990) The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth Res 25:249–257
Giannopolitis CN, Reis SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gossett DR, Millhollon EP, Cran LM (1994) Antioxidant response to NaCl stress in salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Gowd SS, Reddy MR, Govil PK (2010) Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J Hazard Mater 174:113–121
Hayat S, Hayat Q, Alyemeni MN, Ahmad A (2014) Salicylic acid enhances the efficiency of nitrogen fixation and assimilation in Cicer arietinum plants grown under cadmium stress. J Plant Interact 9:35–42
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hou J, Nanliu G, Xue W, Fu WJ, Liang BC, Liu XH (2014) Seed germination, root elongation, root-tip mitosis, and micronucleus induction of five crop plants exposed to chromium in fluvo-aquic soil. Environ Toxicol Chem 33:671–676
Hui R, Li X, Chen C, Zhao X, Jia R, Liu L, Wei Y (2013) Responses of photosynthetic properties and chloroplast ultrastructure of Bryum argenteum from a desert biological soil crust to elevated ultraviolet-B radiation. Physiol Plant 147:489–501
Kalinowska R, Pawlik-Skowrońska B (2010) Response of two terrestrial green microalgae (Chlorophyta, Trebouxiophyceae) isolated from Cu-rich and unpolluted soils to copper stress. Environ Pollut 158:2778–2785
Kuźniak E, Kaźmierczak A, Wielanek M, Głowacki R, Kornas A (2013) Involvement of salicylic acid, glutathione and protein S-thiolation in plant cell death-mediated defence response of Mesembryanthemum crystallinum against Botrytis cinerea. Plant Physiol Biochem 63:30–38
Lavola A, Nybakken L, Rousi M, Pusenius J, Petrelius M, Kellomäki S, Julkunen-Tiitto R (2013) Combination treatment of elevated UVB radiation, CO2 and temperature has little effect on silver birch (Betula pendula) growth and phytochemistry. Physiol Plant 149:499–514
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liu B, Liu XB, Li YS, Herbert SJ (2013) Effects of enhanced UV-B radiation on seed growth characteristics and yield components in soybean. Field Crops Res 154:158–163
Liu S, Dong Y, Xu L, Kong J (2014) Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul 73:67–78
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mishra V, Srivastava G, Prasad SM (2009) Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic 120:373–378
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4. doi:10.3389/fpls.2014.00004
Nakai Y, Nakahira Y, Sumida H, Takebayashi K, Nagasawa Y, Yamasaki K, Akiyama M, Ohme-Takagi M, Fujiwara S, Shiina T, Mitsuda N, Fukusaki E, Kubo Y, Sato MH (2013) Vascular plant one-zinc-finger protein 1/2 transcription factors regulate abiotic and biotic stress responses in Arabidopsis. Plant J 73:761–775
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawkar GM, Maibam P, Park JH, Sahi VP, Lee SY, Kang CH (2013) UV-induced cell death in plants. Int J Mol Sci 14:1608–1628
O’Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ (2001) Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J 25:315–323
Pandey N, Pandey-Rai S (2014) Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of Artemisia annua L. against short-term UV-B radiation. Planta 240:611–627
Patnaik AR, Achary VMM, Panda BB (2013) Chromium (VI)-induced hormesis and genotoxicity are mediated through oxidative stress in root cells of Allium cepa L. Plant Growth Regul 71:157–170
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Sairam RK, Veerabhadra RK, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schiavon M, Pilon-Smits EAH, Wirtz M, Hell R, Malagoli M (2008) Interaction between chromium and sulfur metabolism in Brassica juncea. J Environ Qual 37:1536–1545
Shanker AK, Cervantes C, Tavera-Loza H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sharma DC, Sharma CP, Tripathi RD (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63–68
Shine MB, Guruprasad KN (2012) Oxyradicals and PSII activity in maize leaves in the absence of UV components of solar spectrum. J Biosci 37:703–712
Singh VP, Srivastava PK, Prasad SM (2012) Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol Biochem 61:61–70
Singh VP, Srivastava PK, Prasad SM (2013) Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol Biochem 71:155–163
Sullivan JH, Teramura AH (1989) The effects of ultraviolet-B radiation on loblolly pine: 1. Growth, photosynthesis and pigment production in greenhouse-grown saplings. Physiol Plant 77:202–207
Teramura AH, Sullivan JH (1991) Potential impacts of increased solar UV-B on global plant productivity. In: Riklis E (ed) Photobiology. Plenum Press, New York, pp 625–634
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Plant Sci 151:59–66
WMO (2007) Scientific assessment of ozone depletion: 2006, global ozone research and monitoring project. Technical Report 50. World Meteorological Organization
Xing W, Li D, Liu G (2010) Antioxidative responses of Elodea nuttallii (Planch.) H. St. John to short-term iron exposure. Plant Physiol Biochem 48:873–878
Yang Y, Qi M, Mei C (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40:909–919
Yin LN, Wang SW (2012) Modulated increased UV-B radiation affects crop growth and grain yield and quality of maize in the field. Photosynthetica 50:595–601
Yu GH, Li W, Yuan ZY, Cui HY, Lv CG, Gao ZP, Han B, Gong YZ, Chen GX (2013) The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica 51:33–44
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Acknowledgments
The authors are grateful to the University Grants Commission, New Delhi for providing financial assistance to carry out this study. One of the authors, Jitendra Kumar, is thankful to UGC for providing financial assistance as RGNF (JRF). Authors are also thankful to Dr. Devinder Kaur, Center of Food Technology, University of Allahabad for performing HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, V.P., Kumar, J., Singh, M. et al. Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul 78, 79–91 (2016). https://doi.org/10.1007/s10725-015-0076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0076-4