Abstract
Autism spectrum disorder (ASD) is a developmental disorder of the brain characterized by shortfall in the social portfolio of an individual and abbreviated interactive and communication aspects rendering stereotypical behavior and pitfalls in a child’s memory, thinking, and learning capabilities. The incidence of ASD has accelerated since the past decade, portraying environment as one of the primary assets, comprising of metallic components aiming to curb the neurodevelopmental pathways in an individual. Many regulations like Clean Air Act and critical steps taken by countries all over the globe, like Sweden and the USA, have rendered the necessity to study the effects of environmental metallic components on ASD progression. The review focuses on the primary metallic components present in the environment (aluminum, lead, mercury, and arsenic), responsible for accelerating ASD symptoms by a set of general mechanisms like oxidative stress reduction, glycolysis suppression, microglial activation, and metalloprotein disruption, resulting in apoptotic signaling, neurotoxic effects, and neuroinflammatory responses. The effect of these metals can be retarded by certain protective strategies like chelation, dietary correction, certain agents (curcumin, mangiferin, selenium), and detoxification enhancement, which can necessarily halt the neurodegenerative effects.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) refers to the series of brain developmental pathologies, encompassing varying and lifelong symptoms, revolving around defiant interactive and communicative skills, poor social attitude, and repetitive restricted responses, behaviors, and interests, occurring in children of 3 or less than 3 years of age (Bjørklund et al. 2018; Kelly et al. 2020). ASD portrays typical destruction of social interaction portfolio restricted to the children leading to behavioral symptoms. The difference between the two is based upon repetition, communication, and diagnostic portfolio, where atypical autism accounts for communication and interaction disabilities, with repetitive occurrence along with inability to conform to the diagnostic criteria unlike childhood autism (WHO 1993). Childhood autism has 3 times more prevalence than atypical autism as per a Danish study (Lauritsen et al. 2004). An increase of 30% in ASD progression was reported in the past 20 years (Calabrese et al. 2016). Between 2011 and 2012, the occurrence of ASD in children of 6–17 years of age was 2% and a significant acceleration in ASD cases began from 2007 by 1.16% (Blumberg et al. 2013). The ASD comprises of three functional levels, where the first level is characterized by negligible social interactions and planning capabilities. The second level involved limited interactions revolving around a limited social space and the third level diagnosed verbal and non-verbal interaction problems. Furthermore, ASD patients have been reported to make unusual sensorimotor predictions (Ernst et al. 2019; Kinard et al. 2020). Additionally, ASD patients demonstrating difficulty in generating accuracy in predictions depict greater symptom severity (Greene et al. 2019).
There has been a tremendous increase in ASD prevalence rate, especially in countries like the USA, which are considered to harbor 1 child in 45, diagnosed with ASD (Zablotsky et al. 2015). The children with ASD develop developmental complications, ascending from mild language and speech problems to severe autistic disorders and lifelong disabilities (Green et al. 2020). There are many factors or complications that might play a role in ASD occurrence like obstetrics complications, fetal hypoxia, age of the parents, bleeding of diabetic complications during pregnancy, medicine administration during prenatal period, enhanced glutamine levels, neuroinflammation, oxidative stress, and pollutants occurring in the environment. One of the possible effects of environmental toxins is the genetic mutations caused by the metallic components of the environment, altering the genetic sequence for fetal development. According to the WHO report of 2016, environmental pollution is the major cause of deaths across the globe featuring about 12.6 million people, out of which 1.7 million are the children less than 5 years of age. Maximum environment-related mortality is associated with the Asian countries as per the WHO, because the children residing in the Asian nations are subjected to diverse ethnic, geographical, cultural, socio-developmental, and cultural surroundings that poses a challenge for the researchers to study the associations between environmental toxins and novel emerging threats, to control the health hazards. Many researchers exhibit birth cohort studies to examine the health aspects of the children such as the Japan Environment and Children’s Study (JECS), Korean Children’s Environmental Health Study (KoCHENS), and the China National Birth Cohort (CNBC) (Tsai et al. 2019). In order to prevent the effects of environmental hazards on living beings, which occur as a result of genetic alterations with the geographical and tribal variations, there is a dire need of cooperation among the nations to facilitate cohort studies effectively, promoting development of successful prevention strategies. Such collaborative studies, carried out at a global level, synergistically provide a mechanistic approach to retard the role of environmental pollutants in negatively affecting the health of individuals. Many substances like POPs, polychlorinated biphenyls, mercury, perfluoroalkyl substances, organochlorine pesticides, phthalates, and environmental tobacco smoke are responsible for triggering the release of cytotoxic substances, immune reactions, and neuroinflammation rendering harmful effects of the brain developmental processes of the individuals (Brockmeyer and D’Angiulli 2016). Presence of harmful pollutants in the environment is responsible for one-third premature deaths globally (Mannucci and Franchini 2017), and such pollutants are designated as “new tobacco” by the WHO (Tsai et al. 2019). Heavy metals like cadmium (Cd), mercury (Hg), arsenic (As), and lead (Pb) have exerted neurotoxic effects on children, rendering disturbed psychomotor, learning, cognitive, and intellectual abilities.
Although the exact cause of this disease is still unknown, yet, there has been an evident link between environmental components and ASD prevalence, primarily involving metals like mercury (Hg), lead (Pb), and aluminum (Al) that may penetrate the brain barriers and facilitate oxidative stress-like situations that significantly lead to ASD progression. The neuro-metabolic alterations are significantly associated with ASD patients causing variations in cerebrospinal fluid (CSF). The active blood brain barrier is present, distinguishing blood form CSF, yet the two fluids are intimately related. ASD is associated with multiple risks and pathologies in children, which interfere with the neurodevelopmental progression of the child, initiating conditions like depression, auditory and other sensory disturbances, attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), obsessive compulsive disorder (OCD), anxiety and stress, Tourette’s syndrome, discrepancies in learning, and coordination problems (Skalny et al. 2016). The environmental effects on ASD progression, specifically metals like mercury, lead, and aluminum, are of critical consideration in the present times. All these components are present in the environment, either released by the industries or automobiles, as well as in day to day devices, objects, and equipment that we use, therefore, have become a primary focus in ASD studies. The possible sources of exposure of primary toxic metals are necessary to understand in order to facilitate effective prevention. The primary sources of toxic metallic components include household utensils, wrapping foils, alloys, fuels, paints, ammunitions, batteries, and medicinal substances like antacids, contraceptives, cosmetics, vaccines, astringents, antiseptives, and fungicides. Exposure to metallic components in the environment might cause mutations or imbalance in intracellular metallic concentrations, which might lead to significant degeneration of brain tissue. The human brain is susceptible to all these adverse effects, only during the early developmental and embryonic phases (especially during first 3 months of pregnancy) (Grandjean and Landrigan 2006). Many patients with ASD are diagnosed with genetic alterations as a result of gene interactions with such environmental toxins. Such susceptibilities can either be inherited or induced (Bjørklund et al. 2018).
Role of metallic pollutants in the environment in brain and ASD progression
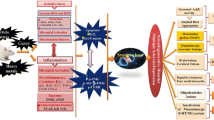
The metallic impurities present in the environment play a significant role in altering the basic neuronal pathways and degenerating the neural processes. Stimulation of inflammatory mediators and vascular endothelial growth factors, microglial activation, oxidative stress, abnormal astrogliogenesis, glutathione upregulation, cell organelle dysfunction, and autoimmune responses are the primary mechanisms evidently signifying the effect of metallic components on neuronal development (Bjørklund et al. 2018). There are many factors that can possibly generate harmful effects on the brain tissue, such as genetic and environment factors (Fig. 1). Both the factors synergize to exert a harmful effect on the human brain at a young age. In many cases, the environmental factors might activate and aggravate the genetic mutations responsible for the neuronal alterations.
The mitochondria are the powerhouse of the cell which fulfills the energy requirements of the cell. Mitochondrial dysfunction might cause neuronal damage. Similarly defects in protein aggregation and degradation mechanisms can accelerate the neurodegeneration processes. Free radical production in the body is responsible for maximum diseases and complications due to the induction of oxidative stress. Release of inflammatory mediators and microglial activation can also result in harmful effects on the biotic brain. All these effects can either be due to genetic mutations or alterations in the genetic sequences, or due to environmental pollutants, that exert effects either independently or by induction of genetic mutations. Various studies have been carried out to determine the role of metallic components in ASD and the alterations in the neuronal processes. Some of them have been discussed in Table 1.
Many metals have been associated with ASD; therefore, it is extremely important to study their percentage occurrence in the environment. The primary metals that play a significant role in ASD occurrence are aluminum, lead, and mercury as discussed in this review. Figure 2 below depicts the percentage contribution of metals to total health index, where arsenic and mercury are prime contributors (Gebeyehu and Bayissa 2020).
Aluminum
The Earth’s crust is a home to many metallic components with aluminum as the third most abundant one. From rocks of cryolite, bauxite, and silicates (Tomljenovic et al. 2014), to our households in cooking equipment, cans, boats, foil, airplanes, cars, electrical devices, packaging and building materials, alloys, fuel additives, explosives, additives, antacids, propellants, astringents, and anti-perspirants, it plays an essential role (Bjørklund et al. 2018). Moreover, aluminum is a metal full of superiorities that possesses many distinguishing characteristics. Even, the vaccine adjuvants comprise of hydroxylated aluminum salts (Tomljenovic et al. 2014). But, despite its presence, the metal forms salts, which are slightly soluble and have toxic effects on the biotic beings of the environment, including the microflora, marine life, plants, animals, and humans. Aluminum is the most durable, light metal that has the ability to oxidize very easily (Bjørklund et al. 2018). It is a strong metal, highly resistant to corrosion. It is highly reactive and can form compounds like aluminum lactate and aluminum citrate, which have potent roles in ASD progression. Aluminum lactate is associated with enhanced TNF-α and IL-1-α levels in the brain, causing apoptotic signaling (Lukiw et al. 2005). Aluminum citrate exerts toxic effects on glial cells of hippocampal cultures (Platt et al. 2007).
Numerous studies have depicted the role of aluminum (in elemental or salt form) in ASD prevalence, when taken via oral route or as an adjuvant. Increased brain levels of aluminum were reported in ASD patients (Mold et al. 2018). Aluminum, in combination with fluorine, synergizes to produce critical health effects such as improvement of behavioral and mental conditions in patients with ASD (Sealey et al. 2016). Exposure to vaccine-derived aluminum generated great risks of developing ASD symptom (Shaw and Tomljenovic 2013).
What does aluminum do when it enters our body?
The sarcoplasmic glycolytic enzymes are affected by the presence of aluminum in the body which leads to a halt in the glycolysis process (Bjørklund et al. 2018). Glycolytic enzymes convert glucose-6-phosphate and nicotinamide adenine dinucleotides to pyruvate and NADH by producing two ATP molecules. Upon entry of aluminum in the system, the glycolysis enzyme action is affected, causing glycolysis suppression, thereby causing reduction in energy production mechanisms.
An Egyptian study revealed the presence of aluminum in the hair of the affected children unlike normal children, but the blood aluminum levels were almost similar to the normal subjects (Mohamed et al. 2015). The presence of aluminum was also detected in the hair (Adams et al. 2006). A higher aluminum content was diagnosed in the urine of patients (Adams et al. 2006), unlike their neurotypical siblings, whereas increased levels of aluminum in blood were diagnosed in ASD patients (Rahbar et al. 2016). Therefore, a childhood autism rating scale (CARS) assessed-study deposited a report elaborating the relationship between aluminum content and ASD progression (Metwally et al. 2015). All the studies for aluminum were conducted at different parameters and the positive studies corresponding to the levels of aluminum in different areas are depicted in Table 2.
Another effect of this toxic metal on neurodevelopmental progress is reactive gliosis induced by aluminum that enhances the GFAP levels and abbreviates the level of anti-oxidant enzymes leading to increased levels of TNF-α and IL-1β, iNOS, and calcium binding adapter molecule 1 (Prakash et al. 2013). Moreover, all these responses induced oxidative stress that led to altered functioning of the powerhouse of the cell, i.e., mitochondria, producing homeostasis imbalance in the brain (Kumar and Gill 2014). Neurogenesis and neurodevelopment might be affected by microglial cells, promoting neuronal proliferation, that might aggravate ASD symptoms (Edmonson et al. 2016). Curcumin and mangiferin had protective actions in such cases (Sood et al. 2012). Not only the elemental form but also the compound form of aluminum (aluminum lactate, aluminum citrate) aggravates the ASD conditions. Moreover, the association of Al3+ ions with oxygen-based ligands or with superoxide anions may be responsible for neurotoxic outcomes (Exley 2012). Cations like Mg 2+ and Ca2+, in association with Al3+, induce neurotoxic and excitotoxic effects (Exley and House 2011).
Mercury
Mercury is a universal environmental pollutant that can be obtained from sea food as well as dental amalgams (Dadar et al. 2014; Bjørklund et al. 2017d). Therefore, not only children but even the adults and the elderly should be cautious against mercury effects. It occupies the position among the top 10 pollutants in the WHO list with a threefold increase in industrial emissions of mercury, and acceleration of atmospheric Hg levels by 1.5% on annual basis (Rice et al. 2014). With every kg of mercury emitted into the environment, an economic loss of 22,937–52,129 Euros is suffered (Nedellec and Rabl 2016). A few studies have indicated the occurrence of ASD in the grandchildren of patients with acrodynia (Shandley and Austin 2011). Therefore, mercuric effects can be long term as an outcome of genetic levels in patients. All the possible effects of mercury can contribute to ASD symptoms. Among several studies carried out between 1999 and 2016, 74% of them revealed an essential link between ASD progression and mercury levels (Kern et al. 2016). Some studies revealed reports of increased mercury levels in teeth of ASD patients and demonstrated that enhanced mercury levels in the body might be due to reduced rate mercury excretion by excessive administration of oral antibiotics (Adams and Romdalvik 2007). An Egyptian study revealed greater mercury levels in the hair and blood samples of ASD patients (Yassa 2014). Another study reported greater mercury levels in blood of pregnant women in late pregnancy and children aged less than 5 years of age, which were found to exhibit autistic symptoms (Ryu et al. 2017). Not only blood and teeth but also endocrine glands and sex hormones, if associated with increased mercury levels, may deteriorate the child developmental procedures, causing ASD-like symptoms (Ryu et al. 2017).
Action of mercury in the brain
Abbreviated levels of glutathione in the body promote mercury retention and deteriorated detoxification processes pave way for mercury toxicity (Jafari et al. 2017). Another pathway of mercury toxicity was revealed by studies depicting exposure of mercury from thimerosal (preservative used in vaccines), leading to atypical autistic symptoms (Geier et al. 2017). Selenium was found to exert protective actions against mercury-induced toxicity (El-Ansary et al. 2017). A study revealed increased concentration of mercury in the urine samples of ASD children unlike normal subjects (Bradstreet et al. 2003). Another risk accounts for mercury placental transfer to the fetus in pregnant mothers with amalgam dental fillings (Bose-O’Reilly et al. 2010). Mercury transfer via breast milk is limited as the fetal digestive system develops metallothioneins to sequester mercury from breast milk (Aschner et al. 2006).
Mercury toxicity indicators, porphyrins, were significantly evaluated in an Egyptian study and enhanced levels of toxic metals like mercury were revealed in ASD patients. Porphyrins like coproporphyrin and precoproporphyrin are relevant biomarkers for mercury toxicity that might be due to excessive exposure to mercury or retarded excretion of the metal from the body, leading to neurological, motor, and sensory differences (Lewis et al. 1992). Neurodevelopmental and cognitive problems were observed in certain studies that highlighted the presence of toxic metals like mercury in amniotic fluid in the fourth and fifth months of pregnancy (Lewis et al. 1992). The study parameters of mercury and the positive results are depicted in Table 3.
Mercury might exert destructive effects on cellular organelles. Hg mediated modulation of cytokine production (IL-6, TNF-α), which may have an adverse impact on ASD patients leading to autoimmune brain response (Curtis 2011), IgG accumulation in brain, and CD4+T cell infiltration (Zhang et al. 2010). Other than this, VEGF activation and IL-6 release from mast cell are also a part of mercury interference protocol which promotes destruction of blood brain barrier (Kempuraj et al. 2010). Another site of mercury toxicity is microglial stimulation-mediated neurotoxicity (Bjørklund et al. 2017d). Mercury toxicity profile resembles that of aluminum in case of oxidative stress induction (Farina et al. 2011). Mercury targets the selenoproteins significantly, on account of their affinity for selenol groups (Farina et al. 2011). Along with glutamate excitotoxicity, oxidative stress primarily leads to cytoskeletal changes as a result of Hg exposure.
Lead
One of the major toxic metals that have adverse effects in nature is lead. The increase in lead levels has been prevalent since 1990s, when lead concentration in soil was increased from 2 to 55 mg/kg (Chen 2016). From 1990 to 2009, lead emissions were approximately 200,000 tons in China (Li et al. 2012). The metal is characterized with many uses in daily life like in ammunitions, car batteries, paints, tin toys, and in automobile and aircraft fuels as anti-knock agents (Bjørklund et al. 2018). Lead obtained from various sources like paints, fuels, batteries, and lead-contaminated water can prove to be disastrous for human consumption (Bellinger 2012). On the Wechsler scale, a loss of 29 million IQ points, on lead exposure, revealed its adverse effects on children (Bellinger 2012). In the USA, lead paints were banned in 1978, and lead present in gasoline and some devices is of critical concern in many areas of globe like Sweden, which is susceptible to periodic surveys in this case (Strömberg et al. 1995). In response to the clean air act, the USA banned the use of leaded gasoline for automobiles in 1996 on account of its role in soil contamination and damage to biotic beings, but as an anti-knock agent, it is still being used (Bjørklund et al. 2018).
Moreover, lead-contaminated water (due to its interaction with lead pipes) alone and when used for processing food and beverages poses a hazardous threat to neurodevelopmental processes in children. Lead synergized with mercury and arsenic lead to neurodevelopmental problems as observed in ASD (Dickerson et al. 2015). The disastrous effect of lead was revealed when a study demonstrated the occurrence of autistic symptoms in children of 8 years of age, even at low concentrations (Kim et al. 2016). Egyptian studies revealed lead levels in blood samples unlike neurotypical children (Khaled et al. 2016). The percentage increase in the levels of blood lead corresponding to different exposure sources is depicted in the Fig. 3 (Lanphear et al. 2002).
Some researchers have depicted a successful study associating the urban emissions of toxic metals and ASD prevalence in such areas, generating a potent evidence for role of lead in ASD progression. Moreover, the blood samples of ASD patients were found to possess increased levels of lead and mercury, with retarded anti-oxidant levels, like glutathione and vitamin E, in some studies (Alabdali et al. 2014). Lead levels were observed in hair and nail samples of ASD patients, as per the data generated in a few studies (Priya and Geetha 2011). An auto-immunity and neuroinflammation stimulatory effect was observed in further studies, due to presence of lead in the system, with certain studies revealing serum anti-ribosomal P antibodies production, as a result of increased lead content (Mostafa et al. 2016b). Different study parameters of lead with the positive results are discussed in Table 4.
Many studies conducted in this behalf observed a variation in the level of lead in ASD patients. What could possibly be the reason?
-
Variable geographical exposure to lead
-
Air pollution
-
Socio-demographic differences
-
Lead-contained paints and old houses
-
Lead-contaminated water consumption
-
Leaded gasoline
-
Industrial exposures
Along with being a neuroinflammatory mediator, lead regulates the concentrations of IL-6, IL-18, and TNF-α in specified brain areas, specifically in the cerebral cortex and hippocampus, promoting learning and memory problems. Lead is also associated with upregulation of VEGF, leading to brain toxicity and neuroinflammation (Kasten Jolly et al. 2011). Similar to mercury and aluminum, lead is also associated with microglial activation, which regulates pro-inflammatory cytokine production, causing destruction to the signaling pathways in the brain and promoting autistic behavior (Strużyńska et al. 2006). Astrogliosis was found to be induced by exposure to lead in a study involving young mice (Bjørklund et al. 2018). Oxidative stress is another factor that potentiates neurotoxicity by lead, that is, initiated by SOD (superoxide dismutase) decrease along with retarded actions of glutathione peroxidase and disulfide reductase enzymes, causing damage to the blood brain barrier, thus leaving the brain viable to the destructive effects of toxic metals (Baranowska-Bosiacka et al. 2012). In some studies, the creatine and pyruvate kinase inhibiting effects of lead have been considered to produce neurotoxicity on account of damaged energy homeostasis in the brain (Lepper et al. 2010). Lead-based alterations in lipid profiles of the body were other possible pathways of lead toxicity (Jung et al. 2017). Some studies concluded the role of N-methyl-D-aspartate receptor (target template for lead toxic effects), in promoting BDNF (brain-derived neurotropic factor) inhibition by lead, altering synaptic formation and function, during the developmental stage, leading to neuronal impairment (Neal et al. 2010).
Arsenic
Exposure to arsenic containing compounds poses a significant threat to the biological system in humans (Rosen and Liu 2009). One of the most common sources of arsenic poisoning is the As-contaminated groundwater (Bjørklund et al. 2017e). The excessive presence of As in drinking water, exceeding the limit recommended by the WHO, has exposed about 140 million people to arsenic poisoning. Most people with arsenic poisoning are found in countries like Bangladesh, India, and Argentina (Rahman et al. 2016). However, arsenic is present in the Earth’s crust (in small amounts) and in minerals. It also occurs in the form of salts and organometallic compounds. The industrial production of arsenic is the major source of arsenic poisoning with arsine (H3As) as the most toxic reagent used in the industries. About 4.53 million tons of As was estimated to be produced in 2000 (Han et al. 2003). Furthermore, exposure to As via drinking water is another important consideration in management of As poisoning, with more than 200 million people exposed to As through drinking water across the globe (Kabay et al. 2010). About $3742 is the total cost of 1 kg of As emissions, out of which 20% is related to psychiatric problems and loss of intelligence, therefore, targeting brain as the most affected area of arsenic poisoning (Nedellec and Rabl 2016).
Exposure induces neurodegeneration events and gliosis, deteriorating the morphology of the brain and BBB disruption (Selim et al. 2012). The arsenic compounds are capable of activating JNK and p38 signaling pathway, resulting in apoptosis in the brain (Namgung and Xia 2001), followed by glutathione depression and lipid peroxidation, contributing to the increased oxidative stress in the brain (Yen et al. 2011). The oxidative stress was shown to mediate neurotoxic effects in the brain (Liu et al. 2013). It was reported that As exposure to mice resulted in elevated levels of Bax and Bak, along with retarded Mcl-1 levels in the cerebral cortex of mice. Disabled neurite growth occurs due to As exposure, on account of its ability to suppress activation of AMPK kinase (Wang et al. 2010). Increased level of pro-inflammatory cytokines in astrocytes is produced by exposure to monomethylarsonous acid (Escudero-Lourdes et al. 2016). Arsenic-mediated inflammatory and neuronal damage occurs, due to alterations in arachidonic acid (Anwar-Mohamed et al. 2014). Arsenic exposure in mice led to enhanced expression of glial fibrillation acidic protein, resulting in cognitive defects and impaired learning abilities (Jiang and Sun 2011). It has also been reported that, due to enhanced expression of GRP94, GRP78, and CHOP, As-exposure is capable of mediating endoplasmic stress (Yen et al. 2011). Mitochondria have been observed as the major target of As-mediated toxicity, resulting in apoptotic death in microglial cells (Prakash et al. 2016; Kharroubi et al. 2017). Neurotransmitter metabolism is also altered by As, resulting in neuronal damage due to disruption of release of glutamate-induced glio-transmitter (Wang et al. 2012). Glutamate transport is also affected due to alterations in EAAT1/GLAST activity in glial cells (Castro-Coronel et al. 2011). Moreover, As also mediates neurotoxicity in dopaminergic signaling in the brain (Shavali and Sens 2007). As-mediated cholinergic alterations lead to impaired memory and learning capabilities in rats (Chandravanshi et al. 2014). Epigenetic modification is another mechanism through which As toxicity prevails, which induces his tone modifications in the CNS (Bjørklund et al. 2017e).
Numerous studies have reported the harmful effects of arsenic in children (Lonsdale et al. 2011; Skalny et al. 2017). Obrenovich et al. (2011), in his study conducted on 39 neurotypical children and 26 children with ASD, reported that the As concentration in ASD patients was higher than in neurotypical patients. Similar study was conducted in ASD patients from Saudi, where enhanced levels of As was found in the patients unlike normal ones (Al-Ayadhi 2005). High levels of As were found in hair samples of ASD patients in a study, but there was no significant difference in the concentration of As levels in the urine of ASD and neurotypical children (Blaurock-Busch et al. 2011). Table 5 summarizes the effects and action mechanism of aluminum, lead, mercury, and arsenic, as discussed in the review.
Reducing the metallic toxicity effects
One of the major control parameters of reducing the toxic effects of metals is definitely the reduction of exposure to metallic components and diminishing the possible effects of such exposures. Potentiating the natural detoxification mechanisms might develop appreciable defense against metal toxicity. Use of chelating agents like EDTA, which successfully combines with the metals and promotes their excretion out of the body, can also serve to lower the metal effects (Bjørklund et al. 2017c). Moreover, dietary supplements have proved to be quite beneficial and significant in reducing the effects of metals on the biological systems. Supplements containing zinc and selenium can significantly curb the effects of metallic toxins by promoting GSH upregulation (Rahman et al. 2017). Similarly, vitamin C, methionine, and tocopherol supplementations lower the lead induces oxidative stress in primary areas of the body (Patra et al. 2001).
Conclusion
The alarming increase in incidence of autism spectrum disorder among children has encouraged various researchers to study the possibilities behind the cause of this condition in order to curb the progression of behavioral differences. Most of the times, we focus on physiological, biological, mental, and social causes, but it is very rare that we consider the role of environment in which we breathe, to be responsible. The environment we live in comprises of a number of components, metallic, non-metallic, microbial, and so on that might induce certain biological alterations in the human body and makes it susceptible to various diseases. The metallic toxins like aluminum, cadmium, arsenic, lead, mercury, manganese, and iron are responsible in some or another for such biological alterations. Over the last few years, many studies have revealed the relationship between ASD and toxic metals, due to which is necessary to study the effects and occurrences of such metals. The mechanisms involved in the toxic metals mediated neurotoxicity primarily involve oxidative stress induction, microglial activation, metalloprotein disruption, endoplasmic reticulum stress, autoimmune stimulation, and glycolysis suppression, which curb the neurodevelopmental processes and accelerate the neurodegeneration pathway, aiding neurotoxic and excitotoxic effects, apoptosis, and neuroinflammation. Various studies have evidently depicted the comparison between ASD patients and neurotypical subjects, by using biomarkers, to provide metallic occurrence in urine, erythrocyte, hair, and teeth.
Moreover, parallel to the effect of metallic toxins on biological systems, the treatment strategies form another protocol that needs to be focused upon, involving use of chelating agents, dietary corrections, glutamate antagonists, anti-inflammatory, and antioxidant therapies, enhancing the detoxification ability of the primarily by glutathione enhancement, and ameliorating antibiotics usage during pregnancy, as well as administering protective agents like curcumin, magniferin, selenium, and chelating agents. With the accelerated risks of ASD in the modern world of industrial and technological advancement, it is tremendously necessary to consider the metallic components in the environment critically, and take necessary actions to curb the risks of neurodevelopmental disruptions in the children, in order to promote safe and effective environment for the growing generation of the modern world.
Future perspectives
There is a dire need to understand the effects of environmental pollutants on brain development and their tendency to aggravate ASD symptoms. Currently, there is not enough focus on the environmental metals and their effects on ASD patients. The link between metallic pollutants and brain disorders is faded in the minds of the people globally. Thus, there should be a proper awareness among the people regarding the harm such pollutants can cause in the neuro-developmental processes in children. Such gaps in knowledge should be filled and significant precautionary approaches can be made by developing certain models and undergoing research studies to understand various etiological variations and possible causes of ASD progression, evaluating neurobehavioral responses in different environmental mediums, investigating the effect of environmental pollutants and their exposure in pregnant mothers, lactating mothers, infants, and early childhood and analysis of neurotoxic metals in the environment affecting the brain developmental pathways in children. Other than the three major metals discussed in the review, there are many other metallic components responsible for aggravation of ASD symptoms, especially during early years of life, when the human body is highly susceptible to environmental toxins. Therefore, in order to facilitate proper growth and developmental processes, primary focus is required towards the neurotoxins in the environment, and effective measures should be taken to retard the growing risks of ASD in children due to the harmful pollutants in the environment.
References
Adams JB, Romdalvik J (2007) Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A 70:1046–1051. https://doi.org/10.1080/15287390601172080
Adams JB, Holloway CE, George F, Quig D (2006) Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol Trace Elem Res 110:193–209. https://doi.org/10.1385/BTER:110:3:193
Alabdali A, Al-Ayadhi L, El-Ansary A (2014) A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav Brain Funct 10:14. https://doi.org/10.1186/1744-9081-10-14
Al-Ayadhi LY (2005) Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosci. 10:213–218
Albizzati A, More L, Di Candia D, Saccani M, Lenti C (2012) Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr 64:27–31
Alexandrov PN, Pogue AI, Lu WJ (2018) Synergism in aluminum and mercury neurotoxicity. Integr Food NutrMetab 5:1–7. https://doi.org/10.15761/IFNM.1000214
Al-Farsi YM, Waly MI, Al-Sharbati MM, Al-Shafaee MA, Al-Farsi OA, Al-Khaduri MM, Gupta I, Ouhtit A, Al-Adawi S, Al-Said MF, Deth RC (2013) Levels of heavy metals and essential minerals in hair samples of children with autism in Oman: a case-control study. Biol Trace Elem Res 151:181–186. https://doi.org/10.1007/s12011-012-9553-z
Anwar-Mohamed A, Elshenawy OH, El-Sherbeni AA, Abdelrady M, El-Kadi AO (2014) Acute arsenic treatment alters arachidonic acid and its associated metabolite levels in the brain of C57Bl/6 mice. Can J Physiol Pharmacol 92:693–702
Aschner M, Syversen T, Souza DO, Rocha JBT (2006) Metallothioneins: mercury species specific induction and their potential role in attenuating neurotoxicity. Exp Biol Med (Maywood) 231:1468–1473. https://doi.org/10.1177/153537020623100904
Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, Marchetti C, Kurzawski M, Dziedziejko V, Nowacki P (2012) Disrupted pro-and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res 1435:56–71. https://doi.org/10.5114/ninp.2012.31607
Bellinger DC (2012) Comparing the population neurodevelopmental burdens associated with children’s exposures to environmental chemicals and other risk factors. Neurotoxicology. 33:641–643
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp 73:225–236
Bjørklund G, Mutter J, Aaseth J (2017c) Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol. https://doi.org/10.1007/s00204-017-2100-0
Bjørklund G, Dadar M, Mutter J, Aaseth J (2017d) The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554. https://doi.org/10.1016/j.envres.2017.08.051
Bjørklund G, Aaseth J, Chirumbolo S, Urbina MA, Uddin R (2017e) Effects of arsenic toxicity beyond epigenetic modifications. Environ Geochem Health. https://doi.org/10.1007/s10653017-9967-9
Bjørklund G, Skalny AV, Rahman MM, Dadar M et al (2018) Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ Res 166:234–250. https://doi.org/10.1016/j.envres.2018.05.020
Blaurock-Busch E, Amin OR, Rabah T (2011) Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica (Buchar) 6:247–257
Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC (2013) Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. Natl Health Stat Rep 20:1–11
Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B (2010) Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care 40:186–215. https://doi.org/10.1016/j.cppeds.2010.07.002
Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR (2003) A case-control study of mercury burden in children with autistic spectrum disorders. J Am Phy Surg 8:76–79
Brockmeyer S, D’Angiulli A (2016) How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci 7:24–30. https://doi.org/10.1515/tnsci-2016-0005
Calabrese V, Giordano J, Ruggieri M, Berritta D, Trovato A, Ontario ML, Bianchini R, Calabrese EJ (2016) Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J Neurosci Res 94:1488–1498. https://doi.org/10.1002/jnr.23893
Castro-Coronel Y, Del Razo LM et al (2011) Arsenite exposure downregulates EAAT1/GLAST transporter expression in glial cells. Toxicol Sci 122:539–550
Chandravanshi LP et al (2014) Reversibility of changes in brain cholinergic receptors and acetylcholinesterase activity in rats following early life arsenic exposure. Int J Dev Neurosci 34:60–75
Chen M (2016) A century long sedimentary record of anthropogenic lead (Pb), Pb isotopes and other trace metals in Singapore. Environ Pollut 213:446–459. https://doi.org/10.1016/j.envpol.2016.02.040
Curtis JT (2011) Chronic inorganic mercury exposure induces sex-specific changes in central TNFα expression: importance in autism? Neurosci Lett 504:40–44. https://doi.org/10.1016/j.neulet.2011.08.053
Dadar M, Peyghan R, Memari HR (2014) Evaluation of the bioaccumulation of heavy metals in white shrimp (Litopenaeusvannamei) along the Persian Gulf coast. Bull Environ Contam Toxicol 93:339–343. https://doi.org/10.1007/s00128-014-1334-2
Dickerson AS et al (2015) Autism spectrum disorder prevalence and proximity to industrial facilities releasing arsenic, lead or mercury. Sci Total Environ 536:245–251. https://doi.org/10.1016/j.scitotenv.2015.07.024
Edmonson CA, Ziats MN, Rennert OM (2016) A non-inflammatory role for microglia in autism spectrum disorders. Front Neurol 7:9. https://doi.org/10.3389/fneur.2016.00009
El-Ansary A et al (2017) Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis 32:1073–1080. https://doi.org/10.1007/s11011-017-9996-1
Ernst TM, Brol AE, Gratz M, Ritter C, Bingel U, Schlamann M, Maderwald S, Quick HH, Merz CJ, Timmann D (2019) The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. Elife. 8:e46831
Escudero-Lourdes C, Uresti-Rivera EE, Oliva-González C, Torres-Ramos MA, Aguirre-Bañuelos P, Gandolfi AJ (2016) Cortical astrocytes acutely exposed to the monomethylarsonous acid (MMAIII) show increased pro-inflammatory cytokines gene expression that is consistent with APP and BACE-1: over-expression. Neurochem Res 41:2559–2572
Exley C (2012) The coordination chemistry of aluminium in neurodegenerative disease. Coord Chem Rev 256:2142–2146. https://doi.org/10.1016/j.ccr.2012.02.020
Exley C, House ER (2011) Aluminium in the human brain. Monatsh Chem 142:357–363. https://doi.org/10.1007/s00775-019-01710-0
Farina M, Aschner M, Rocha JB (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256:405–417. https://doi.org/10.1016/j.taap.2011.05.001
Fuentes-Albero M, Puig-Alcaraz C, Cauli O (2015) Lead excretion in spanish children with autism spectrum disorder. Brain Sci 5:58–68. https://doi.org/10.3390/brainsci5010058
Gadad BS, Li W et al (2015) Administration of Thimerosal-containing vaccines to infant rhesus macaques does not result in autism-like behavior or neuropathology. Proc Natl Acad Sci U S A 112:12498–12503. https://doi.org/10.1073/pnas.1500968112
Gebeyehu HR, Bayissa LD (2020) Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS One 15(1):e0227883. https://doi.org/10.1371/journal.pone.0227883
Geier DA, Kern JK, Geier MR (2017) Increased risk for an atypical autism diagnosis following Thimerosal-containing vaccine exposure in the United States: a prospective longitudinal case-control study in the Vaccine Safety Datalink. J Trace Elem Med Biol 42:18–24. https://doi.org/10.1177/1559325817690849
Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals: a silent pandemic. Lancet 368:2167–2178. https://doi.org/10.1016/S0140-6736(06)69665-7
Green HL, Shuffrey LC, Levinson L, Shen G, Avery T, Wagner MR, Sepulveda DM, Garcia P, Maddox C, Garcia F, Hassan S, Froud K (2020) Evaluation of mismatch negativity as a marker for language impairment in autism spectrum disorder. J Commun Disord 87:105997. https://doi.org/10.1016/j.jcomdis.2020.105997
Greene RK, Zheng S, Kinard JL, Mosner MG, Wiesen CA, Kennedy DP, Dichter GS (2019) Social and nonsocial visual prediction errors in autism spectrum disorder. Autism Res 12:878–883
Han FX et al (2003) Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 90:395–401
Jafari T, Rostampour N, Fallah AA, Hesami A (2017) The association between mercury levels and autism spectrum disorders: a systematic review and meta-analysis. J Trace Elem Med Biol 44:289–297. https://doi.org/10.1016/j.jtemb.2017.09.002
Jiang D, Sun BF (2011) Effect of chronic arsenic poisoning on astrocyte in hippocampal CA1 area of mouse. J Reg Anat Oper Surg 3:239–241
Jung JM, Lee J et al (2017) The effect of lead exposure on fatty acid composition in mouse brain analyzed using pseudo-catalytic derivatization. Environ Pollut 222:182–190. https://doi.org/10.1016/j.envpol.2016.12.058
Kabay N, Bundschuh J et al (2010) The global arsenic problem: challenges for safe water production. CRC Press, Boca Raton
Kasten Jolly J, Heo Y, Lawrence DA (2011) Central nervous system cytokine gene expression: modulation by lead. J Biochem Mol Toxicol 25:41–54. https://doi.org/10.1002/jbt.20358
Kelly E, Escamilla CO, Tsai PT (2020) Cerebellar dysfunction in autism spectrum disorders: deriving mechanistic insights from an internal model framework. Neurosci. 0306-4522. https://doi.org/10.1016/j.neuroscience.2020.11.012.
Kempuraj D et al (2010) Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation 7:20. https://doi.org/10.1186/1742-2094-7-20
Kern JK, Geier DA, Sykes LK, Haley BE, Geier MR (2016) The relationship between mercury and autism: a comprehensive review and discussion. J Trace Elem Med Biol 37:8–24. https://doi.org/10.1016/j.jtemb.2016.06.002
Khaled EM et al (2016) Altered urinary porphyrins and mercury exposure as biomarkers for autism severity in Egyptian children with autism spectrum disorder. Metab Brain Dis 31:1419–1426. https://doi.org/10.1007/s11011-016-9870-6
Kharroubi W, Ahmed SH, Nury T, Andreoletti P, Sakly R, Hammami M, Lizard G (2017) Mitochondrial dysfunction, oxidative stress and apoptotic induction in microglial BV-2 cells treated with sodium arsenate. J Environ Sci 51:44–51
Kim KN, Kwon HJ, Hong YC (2016) Low-level lead exposure and autistic behaviors in school-age children. Neurotoxicology 53:193–200. https://doi.org/10.1016/j.neuro.2016.02.004
Kinard JL, Mosner MG, Greene RK, Addicott M, Bizzell J, Petty C, Cernasov P, Walsh E, EisenlohrMoul T, Carter RM (2020) Neural mechanisms of social and nonsocial reward prediction errors in adolescents with autism spectrum disorder. Autism Res 13:715–728
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166. https://doi.org/10.1016/j.neuro.2014.02.004
Lanphear BP et al (2002) J Pediatr 140:40–47
Lauritsen MB, Pedersen CB, Mortensen PB (2004) The incidence and prevalence of pervasive developmental disorders: a Danish population-based study. Psychol Med 34:1339–1346. https://doi.org/10.1017/s0033291704002387
Lepper TW, Oliveira E, Koch GDW, Berlese DB, Feksa LR (2010) Lead inhibits in vitro creatine kinase and pyruvate kinase activity in brain cortex of rats. Toxicol in Vitro 24:1045–1051
Lewis M, Worobey J, Ramsay DS, McCormack MK (1992) Prenatal exposure to heavy metals: effect on childhood cognitive skills and health status. Pediatrics 89:1010–1015
Li Q, Cheng H, Zhou T, Lin C, Guo S (2012) The estimated atmospheric lead emissions in China, 1990–2009. Atmos Environ 60:1–8
Liu X et al (2013) Neuroglobin involvement in the course of arsenic toxicity in rat cerebellar granule neurons. Biol Trace Elem Res 155:439–446
Lonsdale D, Shamberger RJ, Obrenovich ME (2011) Dysautonomia in autism spectrum disorder: case reports of a family with review of the literature. Autism Res Treat. https://doi.org/10.1155/2011/129795
Lukiw WJ, Percy ME, Kruck TP (2005) Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem 99:1895–1898. https://doi.org/10.1016/j.jinorgbio.2005.04.021
Mannucci PM, Franchini M (2017) Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health 14:1048. https://doi.org/10.3390/ijerph14091048
Metwally FM, Abdelraoof ER et al (2015) Toxic effect of some heavy metals in Egyptian autistic children. Int J Pharm Clin Res 7:206–211
Miller N (2016) Aluminum in childhood vaccines is unsafe. J Am Phys Surg 21:109–117
Mohamed FE et al (2015) Assessment of hair aluminum, lead, and mercury in a sample of autistic Egyptian children: environmental risk factors of heavy metals in autism. Behav Neurol 2015:545674. https://doi.org/10.1155/2015/545674
Mold M, Umar D, King A, Exley C (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol 46:76–82. https://doi.org/10.1016/j.jtemb.2017.11.012
Mostafa GA, Bjørklund G, Urbina MA, AL-Ayadhi LY (2016a) The levels of blood mercury and inflammatory-related neuropeptides in the serum are correlated in children with autism spectrum disorder. Metab Brain Dis 31:593–599. https://doi.org/10.1007/s11011-015-9784-8
Mostafa GA, Bjørklund G, Urbina MA, AL-Ayadhi LY (2016b) The positive association between elevated blood lead levels and brain-specific autoantibodies in autistic children from low lead-polluted areas. Metab Brain Dis 31:1047–1054
Namgung UK, Xia Z (2001) Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol Appl Pharmacol 174:130–138
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor–dependent BDNF signaling. Toxicol Sci 116:249–263
Nedellec V, Rabl A (2016) Costs of health damage from atmospheric emissions of toxic metals. Part 2: analysis for arsenic and cadmium. Risk Anal 36:2096–2104. https://doi.org/10.1111/risa.12598
Obrenovich ME, Shamberger RJ, Lonsdale D (2011) Altered heavy metals andtransketolase found in autistic spectrum disorder. Biol Trace Elem Res 144:475–486. https://doi.org/10.1007/s12011-011-9146-2
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162:81–88. https://doi.org/10.1016/s0300-483x(01)00345-6
Platt B, Drysdale AJ, Nday C, Roloff EVL, Drever BD, Salifoglou A (2007) Differential toxicity of novel aluminium compounds in hippocampal culture. Neurotoxicology 28:576–586
Prakash D, Gopinath K, Sudhandiran G (2013) Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride induced neurotoxicity. NeuroMolecular Med 15:192–208. https://doi.org/10.1007/s12017-012-8210-1
Prakash C, Soni M, Kumar V (2016) Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol 36:179–188
Priya MD, Geetha A (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 142:148–158. https://doi.org/10.1007/s12011-010-8766-2
Rahbar MH, Samms-Vaughan M et al (2016) Role of metabolic genes in blood aluminum concentrations of Jamaican children with and without autism spectrum disorder. Int J Environ Res Public Health 13:1095. https://doi.org/10.3390/ijerph13111095
Rahman MM, Shammi M et al (2016) Assessment of the status of groundwater arsenic at Singair Upazila, Manikganj Bangladesh; exploring the correlation with other metals and ions. Expo Health 8:217–225. https://doi.org/10.1007/s12403-016-0196-8
Rahman MM, Ukiana J, Lopez RU, Sikder MT, Saito T, Kurasaki M (2017) Cytotoxic effects of cadmium and zinc co-exposure in PC12 cells and the underlying mechanism. Chem Biol Interact 269:41–49. https://doi.org/10.1016/j.cbi.2017.04.003
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47:74–83. https://doi.org/10.3961/jpmph.2014.47.2.74
Rosen BP, Liu Z (2009) Transport pathways for arsenic and selenium: a mini review. Environ Int 35:512–515
Ryu J, Ha EH, Kim BN, Ha M, Kim Y, Park H, Hong YC, Kim KN (2017) Associations of prenatal and early childhood mercury exposure with autistic behaviors at 5years of age: the Mothers and Children’s Environmental Health (MOCEH) study. Sci Total Environ 605–606:251–257
Sealey LA, Hughes BW, Sriskanda AN, Guest JR, Gibson AD, Johnson-Williams L, Pace DG, Bagasra O (2016) Environmental factors in the development of autism spectrum disorders. Environ Int 88:288–298. https://doi.org/10.1016/j.envint.2015.12.021
Selim SA, Selim AO, Askar EM (2012) Harmful effects of arsenic on the cerebral cortex of adult male albino rats: light and electron microscopic studies. Egypt J Histol 35:249–258
Shandley K, Austin DW (2011) Ancestry of pink disease (infantile acrodynia) identified as a risk factor for autism spectrum disorders. J Toxicol Environ Health A 74:1185–1194. https://doi.org/10.1080/15287394.2011.590097
Shavali S, Sens DA (2007) Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol Sci 102:254–261
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56:304–316. https://doi.org/10.1007/s12026-013-8403-1
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR et al (2016) Assessment of serum trace elements and electrolytes in children withchildhood and atypical autism. J Trace Elem Med Biol 43:9–14. https://doi.org/10.1016/j.jtemb.2016.09.009
Skalny AV et al (2017) Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis 32:195–202
Sood PK, Nahar U, Nehru B (2012) Stress proteins and glial cell functions during chronic aluminium exposures: protective role of curcumin. Neurochem Res 37:639–646. https://doi.org/10.1007/s11064-011-0655-3
Strömberg U, Schütz A, Skerfving S (1995) Substantial decrease of blood lead in Swedish children, 1978-94, associated with petrol lead. Occup Environ Med 52:764–769. https://doi.org/10.1136/oem.52.11.764
Strużyńska L, Dąbrowska-Bouta B, Koza K, Sulkowski G (2006) Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci 95:156–162. https://doi.org/10.1093/toxsci/kfl134
Tomljenovic L, Blaylock RL, Shaw CA (2014) Autism spectrum disorders and aluminum vaccine adjuvants. In: In: Comprehensive Guide to Autism. Springer, New York, pp 1585–1609
Tsai MS, Chen MH, Lin CC, Liu CY, Chen PC (2019) Children’s environmental health based on birth cohort studies of Asia (2) – air pollution, pesticides, and heavy metals. Environ Res 179:108754. https://doi.org/10.1016/j.envres.2019.108754
Wang X, Meng D, Chang Q, Pan J, Zhang Z, Chen G, Shi X (2010) Arsenic inhibits neurite outgrowth by inhibiting the LKB1–AMPK signaling pathway. Environ Health Perspect 118:627–634
Wang Y, Zhao F, Liao Y, Jin Y, Sun G (2012) Arsenic exposure and glutamateinduced gliotransmitter release from astrocytes. Neural Regen Res 7:2439–2445
World Health Organization (1993) The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research, Geneva.
Yassa HA (2014) Autism: a form of lead and mercury toxicity. Environ. Toxicol Pharmacol 38:1016–1024
Yasuda H, Yoshida K, Yasuda Y, Tsutsui T (2011) Infantile zinc deficiency: association with autism spectrum disorders. Sci Rep 1:129. https://doi.org/10.1038/srep00129
Yen CC, Ho TJ, Wu CC, Chang CF, Su CC, Chen YW, Liu SH (2011) Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch Toxicol 85:565–575
Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ (2015) Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. Natl Health Stat Rep 87:1–20
Zhang Y, Gao D, Bolivar VJ, Lawrence DA (2010) Induction of autoimmunity to brain antigens by developmental mercury exposure. Toxicol Sci 119:270–280. https://doi.org/10.1093/toxsci/kfq334
Author information
Authors and Affiliations
Contributions
IK and TB, conceived the idea and wrote the first draft; MAR, data compilation; AK, SA, figure work; RA and LA, proof read.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
All the authors have given the consent to publish the paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Autistic behavioral symptoms in certain cases are associated with the metallic components in the environment.
• Metals like aluminum, mercury, and lead play a vital role in aggravating autistic conditions.
• Review focuses on the actions of the metallic pollutants and agents to curb their effects.
Rights and permissions
About this article
Cite this article
Kaur, I., Behl, T., Aleya, L. et al. Role of metallic pollutants in neurodegeneration: effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ Sci Pollut Res 28, 8989–9001 (2021). https://doi.org/10.1007/s11356-020-12255-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12255-0