Abstract
Hydrocarbon is a primary source of energy in the current urbanized society. Considering the increasing demand, worldwide oil productions are declining due to maturity of oil fields and because of difficulty in discovering new oil fields to substitute the exploited ones. To meet current and future energy demands, further exploitation of oil resources is highly required. Microorganisms inhabiting in these areas exhibit highly diverse catabolic activities to degrade, transform, or accumulate various hydrocarbons. Enrichment of hydrocarbon-utilizing bacteria in oil basin is caused by continuous long duration and low molecular weight hydrocarbon microseepage which plays a very important role as an indicator for petroleum prospecting. The important microbial metabolic processes in most of the oil reservoir are sulfate reduction, fermentation, acetogenesis, methanogenesis, NO3− reduction, and Fe (III) and Mn (IV) reduction. The microorganisms residing in these sites have critical control on petroleum composition, recovery, and production methods. Physical characteristics of heavy oil are altered by microbial biotransformation and biosurfactant production. Considering oil to be one of the most vital energy resources, it is important to have a comprehensive understanding of petroleum microbiology. This manuscript reviews the recent research work referring to the diversity of bacteria in oil field and reservoir sites and their applications for enhancing oil transformation in the target reservoir and geomicrobial prospecting scope for petroleum exploration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuel supports most of the global energy demand, and this continues to rise with increasing population and urbanization. Among non-renewable energy sources, hydrocarbon is viewed as an essential source of energy in the current urbanized society. Petroleum is a natural hydrocarbon product ensuing due to the anaerobic biotransformation under high temperature and pressure. This product enters our biosphere by natural seepage but at a very slow rate than forceful recovery operations. Considering the increasing demand, worldwide oil productions are declining due to maturity of oil field and also because of difficulty in discovering new oil fields to substitute the exploited ones (Head et al. 2003). Aging of oil fields is a persistent and vital concern faced by the global oil industry; thus, strategies for recovery of oil from aged reservoirs is highly required (Suthar et al. 2008). The process of exploration relies on the success and potential of detection and drilling process. Discovery of oil, determining its amount, quality, existing reservoir physico-chemical conditions, and the grounding of the field for production and development, is dependent on the exploration process (Rovere et al. 2020). Thus, the process of oil exploration is a comprehensive tool which involves applied science and technique in a coordinated manner. Biodegraded heavy oils dominate the world petroleum inventory mostly in shallow reservoir basins (Bourdet et al. 2014). Oil fields are not distributed uniformly throughout the world. Nearly 50% of the worlds confirmed reserves are situated in the Middle East and the area which includes Russia, Kazakhstan, and nations formerly in the Soviet Union (Visser and Spoor 2011). The amount of recoverable oil and flammable gas from an oil well may be much lower than the total amount of existing reserves. For instance, the Middle East contains around 50% of the world’s known reserves; however, it represents just around 30% of worldwide oil yield (Suthar et al. 2008). The USA, on the other hand, makes a case for less than 2% of the world’s total petroleum resource, but it is able to meet about 16% of the world’s oil demand. To meet present and future energy demand, further exploitation of oil resources is required to maximize recovery in mature and aged reserves (Suthar et al. 2008). Ever since the discovery of the primary oil well in 1859, almost fifty thousand oil fields have been found. However, 90% of these fields do not have an important role in oil generation and supply. Based on the total prospected oil reverse, these wells are classified as supergiants (reserve having more than 1 billion barrels) and mammoths (having 500 million–5 billion barrels) of recoverable oil (Horn 2007). The Arabian-Iranian sedimentary basin in the Persian Gulf area includes 66% of these supergiant fields. The rest of the supergiants are located in the USA, Russia, Mexico, Libya, Algeria, Venezuela, China, and Brazil (Safdel et al. 2017). The hydrocarbon asset in India is generally focused on the states of Maharashtra, Gujrat, Assam, Tamilnadu, Rajasthan, and Arunachal Pradesh (Panda 2017). The first oil reserve discovered in India was in 1859 in Digboi, Assam. As of 31 March 2018, India has assessed crude petroleum reserve of 594.49 million tons. Thus, building oil stores like underground tank stockpiling and over the ground tank stockpiling and using potential techniques to exploit in situ reserves are a worthwhile suggestion for an oil bringing in any nation.
Microorganisms inhabiting these oil reserve sites exhibit highly diverse catabolic activity to degrade, transform, or accumulate various hydrocarbon compounds. The ultimate fate of most of the petroleum hydrocarbons in the environment is degradation or catabolism by inhabiting bacteria in order to meet energy and carbon requirements for their growth and reproduction (Head et al. 2003; Timmis 2010). Indigenous microorganisms in oil field sites exhibit interesting interaction mechanisms to flourish in these extreme conditions. The native bacteria in such sites biotransform/degrade hydrocarbon compounds producing undesirable heavy oil (Head et al. 2003). Microbe-mediated degradation of petroleum hydrocarbons leads to decline in paraffin content and often increase density, sulfur content, acidity, and viscosity of crude oil. This has harmful economic consequences for oil production and refining operations, whereas on the other hand, inhabiting bacteria also have a significant role in improving the physical characteristics of heavy oil by biotransforming and decreasing its average molecular weight as well as by producing biosurfactant which can emulsify the hydrocarbon and substantially reduce the viscosity of heavy oil (Shibulal et al. 2014). It is not easy to get a complete understanding of microorganisms inhabiting oil reservoir sites under in situ conditions. A diverse group of bacteria and archaea, such as mesophiles, strictly anaerobic thermophiles, hyperthermophiles, and halophiles, have been isolated and characterized from oil microbiomes. Culture-based techniques have been extensively applied to investigate the diversity of microorganisms in different natural environments (Thatoi et al. 2013; Arroua et al. 2018; Gao et al. 2019; Chen et al. 2020). Culturable bacteria from such extreme sites have been characterized for their physiology and metabolic potential which favor their survival and growth. However, these isolates do not provide a complete picture of the microbial community structure and function. Microbial ecology is a recent discipline, and it has drawn attention of researches with the advances in culture-independent molecular tools and techniques. Metagenomic analysis has helped in the exploration of microbial community in diverse aquatic, surface, subsurface, and deep oil-containing environments (Gittel et al. 2009). Oil field and reservoirs are environments with extreme physico-chemical characteristics such as toxicity, hydrophobicity, high temperature, salinity, and pressure. These environments are rich in various organic (alkanes, cycloalkanes, aromatic hydrocarbons) and inorganic (sulfate, nitrate, metal containing) compounds which can be metabolized by microorganisms either directly or indirectly by cometabolism and syntrophism. Thus, the microorganisms residing in these sites have very sophisticated metabolic capabilities to sustain and flourish under such conditions. The microbiology of hydrocarbon-containing sites has been extensively studied, and the role of microorganisms on petroleum composition, recovery, and production methods has been reported (Li et al. 2014; Arroua et al. 2018; Laso-Pérez et al. 2019). In any hydrocarbon-impacted sites, the key biochemical processes exhibited by microorganisms include sulfate reduction, fermentation, acetogenesis, methanogenesis, NO3− reduction, and Fe and Mn reduction (Dolfing and Hubert 2017; Zhao et al. 2018). Considering oil to be one of the most vital energy resources, it is important to have a comprehensive understanding of petroleum microbiology. However, microbial ecology of oil reservoirs is still subject to accessibility of undisturbed samples from surface and deep sites (Li et al. 2018).
Microorganisms play a key role in controlling the composition, physico-chemical properties, and recovery of crude oil from wells and reservoirs. Thus, it is suggested that the activity of inhabiting microorganisms in an oil reservoir ecosystem in fact has a control on the crude oil production, recovery, and its fiscal value. Therefore, it is highly significant to investigate the oil reservoir ecology with special emphasis on microbial community structure and function. This review presents a critical understanding of petroleum oil field reservoir ecosystems and the potential role of the inhabiting bacteria in hydrocarbon degradation, oil recovery, and bioprospecting.
Nature of oil reservoirs: origin and distribution of microorganisms
Oil reservoir environments are characterized by extremes of temperature, pressure, salinity, and strictly anaerobic conditions. The nature of oil reservoir plays a pivotal role in the survival and growth of indigenous microorganisms. Several parameters such as temperature, pressure, pH, salinity, presence of electron donating, and electron accepting groups shape the physico-chemical characteristics of the reservoir system. Temperature being one of the limiting factors in reservoir environments varies from low (10 °C) to high (120 °C) range (Varjani and Upasani 2017; Kim et al. 2018). An increment of 3 °C/100 m depth is observed in deep environments. In deep oil reservoirs up to 5000 m, temperature ranges from 130 to 150 °C; in these conditions, there is a meager chance of survival of life due to reduced stability of biological compounds (Bennett et al. 2013). Several thermophilic bacteria having a role in hydrocarbon degradation has been reported at reservoir temperature of 80–90 °C (Meckenstock et al. 2014; Zhu and Dittrich 2016; Kim et al. 2018). Natural water associated with the oil and gas reservoir is called formation water, and it has exceptional chemical characteristics. Formation waters can be of low salinity to salt-saturated brine and pH ranges from acidic to slightly alkaline conditions; these factors affect microbial activity and diversity (Kim et al. 2018). Along with pH and salinity, reservoir and oil well pressure is also very typical (Varjani and Upasani 2017). The indigenous bacteria can tolerate pressure up to 500 atm (Bennett et al. 2013). The composition of electron donors and acceptors in these environments has crucial control on bacterial metabolic activity (Head et al. 2014). The majority of oil field sites have low reduction potential with presence of electron donors like carbon dioxide, hydrogen, and some organic molecules; however, it is scarce in electron acceptors (Fe (III), Mn (IV), NO3−) (Meckenstock et al. 2014). Presence of sulfate and carbonate along with organic acids (acetate, benzoate, butyrate, etc.) in reservoir water agree with methanogenesis, sulfate reduction, fermentation, and acetogenesis as the dominant biochemical processes (Yang et al. 2016). Obligate anaerobes are dependent on crude oil components as their sole carbon and energy source. Carbon content in oil reservoirs is unlikely to be a limiting substrate; however, essential nutrients (N and P) are scarcely available, and it directs the biodegradation process. The reservoir constituents itself can provide nitrogen sources in the form of the heterocyclic N-containing compounds. Considering nitrogen to be a limiting factor, microflora having a role in nitrogen biogeochemical cycling in oil wells and reservoirs has been studied. Anaerobic ammonium-oxidizing (anammox), nitrate reducing bacteria have been observed in high temperature oil reservoirs (Gittel et al. 2009).
Since the earliest description of microbial diversity in oil reserves, there has been subsequent development in its microbiology along with exploration of petroleum. One of the challenges faced in petroleum microbiology is to identify the autochthonous bacteria in oil fields and reservoir sites. Very often sampling and oil recovery methods such as water flooding result in inoculation of allochonthous microorganisms into the oil reservoir ecosystems (Varjani and Upasani 2017; Li et al. 2018). The introduction of exogenous microorganisms along with injected water or during enhanced oil recovery operations and other processes makes it challenging to determine whether a bacterium is native to the site. These exogenous microorganisms disturb reservoir biogeochemistry and may permanently shape the microbial community structure and function (Bennett et al. 2013). Many of the exogenous microorganisms introduced in these extreme environments have been detected via culturable and non-culturable techniques. In order to differentiate indigenous microorganisms found in oil reservoir microbiomes, reservoir in situ conditions are replicated in the lab, and their worldwide distribution under similar environment is taken into account (Varjani and Upasani 2017; Kim et al. 2018). However, optimum growth conditions for a bacterium in culture may not be a correct manifestation of the in situ physico-chemical and biotic conditions (Vreeland et al. 2007; Varjani and Upasani 2017). For instance, thermophilic, nitrate ammonifying, and sulfur oxidizing bacteria showed a lower optimum temperature than its indigenous conditions in deep-sea hydrothermal vents. Similar variation in salt tolerance property has been observed for halophilic and halotolerant microorganisms derived from these sites (Vreeland et al. 2007). Thus, the existence of evolutionary-related microorganisms across spatially distinct petroleum oil fields and reservoirs indicates their indigenous origin (Kim et al. 2018). However, this criterion is limited to commonly found microbiota and often overlook the novel groups occupying a specialized niche in specific reservoir and oil field sites (Parnell et al. 2017). Distinct bacterial and archaeal diversity was reported across 22 different reservoirs in China; this variation was mainly correlated with exploration patterns, temperature, salinity, and pH of formation waters (Gao et al. 2016). Oil reservoirs comprise multiple oil-containing strata with reduced porosity and permeability, providing oligotrophic habitat and niche for extremophilic microorganisms. The biotransformation process is dependent on electron donating and accepting functional groups from oil-water transition zone in deep reservoir sites. Thus, this transition zone is considered to be a favorable hot spot for inhabiting microorganisms and the associated degradation process (Pannekens et al. 2019). It is understood that biodegradation generally occurs near the oil-water transition zone, and saturated hydrocarbon content has been reported within an area of 100 m away from this zone (Wang et al. 2012). Variation in water content is observed among diverse oil reservoir sites, and bacteria abundance is generally several folds higher in locations with high water content (Orcutt et al. 2010). Small water inclusions in the oil phase of the Pitch Lake in Trinidad and Tobago had extremely diverse and abundant microbial composition. This finding suggested that small water-filled pockets on solid rock surfaces provide a favorable microenvironment to microorganisms (Meckenstock et al. 2014). Thus, based on abundance and distribution of microorganisms, a gradient model could be proposed for degradation of crude oil in subsequent zones of oil reservoir ecosystems (Fig. 1). Such graded patterns of biotransformed oil have been observed in reservoirs across varying depths, with non-degraded oil at the surface sites (Pannekens et al. 2019). During recovery and production process, the pumped oil is actually a mixture of water, oil, and gas. This mixture do not always gives a true reflection of microbial diversity and composition of non-planktonic population, which remains attached to rocky material or lodged in pores and inclusions. Various studies targeted metagenome from formation water in reservoirs and assumed it to represent the complete diversity of microorganisms; however, separate studies of water and oil phases have revealed considerable variation in their microbial diversity (Cheng et al. 2014; Wang et al. 2014). Variation in microbial community composition in water and oil phases suggests exploration of both the phases to have a more elaborate understanding of indigenous bacteria and their potential role in these ecosystems.
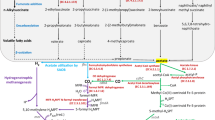
Geomicrobial prospecting for a deep subsurface oil reservoir. Scheme depicting different zones of a deep oil reservoir and microseepage of light hydrocarbons through fissures and faults to surface and subsurface soils and enrichment of hydrocarbon-utilizing bacteria. Seeped hydrocarbon products provide potential for geochemical and microbial prospecting in surface and subsurface zones of deep oil reserve site
Microbial community dynamics
Characterization of microbial communities in extreme environments such as oil fields and reservoir is a big challenge due to the presence of varied, complex organic and inorganic compounds, extreme physico-chemical characteristics, and above all highly intricate metabolic networks of inhabiting bacteria. Regardless of the complication of reservoir ecosystem, advanced molecular, genomics, transcriptomics, metabolomics, and above all next-generation sequencing techniques are being applied to explore the in situ microbial diversity, function, and distribution. The abovementioned sophisticated community analysis tools are used to decipher the role of microorganisms in petroleum bioprospecting, bioremediation of oil contaminated sites, and in studying microbial activities for enhanced oil recovery (Akondi and Lakshmi 2013; Hu et al. 2016; Shekhar et al. 2020). Microbial communities in oil field and reservoir sites have been explored by culture-based and omics-mediated culture-free approach. Culture-based technique employs characterization of bacteria on the basis of morphology and biochemical test followed by molecular analysis to define their taxonomical position and metabolic activity. However, these cultured isolates represent less than 1% of total microorganisms, and their role in ecosystem function is not completely understood. Diverse groups of bacteria and archaea with hydrocarbon-degrading activity have been derived from various hydrocarbon-containing habitats (Table 1). More than 100 bacterial genera from diverse sites have been extensively studied for the transformation of both lighter hydrocarbons (C12–C32) and heavier hydrocarbons (C36–C40) (Hasanuzzaman et al. 2007; Chen et al. 2020). These potential degraders are either able to degrade a wide spectrum of hydrocarbon component or may be reliant on a highly specific substrate which is dependent on the catabolic enzymes produced by them (Nie et al. 2014; Koshlaf and Ball 2017). Various degradation screening tests, oil emulsification capability, and hydrocarbon utilization potential are studied to characterize their physiology and metabolism. These potential degraders are unique with respect to their metabolism (oxidation, mineralization, or removal) and could oxidize at least one intermediate product of polyaromatic hydrocarbon degradation pathway. These isolates from different oil fields have been evaluated for enhanced oil recovery (Rathi et al. 2018; Nikolova and Gutierrez 2020). Microbial consortium-based study has been done to evaluate the biodegradation potential of several bacteria for in situ applications in hydrocarbon-contaminated sites (Al-Bahry et al. 2013). Consortia of alkane (Acinetobacter spp., Rhodococcus spp., Pseudomonas aeruginosa, and Bacillus subtilis) and aromatic hydrocarbon (Pseudomonas putida, Sphingomonas, Paenibacillus) degraders have been studied to get a better understanding of their function and physiology in native habitats. Several potent degrading bacteria have been used in successful field trials for the development of microbially enhanced oil recovery (MEOR) systems (Patel et al. 2015; Cui et al. 2019). In a petroleum reservoir in the Daqing Oilfield, China, biosurfactant-producing indigenous Bacillus strains have been utilized in the transformation of the crude oil and enhancement of its flow characteristics (She et al. 2011). Reservoir temperature and pH play an important role in oil recovery, which has been demonstrated by microbial flooding process using Clostridium sp. and Bacillus sp. (Sivasankar and Govindarajan 2016). A rhamnolipid-producing Pseudomonas sp. has been widely used for bioremediation and MEOR application (Li et al. 2019). Diverse genera of fungi (Aspergillus, Penicillium, Fusarium, Amorphotheca, Neosartorya, Paecilomyces, Talaromyces) have been characterized to decipher their role in degradation of hydrocarbon-containing compounds (Al-Hawash et al. 2018). The aerobic degradation by fungi involves the ability of monooxygenase to decrease the molecular oxygen in order to activate fungi. Role of lignin-degrading enzymes and non-liginolytic enzymes (cytochrome P450 monooxygenase) from fungal species (Aspergillus, Penicillium, and Cunninghamella) has been extensively studied for degradation of polyaromatic hydrocarbons, n-alkanes, and crude oil (Elshafie et al. 2007; Lee et al. 2015; Aydin et al. 2017). Anaerobic degradation of hydrocarbon by fungi has been reported mostly in the consortium of anaerobic fungal strains or a consortium of anaerobic fungi and bacteria to reach maximum efficiency in degradation (Aydin et al. 2017).
Native microbial population contains catabolic genes encoding the enzymes required for degrading petroleum hydrocarbons. Initially for real-time detection of specific degrading bacteria, immune assay-based techniques, mostly tagged with a fluorescent dye or enzyme linked, have been used (Shekhar et al. 2020). These techniques of antigen-antibody reactivity were mostly used for rapid screening and detection of microorganisms of specific metabolic activity under in situ conditions. In recent years, molecular technologies (real-time PCR, qPCR, microarrays, etc.) targeting environmentally important functional genes or specific biodegrading organisms are under rapid development (Embree et al. 2014; Olowomofe et al. 2019; Cao et al. 2020). Research in this area is mostly focused on the identification and monitoring of the key microorganisms and their biocatalytic efficiencies by targeting 16S rRNA as well as various catalytic genes involved in relevant microbial processes. However, based on the culture-dependent techniques, it is difficult to generate a system-based model to represent the overall ecosystem structure and function in these extreme environments; thus, culture-independent molecular tools came into play. In order to study microbial communities via culture-free techniques, the simplest mode employed was to enumerate and differentiate metabolically active strain in environmental samples by epifluorescence microscopy, fluorescence in situ hybridization, and differential staining techniques. Apart from whole cell studies, other commonly used techniques involved total protein banding and phospholipid fatty acid analysis. In recent times by means of advanced molecular tools, researchers are more reliant on PCR-based metagenomic and metatranscriptomics approaches. Microarray-based high-throughput methods have been used to track microbial diversity based on rRNA and functional genes relevant to the specific degradation process. Methods to analyze structural diversity of a community includes DNA-based denaturing and temperature gradient gel electrophoresis, ribosomal intergenic spacer analysis, single-strand conformation polymorphism, internal transcribed spacer-restriction fragment length polymorphism, random amplified polymorphic DNA, and amplified ribosomal DNA restriction analysis. In hydrocarbon-containing sites, microbial diversity has been explored by metagenomics approach. Bacterial and archaea community in different oil and gas field sites in China has been explored by high-throughput sequencing of 16S rRNA genes (Nazina et al. 2013; Tan et al. 2015; Deng et al. 2016; Liu et al. 2019). Diversity of microbial communities associated with the crude-oil and formation-water components of the reservoir fluid from a high-temperature petroleum reservoir in Niibori oilfield, Japan, revealed an abundance of methanogens. Bacterial floras of 49 phylotypes were characterized with greater diversity of the Firmicutes. In microbial communities associated with the crude oil and large insoluble particles, acetate metabolizing Methanosaeta was dominant; on the other hand, methanogen sequences were most abundant in the library of the formation-water-associated community (Kobayashi et al. 2012). Microbial succession has been reported in Halfdan oil field in the North Sea. Shifts in microbial community structure and function were observed in response to the time of production, changing ecological conditions due to the anthropogenic unrest of the oil field ecosystem. Initially slow-growing anaerobic microorganisms (Thermotogales and Clostridiales) were dominant, but during secondary oil recovery injection of sea water and nitrate led to modification of microbial communities with fast-growing opportunistic microorganisms (Deferribacteres, Delta-, Epsilon-, and Gammaproteobacteria) capable of nitrate reduction, H2S, sulfide, and sulfur oxidation (Vigneron et al. 2017). High-salinity oil wells in the Gulf of Mexico showed limited structural and functional diversity of microorganisms with methanogens, Halanaerobium and other Firmicutes lineages being dominant and reduced abundance of Deltaproteobacteria (Christman et al., 2020). In the Bakken Shale, oil, and gas reservoir in the USA, influence of reservoir geochemistry and microbiota on the potential biodegradation activity was observed. Geochemical, quantitative PCR, and 16S rRNA gene-based analysis revealed 19 bacterial families. Here dominance of Halanaerobiaceae, Pseudomonadaceae, and Desulfohalobiaceae having a role in biofilm formation and sulfate reduction was observed (Tinker et al. 2020).
Detection and quantification of specific structural and functional genes targeted by real-time PCR, qPCR, and microarrays provide a complete depiction on the role of inhabiting bacteria in degradation mechanisms and its relation to ecosystem function. Key functional genes responsible for degradation include alkane hydroxylase genes, ring-hydroxylating dioxygenase (RHDα) genes, alky succinate synthase gene (assA), and benzylsuccinate synthase gene (bssA) (Muangchinda et al. 2015). Development in functional genomics along with bioinformatics has further facilitated understanding on the physiology and activity of microorganisms catalyzing critical environmental processes such as oil degradation (Embree et al. 2014; Godheja et al. 2014; Muangchinda et al. 2015; Liu et al. 2016; Vigneron et al. 2017). Thus, cultivation-independent techniques are presently gathering huge data sets of microbial communities inhabiting in diverse natural environments. The application of statistics and data mining and recovery tools helps to understand the complex system of varied microbial interactions occurring in microbial communities (Centler et al. 2020).
Crude oil and its biodegradation
Unprocessed petroleum is a complex mix of natural substances which mainly include solid, liquid, and gaseous hydrocarbons (straight and branch chain alkanes, cycloalkanes, aromatics, and other non-hydrocarbon compounds such as phenols, thiols, naphthenic acids, heterocyclic compounds containing N, O, and S, and trace amounts of metals such as Fe, Ni, Cu, and V). The crude oil contains 15–60% alkanes, 15–30% aromatic hydrocarbon, and the rest comprises resins and asphaltenes. The aromatic compound includes polycyclic hydrocarbons (PAHs), low molecular weight (benzene, naphthalene, and anthracene), and high molecular weight (pyrene and fluoranthenes) compounds (Bacosa and Inoue 2015). Oil-degrading organisms belong to 3 areas of life: bacteria, archaea, and eukaryotes. The ultimate fate of most of the hydrocarbons in the nature is degradation or catabolism by inhabiting microorganisms in order to meet energy and carbon source for their growth and reproduction (Head et al. 2003; Kleindienst et al. 2015). Mostly hydrocarbon compounds of up to 40 carbon atoms can be biologically transformed in few months time. However, complex constituents are more recalcitrant to degradation. The principal pathway for biodegradation of hydrocarbons has a strict requirement of molecular oxygen to deliver water and CO2 catalyzed by monooxygenases and dioxygenases (Fig. 2) (Austin and Callaghan 2013). The basic mechanism is different for hydrocarbon utilization in aerobic and anaerobic microorganisms. Under aerobic conditions, mostly aliphatic compounds are degraded by monooxygenase activity via terminal or subterminal oxidation, whereas aromatics are degraded by dioxygenase activity. In this reaction, molecular oxygen is used as a co-substrate with monooxygenase- or dioxygenase-mediated hydroxylation of aliphatic chains or aromatic rings. The aerobic utilization of hydrocarbon has been studied in bacteria belonging to diverse phyla gamma proteobacteria, alpha proteobacteria, and beta proteobacteria (Head et al. 2006). Microbe-mediated hydrocarbon metabolism under anoxic conditions involves several enzymatic reactions such as toluene-activating benzylsuccinate synthase (BSS) and other related glycyl radical-bearing alkyl-/arylalkylsuccinate synthases adding hydrocarbons to fumarate, ethylbenzene dehydrogenase (EBDH), and other Mo-cofactor-containing dehydrogenase enzymes are involved in hydroxylation of ethylbenzene and other ring-substituted mono- and bicyclic aromatic compounds. Some other important reactions include glycyl radical enzyme 4-hydroxyphenylacetate decarboxylase (4Hpad) which converts to 4-hydroxyphenylacetate to p-cresol. Anaerobic metabolism of benzene and naphthalene has been characterized for sulfate- or nitrate-reducing microorganisms (Rabus et al., 2016). The rate of anaerobic hydrocarbon transformation is a delayed process as compared to oxygen utilizing degradative pathway. The anaerobic microbial degradation of crude oil mostly occurs under methanogenic, sulfate, and iron-reducing conditions (Foght 2008; Fida et al. 2016; Chen et al. 2020). Microorganisms adjusted to oxygen-restricting conditions include sulfate-reducing, denitrifiers and dissimilatory nitrate-reducing, phototrophic, and metal-reducing bacteria, with the capacity of utilizing hydrocarbons as simple alkanes and alkenes to highly complex mixtures (Siegert et al. 2013). Resins and asphaltenes are petroleum compounds containing polycyclic aromatic or naphthenoaromatic molecules with heteroatoms and alkyl side chains. Resins and asphaltenes could be differentiated due to their solubility differences in organic solvents. The asphaltene fraction of crude oil is insoluble in solvents such as n-heptane or n-hexane, while resins are soluble (Liao et al., 2009). Although the resins and asphaltenes are mostly recalcitrant to biodegradation, few studies have reported microbial degradation of these compounds. It has been observed that alteration in the core nuclei and oxidation of alkyl side chain do occur as revealed by spectroscopic techniques such as FTIR and GC-MS. Mostly oxygen contents of resins and asphaltenes are reduced by anaerobic degradation process, and N/C ratio keeps on increasing with biodegradation (Jenisch-Anton et al. 2000; Kim et al. 2005). Similar changes in composition of resin and asphaltenes were reported in biodegraded oils extracted from reservoir cores of the Lengdong oilfield in the Liaohe Basin, NE China (Liao et al., 2009). Asphaltene degradation has been reported by bacteria such as Pseudomonas aeruginosa, Micrococcus sp., and Bacillus sp. isolated from oil-containing habitats (Ali et al., 2012; Gao et al., 2017).
Microbe-mediated hydrocarbon utilization pathways in the oil reservoirs: aerobic (surface and subsurface)
degradation, anaerobic deep site degradation along with metabolite utilization (acetate, hydrogen) via methanogenesis (CoA, coenzyme A; CoM, coenzyme M; H4MPT, coenzyme 788 M methyltransferase; MFR,
macrophage fusion receptor; THF, tetrahydrofolate; TCA, tricarboxylic acid; SOA, syntrophic acetate oxidation)
A minor oil spill if left undergoes natural attenuation by biological and nonbiological mechanisms. Autoxidation in the absence of light plays a minor role in marine environment due to low temperature; however, photooxidation do contributes substantially to autocleaning. In a marine environment, oil degradation activity leads to good amount of carbon availability which supports other organisms in the habitat. However, often P and N shortage retards the degradation activity of microorganisms. Some of the reports are available on enhancing biotransformation ability by supplementing with essential nutrients (Head et al. 2014; Alegbeleye et al. 2017). The quality of crude oil is controlled by the degree of biodegradation process occurring in the reservoir. Thus, microorganisms majorly regulate the initial steps of degradation and have a significant effect on the crude oil composition. Biochemical oxidation occurs naturally to degrade the different hydrocarbon compounds under in situ conditions provided the process of degradation is not limited by a paucity of nutrients (nitrogen, phosphorus, potassium), electron acceptors, trace elements, and moisture (Alegbeleye et al. 2017). Another important factor which controls the process is the physico-chemical condition of the reservoir site. Microorganism native to these sites are utilizing the carbon and energy source from the petroleum compounds this often leads to depletion of lighter hydrocarbons (C1–C6). The results in enrichment of heavy oil fractions and increase in its viscosity (Head et al. 2003). This is one of the bottlenecks which limit the oil mining and recovery operations. The geothermal property of oil reservoir does affect the quality and composition of oil fractions. Mostly lighter oil fractions are available at depth more that 3.5 km with temperature more than 80 °C (Head et al. 2003). The rapid proliferation of hydrocarbon degraders in these sites leads to depletion of essential nutrients (N, P, Fe). Thus, nutrient depletion reduces the microbial growth and propagation which in turn limits the biodegradation process. To increase the in situ biodegradation process application of nutrients (N, P), terminal electron acceptors and additives have been practiced in various studies (Alegbeleye et al. 2017). Thus, biostimulation efforts lead to increase in microbial count and activity with direct increase in degradation process. In several studies, bioaugmentation with suitable potent microorganism or consortia along with biostimulation is used as combined strategy to enhance the biodegradation process (Ortega et al. 2018). Considering the complexity of hydrocarbon compounds, it is often desirable to harness consortia of microorganism with unique biochemical capabilities to transform/ degrade wide groups of compounds present in crude oil.
Common bacterial communities playing major role in oil reservoir ecosystem function
Sulfate-reducing bacteria
Various reports on oxidation of non-methane hydrocarbons by sulfate-reducing bacteria suggest their significant role shaping petroleum biogeochemistry (Ma et al. 2017). The anaerobic degradation in oil fields involves major role of absolute anaerobes such as sulfate reducers, methanogens, and fermentative (acetogenic) bacteria. However, sulfate reduction is a dominant biochemical process over methanogenesis in oil microbiome when its concentration is greater than 50 μM. These microorganisms use oxygen containing sulfur compounds (SO32−, S2O22−, S3O62−, and S4O62−) or elemental sulfur as electron acceptors for anaerobic respiration of organic compounds (Davidova et al. 2012). Oxidized sulfur compounds can be used as terminal electron acceptor for the anaerobic respiration of organic matter by SO42− and S-reducing bacteria. These bacteria are extremophiles (halophiles, barophiles, and thermophiles) based on in situ physico-chemical conditions such as salinity, pressure, and temperature (Jebbar et al. 2015; Jebbar et al. 2020). The presence of SRB in oil reservoirs is a major concern for oil industry. This group of microorganism is considered to be wasteful to petroleum production and recovery process because corrosion and souring of reservoir occur due to production of H2S (Hussain et al. 2016). However, co-habitation of diverse bacterial groups in these sites often alters or modifies the activity of SRB. The coexisting bacterial groups often have a positive control over oil field sulfur cycle and provide possible solution to detrimental effect of SRB in oil recovery operations. Various investigators have studied not only the presence and abundance of sulfur reducers in oil field sites but also bacterial population that coexists with them in sulfate-reducing enrichment cultures or in situ conditions via culturable as well as metagenomic approach (Grigoryan and Voordouw 2008; Tüccar et al. 2019). Some of the SRB isolates from the oil field and reservoir sites are mesophiles and moderate halophiles of genus Desulfovibrio, Desulfobacterium, Desulfovermiculus, and Desulfotomaculum which could utilize variable C sources (pyruvate, malate, fumarate, succinate, and ethanol) in presence of sulfate (Aullo et al. 2013). The nature of these isolates has been determined by laboratory optimal condition, and it might be different from their in situ indigenous nature. Investigation of SRB communities at several marine gas and oil seeps showed good abundance and diversity of acetate oxidizing Desulfosarcina and Desulfococcus with significant contribution in carbon cycling (Orcutt et al. 2010). Diverse groups of thermopiles are widely distributed across geographically distant oil fields and reservoirs; these include both bacteria and archaea. Some the thermophiles which have been commonly reported belong to following genera Desulfotomaculum, Archaeoglobus, Desulfacinum, Thermodesulforhabdus, Thermodesulfobacterium, Thermodesulfobacterium, and Desulfonauticus (Mayilraj et al. 2009).
Methanogens
The anaerobic bacteria and archaea have been very well studied for methane generation by degradation of simple compounds such as carbohydrates and lipids. Methanogenic degradation of hydrocarbons compounds in anoxic conditions has been extensively studied (Zengler et al. 1999; Haroon et al. 2013). Methanogens are one of the significant colonizers in subterrestrial oil reservoir sites. Methanogens metabolize hydrogen, carbon dioxide, acetate, methylamines, and dimethylsulfides producing CH4 as the end product (Fig. 2) (Lv et al. 2016; Okoro and Amund 2018). This group of microorganisms plays a crucial role in degradation of deep methanogenic oil reservoirs (Shelton et al. 2016; Pannekens et al. 2019). Growth and metabolism of methanogens in oil field environment are subjective to physico-chemical factors where salt and oxygen content play vital role (Tan et al. 2015; Liang et al. 2016). The anaerobic oxidation of methane is carried out by diverse taxa of anaerobic methanotrophic archaea in presence of electron acceptors (Fe, Mn, and NO3−), or it may be coupled to SO42− reduction in coordination with other bacteria. In oil reservoir, syntrophism is often observed, where a syntrophic bacteria breaks down crude oil into suitable substrates (acetate, formate, CO2) for methanogens (Shelton et al. 2016). Syntrophic methanogenesis is a dominant mechanism of crude oil degradation in absence of electron acceptor such as oxygen, nitrate, and sulfate. Based on substrate specificity, methanogens are classified into 3 groups, hydrogenotrophs, methylotrophs, and acetoclastic methanogens (Okoro and Amund 2018). Diverse mesophilic, hydrogenotrophic, and acetoclastic methanogens have been identified in oil-producing reservoir sites in China, Japan, and the USA (Grabowski et al. 2005b; Mayumi et al. 2011; Shelton et al. 2016). Methylotrophs such as Methanosarcina siciliae and Methermicoccus shengliensis have been recovered from oil-containing sites (Obraztsova et al. 1987; Cheng et al. 2007). Methanogenesis and anaerobic methane oxidation mediated by methyl-coenzyme M reductase (mcrA gene) has been reported for various oil- and gas-containing natural environments either in consortia of alkane-degrading bacteria and methanogenic archaea or by a clade of uncultured archaea alone (Orcutt et al. 2010). Archaea having the potential to oxidize non-methane compounds such alkanes (ethane, butane) have been characterized from different environments. These microorganisms use the enzymatic machinery similar to MCR and are called alkyl coenzyme M reductase (ACR) (Thauer 2019). The anaerobic methane-oxidizing and multi-carbon alkane-oxidizing archaea are distributed in diverse environments, and high degree of conservation of methyl-coenzyme M reductase and alkyl coenzyme M reductase-encoding genes has been observed (Evans et al. 2015; Wang et al., 2020).
Fermentative bacteria
Diverse fermentative bacterial groups have been characterized from oil fields and reservoirs. These microorganisms are able to metabolize a variety of substrates (carbohydrates, proteins, and hydrocarbons) which act as electron donors leading to end products such as organic acids, H2 and CO2 (Grabowski et al. 2005a; Nazina et al. 2007). Numerous fermentative microorganisms have double fermentative and respiratory metabolic capacities and are also able to reduce sulfur and thiosulfate (Nazina et al. 2013). Isolation of fermentative bacteria (Haloanaerobium spp.) from oil reservoirs with high salinity has been reported (Rainey et al. 1995; Nazina et al. 2007). Fermentative thermophilic bacteria isolated from reservoirs with high temperatures mostly include phyla Thermotogae and Firmicutes. Genera Thermotoga, Thermosipho, Geotoga, and Petrotoga of phylum Thermotogae have been characterized from diverse oil-rich habitats (Grabowski et al. 2005b). These thermophiles could metabolize sugars producing acetate and L-alanine and have been put to industrial application for production of thermostable enzymes. Fermentative bacteria (Geotoga and Petrotoga) which are moderate thermophiles and could survive in saline conditions have been reported from Oklahoma and Texas oil reservoirs (Davey et al., 1993). Thermoanaerobacteriales include members from genera Thermoanaerobacter, Thermoanaerobacterium, Caldanaerobacter, and Mahella derived from oil harboring sites (Grassia et al. 1996; Fardeau et al. 2004). Fermentative firmicutes Anaerobaculum thermoterrenum (organic acid fermenter), Fusibacter paucivorans, and Thermovirga lienii (amino acid degrader) have been derived from oil field environments (Ravot et al. 1999; Dahle and Birkeland 2006). Peptone and amino acid degrader Dethiosulfovibrio peptidovorans have been derived from African oil fields, Congo (Magot et al. 1997).
Metal- and nitrate-reducing bacteria
Iron-, nitrate-, and manganese-reducing bacteria have been studied from diverse oil reserve sites (Vetriani et al. 2004; Nazina et al. 2013). Deferribacter and Geobacillus derived from these sites could use various electron acceptors (Greene et al. 1997). Geobacillus is a thermophile microaerophilic microbe which could degrade alkanes strictly under oxic conditions, whereas some strains are nitrate reducers under anoxic conditions. A thermophilic Fe and Mn reducer Deferribacter thermophilus isolated from oil fields of North Sea, UK, can utilize diverse substrates such as lactate, acetate, tryptone, peptone, and hydrogen as energy source with Fe3+ or Mn4+as terminal electron acceptors (Greene et al. 1997). Another facultative anaerobic isolate Shewanella putrefaciens from oil field site has been very well characterized for its sulfur- and iron-reducing property (Nazina et al. 2007). Nitrate-reducing bacteria of diverse metabolic potential (heterotrophs, chemolithotrophs, and autotrophs) have been derived from different oil microbiomes (Myhr and Torsvik 2000; Vetriani et al. 2004). Sulfurospirillum spp., Arcobacter spp., and the Deferribacter spp. have been frequently observed in oil field sites (Gittel et al. 2009). Nitrate reducers have great contribution in enhancing oil recovery and production, as they could mitigate the hazardous effect of sulfate reducers. Many studies have utilized nitrate amendment in reservoirs to solve the problem of reservoir corrosion and souring due to accumulation of sulfate-reducing biomass and precipitation of metal sulfides (Davidova et al. 2001; Bødtker et al. 2008). This amendment in high temperature Halfdan oil field, Denmark, leads to enrichment of nitrate-reducing and nitrate-reducing sulfur-oxidizing bacteria, thus inhibiting the sulfate reducers and decreased H2S production (Gittel et al. 2009).
Significance of petroleum microbiology: microbially enhanced oil recovery and geomicrobial prospecting
Increasing cost of crude oil and exhaustion of oil reserves needs further exploitation of existing resources in mature reservoirs to meet future energy demands. There is an utmost requirement to develop new technologies for upgradation of heavy crude oil for its easy recovery from subterranean reservoirs. The role of bacteria in degrading heavy crude oil with reduced viscosity and enhanced flow characteristics is considered very effective. Microbial enhanced oil recovery (MEOR) is an efficient system to get the residual oil trapped in the reservoirs. Taxonomically divergent bacteria with varied metabolic potential and physiology have been characterized from oil reservoirs. Initially MEOR practiced in several oil-producing nations included three phases, introduction of relevant bacteria/consortia into reservoir which was followed by dispersal and proliferation of microorganisms in petroleum reservoirs with production of relevant metabolites to aid degradation process. Biological processing of heavy oil is considered a cost-effective and eco-friendly approach which provides a higher selectivity to specific reactions to upgrade heavy oil. Microbial systems which are capable of transforming oil fractions via enzyme-catalyzed reaction are used in heavy oil reservoirs for increased oil recovery (MEOR). Inhabiting bacteria have significant role in improving the physical characteristics of heavy oil by biotransforming and decreasing its viscosity and average molecular weight. Many biosurfactant-producing bacteria derived from oil fields have been utilized in emulsification and reduction of viscosity of heavy oil, thus aiding recovery (Shibulal et al. 2014). Several successful field trials for enhanced oil recovery systems using hydrocarbon-degrading bacteria have been reported from many oil-producing countries (Li et al. 2002; Horn 2007; Head et al. 2014; Gao et al. 2019). The potential of biosurfactant-producing indigenous Bacillus strains to degrade the heavy crude oil and assist in the improvement of its flow characteristics has been investigated in a petroleum reservoir in the Daqing Oilfield, China (She et al. 2011). Reservoir temperature and pH plays an important role in oil recovery, which has been demonstrated by microbial flooding process using Clostridium sp. and Bacillus sp. (Sivasankar and Govindarajan 2016). A rhamnolipid-producing Pseudomonas sp. has been widely used for bioremediation and MEOR application (Li et al., 2019). Consortia of bacteria with wide range of metabolic activity can be more efficient for microbial oil recovery in comparison to a lone bacterial species. The MEOR team in the Sultan Qaboos University, Oman, found that a consortia of Bacillus strains form oil-contaminated soil degraded heavy chain oil (C50–C70) to (C11–C20) (Al-Bahry et al. 2013). Microbially enhanced oil recovery (MEOR) has been widely applied in laboratory experiments and field trials. However, its potential in recovery operations in oil industry has remain underutilized due to several limitations in its application. One major issue is the difficulty in complete replication of all conditions in natural oil field and reservoir environment as established under lab or pilot scale studies. In recent times, the potential of biosurfactant-mediated MEOR approach has been promising as evident from several studies with microorganisms derived from oil-containing sites (Kryachko 2018). A biosurfactant-producing microbial consortium was developed with microorganisms derived from high-temperature reservoir of Gujarat, India. Oil recovery enhancement potential was analyzed by core flooding assay with promising 19% increase in oil recovery (Sharma et al., 2020). In another sand pack flooding study, the enhancement in oil recovery efficiency was found to be 19.2% following bioaugmentation with Bacillus licheniformis DM 1 isolated from a mature reservoir in Dagang Oilfield of China (Fan et al., 2020). In most of the biosurfactant-mediated MEOR operations, there are issues with respect to wettability alterations in water flooding-driven oil recovery. It has been suggested that pore size of rocks, along with activity of microorganism/biosurfactant molecules, controls the success of oil recovery under in situ reservoir conditions. Nitrate-mediated biostimulation has been practiced in MEOR activities in order to inhibit corrosion and souring of reservoir due to production of H2S by sulfate-reducing bacteria (Dennis and Hitzman, 2007; Bao et al., 2009; da Silva et al., 2014; Kryachko and Voordouw, 2014; Gassara et al., 2015, 2017). Anaerobic nitrate-dependent Fe(II) oxidation in packed-bed column showed effective modifications in rock wettability and homogenized the heterogeneous flowpaths. This technique led to 100% improvement in oil recovery from hydrocarbon-saturated packed-bed columns (Zhu et al. 2013). Methanogens have been beneficial for MEOR, but risk of microbial-mediated metal corrosion prevails (Kryachko and Hemmingsen, 2017). Thus, biofilm-forming and metal oxidation potential of microorganism may be assessed before their applications in biostimulation for MEOR. The potential of native microorganisms in oil fields to degrade heavy crude oil is considered to be very effective in MEOR. Even though MEOR offers environmental advantages over chemical-based enhanced recovery methods, it does face some application challenges. One of the challenges is the difficulty in maintaining activity and growth of microorganisms relevant to MEOR over time. Another challenge is the capability of specific microorganisms to grow and reproduce in the hostile environment of oil wells, which are inevitably not similar in different oil fields. In some of the heterogeneous oil reservoirs with varying pore sizes, it may be suggested to employ chemical as well as microbial oil recovery strategies and also consider rock wettability and emulsion shifts. On the other hand, it is also essential to characterize metal oxidation and biofilm-forming ability to rule out the side effects of biocorrosion in MEOR operations. Therefore, knowledge about native bacteria in these extreme subterranean environments and the underlying mechanisms of their interaction with the hydrocarbons would be used to develop appropriate strategies for MEOR in respective oil fields. Continuous research and successful applications establish the fact that MEOR can be viewed as a potent technology.
Geomicrobial prospecting system is well recognized in the area of hydrocarbon exploration. This technique involves an integration of geochemical, surface geophysical, and microbial techniques of hydrocarbon prospecting. The use of microbial prospecting involves evaluation of hydrocarbon reserves of an area and leads to enhanced achievement in petroleum exploration (Tucker and Hitzman 1994; Schumacher 2000). Microbial prospecting of hydrocarbons relies on the detection of anomalous group of hydrocarbon-oxidizing bacteria in the surface soils indicating the presence of subsurface oil and gas reserve (Fig. 1). Due to the pressure of subterranean gas and oil reserves, lighter hydrocarbons (CH4, C2H6, C3H8, and C4H10) break through fissures and faults in reservoirs and move upward to the surface and subsurface soils. These microseepages were detected by various analytical techniques and geochemical surface prospecting methods before planning drilling and recovery operations. On the other hand, indigenous microorganisms use these hydrocarbons as substrate for carbon and energy sources. Thus, such strange enrichment of hydrocarbon-utilizing bacteria on the surface soils caused by continuous long-duration low molecular weight hydrocarbon microseepage has very important role as an indicator for petroleum prospecting (Deng et al. 2016). Studies of the physiological characteristics of bacteria present in these ecosystems are highly significant for application in petroleum prospecting technology. Microbial prospecting assessment has been commonly practiced since 60 years, and good success rate has been reported. The first survey of surface microorganisms for petroleum prospecting was initiated by USSR (Mogilewskii 1940). This survey further led to the identification of several potential oil reserves sites in Soviet Union. Thus, the success of microbial prospecting studies created lot of interest in geochemists and geophysicists. Similar operation was carried out by other oil-producing countries (USA, Germany), and nearly more than 80% correlation was observed in surface microbe population and hydrocarbon reserves (Sealy 1974; Miller 1976). Case studies from sedimentary basins in India (Cambay and Krishna-Godavari) also indicated high abundance of hydrocarbon-oxidizing bacteria in surface soils. Recovery operations confirmed presence of good amount of petroleum reverses in these basins. In Keitz oil field, Germany microbial prospecting method has been successful in discovering two oil-producing wells KiSe 2 and KiSe 5. Soil samples were collected over a wide area and characterized for hydrocarbon-degrading bacterial load. Microbial prospecting results showed high abundance of hydrocarbon-degrading bacteria in oil-producing wells, whereas dry wells were observed in zones with reduced hydrocarbon-utilizing bacterial concentration (Wagner et al. 2002). Another successful microbial prospecting result was obtained for Conglomerate field in Montague County, Texas. Hydrocarbon indicating microbial signatures obtained in this site was found to be consistent with geophysical 3-D seismic data for oil reserve (Rasheed et al., 2015). Thus, considering the limitations of geochemical surface soil gas analysis technique in an environment where high microbial activity leads to utilization of these carbon substrates, it is very important to apply a sustained petroleum prospecting technology which includes microbial technique. The microbial oil survey techniques (MOST) method for reservoir characterization provides key indication for the occurrence of hydrocarbon anomaly. Subsequent application of geochemical and geophysical techniques could be focused in these regions. Thus, an integrated approach applying geological, geochemical, and microbial prospecting technique would lead to success of exploration and reduce risk of dry wells and wildcat drilling operations.
Conclusions
Oil fields and reservoir sites are extreme habitat to metabolically diverse microbial communities such as methanogens, NO3−, SO42−, and Fe3+ reducers. The microbial assemblage varies in various locales of reservoir site based on specific temperature, water salinity, and oil’s physico-chemical properties. Both culture-dependent and culture-independent approaches have contributed extensively to study microbial community dynamics in these sites. It is very well established that inhabiting microorganisms can adapt to specific environmental niches and control reservoir characteristics and function. The process of microbe-mediated biodegradation in oil reservoirs impacts the quantity and quality of oil and gas reserve. Thus, the study of degraders and their metabolic potential is of vital geological and industrial significance. Therefore, it is crucial to explore the microbial community structure and function in surface, subsurface, and subterranean oil reservoir sites. Thus, in-depth knowledge of microbial diversity leads to establishment of techniques for enhancing oil displacement for the target reservoir before establishing effective MEOR strategies. Surface and subsurface microorganisms in prospected oil reserve sites contribute to geomicrobial prospecting for hydrocarbon and oil exploration with reduction in fruitless/unsuccessful drilling activity in dry and wildcat wells.
Data availability
All data generated or analyzed during this study are included in this published article
References
Adkins JP, Madigan MT, Mandelco L, Woese CR, Tanner RS (1993) Arhodomonas aquaeolei gen. nov., sp. nov., an Aerobic, Halophilic Bacterium Isolated from a Subterranean Brine. Int J Syst Bacteriol 43(3):514–520
Aeckersberg F, Bak F, Widdel F (1991) Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol 156:5–14
Agrawal A, Vanbroekhoven K, Lal B (2010) Diversity of culturable sulfidogenic bacteria in two oil–water separation tanks in the north-eastern oil fields of India. Anaerobe 16(1):12–18
Akondi KB, Lakshmi VV (2013) Emerging trends in genomic approaches for microbial bioprospecting. Omics: a journal of integrative biology 17(2):61–70
Al-Bahry SN, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ, Al-Makhmari HS, Al-Sulaimani HS (2013) Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int Biodeterior Biodegradation 81:141–146
Alegbeleye OO, Opeolu BO, Jackson VA (2017) Polycyclic aromatic hydrocarbons: a critical review of environmental occurrence and bioremediation. J Environ Manag 60(4):758–783
Al-Hawash AB, Dragh MA, Li S, Alhujaily A, Abbood HA, Zhang X, Ma F (2018) Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res 44:71–76
Ali HR, El-Gendy NS, Moustafa YM, Roushdy MI, Hashem AI (2012) Degradation of Asphaltenic Fraction by Locally Isolated Halotolerant Bacterial Strains. ISRN Soil Sci 2012:1–11
Arroua B, Grimaud R, Hirschler-Réa A, Bouriat P, Magot M, Urios L, Ranchou-Peyruse A (2018) Pleomorphochaeta naphthae sp. nov., a new anaerobic fermentative bacterium isolated from an oil field. Int J Syst Evol Microbiol 68(12):3747–3753
Aullo T, Ranchou-Peyruse A, Ollivier B and Magot M (2013) Desulfotomaculum spp. and related gram-positive sulfate-reducing bacteria in deep subsurface environments. Front Microbiol 4:362
Austin RN, Callaghan AV (2013) Microbial enzymes that oxidize hydrocarbons. Front Microbiol 4:338
Aydin S, Karacay HA, Shahi A, Gokce S, Ince B, Ince O (2017) Aerobic and anaerobic fungal metabolism and Omics insights for increasing polycyclic aromatic hydrocarbons biodegradation. Fungal Biol Rev 31:61–72
Bacosa HP, Inoue C (2015) Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi, Japan. J Hazard Mater 283:689–697
Bao M, Kong X, Jiang G, Wang X, Li X (2009) Laboratory study on activating indigenous microorganisms to enhance oil recovery in Shengli Oilfield. J Pet Sci Eng 66(1-2):42–46
Bennett B, Adams JJ, Gray ND, Sherry A, Oldenburg TBP, Huang H, Larter SR, Head IM (2013) The controls on the composition of biodegraded oils in the deep subsurface–Part 3. The impact of microorganism distribution on petroleum geochemical gradients in biodegraded petroleum reservoirs. Org Geochem 56:94–105
Bødtker G, Thorstenson T, Lillebø BLP, Thorbjørnsen BE, Ulvøen RH, Sunde E, Torsvik T (2008) The effect of long-term nitrate treatment on SRB activity, corrosion rate and bacterial community composition in offshore water injection systems. J Ind Microbiol Biotechnol 35(12):1625–1636
Bourdet J, Burruss RC, Chou IM, Kempton R, Liu K, Hung NV (2014) Evidence for a palaeo-oil column and alteration of residual oil in a gas-condensate field: integrated oil inclusion and experimental results. Geochim Cosmocim Ac 142:362–385
Cao Y, Yu M, Dong G, Chen B, Zhang B (2020) Digital PCR as an emerging tool for monitoring of microbial biodegradation. Molecules 25(3):706
Cayol JL, Ollivier B, Patel BKC, Ravot G, Magot M, Ageron E, Grimont PA, Garcia JL (1995) Description of Thermoanaerobacter brockii subsp. lactiethylicus subsp. nov., isolated from a deep subsurface French oil well, a proposal to reclassify Thermoanaerobacter finnii as Thermoanaerobacter brockii subsp. finnii comb. nov., and an emended description of Thermoanaerobacter brockii. Int J Syst Bacteriol 45:783–789
Centler F, Günnigmann S, Fetzer I, Wendeberg A (2020) Keystone species and modularity in microbial hydrocarbon degradation uncovered by network analysis and association rule mining. Microorganisms 8(2):190
Chen J, Liu YF, Zhou L, Irfan M, Hou ZW, Li W, Mbadinga SM, Liu JF, Yang SZ, Wu XL, Gu JD (2020) Long-chain n-alkane biodegradation coupling to methane production in an enriched culture from production water of a high-temperature oil reservoir. AMB Express 10:1–11
Cheng L, Qiu TL, Yin XB, Wu XL, Hu GQ, Deng Y, Zhang H (2007) Methermicoccus shengliensis gen. nov., sp. nov., a thermophilic, methylotrophic methanogen isolated from oil-production water, and proposal of Methermicoccaceae fam. nov. Int J Syst Evol Microbiol 57:2964–2969
Cheng L, Shi S, Li Q, Chen J, Zhang H, Lu Y (2014) Progressive degradation of crude oil n-alkanes coupled to methane production under mesophilic and thermophilic conditions. PLoS One 9(11):e113253
Cui K, Zhang Z, Zhang Z, Sun S, Li H, Fu P (2019) Stimulation of indigenous microbes by optimizing the water cut in low permeability reservoirs for green and enhanced oil recovery. Sci Rep 9(1):1–12
Dahle H, Birkeland NK (2006) Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int J Syst Evol Microbiol 56(7):1539–1545
da Silva MLB, Soares HM, Furigo A Jr, Schmidell W, Corseuil HX (2014) Effects of nitrate injection on microbial enhanced oil recovery and oilfield reservoir souring. Appl Biochem Biotechnol 174:1810–1821
Davey ME, Wood WA, Key R, Nakamura K, Stahl DA (1993) Isolation of Three Species of Geotoga and Petrotoga: Two New Genera, Representing a New Lineage in the Bacterial Line of Descent Distantly Related to the “Thermotogales”. Syst Appl Microbiol 16(2):191–200
Davidova I, Hicks MS, Fedorak PM, Suflita JM (2001) The influence of nitrate on microbial processes in oil industry production waters. J Ind Microbiol Biotechnol 27(2):80–86
Davidova IA, Duncan KE, Perez-Ibarra BM, Suflita JM (2012) Involvement of thermophilic archaea in the biocorrosion of oil pipelines. Environ Microbiol 14(7):1762–1771
Deng C, Yu X, Yang J, Li B, Sun W, Yuan H (2016) Universal indicators for oil and gas prospecting based on bacterial communities shaped by light-hydrocarbon microseepage in China. J Microbiol Biotechnol 26(7):1320–1332
Dennis DM, Hitzman DO (2007) Advanced Nitrate-Based Technology for Sulfide Control and Improved Oil Recovery. Soc Pet Eng. https://doi.org/10.2118/106154-MS
DiPippo JL, Nesbø CL, Dahle H, Doolittle WF, Birkland N-K, Noll KM (2009) Kosmotoga olearia gen. nov., sp. nov., a thermophilic, anaerobic heterotroph isolated from an oil production fluid. Int J Syst Evol Microbiol 59:2991–3000
Dolfing J, Hubert CR (2017) Using thermodynamics to predict the outcomes of nitrate-based oil reservoir souring control interventions. Front Microbiol 8:2575
Elshafie A, AlKindi YA, Al-Busaidi S, Bakheit C, Albahry SN (2007) Biodegradation of crude oil and n-alkanes by fungi isolated from Oman. Mar Pollut Bull 54:1692–1696
Embree M, Nagarajan H, Movahedi N, Chitsaz H, Zengler K (2014) Single-cell genome and metatranscriptome sequencing reveal metabolic interactions of an alkane-degrading methanogenic community. ISME J 8(4):757–767
Esmaeil AS, Akbar A (2015) Occurrence of Pseudomonas aeruginosa in Kuwait soil. Chemosphere 120:100–107
Evans PN, Parks DH, Chadwick GL, Robbins SJ, Orphan VJ, Golding SD, Tyson GW (2015) Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350(6259):434–438
Fan Y, Wang J, Gao C, Zhang Y, Wen D (2020) A novel exopolysaccharide-producing and long-chain n-alkane degrading bacterium Bacillus licheniformis strain DM-1 with potential application for in-situ enhanced oil recovery. Sci Rep 10(1)
Fardeau ML, Salinas MB, l'Haridon S, Jeanthon C, Verhé F, Cayol JL, Patel BK, Garcia JL, Ollivier B (2004) Isolation from oil reservoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. Int J Syst Evol Microbiol 54(2):467–474
Fardeau ML, Magot M, Patel BKC, Thomas P, Garcia JL, Ollivier B (2000) Thermoanaerobacter subterraneus sp. nov., a novel thermophile isolated from oilfield water. Int J Syst Evol Microbiol 50:2141–2149
Fida TT, Chen C, Okpala G, Voordouw G (2016) Implications of limited thermophilicity of nitrite reduction for control of sulfide production in oil reservoirs. Appl Environ Microbiol 82(14):4190–4199
Foght J (2008) Anaerobic biodegradation of aromatic hydrocarbons: pathways and prospects. J Mol Microbiol Biotechnol 15(2-3):93–120
Gam ZBA, Abdelkafi S, Casalot L, Tholozan JL, Oueslati R, Labat M (2007) Modicisalibacter tunisiensis gen. nov., sp. nov., an aerobic, moderately halophilic bacterium isolated from an oilfield-water injection sample, and emended description of the family Halomonadaceae Franzmann et al. 1989 emend Dobson and Franzmann 1996 emend. Ntougias et al. 2007. Int J Syst Evol Microbiol 57(10):2307–2313
Gao P, Tian H, Wang Y, Li Y, Li Y, JinxiaXie J, Zeng B, Zhou J, Li G, Ma T (2016) Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. Sci Rep 6:20174. https://doi.org/10.1038/srep20174
Gao H, Zhang J, Lai H, Xue Q (2017) Degradation of asphaltenes by two Pseudomonas aeruginosa strains and their effects on physicochemical properties of crude oil. Int Biodeterior Biodegradation 122:12–22
Gao P, Wang H, Li G, Ma T (2019) Low-abundance dietzia inhabiting a water-flooding oil reservoir and the application potential for oil recovery. Biomed Res Int 2019:2193453
Gassara F, Suri N, Stanislav P, Voordouw G (2015) Microbially enhanced oil recovery by sequential injection of light hydrocarbon and nitrate in low- and high-pressure bioreactors. Environ Sci Technol 49:12594–12601
Gassara F, Suri N, Voordouw G (2017) Nitrate-Mediated Microbially Enhanced Oil Recovery (N-MEOR) from model upflow bioreactors. J Hazard Mater 324:94–99
Genouw GFPHWW, De Naeyer F, Van Meenen P, Van de Werf H, De Nijs W, Verstraete W (1994) Degradation of oil sludge by landfarming—a case-study at the Ghent harbor. Biodegradation 5(1):37–46
Godheja J, Shekhar SK, Modi DR (2014) Advances in molecular biology approaches to guage microbial communities and bioremediation at contaminated sites. Int J Environ Bioremediat Biodegrad 2(4):167–177
Gittel A, Sørensen KB, Skovhus TL, Ingvorsen K, Schramm A (2009) Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Appl Environ Microbiol 75(22):7086–7096
Grabowski A, Nercessian O, Fayolle F, Blanchet D, Jeanthon C (2005a) Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol Ecol 54(3):427–443
Grabowski A, Tindall BJ, Bardin V, Blanchet D, Jeanthon C (2005b) Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int J Syst Evol Microbiol 55:1113–1121
Grassia GS, McLean KM, Glénat P, Bauld J, Sheehy AJ (1996) A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol Ecol 21(1):47–58
Greene AC, Patel BK, Sheehy AJ (1997) Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese-and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Evol Microbiol 47(2):505–509
Grigoryan A, Voordouw G (2008) Microbiology to help solve our energy needs: methanogenesis from oil and the impact of nitrate on the oil-field sulfur cycle. Ann N Y Acad Sci 1125(1):345–352
Hackbusch S, Noirungsee N, Viamonte J, Sun X, Bubenheim P, Kostka JE, Müller R, Liese A (2020) Influence of pressure and dispersant on oil biodegradation by a newly isolated Rhodococcus strain from deep-sea sediments of the gulf of Mexico. Mar Pollut Bull 150:110683
Hamzah AF, Abd-Alsahib WH, Mahdi SS (2020) Isolation and identification new bacterial strains isolated from different sources of Al-Rafidiyah oil field in Iraq. Int J Environ Sci 21:15–22
Hao DH, Lin JQ, Song X, Lin JQ, Su YJ, Qu YB (2008) Isolation, identification, and performance studies of a novel paraffin-degrading bacterium of Gordonia amicalis LH3. Biotechnol Bioproc Eng 13(1):61–68
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464):567–570
Hasanuzzaman M, Ueno A, Ito H, Ito Y, Yamamoto Y, Yumoto I, Okuyama H (2007) Degradation of long-chain n-alkanes (C36 and C40) by Pseudomonas aeruginosa strain WatG. Int Biodeterior Biodegradation 59(1):40–43
Hassanshahian M, Zeynalipour MS, Musa FH (2014) Isolation and characterization of crude oil degrading bacteria from the Persian Gulf (Khorramshahr provenance). Mar Pollut Bull 82(1-2):39–44
Head IM, Jones DM, Larter SR (2003) Biological activity in the deep subsurface and the origin of heavy oil. Nature 426(6964):344–352
Head IM, Jones DM, Röling WF (2006) Marine microorganisms make a meal of oil. Nat Rev Microbiol 4(3):173–182
Head IM, Gray ND, Larter SR (2014) Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front Microbiol 5:566
Horn MK (2007) Relationship between giant field data and ultimate recoverable oil. AAPG 12(5):1–13
Hu P, Tom L, Singh A, Thomas BC, Baker BJ, Piceno YM, Andersen GL, Banfield JF (2016) Genome-resolved metagenomic analysis reveals roles for candidate phyla and other microbial community members in biogeochemical transformations in oil reservoirs. MBio 7(1):01669–01615
Hussain A, Hasan A, Javid A and Qazi JI (2016) Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: a mini review. 3 Biotech 6(2):119
Jamal MT (2020) Enrichment of potential halophilic Marinobacter consortium for mineralization of petroleum hydrocarbons and also as oil reservoir indicator in Red Sea, Saudi Arabia. Polycycl Aromat Comp 1-12
Jayasinghearachchi HS, Lal B (2011) Oceanotoga teriensis gen. nov., sp. nov., a thermophilic bacterium isolated from offshore oil-producing wells. Int J Syst Evol Microbiol 61:554–560
Jebbar M, Franzetti B, Girard E, Oger P (2015) Microbial diversity and adaptation to high hydrostatic pressure in deep-sea hydrothermal vents prokaryotes. Extremophiles 19(4):721–740
Jebbar M, Hickman-Lewis K, Cavalazzi B, Taubner RS, Simon KMR, Antunes A (2020) Microbial diversity and biosignatures: an icy moons perspective. Space Sci Rev 216(1):10
Jenisch-Anton A, Adam P, Michaelis W, Connan J, Herrmann D, Rohmer M, Albrecht P (2000) Molecular evidence for biodegradation of geomacromolecules. Geochim Cosmochim Acta 64(20):3525–3537
Kato T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2001) Isolation and characterization of long-chain-alkane degrading Bacillus thermoleovorans from deep subterranean petroleum reservoirs. J Biosci Bioeng 91(64-70):270
Kim S, Stanford LA, Rodgers RP, Marshall AG, Walters CC, Qian K, Wenger LM, Mankiewicz P (2005) Microbial alteration of the acidic and neutral polar NSO compounds revealed by Fourier transform ion cyclotron resonance mass spectrometry. Org Geochem 36(8):1117–1134
Kim DD, O'Farrell C, Toth CRA, Montoya O, Gieg LM, Kwon T-H, Yoon S (2018) Microbial community analyses of produced waters from high-temperature oil reservoirs reveal unexpected similarity between geographically distant oil reservoirs. Microb Biotechnol 11(4):788–796
Kobayashi H, Endo K, Sakata S, Mayumi D, Kawaguchi H, Ikarashi M, Miyagawa Y, Maeda H, Sato K (2012) Phylogenetic diversity of microbial communities associated with the crude-oil, large-insoluble-particle and formation-water components of the reservoir fluid from a non-flooded high-temperature petroleum reservoir. J Biosci Bioeng 113(2):204–210
Koshlaf E, Ball AS (2017) Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS microbiology 3(1):25–49
Kryachko Y (2018) Novel approaches to microbial enhancement of oil recovery. J Biotechnol 266:118–123
Kryachko Y, Voordouw G (2014) Microbially enhanced oil recovery from miniature model columns through stimulation of indigenous microflora with nitrate. Int Biodeterior Biodegradation 96:135–143
Kryachko Y, Hemmingsen SM (2017) The Role of Localized Acidity Generation in Microbially Influenced Corrosion. Curr Microbiol 74(7):870–876
Kleindienst S, Seidel M, Ziervogel K, Grim S, Loftis K, Harrison S, Malkin SY, Perkins MJ, Field J, Sogin ML, Dittmar T (2015) Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. P Natl Acad Sci Usa 112(48):14900–14905
Laso-Pérez R, Hahn C, van Vliet DM, Tegetmeyer HE, Schubotz F, Smit NT, Pape T, Sahling H, Bohrmann G, Boetius A, Knittel K (2019) Anaerobic degradation of non-methane alkanes by “Candidatus Methanoliparia” in hydrocarbon seeps of the Gulf of Mexico. mBio 10(4):01814–01819
Li Q, Kang C, Wang H, Liu C, Zhang C (2002) Application of microbial enhanced oil recovery technique to Daqing Oilfield. Biochem Eng J 11(2-3):197–199
Li G, Gao P, Wu Y, Tian H, Dai X, Wang Y, Cui Q, Zhang H, Pan X, Dong H, Ma T (2014) Microbial Abundance and Community Composition Influence Production Performance in a Low-Temperature Petroleum Reservoir. Environ Sci Technol 48(9):5336–5344
Li G, Gao P, Zhi B, Fu B, Gao G, Chen Z, Gao M, Wu M, Ma T (2018) The relative abundance of alkane-degrading bacteria oscillated similarly to a sinusoidal curve in an artificial ecosystem model from oil-well products. Environ Microbiol 20(10):3772–3783
Liang B, Wang LY, Zhou Z, Mbadinga SM, Zhou L, Liu JF, Yang SZ, Gu JD, Mu BZ (2016) High frequency of Thermodesulfovibrio spp. and Anaerolineaceae in association with Methanoculleus spp. in a long-term incubation of n-alkanes-degrading methanogenic enrichment culture. Front Microbiol 7:1431
Liao Y, Geng A, Huang H (2009) The influence of biodegradation on resins and asphaltenes in the Liaohe Basin. Org Geochem 40:312–320
Liu B, Ju M, Liu J, Wu W, Li X (2016) Isolation, identification, and crude oil degradation characteristics of a high-temperature, hydrocarbon-degrading strain. Mar Pollut Bull 106(1-2):301–307
Liu J, Wu J, Lin J, Zhao J, Xu T, Yang Q, Zhao J, Zhao Z, Song X (2019) Changes in the microbial community diversity of oil exploitation. Genes 10(8):556
Lv Y, Liang Z, Ge M, Qi W, Zhang T, Lin F, Peng Z, Zhao H (2016) Genome-wide identification and functional prediction of nitrogen-responsive intergenic and intronic long non-coding RNAs in maize (Zea mays L.). BMC Genomics 17(1):350
Ma TT, Liu LY, Rui JP, Yuan Q, Feng DS, Zhou Z, Dai LR, Zeng WQ, Zhang H, Cheng L (2017) Coexistence and competition of sulfate-reducing and methanogenic populations in an anaerobic hexadecane-degrading culture. Biotechnol Biofuels 10(1):207
Magot M, Ravot G, Campaignolle X, Ollivier B, Patel BKC, Fardeau ML, Thomas P, Crolet JL, Garcia JL (1997) Dethiosulfovibrio peptidovorans gen. nov., sp. nov., a new anaerobic, slightly halophilic, thiosulfate-reducing bacterium from corroding offshore oil wells. Int J Syst Evol Microbiol 47(3):818–824
Mayilraj S, Kaksonen AH, Cord-Ruwisch R, Schumann P, Spröer C, Tindall BJ, Spring S (2009) Desulfonauticus autotrophicus sp. nov., a novel thermophilic sulfate-reducing bacterium isolated from oil-production water and emended description of the genus Desulfonauticus. Extremophiles 13(2):247–255
Mayumi D, Mochimaru H, Yoshioka H, Sakata S, Maeda H, Miyagawa Y, Ikarashi M, Takeuchi M, Kamagata Y (2011) Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high temperature petroleum reservoir of Yabase oil field (Japan). Environ Microbiol 13(8):1995–2006
Meckenstock RU, von Netzer F, Stumpp C, Lueders T, Himmelberg AM, Hertkorn N, Schmitt-Kopplin P, Harir M, Hosein R, Haque S, Schulze-Makuch D (2014) Water droplets in oil are microhabitats for microbial life. Science 345(6197):673–676
Miller GH (1976) Microbial survey helps to evaluate oil and gas. Oil Gas J 4:192
Miranda-Tello E, Fardeau M-L, Sepulveda J, Fernandez L et al (2003) Garciella nitratireducens gen. nov., sp. nov., an anaerobic, thermophilic, nitrate- and thiosulfatereducing bacterium isolated from an oilfield separator in the Gulf of Mexico. Int J Syst Evol Microbiol 53:1509–1514
Mnif S, Chamkha M, Labat M, Sayadi S (2011) Simultaneous hydrocarbon biodegradation and biosurfactant production by oilfield-selected bacteria. J Appl Microbiol 111(525-536):268
Mogilewskii GA (1940) The bacterial method of prospecting for oil and natural gases. Razvedka Nedr 12:32–43
Muangchinda C, Chavanich S, Viyakarn V, Watanabe K, Imura S, Vangnai AS, Pinyakong O (2015) Abundance and diversity of functional genes involved in the degradation of aromatic hydrocarbons in Antarctic soils and sediments around Syowa Station. Environ Sci Pollut Res 22(6):4725–4735
Myhr S, Torsvik T (2000) Denitrovibrio acetiphilus, a novel genus and species of dissimilatory nitrate-reducing bacterium isolated from an oil reservoir model column. Int J Syst Evol Microbiol 50(4):1611–1619
Nazina TN, Sokolova DS, Grigoryan AA, Shestakova NM, Mikhailova EM, Poltaraus AB, Tourova TP, Lysenko AM, Osipov GA, Belyaev SS (2005) Geobacillus jurassicus sp. nov., a new thermophilic bacterium isolated from a high-temperature petroleum reservoir, and the validation of the Geobacillus species. Syst Appl Microbiol 28:43–53
Nazina TN, Grigor’Yan AA, Shestakova NM, Babich TL, Ivoilov VS, Feng Q, Ni F, Wang J, She Y, Xiang T, Luo Z (2007) Microbiological investigations of high-temperature horizons of the Kongdian petroleum reservoir in connection with field trial of a biotechnology for enhancement of oil recovery. Microbiology 76(3):287–296
Nazina TN, Shestakova NM, Pavlova NK, Tatarkin YV, Ivoilov VS, Khisametdinov MR, Sokolova DS, Babich TL, Tourova TP, Poltaraus AB, Belyaev SS (2013) Functional and phylogenetic microbial diversity in formation waters of a low-temperature carbonate petroleum reservoir. Int Biodeterior Biodegradation 81:71–81
Nie Y, Liang JL, Fang H, Tang YQ, Wu XL (2014) Characterization of a CYP153 alkane hydroxylase gene in a Gram-positive Dietzia sp. DQ12-45-1b and its “team role” with alkW1 in alkane degradation. Appl Microbiol Biotechnol 98(1):163–173
Nikolova C, Gutierrez T (2020) Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Front Microbiol 10:2996
Nilsen RK, Torsvik T (1996) Methanococcus thermolithotrophicus isolated from North Sea oil field reservoir water. Appl Environ Microbiol 62:728–731
Nkem BM, Halimoon N, Yusoff FM, Johari WLW, Zakaria MP, Medipally SR, Kannan N (2016) Isolation, identification and diesel-oil biodegradation capacities of indigenous hydrocarbon-degrading strains of Cellulosimicrobium cellulans and Acinetobacter baumannii from tarball at Terengganu beach, Malaysia. Mar Pollut Bull 107(1):261–268
Obraztsova AY, Laurinavinchus KS, Bezrukova LV, TSYBAN V, Belyaev SS (1987) Biological properties of methanosarcina not utilizing carbonic-acid and hydrogen. Microbiology 56(6):807–812
Okoro CC, Amund OO (2018) Microbial community structure of a low sulfate oil producing facility indicate dominance of oil degrading/nitrate reducing bacteria and methanogens. Pet Sci Technol 36(4):293–301
Olowomofe TO, Oluyege JO, Aderiye BI, Oluwole OA (2019) Degradation of poly aromatic fractions of crude oil and detection of catabolic genes in hydrocarbon-degrading bacteria isolated from Agbabu bitumen sediments in Ondo State. AIMS Microbiol 5(4):308–323
Ollivier B, Fardeau ML, Cayol JL, Magot M, Patel BK, Prensier G, Garcia JL (1998) Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. Int J Syst Evol Microbiol 48(3):821–828
Orcutt BN, Joye SB, Kleindienst S, Knittel K, Ramette A, Reitz A, Samarkin V, Treude T, Boetius A (2010) Impact of natural oil and higher hydrocarbons on microbial diversity, distribution, and activity in Gulf of Mexico cold-seep sediments. Deep Sea Res Part II Top Stud Oceanogr 57(21-23):2008–2021
Ortega MF, Guerrero DE, García-Martínez MJ, Bolonio D, Llamas JF, Canoira L, Gallego JLR (2018) Optimization of landfarming amendments based on soil texture and crude oil concentration. Water Air Soil Pollut 229(7):234
Panda H (2017) Political economy of energy policy in India: Electricity and LPG.
Pannekens M, Kroll L, Müller H, Mbow FT, Meckenstock RU (2019) Oil reservoirs, an exceptional habitat for microorganisms. New Biotechnol 49:1–9
Parnell J, Bowden S, Muirhead D (2017) Subsurface biodegradation of crude oil in a fractured basement reservoir, Shropshire, UK. J Geol Soc India 174(4):655–666
Patel J, Borgohain S, Kumar M, Rangarajan V, Somasundaran P, Sen R (2015) Recent developments in microbial enhanced oil recovery. Renew Sust Energ Rev 52:1539–1558
Rabus R, Boll M, Heider J, Meckenstock R, Buckel W, Einsle O, Ermler U, Golding B, Gunsalus R, Kroneck P, Krüger M, Lueders T, Martins B, Musat F, Richnow H, Schink B, Seifert J, Szaleniec M, Treude T, Wilkes H (2016) Anaerobic Microbial Degradation of Hydrocarbons: From Enzymatic Reactions to the Environment. J Mol Microbiol Biotechnol 26:5–28
Rainey FA, Zhilina TN, Boulygina ES, Stackebrandt E, Tourova TP, Zavarzin GA (1995) The taxonomic status of the fermentative halophilic anaerobic bacteria: description of Haloanaerobiales ord. nov., Halobacteroidaceae fam. nov., Orenia gen. nov. and further taxonomic rearrangements at the genus and species level. Anaerobe 1(4):185–199
Rasheed MA, Hasan SZ, Srinivasa Rao PL, Annapurna B, Sudarshan V, Kumar B, Harinarayana T (2015) Application of geo-microbial prospecting method for finding oil and gas reservoirs. Front Earth Sci 9(1):40–50
Rathi R, Lavania M, Kukreti V, Lal B (2018) Evaluating the potential of indigenous methanogenic consortium for enhanced oil and gas recovery from high temperature depleted oil reservoir. J Biotechnol 283:43–50
Ravot G, Magot M, Ollivier B, Patel BKC, Ageron E, Grimont PAD, Thomas P, Garcia JL (1997) Haloanaerobium congolense sp. nov., an anaerobic, moderately halophilic, thiosulfate- and sulfur-reducing bacterium from an African oil field. FEMS Microbiol Lett 147:81–88
Ravot G, Magot M, Fardeau ML, Patel BK, Thomas P, Garcia JL, Ollivier B (1999) Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate-reducing bacterium from an oil-producing well. Int J Syst Evol Microbiol 49(3):1141–1147
Rees GN, Patel BKC, Grassia GS, Sheehy AJ (1997) Anaerobaculum thermoterrenum gen. nov., sp. nov., a novel, thermophilic bacterium which ferments citrate. Int J Syst Bacteriol 47(150-154):269
Révész F, Figueroa-Gonzalez PA, Probst AJ, Kriszt B, Banerjee S, Szoboszlay S, Maróti G, Táncsics A (2020) Microaerobic conditions caused the overwhelming dominance of Acinetobacter spp. and the marginalization of Rhodococcus spp. in diesel fuel/crude oil mixture-amended enrichment cultures. Arch Microbiol 202(2):329–342
Rovere M, Mercorella A, Frapiccini E, Funari V, Spagnoli F, Pellegrini C, Bonetti AS, Veneruso T, Tassetti AN, Dell’Orso M, Mastroianni M (2020) Geochemical and geophysical monitoring of hydrocarbon seepage in the Adriatic Sea. Sensors 20(5):1504
Roy AS, Baruah R, Borah M, Singh AK, Boruah HPD, Saikia N, Deka M, Dutta N, Bora TC (2014) Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int Biodeterior Biodegradation 94:79–89
Safdel M, Anbaz MA, Daryasafar A, Jamialahmadi M (2017) Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew Sust Energ Rev 74:159–172
Salinas MB, Fardeau M-L, Cayol J-L, Casalot L, Patel BKC, Thomas P, Garcia JL, Ollivier B (2004) Petrobacter succinatimandens gen. nov., sp. nov., a moderately thermophilic, nitrate-reducing bacterium isolated from an Australian oil well. Int J Syst Evol Microbiol 54:645–649
Schippers A, Schumann P, Sproer C (2005) Nocardioides oleivorans sp. nov., a novel crudeoil-degrading bacterium. Int J Syst Evol Microbiol 55:1501–1504
Schumacher DD (2000) Surface geochemical exploration for oil and gas: new life for an old technology. Lead Edge 19(3):258–261
Sealy JR (1974) A geomicrobial method of prospecting for oil. Oil Gas J 15:98–102
Sharma N, Lavania M, Kukreti V, Lal B, Gupta V (2020) Instigation of indigenous thermophilic bacterial consortia for enhanced oil recovery from high temperature oil reservoirs. PLoS One 15(5):e0229889
She YH, Zhang F, Xia JJ, Kong SQ, Wang ZL, Shu FC, Hu JM (2011) Investigation of biosurfactant-producing indigenous microorganisms that enhance residue oil recovery in an oil reservoir after polymer flooding. Appl Biochem Biotechnol 163(2):223–234
Shekhar SK, Godheja J, Modi DR (2020) Molecular technologies for assessment of bioremediation and characterization of microbial communities at pollutant-contaminated sites. In: In Bioremediation of Industrial Waste for Environmental Safety. Springer, Singapore, pp 437–474
Shelton JL, Akob DM, McIntosh JC, Fierer N, Spear JR, Warwick PD, McCray JE (2016) Environmental drivers of differences in microbial community structure in crude oil reservoirs across a methanogenic gradient. Front Microbiol 7:1535
Shibulal B, Al-Bahry SN, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ (2014) Microbial enhanced heavy oil recovery by the aid of inhabitant spore-forming bacteria: an insight review. Sci World J 2014:1–12. https://doi.org/10.1155/2014/309159
Shibulal B, Al-Bahry SN, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ (2018) Microbial-enhanced heavy oil recovery under laboratory conditions by Bacillus firmus BG4 and Bacillus halodurans BG5 isolated from heavy oil fields. Colloids Interfaces 2(1):1
Sivasankar P and Govindarajan SK (2016) Modelling the coupled effects of temperature, injection rate and microbial kinetic parameters on oil recovery by microbial flooding. In SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition. Soc Pet Eng J.
Siegert M, Sitte J, Galushko A, Krüger M (2013) Starting up microbial enhanced oil recovery. In: In Geobiotechnology II. Springer, Berlin, pp 1–94
Suthar H, Hingurao K, Desai A, Nerurkar A (2008) Evaluation of bioemulsifier mediated microbial enhanced oil recovery using sand pack column. J Microbiol Methods 75(2):225–230
Tan B, Fowler SJ, Laban NA, Dong X, Sensen CW, Foght J, Gieg LM (2015) Comparative analysis of metagenomes from three methanogenic hydrocarbon-degrading enrichment cultures with 41 environmental samples. ISME J 9(9):2028–2045
Thatoi H, Behera BC, Mishra RR, Dutta SK (2013) Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann Microbiol 63(1):1–19
Thauer RK (2019) Methyl (alkyl)-coenzyme M reductases: nickel F-430-containing enzymes involved in anaerobic methane formation and in anaerobic oxidation of methane or of short chain alkanes. Biochemistry 58(52):5198–5220
Timmis KN (ed) (2010) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, p 4716
Tinker K, Gardiner J, Lipus D, Sarkar P, Stuckman M, Gulliver D (2020) Geochemistry and microbiology predict environmental niches with conditions favoring potential microbial activity in the Bakken shale. Front Microbiol 11:1781
Tüccar T, Ilhan-Sungur E, Abbas B, Muyzer G (2019) Coexistence of sulfate reducers with the other oil bacterial groups in Diyarbakır oil fields. Anaerobe 59:19–31
Tucker J, Hitzman D (1994) Detailed microbial surveys help improve reservoir characterization. Oil Gas J 92(23)
Varjani SJ, Upasani VN (2017) A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad 120:71–83
Vetriani C, Speck MD, Ellor SV, Lutz RA, Starovoytov V (2004) Thermovibrio ammonificans sp. nov., a thermophilic, chemolithotrophic, nitrate-ammonifying bacterium from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 54(1):175–181
Veerapagu M, Jeya KR, Kalaivani R, Jeyanthi KA, Geethanjali S (2019) Screening of hydrocarbon degrading bacteria isolated from oil contaminated soil. Pharm Innov J 8(6):69–72
Viesser JA, Sugai-Guerios MH, Malucelli LC, Pincerati MR, Karp SG, Maranho LT (2020) Petroleum-tolerant rhizospheric bacteria: isolation, characterization and bioremediation potential. Sci Rep 10(1):1–11
Vigneron A, Alsop EB, Cruaud P, Philibert G, King B, Baksmaty L, Lavallée D, Lomans BP, Kyrpides NC, Head IM, Tsesmetzis N (2017) Comparative metagenomics of hydrocarbon and methane seeps of the Gulf of Mexico. Sci Rep 7(1):1–12
Visser O, Spoor M (2011) Land grabbing in post-Soviet Eurasia: the world’s largest agricultural land reserves at stake. J Peasant Stud 38(2):299–323
Vreeland RH, Jones J, Monson A, Rosenzweig WD, Lowenstein TK, Timofeeff M, Satterfield C, Cho BC, Park JS, Wallace A, Grant WD (2007) Isolation of live Cretaceous (121–112 million years old) halophilic Archaea from primary salt crystals. Geomicrobiol J 24(3-4):275–282
Wagner M, Wagner M, Piske J and Smit R (2002) AAPG Studies in Geology No. 48/SEG Geophysical References Series No. 11, Chapter 19: Case histories of microbial prospection for oil and gas, onshore and offshore in Northwest Europe
Wang X-B, Chi C-Q, Nie Y, Tang Y-Q, Tan Y, Wu G, Wu XL (2011) Degradation of petroleum hydrocarbons (C6-C40) and crude oil by a novel Dietzia strain. Bioresour Technol 102:7755–7761
Wang LY, Duan RY, Liu JF, Yang SZ, Gu JD, Mu BZ (2012) Molecular analysis of the microbial community structures in water-flooding petroleum reservoirs with different temperatures. Biogeosci Biogeosci Discuss 9:5177–5203
Wang LY, Ke WJ, Sun XB, Liu JF, Gu JD, Mu BZ (2014) Comparison of bacterial community in aqueous and oil phases of water-flooded petroleum reservoirs using pyrosequencing and clone library approaches. J Mol Microbiol Biotechnol 98(9):4209–4221
Wang Y, Wegener G, Ruff SE, Wang F (2020) Methyl/alkyl-coenzyme M reductase-based anaerobic alkane oxidation in archaea. Environ Microbiol. https://doi.org/10.1111/1462-2920.15057
Yang ZH, Xu R, Zheng Y, Chen T, Zhao LJ, Li M (2016) Characterization of extracellular polymeric substances and microbial diversity in anaerobic co-digestion reactor treated sewage sludge with fat, oil, grease. Bioresour Technol 212:164–173
Ying J-Y, Wang B-J, Yang S-S, Liu S-J (2006) Cyclobacterium lianum sp. nov., a marine bacterium isolated from sediment of an oilfield in the South China Sea, and emended description of the genus Cyclobacterium. Int J Syst Evol Microbiol 56:2927–2930
Ying J-Y, Wang B-J, Dai X, Yang S-S, Liu S-J, Liu Z-P (2007) Wenxinia marina gen. nov., sp. nov., a novel member of the Roseobacter clade isolated from oilfield sediments of the South China Sea. Int J Syst Evol Microbiol 57:1711–1716
Zengler K, Richnow HH, Rosselló-Mora R, Michaelis W, Widdel F (1999) Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401(6750):266–269
Zheng C, Yu L, Huang L, Xiu J, Huang Z (2012) Investigation of a hydrocarbon-degrading strain, Rhodococcus ruber Z25, for the potential of microbial enhanced oil recovery. J Pet Sci Eng 81(49-56):318
Zheng J, Feng JQ, Zhou L, Mbadinga SM, Gu JD, Mu BZ (2018) Characterization of bacterial composition and diversity in a long-term petroleum contaminated soil and isolation of high-efficiency alkane-degrading strains using an improved medium. World J Microbiol Biotechnol 34(2):34
Zhao F, Li P, Guo C, Shi RJ, Zhang Y (2018) Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresour Technol 251:295–302
Zhu H, Carlson HK, Coates JD (2013) Applicability of anaerobic nitrate-dependent Fe (II) oxidation to microbial enhanced oil recovery (MEOR). Environ Sci Technol 47(15):8970–8977
Zhu T, Dittrich M (2016) Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol 4:4
Acknowledgment
The authors would like to acknowledge the Banasthali Vidyapith for providing financial assistance and research facility. Authors also acknowledge the Bioinformatics Center, Banasthali Vidyapith, supported by DBT, India, for providing computation support.
Author information
Authors and Affiliations
Contributions
Sangeeta Choudhary contributed to the study conception and design. The first draft of the manuscript was written by Nishi Kumari Singh, and both the authors commented on previous versions of the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible editor: Robert Duran
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, N.K., Choudhary, S. Bacterial and archaeal diversity in oil fields and reservoirs and their potential role in hydrocarbon recovery and bioprospecting. Environ Sci Pollut Res 28, 58819–58836 (2021). https://doi.org/10.1007/s11356-020-11705-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11705-z