Abstract
A novel moderately thermophilic and halophilic sulfate-reducing bacterium, strain TeStT, was isolated from production water of an oil field in Northern Germany near Hamburg. The cells were Gram-negative, straight to slightly curved rods and motile by a single polar flagellum. Only hydrogen and formate served as electron donors, whereas a wide variety of organic substrates and CO2 could be used as carbon sources. Sulfate, sulfite, thiosulfate and sulfur were used as electron acceptors, but not nitrate or ferric iron. The novel isolate was negative for oxidase, catalase and desulfoviridin enzyme activity. Cytochromes were present and predominantly of the c-type. Whole-cells fatty acid patterns were dominated by the branched-chain fatty acids anteiso-C15:0, iso-C15:0, iso-C17:0 and anteiso-C17:0. As major respiratory lipoquinones partially saturated derivates of menaquinone 6 [MK-6(H2) and probably MK-6(H4)] were identified. The G + C content of the genomic DNA was 41.3 mol% (HPLC method). An analysis of the 16S rRNA gene sequence indicated that strain TeStT belongs to the family Desulfohalobiaceae within the class Deltaproteobacteria. The most closely related species with a sequence similarity of 95.0% was Desulfonauticus submarinus suggesting an affiliation of TeStT to the genus Desulfonauticus. The novel isolate could be clearly distinguished from Desulfonauticus submarinus by its ability to grow chemolithoautotrophically and hence should be assigned to a novel species for which the name Desulfonauticus autotrophicus sp. nov. is proposed. The type strain is TeStT (=DSM 4206T = JCM 13028T).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Formation of hydrogen sulfide is a major concern in oil production and causes severe financial losses by causing corrosive damage to steel equipment and pipelines, reduction of oil quality by souring or by plugging of injection wells with sulfidic precipitates. Sulfate-reducing bacteria that thrive under anoxic conditions and can use hydrogen or various simple organic compounds as electron donors are the main cause of sulfide production and biological corrosion in crude oil production (Hamilton 1985). Among several mechanisms that are currently discussed to contribute to anaerobic corrosion the two most important are production of hydrogen sulfide and removal of electrons from the metal for example via hydrogen (Cord-Ruwisch et al. 1987). Hydrogen sulfide can attack metallic iron indirectly and cause the formation of ferrous sulfide and hydrogen, whereas the removal of an electrochemically formed protective hydrogen film on the cathodic iron surface by sulfate-reducing bacteria represents an indirect mechanism of anaerobic corrosion. In an oil field near Hamburg, Germany, operated by Preussag (Gaz de France since 2003) an increase of hydrogen sulfide production was observed in the early 1980 s. Although serious damage by anaerobic corrosion was not observed, accumulation of hydrogen sulfide in the injection water caused the plugging of injection wells by the formation of FeS flocs. The main source of sulfide was the oil processing unit (treater) that separates crude oil from the production water in order to make it transportable via pipelines. Numerous sulfate-reducing bacteria could be retrieved from the saline water phase of the treater and were tentatively characterized by Cord-Ruwisch et al. (1986). A phylogenetic analysis of one of the isolates, designated TeStT, revealed that it represents a novel lineage within the family Desulfohalobiaceae, currently comprising five genera with validly published names (i.e., Desulfohalobium, Desulfonatronovibrio, Desulfothermus, Desulfonauticus and Desulfonatronospira) and one genus, “Desulfovermiculus” that has no standing in nomenclature. In this study, we present a detailed characterization of strain TeStT as a moderately thermophilic and halophilic sulfate-reducing bacterium being indigenous to brine extracted from a subterrestrial oil reservoir in Northern Germany.
Materials and methods
Source of sample, isolation and cultivation
An oil processing unit (heater treater) separating water from crude oil in a field near Hamburg (Germany) was identified as major source of sulfide that accumulated in the injection water. Water samples for studying sulfate-reducing bacteria were taken from the treater near the bottom of a water compartment. The operating temperature of the treater in the oil phase was 60–70°C, in the water phase it was between 30 and 45°C. The salinity of the treater water was about 100 g/l (mainly NaCl).
Enrichment of sulfate reducers was carried out in artificial sea water medium described by Widdel and Pfennig (1984) except that the NaCl concentration was adjusted to 30 g/l. An enrichment culture incubated at 58°C containing hydrogen gas and 1 mM acetate as substrates resulted in the proliferation of rod-shaped thermophilic bacteria. This culture was purified by repeated serial dilution in agar shake cultures. An individual colony grown in the highest dilution step was obtained and designated strain TeStT. For the routine subcultivation of the novel isolate a complex medium was prepared that yielded higher cell densities as the defined salt water medium used for initial enrichment. It was designated SYAH2 and contained per liter of distilled water: 35.0 g sea salts (SIGMA), 2.5 g NaHCO3, 2.0 g yeast extract, 0.7 g sodium acetate, 1 ml Wolfe’s mineral elixir (DSMZ medium 792), 0.5 mg resazurin and 10 mg sodium dithionite. The medium was prepared anoxically under 80% H2 and 20% CO2 gas atmosphere using the culture technique of Hungate (1950) with the modifications introduced by Bryant (1972). A stock solution of NaHCO3 (10% w/v) was prepared anoxically under 80% N2 and 20% CO2 gas atmosphere and autoclaved. Anoxic stock solutions of vitamins and sodium dithionite (5% w/v) were prepared under 100% N2 and sterilized by filtration. Bicarbonate, vitamins and dithionite were added to the medium after autoclaving. The pH of the completed medium was adjusted to 7.5. After inoculation, culture vessels were pressurized with a sterile gas mixture of 80% H2 and 20% CO2 to 1–2 bar overpressure.
For comparison the strain Desulfonauticus submarinus DSM 15269T was used and cultured in DSM medium 383d at 45°C as indicated in the DSMZ catalogue of strains.
Morphological and physiological characterization
Morphology
Cell dimensions, presence of spores and motility were examined by phase-contrast microscopy. Flagella were visualized according to Heimbrook et al. (1989) and Gram-staining carried out using the kit of Merck (Germany) according to the instructions.
Growth parameters
The influence of temperature on the growth response was determined in SYAH2 medium with 2 bar overpressure of 80% H2 and 20% CO2. For incubation Balch tubes containing 10 ml of anaerobic culture were inserted in a TN-3 temperature gradient incubator (Toyo Kagaku Sangyo, Japan) with constant shaking. The pH range and optimum for growth were determined in buffered SYAH2 medium. The final pH of the medium was adjusted after autoclaving with sterile stock solutions of HCl (1%) or Na2CO3 (5% w/v) to values between 5.0 and 10.0 in increments of approx. 0.5 units. The following buffers were used (each at 10 mM): trisodium citrate (pH 5.0–5.5), MES (pH 5.5–6.5), PIPES (pH 6.5–7.0), MOPS (pH 7.0–7.5), HEPES (pH 7.5–8.0), TRIS (pH 8.0–9.0) and CHES (pH 9.0–10.0). For the determination of the salinity range and optimum sodium chloride was added up to 10% (w/v) to SYAH2 medium prepared without sea salts and supplemented either with 10 mM MgSO4·7H2O or 10 mM (NH4)2SO4. Alternatively, SYAH2 medium was prepared with various amounts of sea salts [0–10% (w/v)] and supplemented with 10 mM MgSO4·7H2O.
The growth response was monitored by inserting Balch tubes containing 10 ml of anaerobic culture directly into an Ultraspec II LKB Biochrom 4050 UV/visible-spectrophotometer and measuring turbidity at 430 nm. Hydrogen sulfide production was detected by the formation of a precipitate with 2 mM CuSO4 in acidic solution and quantified by the colorimetric method of Cord-Ruwisch (1985). The stability of growth parameters was verified by performing experiments in duplicate or triplicate.
Growth requirements and substrate utilization
For the determination of growth factor requirements and substrate utilization patterns the following mineral medium was used (per liter distilled water): 21.0 g NaCl, 2.8 g Na2SO4, 2.5 g NaHCO3, 1.8 g MgCl2·6H2O, 0.5 g NH4Cl, 0.4 g CaCl2·2H2O, 0.15 g K2HPO4, 1 ml Wolfe’s mineral elixir, 0.5 mg resazurin and 10.0 mg sodium dithionite added from a sterile anoxic stock solution (5% w/v) after autoclaving. Suitable sources of nitrogen were determined in mineral medium prepared without ammonium chloride. The utilization of electron donors other than hydrogen was tested in mineral medium prepared under 80% N2 and 20% CO2 gas atmosphere. Possible electron acceptors were tested in mineral medium prepared without sodium sulfate under 80% H2 and 20% CO2 gas atmosphere and supplemented with 0.7 g/l sodium acetate and 0.1 g/l yeast extract as carbon sources. The utilization of carbon sources was determined in the following medium (per liter distilled water): 30.0 g sea salts (SIGMA), 2.1 g MOPS, 1.0 ml trace elements solution SL-12 (DSMZ medium 815), 1.0 ml selenite/tungstate solution (DSMZ medium 385), 0.5 g NH4Cl, 0.15 g K2HPO4, 0.5 mg resazurin and 10 mg sodium dithionite. The medium was prepared under 100% H2 or 80% H2 and 20% CO2 gas atmosphere, if carbon dioxide was tested as carbon source. The pH was adjusted to 7.5. Supplementation of the medium with 10 mM MOPS buffer was necessary to stabilize the medium pH in the absence of carbonate/CO2.
Growth of TeStT with different substrates was determined after the third transfer in liquid medium by measuring the increase in optical density at 430 nm and the production of sulfide. The determination of the substrate utilization pattern was performed in duplicate or triplicate experiments without a significant variation of the results. The presence of fermentation products was analyzed with a Waters 510 liquid chromatograph with a Shodex Sugar SH1011 column (Showa denko K.K., Tokyo, Japan) and a Δn − 1000 refraction index detector (WGE Dr Bures GmbH & Co. KG, Dallgow, Germany) as previously described by Koskinen et al. (2008).
Enzyme activities
Expression of cytochromes and enzyme activities were analyzed with cells grown in SYAH2 medium. Oxidase and catalase activities were determined with freshly harvested cell pellets following the protocols given by Cappuccino and Sherman (1998). Presence of desulfoviridin was determined by the method of Postgate (1959). The cytochrome composition was analyzed by redox-difference spectroscopy of whole-cell suspensions or solubilized extracts. Prior to harvesting, cultures were sparged for 15 min with 100% CO2 gas in order to remove dissolved hydrogen sulfide. Whole-cell extracts were obtained by lyzing cell suspensions with the non-ionic detergent N,N-dimethyldodecylamine-N-oxide (LDAO) added to a final concentration of 0.3% (w/v). The obtained crude extract was cleared from large cell debris by a centrifugation step (14,000 rpm for 5 min) and the resulting supernatant distributed into two quartz cuvettes. Absorption-spectra were recorded with a Perkin Elmer Lambda 2 split beam spectrophotometer.
Chemotaxonomical analysis of the cell membrane
Cellular fatty acid patterns were determined from cells grown to stationary phase in DSMZ medium 383d. The preparation and extraction of fatty acid methyl esters from biomass and their subsequent separation and identification by gas chromatography was done as described by Kaksonen et al. (2006).
Respiratory isoprenoid quinones were extracted from freeze dried cells and analyzed with known standards according to the methods described by Collins and Jones (1981), Monciardini et al. (2003) and Groth et al. (1996) using a Shimadzu HPLC apparatus fitted with a reverse phase C18 column [150 mm × 4.6 mm (ID), 5 μm, porosity 90 Å, Vydac] with UV detection at 269 nm.
DNA base composition and phylogenetic analysis
Genomic DNA for the determination of DNA base composition was obtained by disruption of cells using a French pressure cell and purified by chromatography on hydroxyapatite as described by Cashion et al. (1977). The G + C content was determined by reversed phase HPLC of nucleosides according to Mesbah et al. (1989).
Genomic DNA extraction and PCR-mediated amplification of the 16S rRNA gene were done as described by Rainey et al. (1996). Purification of PCR products, sequencing of DNA and electrophoresis of sequence reactions were done as reported previously (Ramamoorthy et al. 2006). The almost-complete 16S rRNA gene sequence of strain TeStT was aligned with sequences included in the SILVA database (release 95) (Pruesse et al. 2007) using tools implemented in the ARB package (www.arb-home.de). The resulting alignment was inspected visually and potential errors were corrected manually. Phylogenetic trees were reconstructed using neighbor-joining, parsimony and maximum-likelihood algorithms using programs implemented in the ARB software package (Ludwig et al. 2004).
The GenBank/EMBL/DDBJ nucleotide sequence accession number of the 16S rRNA gene of strain TeStT is FJ194951.
Results and discussion
Morphology
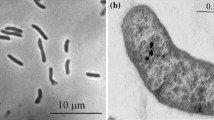
Cells were motile, straight to slightly curved rods occurring singly or in pairs and stained Gram-negative. They had dimensions of 0.6 μm in width and 1.2–4 μm in length (Fig. 1). No conspicuous intracellular inclusions or spores became apparent in phase-contrast microscopy. Motility was conferred by a single polar flagellum. Growth in liquid culture was evenly turbid without formation of visible flocs or aggregates.
Growth parameters
Strain TeStT grew well between 40 and 62°C and showed weak growth at 64°C. The optimum temperature for growth was 58°C (Fig. 2). No growth was observed below pH 6.2 and above pH 8.6, while it was optimal at pH 7.8. Growth occurred between salinities of 1 and 6% (w/v) sea salts with an optimum at 3% (w/v). Suitable NaCl concentrations for growth were in the range from 1 to 4% (w/v) with an optimum at 2.5% (w/v). Strain TeStT did not grow in media without added magnesium salts. Under optimal growth conditions, the mean doubling time was 80 min.
Physiology and biochemistry
Strain TeStT could grow chemoautotrophically in the presence of sulfate with hydrogen as electron donor and carbon dioxide as carbon source. The addition of organic carbon sources or vitamins was not required. However, growth was considerably enhanced by the supplementation of medium with acetate or yeast extract as additional carbon sources. Growth of strain TeStT was always accompanied by the production of sulfide so that it was strictly dependent on the reduction of sulfur compounds. Strain TeStT was strictly anaerobic, but not extremely sensitive to oxygen. It could initiate growth without lag phase in anoxic medium that was not pre-reduced with dithionite or sulfide. With delay growth occurred even at an initial oxygen concentration of 1% (v/v) in the head space gas atmosphere of the culture vessels, but was completely prevented at oxygen concentrations of 2.5% (v/v) or above. In addition, cells lost viability completely after incubation of inoculated cultures in the presence of 2.5 or 5.0% (v/v) oxygen at 55°C for 24 h.
Suitable nitrogen sources for growth were tested in defined mineral medium supplemented with 5 mM acetate as additional carbon source. It was observed that ammonium chloride (0.5 g/l), sodium glutamate (0.9 g/l) and casamino acids (1.0 g/l) were utilized, but sodium nitrate (0.5 g/l) was not. Hence, growth of strain TeStT depends on a reduced source of nitrogen.
Suitable electron donors in the presence of sulfate as electron acceptor and with CO2 as carbon source were only hydrogen and formate. Growth with formate and carbon dioxide was only weak, but could be stimulated by the addition of acetate as additional carbon source. The following compounds were not utilized as electron donor: fructose, glucose, glutamate, rhamnose, sucrose (5 mM each); acetate, butyrate, ethanol, fumarate, glycerol, malate, mannose, methanol, propionate, pyruvate, succinate (10 mM each); lactate (20 mM), casamino acids (1.0 g/l), trypticase peptone (1.0 g/l) and yeast extract (2.0 g/l).
Besides sulfate, strain TeStT was able to utilize the following electron acceptors in the presence of hydrogen: sulfite (2 mM), thiosulfate (20 mM) and sulfur (10 g/l). Nitrate (10 mM), fumarate (10 mM) and ferric iron [provided as 30 mM Fe(III)citrate] were not utilized. No fermentation of carbon compounds and no disproportionation of thiosulfate or sulfur could be detected in medium without sulfate.
Strain TeStT was quite versatile in the utilization of carbon sources in the presence of hydrogen as electron donor and sulfate as electron acceptor. Besides CO2, the following compounds were utilized: glutamate (5 mM); acetate, malate, pyruvate, succinate (10 mM each); casamino acids (1.0 g/l), trypticase peptone (1.0 g/l) and yeast extract (2.0 g/l). The following substrates were tested, but not utilized: fructose, glucose, sucrose (5 mM each); butyrate, ethanol, formate, fumarate, glycerol, mannose, methanol, propionate (10 mM each) and lactate (20 mM). A comparison of growth characteristics of strain TeStT incubated with different substrates is shown in Supplementary Table 1.
Both strains, TeStT and Desulfonauticus submarinus DSM 15269T, were tested negative for oxidase and catalase activity and the presence of a desulfoviridin-type dissimilatory sulfite reductase. An analysis of the cytochrome composition revealed similar patterns in both strains. No reduction of cytochromes was obtained in redox-difference spectra with ascorbate as reductant indicating the presence of only low-potential cytochromes. In dithionite-reduced minus ferricyanide-oxidized difference spectra of cell free extracts only c-type cytochromes could be detected displaying characteristic peaks at 420, 523 and 552 nm in extracts of strain TeStT and at 421, 524 and 553 nm in extracts of Desulfonauticus submarinus. The alpha peaks were symmetrical. When whole-cell suspensions were used the obtained redox-difference spectra were red-shifted by 1–2 nm and the alpha peaks showed some asymmetry with detectable shoulders around 557 nm, which is typical for b-type cytochromes (Supplementary Figure 1). Hence, it can be concluded that in both strains soluble low-potential cytochromes c are dominating, whereas insoluble, probably membrane-bound cytochromes b are present in minor amounts.
Chemotaxonomy
The cellular fatty acid patterns of the isolate TeStT and Desulfonauticus submarinus DSM 15269T were determined (Table 1). The whole-cell fatty acid compositions of both strains were dominated by saturated and branched-chain fatty acids. A further common characteristic was the presence of small amounts of 3-OH hydroxylated fatty acids. The most abundant compounds in both strains were branched-chain C15:0 fatty acids, which appear to be a common feature among members of the family Desulfohalobiaceae. Notable exceptions are so far only Desulfonatronovibrio hydrogenovorans (Zhilina et al. 1997) and Desulfothermus okinawensis (Nunoura et al. 2007), which contain palmitic acid (C16:0) as dominating compound. Despite, an overall similarity the obtained fatty acid patterns allowed a clear distinction of the novel isolate from Desulfonauticus submarinus. For example, strain TeStT contained only 1.6% unsaturated fatty acids compared to 28.7% in Desulfonauticus submarinus DSM 15269T. In addition, branched-chain C17:1 fatty acids were only detected in DSM 15269T. An interesting feature of the cellular fatty acid pattern of strain TeStT was the pairwise occurrence of iso- and anteiso-branched saturated fatty acids with the same chain length. It is known that anteiso fatty acids promote a more fluid membrane structure compared to equivalent iso fatty acids and that some bacteria modify the iso to anteiso ratio of their cellular fatty acids in response to temperature and pH stress (Zhang and Rock 2008). Hence, it is likely that the cellular fatty acid composition of strain TeStT reflects an innate strategy used to balance the stability of the cytoplasmic membrane in response to different temperatures encountered in various ecological niches.
The respiratory lipoquinone composition of TeStT was analyzed and compared with that of Desulfonauticus submarinus. So far, the respiratory lipoquinone composition has been determined only for the strain “Desulfovermiculus halophilus” VKM B-2364 of the family Desulfohalobiaceae. It was found that this species contains only menaquinone 7 (MK-7). In contrast, in both strains, TeStT and DSM 15269T, menaquinone 6 (MK-6) and its partially saturated derivates were identified as dominating compounds. The isoprenoid quinone composition of TeStT was unique and could be clearly distinguished from that of Desulfonauticus submarinus. The type strain of Desulfonauticus submarinus contained 87% MK-6(H2), 12% MK-6 and trace amounts of MK-7, whereas a hitherto unknown quinone compound was detected in strain TeStT, which was tentatively identified as MK-6(H4) based on its retention time during HPLC separation. As shown in Fig. 3 the difference in retention time between MK-6 and MK-6(H2) is nearly the same as between MK-6(H2) and the novel quinone thereby indicating saturation of a further double bond in the isoprenoid unit. To the best of our knowledge this would be the first report of the presence of MK-6(H4) in a sulfate-reducing bacterium. In TeStT this compound is the most abundant isoprenoid quinone (77%), followed by MK-6(H2) (21%) and MK-6 (3%).
The G + C content of the genomic DNA of strain TeStT was 41.3 mol% as determined by HPLC. The reported DNA G + C content of Desulfonauticus submarinus is only 34.4 mol% (Audiffrin et al. 2003). It has been empirically found that the variation in the DNA G + C content of strains belonging to the same species does not exceed 5 mol% (Rosselló-Mora and Amann 2001), thus indicating a separate taxonomic status of strain TeStT.
Phylogeny and ecology
Based on the determined sequence of the almost-complete 16S rRNA gene (1,547 nucleotides) strain TeStT could be affiliated to the family Desulfohalobiaceae within the order Desulfovibrionales, class Deltaproteobacteria. The most closely related species was Desulfonauticus submarinus sharing 95.0% sequence similarity with the 16S rRNA gene of the novel isolate. In several phylogenetic trees, reconstructed using various methods, the type strain of Desulfonauticus submarinus and strain TeStT always formed a common branch, which was also supported by high bootstrap values (Fig. 4). A phylogenetic analysis of TeStT based on the deduced amino acid sequence of the adenosine-5′-phosphosulfate (APS) reductase gene was done previously by Meyer and Kuever (2007). In this study a phylogenetic tree of the AprB subunit of the APS reductase from representatives of several families of sulfate-reducing prokaryotes is shown, which illustrates a common branch of strain TeStT and Desulfonauticus submarinus being supported by 98% bootstrap resampling.
Phylogenetic tree based on almost-complete 16S rRNA gene sequences illustrating the position of strain TeStT within the family Desulfohalobiaceae. The tree was reconstructed by the neighbor-joining method using a phylogenetic distance matrix calculated by the algorithm of Jukes and Cantor. Only bootstrap resampling values above 80% (1,000 replications for each node) are shown at branching points. The sequence of Desulfobacter postgatei (AF418180) was used as outgroup (not shown). Bar = 5% estimated sequence divergence
The close phylogenetic relationship deduced from similar 16S rRNA gene sequences and APS reductase protein sequences confirms the affiliation of strain TeStT to the genus Desulfonauticus. Further phenotypic traits that justify this affiliation are the restricted use of electron donors, an adaptation to moderate thermophilic and halophilic growth conditions, cell morphology and flagellation type. On the other hand, some distinguishing traits to the species Desulfonauticus submarinus could be identified and are listed in Table 2.
Therefore, based on the results presented in this study we propose to establish a novel species within the genus Desulfonauticus to accommodate strain TeStT and to emend the description of the genus Desulfonauticus.
The determined growth parameters of strain TeStT make it likely that members of the proposed novel species are indigenous to a deep subterranean biosphere as suggested by Nilsen et al. (1996) and were not introduced into the oil reservoir along with surface water injected into the oil well. Probably thermophilic subsurface bacteria were dispersed from their natural ecological niche by the flow of injection water so that they penetrated the porous stratum of the oil reservoir and finally entered the oil-production water. Interestingly, enrichments in treater water using H2 as the only electron donor also contained H2 consuming methanogens, similar in phenotype to Methanothermococcus thermolithotrophicus. At enrichment temperatures higher than 50°C the methanogen always overgrew strain TeStT in spite of sufficiently high sulfate concentrations (20 mM). At incubation temperatures between 67 and 72°C only the methanogen enriched. Also increased NaCl concentrations above 60 g/l suppressed the growth of TeStT while the methanogen could still grow. It hence seems that the more extreme conditions favored the growth of methanogens over sulfate reducers and that the injection of cool fresh water into the oil reservoir via the injection well could stimulate sulfate reduction.
Emendation of the genus Desulfonauticus
In addition to the description given by Audiffrin et al. (2003) further characteristics of members of this genus are as follows: formate can be used as electron donor. Some species may use CO2 as carbon source and are facultatively chemolithoautotrophic. Partially saturated derivates of menaquinone 6 occur as the major respiratory lipoquinones and whole-cells fatty acid patterns are dominated by branched-chain C15:0 and C17:0 fatty acids. Desulfoviridin is not present. Cytochromes are present and mainly of the c-type. The G + C content of the genomic DNA is in the range between 34 and 41 mol%.
Description of Desulfonauticus autotrophicus sp. nov.
Desulfonauticus autotrophicus (au.to.tro’phi.cus. Gr. pref. auto self; Gr. n. trophos one who feeds; M.L. masc. adj. autotrophicus self feeding, referring to the ability of the organism to use CO2 as a sole carbon source).
Cells are Gram-negative, straight to slightly curved rods without spores, 0.6 × 1.2–4 μm in size and are motile by a single polar flagellum. Optimum growth occurs at 58°C, pH 7.8 and 2.5% (w/v) NaCl. The mean doubling time under optimal growth conditions is 1.3 h. Magnesium ions are required for growth. Strictly anaerobic metabolism using sulfate, sulfite, thiosulfate and sulfur as electron acceptors and hydrogen or formate as electron donors. Chemolithoautotrophic growth with hydrogen and CO2 possible, but organic carbon sources are preferred. Fermentation of organic substrates or disproportionation of sulfur or thiosulfate were not observed. Suitable nitrogen sources are ammonium and amino acids. Major compounds of the cellular fatty acid pattern are anteiso-C15:0, iso-C15:0, iso-C17:0 and anteiso-C17:0. The main respiratory lipoquinone is a hitherto unknown saturated derivate of menaquinone 6. The DNA G + C content of the type strain is 41.3 mol% (HPLC). The type strain is designated TeStT (= DSM 4206T = JCM 13028T) and was isolated from brine taken from a crude oil–water separation unit (heater treater) installed in an oil field near Hamburg (Germany).
References
Audiffrin C, Cayol JL, Joulian C, Casalot L, Thomas P, Garcia JL, Ollivier B (2003) Desulfonauticus submarinus gen. nov., sp. nov., a novel sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 53:1585–1590
Bryant MP (1972) Commentary on the Hungate technique for the culture of anaerobic bacteria. Am J Clin Nutr 25:1324–1328
Cappuccino JG, Sherman N (1998) Microbiology: a laboratory manual, 5th edn. Benjamin-Cummings Science Publishing, Menlo Park
Cashion P, Holder-Franklin MA, McCully J, Franklin M (1977) A rapid method for the base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Collins MD, Jones D (1981) Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev 45:316–354
Cord-Ruwisch R (1985) A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiol Methods 4:33–36
Cord-Ruwisch R, Kleinitz W, Widdel F (1986) Sulfatreduzierende Bakterien in einem Erdölfeld—Arten und Wachstumsbedingungen. Erdöl Erdgas Kohle 102:281–289
Cord-Ruwisch R, Kleinitz W, Widdel F (1987) Sulfate-reducing bacteria and their activities in oil production. J Pet Technol 39:97–106
Groth I, Schumann P, Weiss N, Martin K, Rainey FA (1996) Agrococcus jenensis gen. nov., sp. nov., a new genus of actinomycetes with diaminobutyric acid in the cell wall. Int J Syst Bacteriol 46:234–239
Hamilton WA (1985) Sulphate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol 39:195–217
Heimbrook ME, Wang WLL, Campbell G (1989) Staining bacterial flagella easily. J Clin Microbiol 27:2612–2615
Hungate RE (1950) The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev 14:1–49
Kaksonen AH, Spring S, Schumann P, Kroppenstedt RM, Puhakka JA (2006) Desulfotomaculum thermosubterraneum sp. nov., a thermophilic sulfate-reducer isolated from an underground mine located in geothermally active area. Int J Syst Evol Microbiol 56:2603–2608
Koskinen PEP, Lay C-H, Beck SR, Tolvanen KES, Kaksonen AH, Örlygsson J, Lin C-Y, Puhakka J (2008) Bioprospecting thermophilic microorganisms from Icelandic hot springs for hydrogen and ethanol production. Energy Fuels 22:134–140
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar BuchnerA, Lai T, Steppi S, Jobb G et al (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G + C content of deoxyribonucleotide acid by high-performance liquid chromatography. Int J Syst Evol Microbiol 39:159–167
Meyer B, Kuever J (2007) Phylogeny of the alpha and beta subunits of the dissimilatory adenosine-5′-phosphosulfate (APS) reductase from sulfate-reducing prokaryotes—origin and evolution of the dissimilatory sulfate-reduction pathway. Microbiology 153:2026–2044
Monciardini P, Cavaletti L, Schumann P, Rohde M, Donadio S (2003) Conexibacter woesei gen. nov., sp. nov., a novel representative of a deep evolutionary line of descent within the class Actinobacteria. Int J Syst Evol Microbiol 53:569–576
Nilsen RK, Beeder J, Thorstenson T, Torsvik T (1996) Distribution of thermophilic marine sulfate reducers in North Sea oil field waters and oil reservoirs. Appl Environ Microbiol 62:1793–1798
Nunoura T, Oida H, Miyazaki M, Suzuki Y, Takai K, Horikoshi K (2007) Desulfothermus okinawensis sp. nov., a thermophilic and heterotrophic sulfate-reducing bacterium isolated from a deep-sea hydrothermal field. Int J Syst Bacteriol 57:2360–2364
Postgate JR (1959) A diagnostic reaction of Desulphovibrio desulphuricans. Nature 183:481–482
Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Ramamoorthy S, Sass H, Langner H, Schumann P, Kroppenstedt RM, Spring S, Overmann J, Rosenzweig RF (2006) Desulfosporosinus lacus sp. nov., a sulfate-reducing bacterium isolated from pristine freshwater lake sediments. Int J Syst Evol Microbiol 56:2729–2736
Rosselló-Mora R, Amann R (2001) The species concept for prokaryotes. FEMS Microbiol Rev 25:39–67
Widdel F, Pfennig N (1984) Dissimilatory sulfate- or sulfur-reducing bacteria. In: Krieg NR (ed) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore, pp 663–679
Zhang Y-M, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Zhilina TN, Zavarzin GA, Rainey FA, Pikuta EN, Osipov GA, Kostrikina NA (1997) Desulfonatronovibrio hydrogenovorans gen. nov., sp. nov., an alkaliphilic, sulfate-reducing bacterium. Int J Syst Bacteriol 47:144–149
Acknowledgments
Ina Kramer and Jennifer Gregor are acknowledged for determining the 16S rRNA gene sequence of strain TeStT. The cellular fatty acid profiles were determined by Marlen Jando and Gabriele Pötter. Shanmugam Mayilraj acknowledges a bilateral exchange program awarded by INSA (India) and DFG (Germany). The Academy of Finland is acknowledged for the financial support for Anna H. Kaksonen for the project “Deep biosphere microbiology” (Decision no. 122394).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mayilraj, S., Kaksonen, A.H., Cord-Ruwisch, R. et al. Desulfonauticus autotrophicus sp. nov., a novel thermophilic sulfate-reducing bacterium isolated from oil-production water and emended description of the genus Desulfonauticus. Extremophiles 13, 247–255 (2009). https://doi.org/10.1007/s00792-008-0212-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0212-4