Abstract
Freshwater fish Cirrhinus mrigala were exposed to tris(2-chloroethyl) phosphate (TCEP) with three different concentrations (0.04, 0.2, and 1 mg/L) for a period of 21 days. During the study period, thyroid-stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4) levels were significantly (p < 0.05) inhibited. The superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), and lipid peroxidation (LPO) levels were increased significantly (p < 0.05) in gills, liver, and kidney tissues, whereas glutathione (GSH) and glutathione peroxidase (GPx) (except liver tissue) activities were inhibited when compared to the control group. Likewise, exposure to TCEP significantly (p < 0.05) altered the biochemical (glucose and protein) and electrolyte (sodium, potassium, and chloride) levels of fish. Light microscopic studies exhibited series of histopathological anomalies in the gills, liver, and kidney tissues. The present study reveals that TCEP at tested concentrations causes adverse effects on fish and the studied biomarkers could be used for monitoring the ecotoxicity of organophosphate esters (OPEs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flame retardants (FRs) are organic chemicals that are used extensively in many polymer-based industrial and house-hold goods to prevent the chances of explosion or to avoid damage of materials during fire accident. The usage of FRs is increasing every year (Mags- Novel; News and Events 2015). The major concern is that FRs do not bind with the polymers; hence, the possibility of leaching into their environment is higher. FRs have been detected in environment including human body fluids up to micrograms or even more; unfortunately, these chemicals have potential to cause toxicological impacts on living organism even at low level (Morgan et al. 2019). Different groups of FRs such as bromine, chlorine, phosphorus, nitrogen, boron, and metallic hydroxide-based organic compounds have been utilized so far. Some of the FRs are listed as persistent organic pollutants and banned for using further (Sugeng et al. 2017; Hao et al. 2018; Hou et al. 2019). Organophosphate flame retardants (OPFRs) with logKow ranges from 1.44 to 9.49 and logKoc from 2.21 to 6.87 have been used as substitute for toxic FRs and additives in combustible products up to 15% (Cristale et al. 2016; Wolschke et al. 2018; Chokwe and Okonkwo 2019). During manufacturing, these OPFRs might leach and reach the environment. The persistence nature of their chlorine atoms in the environment could be a contributor for the formation of various hazardous polychlorinated compounds such as dibenzo-p-dioxins, dibenzofurans, and dioxin-like polychlorinated biphenyls, hexa- and penta-chlorobenzene, and penta-chlorophenol (Matsukami et al. 2014).
Among OPFRs, tris(2-chloroethyl) phosphate (TCEP) is a high production volume chemical, which is extensively used in many polymeric materials such as adhesives, polyvinyl chloride, textiles, baby products, plastics, electric and electronic products, and building materials up to 30% (Liu et al. 2016; Wolschke et al. 2016). Chemically, TCEP are classified as semi-volatile organic compounds that are used as an alternative for toxic polybrominated diphenyl ethers (PBDEs) (Wu et al. 2017; Chen et al. 2018). The global market demand for these compounds has been increased from 5 lakhs tones (in 2011) to 7 lakhs tones (in 2015) and the demand was expected to rise by 15% every year (van der Veen and de Boer 2012; Wang et al. 2015; Li et al. 2016). Like other FRs, their chemical nature (volatilization, leaching, and abrasion properties) increases migration capacity to reach their surrounding environment (Bollmann et al. 2012; Wei et al. 2015). As an evidence, the presence of TCEP in dust (330,000 ng/g), human excreta (2.1 ng/L), and breast milk (2.1 ng/g to 8.2 ng/g) was reported worldwide (Sundkvist et al. 2010; Ding et al. 2015; Zhang et al. 2017; Zhang et al. 2018). TCEP have low removal efficiency and thus, they are predominant contaminant among the chlorinated aliphatic esters in the aquatic ecosystem. Mean concentration of TCEP in the wastewater treatment plant in Germany was ranged up to 370 ng/L (Reemtsma et al. 2008). Highest concentration of TCEP (87.4 mg/L) was recorded in raw water from a Japanese sea-based wastewater disposal site (as mentioned in Du et al. 2019). TCEP have also been detected in various water bodies. OPFR concentration in water ranged 9.6 to 1549 ng/L, among them, TCEP accounted for > 69% with a maximum concentration (268 ng/L) and the most contaminant of Xiaoling River, China (Wang et al. 2015). TCEP have been detected up to 130 ng/L in River Ruhrare, Germany (Andresen et al. 2004), ranged up to 130 ng/L in river water of Austria (Martínez-Carballo et al. 2007), and quantified up to 61 ng/L in lake water of Germany (Regnery and Püttmann 2010). TCEP persist and tend to move between the environments; thus, it can reach the aquatic organisms (Liu et al. 2016). TCEP occurrence has been reported in biological sample collected from the Pearl River Delta region, China (Ma et al. 2013), in fishes from Great Lakes basin, USA (Guo et al. 2017), and in the collected samples such as crab, blue mussel, burbot liver, and cod liver from the regions of Norwegian Arctic (Evens et al. 2009).
TCEP exposure could cause various acute and chronic toxicological effects on organisms, such as reproductive system and fertility abnormalities in fishes (Liu et al. 2012; Ta et al. 2014; Arukwe et al. 2018), tumors in the liver and kidney of rodent (Matthews et al. 1993; Zhang et al. 2017), increases carcinogenic activity, and provokes thalamus neuronal necrosis in the mammalian models (mice and rat) (Moser et al. 2015; Xu et al. 2017). There are also reports on steroidogenic pathways in juvenile salmon (Arukwe et al. 2016; Arukwe et al. 2018), DNA damage, changes in acetylcholinesterase (AChE) activity, alteration in gene expression and oxidative stress in earthworms (Yang et al. 2018a), and genotoxic effects on zebrafish (Wu et al. 2017) under TCEP treatments. Thus, TCEP was categorized as emerging contaminants in the environment (EC 2009; ECH 2009; Liu et al. 2017).
The freshwater system plays a major role in the growth of many aquatic organisms and is known to be an ultimate sink for most of the chemicals. Aquatic organism can act as biological markers to assess the health status of the aquatic environment. Among aquatic organism, fish are used widely in toxicological study because they are highly sensitive to slight environmental alterations. Toxicity of waterborne chemicals could be assessed by using fish bioassay. The long-term toxic effects of waterborne chemicals on fish models could help in establishing standard criteria for risk assessment and safety for aquatic environment (Zhong et al. 2018). Assessment of alterations on morphological and physiological biomarkers of fish reflects the harmful effects of environmental pollutants on aquatic organisms (Austin 1998). Thus, fish biomarkers collectively provide an insight into the overall health status of the aquatic organisms and act as an indicator of the environmental pollution status.
In fish, growth, maturation (Walpita et al. 2009), osmoregulation (Peter 2011), smoltification (Björnsson et al. 2011), and larval metamorphosis (Taillebois et al. 2011) are controlled by the thyroid metabolism. Ecological contaminations also affect the thyroid system and directly block THs (thyroid hormone) synthesis, TH-blood transport, and TH metabolism (Boas et al. 2006). Hormonal assay is a valid technique in the field of toxicology; the hormonal activity is used as an early warning signal (Folmar 1993; Hontela et al. 1993). Moreover, responses of thyroid hormones have been used in toxicological research to understand the growth and metabolism of fish under stress conditions. Previous study indicates that aquatic pollutants alter the level of thyroid hormones, which results in impairment of development and function of thyroid hormones (He et al. 2012; Katuli et al. 2014; Shirdel et al. 2016). FR once reaches the digestive system, it readily enters the circulatory system and accumulates at tissues and acts as potential thyroid hormone disruptors (Zhang et al. 2016; Curran et al. 2017). Generally, organophosphorus esters (OPEs) cause disturbances in thyroid hormone signaling and endocrine disruption action on organisms (Kojima et al. 2013; Greaves and Letcher 2017). However, disruptions in thyroid function by TCEP exposure on fish are not reported.
During metabolic reaction, body produces free radicals that could cause harmful effects. The body itself generates antioxidant to balance the free radicals. Thus, antioxidant enzyme activities are considered an important tool in toxicity studies as their levels reflect the toxicity, concentration, and exposure duration (Pamanji et al. 2016). Numerous studies explain that the antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH)-dependent enzymes (e.g., glutathione S-transferases (GST), glutathione reductase (GR), glutathione peroxidase (GSH-Px), and lipid peroxidation (LPO)) are considered as a valuable biomarker to evaluate chemical-related stress in fishes. Their imbalance will result in tissue damage and alteration in normal homeostasis in an organism (Poopal et al. 2017; Sahu et al. 2018).
Histopathology is an important tool for studying the effects of waterborne chemicals in major organs such as gill, liver, and kidney that are responsible for respiration, metabolism, and excretion in fishes, respectively (Nagarjuna and Mohan 2017; Ramesh et al. 2018). Histological anomalies are indicator for the loss of function (Fanta et al. 2003) and act as a signal of health status of an organism (Hinton and Lauren 1990).
The plasma biomarkers (e.g., glucose, proteins) are widely studied to understand the effects of xenobiotic exposures on organisms. Plasma biomarkers give the relevant data in determining the extent of cell, tissues, and organ damages and provide an early warning signal to animals in concern (Canli and Canli 2015). In addition to the above biomarkers, the measure of ionic levels such as potassium (K+), chloride (Cl−), and sodium (Na+) in the blood of aquatic organisms also aids in monitoring the polluted aquatic ecosystem (Mayer et al. 1992; Sathya et al. 2012; Hemalatha et al. 2016).
Previous literatures report the occurrence, accumulation, and distribution of TCEP in various aquatic organisms (Hou et al. 2017; Arukwe et al. 2018). However, reports on toxicological responses such as primary stress indicator (hormones), oxidative stress, pathological structures, blood biomarkers (glucose and protein), and electrolyte levels with respect to FRs especially on TCEP exposures on freshwater fish are limited. Therefore, this study was undertaken to examine the responses of various biomarkers in a freshwater fish Cirrhinus mrigala exposed (chronic) to different concentrations (0.04, 0.2, and 1 mg/L) of TCEP. The findings of the present study might provide better knowledge on TCEP toxicity on aquatic organisms and contribute to a greater understanding of overall organophosphorus flame retardant toxicity.
Materials and methods
All the laboratory analyses and fish maintenance were performed by following the guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Fish
Freshwater fish (C. mrigala) with an average length 7.0 ± 0.1 cm and weight 12 ± 0.1 g were obtained from Aliyar fish farm, Pollachi, Tamil Nadu, India, and housed at Toxicology Lab, Department of Zoology, Bharathiar University, Coimbatore, Tamil Nadu, India. The fingerlings were adapted to laboratory conditions for 2 weeks in a tank containing dechlorinated tap water with temperature 26 ± 0.1 °C, pH 7.2, dissolved oxygen 6.6 ± 0.02 mg/L, and 12:12-h photoperiods and used as main stock. During the acclimatization periods, rice bran and groundnut oil cakes were fed (once in a day) in the form of dough. The water in the tank was renewed every 24 h to make sure the tank is free from fecal matter and enough oxygen is supplied to fish.
Test chemical
Tris(2-chloroethyl) phosphate was obtained from Sigma-Aldrich (purity 97%, CAS no. 115-96-8). Stock solution was prepared by dissolving 1 g/L of tris(2-chloroethyl) phosphate in Milli-Q water. Fresh stock solutions were used.
Experimental design
For toxicity study, healthy fingerlings were collected randomly from the main stock and kept in a separate rectangular glass aquarium (120 × 80 × 40 cm) and used as test stock. The fish in test stock were not fed for 24 h prior to the commencement of experiment. For the study, three different concentrations of TCEP (treatment-I, 0.04; treatment-II, 0.2; treatment-III, 1 mg/L) were selected based on the previous literature (Arukwe et al. 2016). A 0.04 mg/L of TCEP was mixed in a glass aquarium containing 90 L of water and 40 fish. Similar setup was made for 0.2 and 1 mg/L concentrations of TCEP and a common control (without adding TCEP) were maintained. During the exposure periods, fingerlings were fed ad libitum. Debris (uneaten feed and fecal matters) was removed and water in the aquarium was renewed daily by removing three-fourth of the water. Three replicates were maintained for control and TCEP-treated groups with similar setup.
Sampling frequency and collection of blood and tissue samples
Samplings were done at every 7 days until 21 days. At the end of every stipulated period, 30 fish were euthanized respectively from each group, and blood was collected by cardiac puncture, then centrifuged at 10,000 rpm for 10 min and the plasma was used for the hormonal assay (thyroid-stimulating hormone [TSH], triiodothyronine [T3], and thyroxine [T4]), electrolytes (Na+, K+, and Cl−), and biochemical (glucose and protein) parameters. Simultaneously, tissues such as gills, liver, and kidney were homogenized (Teflon-coated mechanical tissue homogenizer) with phosphate buffer (pH 7.4) in ice cold conditions, then centrifuged at 5000 rpm for 20 min and the supernatant was used for the analysis of antioxidant enzyme activity. A portion of the collected tissues were fixed in Blouin’s fluid for histopathological studies.
Thyroid hormone assay
TSH, T3, and T4 levels in control and TCEP-treated fingerlings were measured by following the manual provided by the TOSOH commercial ELISA kits (product code: 8025-300D) (Hoseini et al. 2014). The optical density (OD) of the samples was measured by using an automatic ELISA plate analyzer (Avecina Touch Plate Reader, Iran) at 450 nm and expressed as nanograms per milliliter (ng/mL).
Antioxidants assay (gills, liver, and kidney)
SOD activity in gills, liver, and kidney of the fish was determined by the method of Marklund and Marklund (1974) and the activity was expressed as U/mg protein. CAT activity was assayed following the method of Aebi (1984) and the activity was expressed as μmol H2O2 utilized/min/mg protein. GST activity was determined by the method of Habig et al. (1974) and the enzyme activity was expressed as μmol GSH-cDNB conjugate formed/min/mg protein. GPx activity was measured by following the method described in Rotruck et al. (1973) and the enzyme activity was expressed as μmol GSH oxidized/min/mg protein. The reduced glutathione levels were determined using the modified method of Ellman (1959) and expressed as micrograms of GSH formed/min/mg protein. LPO was estimated through thiobarbituric acid (TBA) assay following the method of Devasagayam and Tarachand (1987) using malondialdehyde (MDA) in the samples. LPO level was expressed as moles of MDA formed/mg protein. All the assays were carried out using UV-visible spectrophotometer (Jasco, V-530).
Glucose and protein assay
Glucose level in control and TCEP-treated fingerlings was estimated using the O-toluidine method of Cooper and Mc Daniel (1970) and their OD measured against the blank at 630 nm within 30 min using a UV spectrophotometer and expressed as mg/100 mL. Plasma protein was estimated following the method of Lowry et al. (1951). The OD was read after 15 min at 720 nm using a UV spectrophotometer. A standard was also prepared, and the protein level was expressed as micrograms per milliliter.
Electrolytes assay
Na+, K+, and Cl− levels were estimated using standard kit manufactured from Coral Clinical systems and supplied by Tulip Diagnostics (P) Limited, Goa, India. Sodium was estimated using the method of Maruna (1958) and Trinder (1951). Potassium was estimated following the method of Terri and Sesin (1958) and Sunderman and Sunderman (1959). Chloride was estimated by the modified method of Schales and Schales (1941) and Schoenfeld and Lewellen (1964). The levels of Na+, K+, and Cl− in plasma of the fish were expressed as millimoles per liter.
Histopathological analysis
Dissected tissues (gills, liver, and kidney) were fixed in Blouin’s fluid for 2 days, dehydrated with graded ethanol, infiltrated using xylene, embedded in paraffin wax, then sectioned and mounted on clean glass slides, and ended with hematoxylin and eosin staining. The tissues were examined using light microscope with image analysis system connected to a computer.
Statistical analysis
The results of the study are interpreted as mean ± S.E. The significance of the samples between control and TCEP treatments was evaluated by one-way ANOVA (analysis of variance) followed by Duncan multiple range test (DMRT). Different alphabets represent significance levels at p < 0.05.
Results
When fingerlings exposed to TCEP at different concentrations, behavior changes such as fast swimming, movement around the wall of the tank, and inability to feed were observed and finally settled at the bottom. In the control group, no such behavior changes were noticed. No alteration in the schooling behavior was observed (direct observation). Thus, it reveals that fingerlings used in our study are healthy.
Effects of TCEP on thyroid hormones
Plasma thyroid hormone levels in the fish exposed to TCEP concentrations were illustrated in Fig. 1a–c (TSH (a), T4 (b), T3 (c)). When compared to the control group, TSH, T4, and T3 levels were found to be decreased significantly (p < 0.05) in fish exposed to TCEP treatments. Among the treatment groups, thyroid hormone was declined higher at treatment III (1 mg/L). In 0.04 and 0.2 mg/L, the changes in TSH, T4, and T3 levels were found to be decreased with less significance than the control group. A steady decline was noticed in the thyroid hormone levels; among the thyroid hormones, a higher decline was observed in T3.
Effects of TCEP on gills, liver, and kidney antioxidant parameters
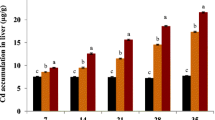
SOD (Fig. 2a–c) and CAT (Fig. 3a–c) activity in gills, liver, and kidney tissues was found to be increased significantly (p < 0.05). A steady increase in the SOD and CAT activity was observed in TCEP treatment-III group. Among the studied tissues, SOD and CAT activity was found higher in liver tissue at the end TCEP (treatment-III) exposure periods. The activity of GPx (Fig. 4a–c) in gills and kidney tissues was declined gradually, whereas in the liver tissue, the activity was found to be increased constantly when compared to the control group. The alterations were statically significant (p < 0.05). GSH activity in studied tissues were inhibited significantly (p < 0.05) in all the TCEP treatments (Fig. 5a–c). Among the tissues, liver tissue was affected higher. The GST activity (Fig. 6a–c) and LPO level (Fig. 7a–c) were elevated significantly (p < 0.05) throughout the TCEP exposure periods, showing a gradual increase towards the end of the study periods in the treatment-III group. Overall, a concentration-based effect was noticed in TCEP treatment groups on antioxidant parameters.
Effects of TCEP on biochemical parameters
Glucose and protein levels in plasma of fish exposed to TCEP treatments were significantly (p < 0.05) altered during the study period. TCEP toxicity resulted in hyperglycemic condition in C. mrigala (Fig. 8a). Among the TCEP treatments, higher level of plasma glucose was measured in treatment III. Protein level in plasma of TCEP-treated fish was found to be declined significantly (p < 0.05) throughout the study period when compared to the control group (Fig. 8b). The biochemical parameters also reveal that TCEP has duration and concentration-dependent effects on fish.
Effects of TCEP on plasma electrolytes
Plasma electrolyte levels of fish exposed to different concentrations of TCEP were illustrated in Fig. 9 (a, Na+; b, K+; c, Cl−). TCEP could influence the electrolyte levels in plasma, and the alterations were found statistically significant (p < 0.05). In higher concentration (1 mg/L), maximum decrease in plasma Na+ and Cl− was observed when compared to the control group, whereas plasma K+ level in TCEP-exposed fish was found to be increased. Maximum alterations in plasma electrolytes were noticed in TCEP treatment-III group. In this study, TCEP induced a concentration-based effect on electrolyte levels.
Histopathology examination
No visible structural abnormalities (gills, liver, and kidney tissues) were examined in the control group (Figs. 10a, 11a, and 12a). TCEP exposure caused epithelial lifting, hyperplasia, degeneration of cells in primary lamellae, lamellar fusion, mucus accumulation, and vacuolation in fish gills tissue (Fig. 10b–d). In liver tissue, pyknotic nuclei, fat deposition, necrosis, increased sinusoids vessels, congestion, and vacuolation were noticed in TCEP treatment groups (Fig. 11b–d). TCEP toxicity also caused hypertrophy in epithelial cells of renal tubules, shrinkage of glomeruli, expansion of Bowman’s capsule, and tubular degeneration in kidney tissues (Fig. 12b–d). TCEP toxic effects on gills, liver, and kidney tissue morphology were in the series of treatment I < treatment II < treatment III. More structural anomalies related to TCEP toxicity were examined in gills and liver tissues than in kidney tissue (Tables 1, 2, and 3). TCEP caused concentration-based structural alterations in selected tissues of C. mrigala.
Histological sections of gills of C. mrigala. a) control group, with a normal gill filament (GF), gill lamella (GL), cartilage (CA), pillar cells (P), erythrocytes (W). A series of morphological anomalies (epithelial lifting (EL), hyperplasia (H), degeneration of cells in primary lamellae (DP), fusion (F), mucous accumulation (M), Vacuolation (V)) were noticed in TCEP treatment (treatment I (b), treatment II (c), and treatment III (d)) groups. (HE × 40)
Histological sections of liver of C. mrigala. a) control group, with a normal hepatocyte (HN), and nucleus (N). A series of morphological anomalies (pyknotic nuclei (PN), fat deposition (FD), necrosis (N), increased sinusoids vessels (ISS), congestion (C) and vacuolation (V)) were noticed in TCEP treatment (treatment I (b), treatment II (c), and treatment III (d)) groups. (HE × 40)
Histological sections of kidney of C. mrigala. a) control group, with a normal Bowman’s capsule (BC), renal tubule (RT), and glomerulus (EC). A series of morphological anomalies (hypertrophied epithelial cells of renal tubes (HER), shrinkage of glomeruli (SG), expansion of Bowman’s capsule (EBC), degenerated tubular epithelium (TD)) were noticed in TCEP treatment (treatment I (b), treatment II (c), and treatment III (d)) groups. (HE × 40)
Discussion
Due to the higher and frequent utilization of TCEP, it occurs ubiquitously in the water bodies. The reported concentration of TCEP in the water system (including wastewater system) ranges several tens of micrograms per liter and a few thousands of nanograms per liter. Arukwe et al. (2016) reported that TCEP have potential to cause toxic effects on fish at mg/L levels (0.04, 0.2, and 1 mg/L). TCEP could also cause alterations on steroidogenesis or estrogen metabolism in H295R cells at different dose (0.001–10 mg/L) (as mentioned in Du et al. 2019). The data on effects of TCEP on aquatic organism are lacking (Fisk et al. 2003; Cristale et al. 2013). Thus, toxicological profiles of TCEP on aquatic organisms are much warranted (Jin et al. 2013). Therefore, to increase the availability of eco-toxicity data of TCEP, we selected different concentrations (0.04, 0.2, and 1 mg/L) and examined the potential responses of hormonal, antioxidants, biochemical, electrolyte, and histological effects on fish.
The change in the level of thyroid hormones indicates the primary stress responses of the organism exposed to toxicants. In the present study, TCEP have potential to reduce the TSH, T3, and T4 levels in the plasma of the fish. Generally, thyroid hormone homoeostasis is maintained through TSH and negative feedback mechanism. The secretion and release of TSH stimulated in the hypothalamus, and TSH stimulates T4 secretion in the thyroid. T4 converted to form T3 a biologically active form (Sabir et al. 2019). Generally, the level of T4 is higher than that of T3 in the blood. The thyroid hormone levels were declined steadily as exposure period increases. This indicates that TSH synthesis was inhibited under long-term TCEP exposure either interfering directly on the structural changes in the thyroid gland, or target on the metabolism, or due to binding with protein molecules in the thyroid hormone. Inhibition in the TSH synthesis could reduce the production, conversion, and release of T4 and T3 in the plasma. Decrease in TSH levels in the blood could be a result of negative regulation at thyroidal axis or disturbance in the synthesis and release of hormone from the thyrotrope cells of pituitary (Raibeemol and Chitra 2020). The activity of UDP-glucuronosyl transferase that are responsible for glucuronidation and clearance of thyroid hormone (T4) could decrease level of thyroid hormone (T4) (Movahedinia et al. 2018). Flame retardants are known to form glucuronide metabolite through UDP-glucuronosyl transferases (Eng et al. 2019). Flame retardants are potential to cause changes in dio (deiodinase) gene transcription that an important enzyme for circulating and peripheral TH levels in the fish (Liu et al. 2019). Dio2 enzyme catalyzes T4 to T3 (Xu et al. 2018). Thus, the disturbance in conversion of T4 to T3 might reduce the T3 levels in the plasma of TCEP-treated groups. A significant decrease in thyroid hormone levels has been reported in Danio rerio exposed to tris(1,3-dichloro-2-propyl)phosphate (Xu et al. 2015). Likewise, a significant decrease in thyroxine (T4) level was reported in zebrafish exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(1,3-dichloroisopropyl)phosphate (TDCPP) (Wang et al. 2013; Kim et al. 2015) which indicates these compounds might be potential endocrine disruptors (Zhang et al. 2016). TDCIPP may alter the gene transcriptions which are responsible for the production of growth hormones (Wang et al. 2013). Tris(2-butoxyethyl)phosphate (TBOEP) altered the whole body T3 and T4 concentrations in zebrafish larvae indicating the disruptive action of the TBOEP (Liu et al. 2017). FRs could displace thyroid hormone competitively from a thyroid-binding protein, transthyretin. The displacement of thyroid hormone from its binding site could result in metabolism and elimination of hormone which results in decline of thyroid hormone for circulation (Osimitz et al. 2016). From our result, we conclude that FRs could cause alterations in thyroid hormones that might affect growth and metabolism of an organism. The alterations of these hormones indicate the disruptive action of TCEP. However, the mode of action on thyroid function or the endocrine disruption potential of TCEP on aquatic organisms needs to be studied (Wu et al. 2017).
Detoxification, excretion, and balanced antioxidants could strengthen immune system to adapt aquatic organisms to different types of waterborne contaminants (Sahu et al. 2018). Generation of reactive oxygen species associates with many health defects including neurological defects, and FRs known to generate reactive oxygen species (Jarosiewicz et al. 2019). Any imbalance occurred between free radicals and antioxidants results in oxidative stress in an organism (Tabassum et al. 2016; Poopal et al. 2017). Among antioxidants, SOD, CAT, and GPx act as a first-line defense system in converting free radicals to hydrogen peroxide, molecular oxygen, and water. Their imbalance could be an indication of oxidative stress caused by stressors in an organism (Yan et al. 2017; Yonar et al. 2014). In the present study, the increased activity of SOD and CAT in gills, liver, and kidney tissues of fish indicates that the body triggered its defense mechanism against the production of free radicals during the detoxification of TCEP.
Arukwe et al. reported that bis(2-chloroethyl)carboxymethyl-phosphate, bis(2-chloroethyl)hydrogen-phosphate, and bis(2-chloroethyl)-2-hydro-xyethyl-phosphate glucuronides were the urinary metabolites of TCEP involved in the metabolic pathways such as oxidative and hydrolytic reactions, as well as glucuronidation through phase-II metabolism (Arukwe et al. 2018). The increase in CAT activity indicates the role of CAT in elimination of H2O2 produced by SOD (Guptha et al. 2016). In contrast to the present study, failure of antioxidant system has been reported in fish exposed to brominated flame retardant (BFR) (Feng et al. 2013) indicating that the capacity of mitigating and scavenging of free radicals by the antioxidants in the organism is governed by the toxic strength of the chemical.
GPx metabolizes H2O2 and reduces fatty acid peroxides. This enzyme can act on a variety of organic peroxides and catalyzes the oxidation of reduced glutathione to glutathione disulfide (Di Giullio and Hinton 2008; Plhalova et al. 2014). In our study, the activity of GPx in gills and kidney tissues of TCEP-exposed fish showed a significant decrease, whereas it was found to be increased in the liver tissue. The significant increase in GPx activity in the liver tissue of fish indicates the activation of defense mechanism or an adaptation of fish to respond the TCEP-related ROS production (John et al. 2001; Li et al. 2009, 2010). In contrast, the decrease in GPx activity in gill and kidney tissues indicates reduced capacity to scavenge hydrogen peroxide produced in these organ/tissues (Melekh et al. 2017). Interference of TCEP on the synthesis of GPx in tissues might be another reason for the disruption of the enzyme activity. Furthermore, differences in the response of GPx activity in gills, liver, and kidney tissues might result from different mechanisms of TCEP and H2O2 and distribution of enzymes in tissue/organs as suggested by Tkachenko et al. (2014).
GSH is a major antioxidant that has a critical role as a co-substrate of GST in phase II biotransformation. It involves in reactive intermediates and free oxide detoxification mechanism (Samanta et al. 2014). The decrease in GSH activity in tissues is an indication of counteract to oxidative stress caused by TCEP, which is due to the imbalance of free radicals and antioxidants resulting in reduction of GSH in fish (Yonar et al. 2014). It has been reported that BFRs could induce oxidative stress, which results in increased utilization of GSH for the detoxification processes (Feng et al. 2013). GST could involve in the protective mechanism against many chemical substances (Bastos et al. 2013). In the present study, the increased activity of GST in tissues of fish is probably due to the action of defensive mechanism against the TCEP toxicity. The increase in the process of biotransformation of chemicals could activate GST in an organism (Modesto and Martinez 2010). The activity of GSH lays antiparallel to GST, i.e., inhibition of GSH results due to their exhaustion in the phase II biotransformation process with an elevation of GST activity (Kaur and Jindal 2017). Our results are in strong agreement with the authors’ statement.
LPO levels could be used as an indication of health status of cell and tissues. Imbalanced antioxidant and free radical could result in alteration of LPO level (Pan et al. 2006). In the present study, we found elevation of LPO level in tissues of TCEP-exposed fish. The elevation of LPO level is an evidence of improper functioning of cells due to oxidative stress caused by TCEP (Monteiro et al. 2006; Feng et al. 2013). Likewise, tris(2-butoxyethyl)phosphate (TBOEP) at 2500 μg/L concentration increased the MDA level and resulted oxidative stress in zebrafish (Jiang et al. 2018). Generally, lipid peroxidation formation will be prevented by SOD activity. In our study, both LPO and SOD activities are elevated; this reveals that antioxidants are intolerable to TCEP-mediated free radicals. FRs could generate higher intercellular reactive oxygen species and damage DNA as concentration increases (Yuan et al. 2019). This reveals that TCEP have potential to generate free radicals and act on lipid profiles.
Glucose level is frequently used as stress response biomarker in toxicology. Fish exposed to TCEP showed hyperglycemic response. Generally, this situation occurs as a result of high energy demand in an organism. TCEP showed increased glucose level at higher concentration and neurological metabolic disorders on experimental mammalian model (Yang et al. 2018b). Thus, the hyperglycemic condition in the present study reveals that the fish utilize glucose to compensate stress caused due to TCEP toxicity. Proteins have significant role in many metabolic actions in the body and their concentrations are widely used as health indicator in the field of toxicology. Proteins are utilized as major among the energy sources under stress conditions (Woo et al. 2018). Hypoproteinemia in the TCEP-exposed fish indicates the catabolism of protein to meet the energy demand generated during elimination or detoxification process. The protein might act as a compensatory action for inefficiency of immune action to tolerate TCEP-mediated stress. Moreover, TCEP may influence citrate cycle by elevating citrate and 2-oxoglutarate which result in energy deficiency (Deng et al. 2018); this could result in gluconeogenesis process, which ultimately alter biochemical (glucose and protein) levels to meet out the energy demand caused in the fish exposed to TCEP.
Electrolyte imbalances are important biomarkers that have been widely studied in toxicity assessment (Ajima et al. 2018). Levels of Na+, K+, and Cl− in circulatory system are widely used to assess the health conditions of aquatic organisms (Poopal et al. 2013; Hemalatha et al. 2016). Na+ and Cl− level in the plasma of TCEP-exposed fish was declined. The K+ level was found higher in TCEP-treated fish. In the present study, accumulation of TCEP in gills and liver may lead to osmoregulatory failure which results in alterations of plasma ions. Accumulation of TCEP has been reported in fish species such as Silurus glanis and Atlantic salmon (Jakimska et al. 2013; Arukwe et al. 2018). The alteration of electrolytes in living organisms might be due to dehydration and damage in liver or kidney tissues (Curran et al. 2017).
Structural changes in the tissues are widely used as an ideal biomarker in toxicology. Adverse changes that occurred in biochemical and physiological parameters could cause histological alterations in tissues of fish (Arellano et al. 2001; Fanta et al. 2003). Most of lipophilic chemical substances tend to cross the cell membrane actively and accumulate in the cell. This could affect the amino acid, fatty acid, and lipid metabolism, which are known to be the major components in energy regulation for normal homeostasis and function of cells. Gill is the primary organ in contact with waterborne contaminants. It also plays a vital role in osmoregulation (Marigoudar et al. 2018). A series of structural anomalies such as epithelial lifting (EL), hyperplasia (H), mucus accumulation (M), and vacuolation (V) were observed in the gills of TCEP-treated fish. Since TCEP is a lipophilic substance, it could cross the epithelial membrane and altered the biochemical component in the layer and that could be the reason for occurring of epithelial lifting and degeneration of cells in primary lamellae. Fusion of cells could generate more free space; thus, vacuolation occurred in TCEP-treated fish. In aquatic animals, OPFRs usually absorbed from the water by gill and tend to accumulate in gill (Saeger et al. 1979; Hou et al. 2016).
Liver is a second target organ for most of the compounds, where the biotransformation and detoxification take place. In the present study, TCEP included increased in fat deposits, pyknotic nuclei, vacuolization, necrosis, enlarged sinusoidal vessels, and congestion. Similarly, structural changes in the liver tissues of Danio rerio exposed to triphenyl phosphate (Du et al. 2016). Tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) concentration caused structural alterations in liver such as vacuolization and apoptosis in zebrafish (Liu et al. 2016). The ability of liver to degrade various toxicants results in subsequent structural damage of liver tissues (Bruslé and Anadon 1996; Tabassum et al. 2016). And also, kidney is a major excretory organ where protein reabsorption occurs, where the compounds either parent or metabolites reach the kidney. In the present study, TCEP induced changes in kidney such as hypertrophy in the epithelial cells of renal tubes (HER), shrinkage of glomeruli (SG), expansion of Bowman’s capsule (EBC), and degenerated tubular epithelium (TD). TCEP at high concentration (10 mg/kg) damage the structure and function of the intestinal epidermal cells, which may lead to multiple biochemical changes in earthworm (Yang et al. 2018a). Several structural anomalies were noted in Xenopus tropicalis embryos upon exposure to FRs (Zhang et al. 2016). Changes in LPO activity observed in this study reveal the action of free radicals on the lipid membrane of the cell, which might result in a series of histological anomalies in TCEP-treated fish. Thus, antioxidants, hormonal, and biochemical alterations observed in the present study might cause some morphological changes in the tissues of TCEP-treated fish.
Conclusion
The findings of the present investigation indicate that the TCEP at tested concentrations induced endocrine disruption especially in the thyroid hormone levels and caused oxidative stress in fish. Furthermore, the structural damage mediated by TCEP resulted in high energy demand that ultimately increased the energy metabolism in fish. The alterations of these parameters can be effectively used to monitor the impact of FRs on aquatic environment. The data of the present study could be used as a toxicity data for other organophosphorus flame retardants on fishes. From the result, we conclude that the levels of FRs in aquatic environment should be monitored.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ajima MNO, Pandey PK, Kumar K, Poojary N (2018) Alteration in DNA structure, molecular responses and Na+-K+-ATPase activities in the gill of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) in response to sublethal verapamil. Ecotoxicol Environ Saf 147:809–816. https://doi.org/10.1016/j.ecoenv.2017.09.050
Andresen JA, Grundmann A, Bester K (2004) Organophosphorus flame retardants and plasticizers in surface waters. Sci Total Environ 332:155–166. https://doi.org/10.1016/j.scitotenv.2004.04.021
Arellano JM, Ortiz JB, Luisa Gonzalez de Canales ML, Sarasquete C (2001) Histopathological alterations and induction of cytochrome P-450 1A in the liver and gills of the gilthead sea bream (Sparus aurata) exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Histo Chem J 33:663–674. https://doi.org/10.1023/a:1016358518743
Arukwe A, Carteny CC, Moder M, Bonini A, Maubach MA, Eggen T (2016) Differential modulation of neuro- and interrenal steroidogenesis of juvenile salmon by the organophosphates – tris(2-butoxyethyl) - and tris(2-cloroethyl) phosphate. Environ Res 148:63–71. https://doi.org/10.1016/j.envres.2016.03.020
Arukwe A, Carteny C, Eggen T, Möder M (2018) Novel aspects of uptake patterns, metabolite formation and toxicological responses in Salmon exposed to the organophosphate esters-Tri (2-butoxyethyl)- and tris(2-chloroethyl) phosphate. Aquat Toxicol 196:146–153. https://doi.org/10.1016/j.aquatox.2018.01.014
Austin B (1998) The effects of pollution on fish health. J Appl Microbiol 85:234S–242S. https://doi.org/10.1111/j.1365-2672.2009.04445.x
Bastos FF, Hauser-Davis RA, Tobar SAL, Campos RC, Ziolli RL, Bastos VC, Bastos JC (2013) Enzymatic GST levels and overall health of mullets from contaminated Brazilian lagoons. Aquat Toxicol 126:414–423. https://doi.org/10.1016/j.aquatox.2012.08.020
Björnsson BT, Stefansson SO, McCormick SD (2011) Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol 170:290–298. https://doi.org/10.1016/j.ygcen.2010.07.003
Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM (2006) Environmental chemicals and thyroid function. Eur J Endocrinol 154(5):599–611. https://doi.org/10.1530/eje.1.02128
Bollmann UE, Möller A, Xie Z, Ebinghaus R, Einax JW (2012) Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res 46:531–538. https://doi.org/10.1016/j.watres.2011.11.028
Bruslé J, Anadon GG (1996) The structure and function of fish liver. In: Dutta HM, Datta-Mushi JS (eds) Fish morphology. Science Publishers, pp 77–93
Canli EG, Canli M (2015) Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ Toxicol Pharmacol 39(2):606–613. https://doi.org/10.1016/j.etap.2014.12.019
Chen H, Wang P, Du Z, Wang G, Gao S (2018) Oxidative stress, cell cycle arrest, DNA damage and apoptosis in adult zebrafish (Danio rerio) induced by tris(1,3-di-chloro-2-propyl) phosphate. Aquat Toxicol 194:37–34. https://doi.org/10.1016/j.aquatox.2017.11.001
Chokwe TB, Okonkwo JO (2019) Occurrence, distribution and ecological risk assessment of organophosphorus flame retardants and plasticizers in sediment samples along the Vaal River catchment, South Africa. Emerg Contam 5:173–178. https://doi.org/10.1016/j.emcon.2019.05.003
Cooper CR, Mc Daniel V (1970) The determination of glucose by the orthotoluidine method. Clin Chem 6:159–170. https://doi.org/10.1016/B978-0-12-609106-9.50021-X
Cristale J, García Vázquez A, Barata C, Lacorte S (2013) Priority and emerging flame retardants in rivers: occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ Int 59:232–243. https://doi.org/10.1016/j.envint.2013.06.011
Cristale J, Hurtado A, Gómez-Canela CG, Lacorte S (2016) Occurrence and sources of brominated and organophosphorus flame retardants in dust from different indoor environments in Barcelona, Spain. Environ Res 149:66–76. https://doi.org/10.1016/j.envres.2016.05.001
Curran IH et al (2017) Toxicologic effects of 28-day dietary exposure to the flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)-cyclohexane (TBECH) in F344 rats. Toxicology 377:1–13. https://doi.org/10.1016/j.tox.2016.12.001
Deng Y, Zhang Y, Qiao R, Bonilla MM, Yang X, Ren H, Lemos B (2018) Evidence that micro plastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J Hazard Mater 357(5):348–354. https://doi.org/10.1016/j.tox.2016.12.001
Devasagayam TPA, Tarachand V (1987) Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun 145:134–138. https://doi.org/10.1016/j.tox.2016.12.001
Di Giullio RT, Hinton DE (2008) The toxicology of fishes, vol 1071. Taylor & Francis
Ding J, Shen X, Liu W, Covaci A, Yang F (2015) Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci Total Environ 538:959–965. https://doi.org/10.1016/j.scitotenv.2015.08.101
Du Z, Zhang Y, Wang G, Pong J, Wang Z, Gao S (2016) TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep 6:218–227. https://doi.org/10.1038/srep21827
Du J, Li H, Xu S, Zhou Q, Jin M, Tang J (2019) A review of organophosphorus flame retardants (OPFRs): occurrence, bioaccumulation, toxicity, and organism exposure. Environ Sci Pollut Res 26:22126–22136. https://doi.org/10.1007/s11356-019-05669-y
EC (2009) European Commission, European Union risk assessment report, tris(2-chloroethyl) phosphate, TCEP.
ECH (2009) Agreement of the members’ state committee on identification of tris(2-chloroethyl) phosphate (TCEP) as a substrate of very high concern.
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Eng ML, Williams TD, Fernie KJ, Karouna Renier NK, Henry PFP, Letcher RJ, Elliott JE (2019) In ovo exposure to brominated flame retardants Part I: Assessment of effects of TBBPA-BDBPE on survival, morphometric and physiological endpoints in zebra finches. Ecotoxicol Environ Saf 179:104–110. https://doi.org/10.1016/j.ecoenv.2019.04.048
Evens A, Leknes H, Christensen GN, Warner N, Remberger M, Gabrielsen GW (2009) Screening of new contaminants in samples from the Norwegian Artic. SPFO Report 1049/2009. TA-2510/2009.
Fanta E, Rios FSA, Romao S, Vianna ACC, Freiberger S (2003) Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf 54:119–130. https://doi.org/10.1016/S01476513(02)00044-1
Feng M, Qu R, Wang C, Wang L, Wang Z (2013) Comparative antioxidant status in freshwater fish Carassius auratus exposed to six current-use brominated flame retardants: a combined experimental and theoretical study. Aquat Toxicol 140-141:314–323. https://doi.org/10.1016/j.aquatox.2013.07.001
Fisk PR, Girling AE, Wildey RJ (2003) Produced for Environment Agency, United Kingdom, 2003.
Folmar LC (1993) The effects of chemical contaminants on the blood chemistry of teleost fish: bibliography and synopsis of selected effects. Environ Toxicol Chem 12:337–375. https://doi.org/10.1002/etc.5620120216
Greaves AK, Letcher RJ (2017) A review of organophosphate esters in the environment from biological effects to distribution and fate. Bull Environ Contam Toxicol 98(1):2–7. https://doi.org/10.1007/s00128-016-1898-0
Guo J, Venier M, Salamova A, Hites RA (2017) Bioaccumulation of dechloranes, organophosphate esters, and other flame retardants in Great Lakes fish. Sci Total Environ 583:1–9. https://doi.org/10.1016/j.scitotenv.2016.11.063
Guptha JS, Renuka M, Suneetha Y, Srinivasulu Reddy M (2016) Evaluation of antioxidant defence system during xenobiotic induced oxidative stress in freshwater fish Oreochromis mossambicus. Int J Fish Aquat 4(1):379–385
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hao C, Helm PA, Morse D, Reiner EJ (2018) Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 191:288–295. https://doi.org/10.1016/j.chemosphere.2017.10.060
He C, Zuo Z, Shi X, Sun L, Wang C (2012) Pyrene exposure influences the thyroid development of Sebastiscus marmoratus embryos. Aquat Toxicol 124:28–33. https://doi.org/10.1016/j.aquatox.2012.07.007
Hemalatha D, Muthukumar A, Rangasamy B, Nataraj B, Ramesh M (2016) Impact of sublethal concentration of a fungicide propiconazole on certain health biomarkers of Indian major carp Labeo rohita. Biocatal Agric Biotechnol 8:321–327. https://doi.org/10.1016/j.bcab.2016.10.009
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fish’s potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination, vol 26. Lewis Publishers, Boca Raton, pp 17–57. https://doi.org/10.1016/S0048-9697(05)80140-8
Hontela A, Rasmussen JB, Chevalier G (1993) Endocrine responses as indicators of sublethal toxic stress in fish from polluted environments. Water Poll Res J Can 28(4):767–780. https://doi.org/10.2166/wqrj.1993.042
Hoseini SM, Hedayati A, Ghelichpour M (2014) Plasma metabolites, ions and thyroid hormones levels, and hepatic enzymes′ activity in Caspian roach (Rutilus rutilus caspicus) exposed to waterborne manganese. Ecotoxicol Environ Saf 107:8489–24862. https://doi.org/10.1016/j.ecoenv.2014.05.002
Hou R, Xu Y, Wang Z (2016) Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153:78–90. https://doi.org/10.1016/j.chemosphere.2016.03.003
Hou R, Liu C, Gao X, Xu Y, Zha J, Wang Z (2017) Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ Pollut 229:548–556. https://doi.org/10.1016/j.envpol.2017.06.097
Hou R, Yuan S, Feng C, Xu Y, Rao K, Wang Z (2019) Toxicokinetic patterns, metabolites formation and distribution in various tissues of the Chinese rare minnow (Gobiocypris rarus) exposed to tri (2 butoxyethyl) phosphate (TBOEP) and tri-n-butyl phosphate (TNBP). Sci Total Environ 668:806–814. https://doi.org/10.1016/j.scitotenv.2019.03.038
Jakimska A, Huerta B, Barganska Z, Kot-Wasik A, Rodriguez-Mozaz S, Barcelo D (2013) Development of a liquid chromatography-tandem mass spectrometry procedure for determination of endocrine disrupting compounds in fish from Mediterranean rivers. J Chromatogr A 26:44–58. https://doi.org/10.1016/j.chroma.2013.07.050
Jarosiewicz M, Krokosz A, Marczak A, Bukowska B (2019) Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere 227:93–99. https://doi.org/10.1016/j.chemosphere.2019.04.008
Jiang F, Liu J, Zeng X, Yu L, Liu C, Wang J (2018) Tris(2-butoxyethyl) phosphate affects motor behavior and axonal growth in zebrafish (Danio rerio) larvae. Aquat Toxicol 198:215–223. https://doi.org/10.1016/j.aquatox.2018.03.012
Jin S et al (2013) An in vivo zebrafish screen identifies organophosphate antidotes with diverse mechanisms of action. J Biomol Screen 18:108–115. https://doi.org/10.1177/1087057112458153
John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem 12:500–504. https://doi.org/10.1016/S0955-2863(01)00160-7
Katuli KK, Amiri BM, Massarsky A, Yelghi S (2014) Impact of a short-term diazinon exposure on the osmoregulation potentiality of Caspian roach (Rutilus rutilus) fingerlings. Chemosphere 108:396–404. https://doi.org/10.1016/j.chemosphere.2014.02.038
Kaur M, Jindal R (2017) Oxidative stress response in liver, kidney and gills of Ctenopharyngodon idellus (Cuvier & Valenciennes) exposed to chlorpyrifos. MOJ Biol Med 1(4):103–112. https://doi.org/10.15406/mojbm.2017.01.00021
Kim S, Jung J, Lee I, Jung D, Youn H, Choi K (2015) Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol 26:188–196. https://doi.org/10.1016/j.aquatox.2015.01.016
Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T (2013) In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314:76–83. https://doi.org/10.1016/j.tox.2013.09.004
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Randak T (2009) Responses of antioxidant status and Na+ K+-ATPase activity in gill of rainbow trout, Oncorhynchus mykiss, chronically treated with carbamazepine. Chemosphere 77:1476–1481. https://doi.org/10.1016/j.etap.2014.04.005
Li X, Li Y, Chen J, Sun J, Li X, Sun X, Kang X (2010) Tetrahydroxystilbene glucoside attenuates MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation. Neurosci Lett 483(1):1–5. https://doi.org/10.1016/j.neulet.2010.07.027
Li WL, Liu LY, Song WW, Zhang Z, Qiao LN, Ma WL, Li YF (2016) Five-year trends of selected halogenated flame retardants in the atmosphere of Northeast China. Sci Total Environ 539:286–293. https://doi.org/10.1016/j.scitotenv.2015.09.001
Liu X, Ji K, Choi K (2012) Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol 114-115:173–181. https://doi.org/10.1016/j.aquatox.2012.02.019
Liu C, Su G, Giesy JP, Letcher RJ, Li G, Agrawal I, Li J, Yu L, Wang J, Gong Z (2016) Acute exposure to tris(1, 3-dichloro-2-propyl) phosphate (TDCIPP) causes hepatic inflammation and leads to hepatotoxicity in zebrafish. Sci Rep 19045. https://doi.org/10.1038/srep19045
Liu J, Ye J, Chen Y, Li C, Ou H (2017) UV-driven hydroxyl radical oxidation of tris(2-chloroethyl) phosphate: intermediate products and residual toxicity. Chemosphere 190:225–233. https://doi.org/10.1016/j.chemosphere.2017.09.111
Liu X, Cai Y, Wang Y, Xu S, Ji K, Choi K (2019) Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenylphosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol Environ Saf 170:25–32. https://doi.org/10.1016/j.ecoenv.2018.11.058
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma Y, Cui K, Zeng F, Wen J, Liu H, Zhu F, Ouyang G, Luan T, Zeng Z (2013) Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography-mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples. Anal Chim Acta 786:47–53. https://doi.org/10.1016/j.aca.2013.04.062
Mags- Novel; News & Events 2015. Copyrights © 2010-13 Mags-Novel.
Marigoudar SR, Nagarjuna A, Karthikeyan P, Mohan D, Sharma KV (2018) Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere 211:89–101. https://doi.org/10.1016/j.chemosphere.2018.07.137
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Martínez-Carballo E, González-Barreiro C, Sitka A, Scharf S, Gans O (2007) Determination of selected organophosphate esters in the aquatic environment of Austria. Sci Total Environ 388:290–299. https://doi.org/10.1016/j.scitotenv.2007.08.005
Maruna RFL (1958) Quantitative estimation of sodium (Na+) and potassium (K+) in human serum by colorimetric method. Clin Chem Acta 2:581–585
Matsukami H, Kose T, Watanabe M, Takigami H (2014) Pilot-scale incineration of wastes with high content of chlorinated and non-halogenated organophosphorus flame retardants used as alternatives for PBDEs. Sci Total Environ 493:672–681. https://doi.org/10.1016/j.scitotenv.2014.06.062
Matthews H, Eustis S, Haseman J (1993) Toxicity and carcinogenicity of chronic exposure to tris(2-chloroethyl) phosphate. Toxicol Sci 20:477–485. https://doi.org/10.1093/toxsci/20.4.477
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, Mc Cume DC, Ratner BA (1992) Physiological and nonspecific biomarkers. In: Huggert RF, Kimerle RA, Mehrle PM, Bergman LH (eds) Biomarkers: biochemical, physiological and histological markers of anthropogenic stress, Lewis, Boca Raton, pp 5–85
Melekh B, Ilkiv I, Lozynskyi A, Sklyarov A (2017) Antioxidant enzyme activity and lipid peroxidation in rat liver exposed to celecoxib and lansoprazole under epinephrine- induced stress. Int J Appl Pharm Sci Res 7(10):94–99. https://doi.org/10.1016/j.fct.2003.10.005
Modesto K, Martinez CBR (2010) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78(3):294–299. https://doi.org/10.1016/j.chemosphere.2009.10.047
Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL (2006) Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol 143(2):141–149. https://doi.org/10.1016/j.cbpc.2006.01.004
Morgan MA, Griffith CM, Volz DC, Larive CK (2019) TDCIPP exposure affects Artemia franciscana growth and osmoregulation. Sci Total Environ 694:133486–124862. https://doi.org/10.1016/j.scitotenv.2019.07.292
Moser VC, Phillips PM, Hedge JM, McDaniel KL (2015) Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) and tris(2-chloro-2-ethyl) phosphate (TCEP). Neurotoxicol Teratol 52:236–247. https://doi.org/10.1016/j.ntt.2015.08.004
Movahedinia A, Salamat N, Kheradmand P (2018) Effects of the environmental endocrine disrupting compound benzo[a]pyrene on thyroidal status of abu mullet (Liza abu) during short-term exposure. Toxicol Rep 5:377–382. https://doi.org/10.1016/j.toxrep.2018.02.018
Nagarjuna A, Mohan D (2017) Biochemical and histopathological changes induced by nickel in the striped mullet, Mugil cephalus (Linnaeus 1758). Bull Environ Contam Toxicol 98(1):33–40. https://doi.org/10.1007/s00128-016-1961-x
Osimitz TG, Droege W, Hayes AW (2016) Subchronic toxicology of tetrabromobisphenol A in rats. Hum Exp Toxicol 35:11–24862. https://doi.org/10.1177/0960327115627684
Pamanji R, Yashwanth B, Venkateswara Rao J (2016) Profenofos induced biochemical alterations and in silico modelling of hatching enzyme, zhe1 in zebrafish (Danio rerio) embryos. Environ Toxicol Pharmacol 45:123–131. https://doi.org/10.1016/j.etap.2016.05.027
Pan LQ, Ren J, Liu J (2006) Responses of antioxidant systems and LPO level to benzo (a) pyrene and benzo (k) fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ Pollut 141(3):443–451. https://doi.org/10.1016/j.envpol.2005.08.069
Peter MCS (2011) The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol 172:19–210. https://doi.org/10.1016/j.ygcen.2011.02.023
Plhalova L, Zivna D, Bartoskova M, Blahova J, Sevcikova M, Skoric M, Marsalek P, Stancova V, Svobodova Z (2014) The effects of subchronic exposure to ciprofloxacin on zebrafish (Danio rerio). Neuro Endocrinol Lett 35(2):64–70
Poopal RK, Ramesh M, Dinesh B (2013) Short-term mercury exposure on Na+/K+-ATPase activity and ionoregulation in gill and brain of an Indian major carp, Cirrhinus mrigala. J Trace Elem Med Biol 27(1):70–75. https://doi.org/10.1016/j.jtemb.2012.06.002
Poopal RK, Ramesh M, Maruthappan V, Babu Rajendran R (2017) Potential effects of low molecular weight phthalate esters (C16H22O4 and C12H14O4) on the freshwater fish Cyprinus carpio. Toxicol Res 6:505–520. https://doi.org/10.1016/j.jtemb.2012.06.002
Raibeemol KP, Chitra KC (2020) Induction of immunological, hormonal and histological alterations after sublethal exposure of chlorpyrifos in the freshwater fish, Pseudetroplus maculatus (Bloch, 1795). Fish Shellfish Immunol 102:1–12. https://doi.org/10.1016/j.fsi.2020.04.005
Ramesh M, Anitha S, Poopal RK, Shobana C (2018) Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol Rep 5:18–27. https://doi.org/10.1016/j.toxrep.2017.11.006
Reemtsma T, Quintana JB, Rodil R, García-López M, Rodríguez I (2008) Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate. Trends Anal Chem 27:727–737. https://doi.org/10.1016/j.trac.2008.07.002
Regnery J, Püttmann W (2010) Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany. Water Res 44:4097–4104. https://doi.org/10.1016/j.toxrep.2017.11.006
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Sabir S, Akhtar MF, Saleem A (2019) Endocrine disruption as an adverse effect of non-endocrine targeting pharmaceuticals. Environ Sci Pollut Res 26:1277–1286. https://doi.org/10.1007/s11356-018-3774-4
Saeger VW, Hicks O, Kaley RG, Michael PR, Mieure JP, Tucker ES (1979) Environmental fate of selected phosphate esters. Environ Sci Technol 13:840–844. https://doi.org/10.1021/es60155a010
Sahu VK, Karmakar S, Kumar S, Shukla SP, Kundan Kumar K (2018) Triclosan toxicity alters behavioral and hematological parameters and vital antioxidant and neurological enzymes in Pangasianodon hypophthalmus (Sauvage, 1878). Aquat Toxicol 202:145–152. https://doi.org/10.1016/j.aquatox.2018.07.009
Samanta P, Pal S, Mukherjee AK, Ratan Ghosh A (2014) Biochemical effects of glyphosate-based herbicide, Excel Mera 71 on enzyme activities Triclosan toxicity alters behavioral and hematological parameters and vital antioxidant and neurological enzymes in Pangasianodon hypophthalmus (Sauvage, 1878) of acetylcholinesterase (AChE), lipid peroxidation (LPO), catalase (CAT), glutathione-S-transferase (GST) and protein content on teleostean fishes. Ecotoxicol Environ Saf 107:120–125. https://doi.org/10.1016/j.ecoenv.2014.05.025
Sathya V, Ramesh M, Poopal RK, Dinesh KPB (2012) Acute and sublethal effects in an Indian major carp Cirrhinus mrigala exposed to silver nitrate: gill Na+ /K+-ATPase, plasma electrolytes and biochemical alterations. Fish Shellfish Immunol 32:862–868. https://doi.org/10.1016/j.fsi.2012.02.014
Schales N, Schales SS (1941) A simplified and accurate method for the determination of chloride in biological fluids. J Biol Chem 140:879–884
Schoenfeld FG, Lewellen CJ (1964) A colorimetric method for determination of serum chloride. Clin Chem 10:533–539
Shirdel I, Reza Kalbassi M, Shokri M, Olyaei R, Sharifpour I (2016) The response of thyroid hormones, biochemical and enzymological biomarkers to pyrene exposure in common carp (Cyprinus carpio). Ecotoxicol Environ Saf 130:207–213. https://doi.org/10.1016/j.ecoenv.2016.03.023
Sugeng EJ, Cock M, Linda J, Schoonmade LJ, Bor M (2017) Toddler exposure to flame retardant chemicals: Magnitude, health concern and potential risk- or protective factors of exposure: observational studies summarized in a systematic review. Chemosphere 184:820–831. https://doi.org/10.1016/j.chemosphere.2017.06.041
Sunderman FW, Sunderman FW (1959) The rapid colorimetric estimation of potassium. Am J Clin Pathol 29:95
Sundkvist AM, Olofsson U, Haglund P (2010) Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12:943–951. https://doi.org/10.1039/b921910b
Ta N, Li C, Fang Y, Liu H, Lin B, Jin H, Tian L, Zhang H, Zhang W, Xi Z (2014) Toxicity of TDCPP and TCEP on PC12 cell: changes in CAMKII, GAP43, tubulin and NF-H gene and protein levels. Toxicol Lett 227:164–171. https://doi.org/10.1016/j.toxlet.2014.03.023
Tabassum H, Dawood AQ, Sharma P, Khan J, Raisuddin S, Parvez S (2016) Multi-organ toxicological impact of fungicide propiconazole on biochemical and histological profile of freshwater fish Channa punctata Bloch. Ecol Indic 63:359–365. https://doi.org/10.1016/j.ecolind.2015.11.052
Taillebois L, Keith P, Valade P, Torres P, Baloche S, Dufour S, Rousseau K (2011) Involvement of thyroid hormones in the control of larval metamorphosis in Sicyopterus lagocephalus (Teleostei: Gobioidei) at the time of river recruitment. Gen Comp Endocrinol 173(2):281–288. https://doi.org/10.1016/j.ygcen.2011.06.008
Terri AE, Sesin PG (1958) Colorimetric method of potassium estimation using sodium tetraphenylboron. Am J Clin Pathol 29:86–90
Tkachenko H, Kurhaluk N, Grudniewska J (2014) Oxidative stress biomarkers in different tissues of rainbow trout (Oncorhynchus mykiss) exposed to disinfectant-CIP formulated with peracetic acid and hydrogen peroxide. Arch Pol Fish 22:207–219. https://doi.org/10.2478/aopf-2014-0021
Trinder P (1951) A rapid method for the determination of sodium in serum. Analyst 76:596–599. https://doi.org/10.1139/cjms52-038
Van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Walpita CN, Crawford AD, Janssens EDR, Vander Geyten S, Darras VM (2009) Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology 150:530–539. https://doi.org/10.1210/en.2008-0457
Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B (2013) Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic–pituitary–thyroid axis. Aquat Toxicol 126:207–213. https://doi.org/10.1016/j.aquatox.2012.11.009
Wang R, Tang J, Xie Z, Mi W, Chen Y, Wolschke H, Tian C, Pan X, Luo Y, Ebinghaus R (2015) Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ Pollut 198:172–178. https://doi.org/10.1016/j.envpol.2014.12.037
Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, Li JJ, Zhang SJ, Liang ZQ (2015) Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut 196:29–46. https://doi.org/10.1016/j.envpol.2014.09.012
Wolschke H, Sühring R, Mi W, Moller A, Xie Z, Ebinghaus R (2016) Atmospheric occurrence and fate of organophosphorus flame retardants and plasticizer at the German coast. Atmos Environ 137:1–5. https://doi.org/10.1016/j.atmosenv.2016.04.028W
Wolschke H, Sühring R, Massei R, Tang J, Ebinghaus R (2018) Regional variations of organophosphorus flame retardants - fingerprint of large river basin estuaries/deltas in Europe compared with China. Environ Pollut 236:391–395. https://doi.org/10.1016/j.envpol.2018.01.061
Woo SJ, Kim NY, Kim SH, Ahn SJ, Seo JS, Jung SH, Cho MY, Chung JK (2018) Toxicological effects of trichlorfon on hematological and biochemical parameters in Cyprinus carpio L. following thermal stress. Comp Biochem Physiol C Toxicol Pharmacol 209:18–27. https://doi.org/10.1016/j.cbpc.2018.03.001
Wu Y, Su G, Tang S, Liu W, Ma Z, Zheng X, Liu H, Yu H (2017) The combination of in silico and in vivo approaches for the investigation of disrupting effects of tris(2-chloroethyl) phosphate (TCEP) toward core receptors of zebrafish. Chemosphere 168:122–130. https://doi.org/10.1016/j.chemosphere.2016.10.038
Xu T, Wang Q, Shi Q, Fang Q, Guo Y, Zhou B (2015) Bioconcentration, metabolism and alterations of thyroid hormones of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in zebrafish. Environ Toxicol Pharmacol 40:581–586. https://doi.org/10.1016/j.etap.2015.08.020
Xu X, Chen J, Qu R, Wang Z (2017) Oxidation of tris(2-chloroethyl) phosphate in aqueous solution by UV-activated peroxymonosulfate: kinetics, water matrix effects, degradation products and reaction pathways. Chemosphere 185:833–843. https://doi.org/10.1016/j.chemosphere.2017.07.090
Xu C, Li X, Jin M, Sun X, Niu L, Lin C, Liu W (2018) Early life exposure of zebrafish (Danio rerio) to synthetic pyrethroids and their metabolites: a comparison of phenotypic and behavioral indicators and gene expression involved in the HPT axis and innate immune system. Environ Sci Pollut Res 25:12992–13003. https://doi.org/10.1007/s11356-018-1542-0
Yan S, Wu H, Qin J, Zha J, Wang Z (2017) Halogen-free organophosphorus flame retardants caused oxidative stress and multi xenobiotic resistance in Asian freshwater clams (Corbicula fluminea). Environ Pollut 225:559–568. https://doi.org/10.1016/j.envpol.2017.02.071
Yang Y, Xiao Y, Chang Y, Cui Y, Klobučar G, Li M (2018a) Intestinal damage, neurotoxicity and biochemical responses caused by tris(2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotoxicol Environ Saf 158:78–86. https://doi.org/10.1016/j.ecoenv.2018.04.012
Yang YC, Chien MC, Liu HY, Chang YC, Chen CK, Lee WL, Kuo TC, Hsiao M, Hua KT, Cheng TY (2018b) Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett 421:28–40. https://doi.org/10.1016/j.canlet.2018.01.075
Yonar SM, Ural MS, Silici S, EnisYonar M (2014) Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio: protective role of propolis. Ecotoxicol Environ Saf 102:202–209. https://doi.org/10.1016/j.ecoenv.2014.01.007
Yuan S, Han Y, Ma M, Rao K, Wang Z, Yang R, Liu Y, Zhou X (2019) Aryl-phosphorus-containing flame retardants induce oxidative stress, the p53-dependent DNA damage response and mitochondrial impairment in A549 cells. Environ Pollut 250:58–67. https://doi.org/10.1016/j.envpol.2019.03.109
Zhang Q, Ji C, Yin X, Yan L, Lu M, Zhao M (2016) Thyroid hormone-disrupting activity and ecological risk assessment of phosphorus-containing flame retardants by in vitro, in vivo and in silico approaches. Environ Pollut 210:27–33. https://doi.org/10.1016/j.envpol.2015.11.051
Zhang W, Zhang Y, Hou J, Xu T, Yin W, Xiong W, Lu W, Zheng H, Jun Chen J, Yuan J (2017) Tris(2-chloroethyl) phosphate induces senescence-like phenotype of hepatocytes via the p21Waf1/Cip1-Rb pathway in a p53-independent manner. Environ Toxicol Pharmacol 56:68–75. https://doi.org/10.1016/j.etap.2017.08.028
Zhang Y, Li M, Li S, Wang Q, Zhu G, Su G, Letcher RJ, Liu C (2018) Exposure to tris(1,3-dichloro-2-propyl) phosphate for two generations decreases fecundity of zebrafish at environmentally relevant concentrations. Aquat Toxicol 200:178–187. https://doi.org/10.1016/j.aquatox.2018.05.010
Zhong M, Wu H, Mi W, Li F, Ji C, Ebinghaus R, Tang J, Xie Z (2018) Occurrences and distribution characteristics of organophosphate ester flame retardants and plasticizers in the sediments of the Bohai and Yellow Seas, China. Sci Total Environ 615:1305–1311. https://doi.org/10.1016/j.scitotenv.2017.09.272
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sutha, J., Anila, P.A., Umamaheswari, S. et al. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP). Environ Sci Pollut Res 27, 34369–34387 (2020). https://doi.org/10.1007/s11356-020-09527-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09527-0