Abstract
The present study is focused on determining the acute and chronic toxicity of nickel (Ni) to fish fingerlings, Mugil cephalus. The 96-h median lethal concentration (LC50) for Ni was found to be 42.2 ± 3.9 mg L−1. Based on the chronic toxicity test for 30 days, “No Observed Effect Concentration”, “Lowest Observed Effect Concentration” and “Chronic value” were found to be 2.9 ± 0.14, 4.7 ± 0.14 and 3.7 ± 0.14 mg Ni L−1, respectively. The activities of biomarker enzymes including esterase, superoxide dismutase and malate dehydrogenase showed differential expression and cellular anomalies like hyperplasia and detachment of bipolar cells from photoreceptor cells in the retina of eye of mullet. Cellular anomalies in the retina of fish eye affect the primary function of retina, which is to convert light energy into nerve impulses transferred to the brain via the optic nerve, leading to loss or poor vision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Nickel (Ni) is considered to be a nutritionally essential trace metal in animals, microorganisms, plants and has been a constituent of enzymes and proteins and therefore either toxicity symptoms or deficiency can occur when too much or too little Ni is assimilated. Creeks are the intertidal regions developed along the coasts and are sites of major port, industrial, urban and recreational activities (Ridgway and Shimmield 2002). Overall assessment of metal pollution in the coastal regions of Chennai, Tamil Nadu, India revealed that bioaccumulation of heavy metals has increased in all compartments, viz., water, sediment and biota during the past decade (Seshan et al. 2011). Overexploitation, mismanagement and untreated or not properly treated industrial effluents from more than 25 industries were discharged into North Chennai coastal region, which brings a great challenge for the ecosystem balance (Kannan et al. 2007).
The imbalance between generation and neutralization of Reactive Oxygen Species (ROS) by antioxidants in the organism is called oxidative stress (Davies 1995). ROS are induced by substances such as transitional metal ions, pesticides and petroleum pollutants (Slaninova et al. 2009). Gernhofer et al. (2001) studied ultrastructural responses in different organs of fish to be useful tools to characterize the health status and also to assess the impact of environmental contaminants on fish. Biomarker enzymes reflect the stress elicited due to exposure to environmental contaminants (Tu et al. 2010). Consequently, studies on histology of marine organisms exposed to heavy metals suggested that increased exposure concentrations produced significant effects like mortality, while lower concentrations caused physiological stress during chronic exposure (Adil et al. 2011).
The striped mullets (Mugil cephalus) are usually found in schools over sand or mud bottom between 0 and 10 m depths. The species is highly commercial, occurring equally in tropical, sub-tropical and temperate waters. Mainly diurnal, both juvenile and adult fish feed on zooplankton larvae, detritus, micro-algae and benthic organisms. The species were used as the test organism for toxicity studies due to being ecologically important and playing a key role in the food chain. Other considerations behind the choice of the test species were availability, amenability to laboratory testing, and its genetic stability. Adequate background data on the species (i.e. its physiology, genetics, taxonomy, and their place in the environment) were also considered. These criteria are closely matched with the US EPA criteria (US Environmental Protection Agency (USEPA 2002) for selection of organisms for toxicity studies.
The present study was aimed at determining the acute and chronic toxicity of Ni to M. cephalus and deriving respective levels of “No Observed Effect Concentration” (NOEC) and the “Lowest Observed Effective Concentration” (LOEC). The activity of enzymes like esterase, superoxide dismutase, malate dehydrogenase and histopathological changes in the gills and retina of eye of mullet were studied after exposure to Ni for 30 days under controlled laboratory conditions.
Materials and Methods
Fingerlings of M. cephalus (about 3.0–3.5 cm in length), a natural inhabitant of the estuaries, were collected from the mud banks of Kovalam creek located on the southern part of Chennai, Tamil Nadu, India (Lat. 12.792°N, Long. 80.252°E). Water quality parameters like salinity, pH, water temperature and dissolved oxygen (DO) were monitored by using a pre-calibrated Hydrolab water quality probe (Quanta, USA). The background concentration of Ni in ‘Ennore’ seawater was monitored throughout the study period. The same seawater was used for conducting toxicity experiments under flow-through conditions. The stock solution of Ni (1000 mg L−1) was prepared by dissolving 4.05 g of Nickel chloride hexahydrate (NiCl2·6H2O; Merck, Germany) in 1 L of ultrapure deionized water (Millipore-Milli-Q) and the required test concentrations were prepared from the main stock solution. The bioassay for acute and chronic toxicity tests were conducted by using customized continuous flow through test method with fish fingerlings as described by Sprague (1973) and Stephan et al. (1985). For the acute toxicity tests, healthy fish fingerlings were separated from the storage tanks and divided into six groups, viz., 10 numbers each were exposed in duplicate to the definitive test concentrations—(i) control; (ii) 35.0 (iii) 42.0 (iv) 50.0 (v) 60.0 and (vi) 73.0 mg L−1 Ni. The acute exposure period was 96 h and totally three successive flow through tests were performed for confirmation. Similarly, for chronic toxicity tests, healthy fish fingerlings were divided into six groups, viz., 10 numbers each were exposed in duplicate to the following concentrations: (i) control; (ii) 3.0, (iii) 5.1, (iv) 8.7, (v) 14.7 and (vi) 25.1 mg L−1 Ni. The concentrations were selected based on 96 h LC50 already derived for M. cephalus. The chronic toxicity test was continued for 30 days of exposure and it was repeated once for confirmation. The seawater samples were collected at 24 h intervals (i.e. 24, 48, 72 and 96 h) for acute toxicity tests and between 10 days intervals (i.e. 10th, 20th and 30th day) for chronic tests. The experimental seawater samples were filtered through 0.45 µm Millipore filters and acidified with suprapure nitric acid (Fluka) to pH 2–3. The nickel was extracted from the experimental seawater and sea water samples spiked with nickel standard (Merck) and nickel chloride hexahydrate for recovery analysis by using ammonium pyrrolidinedithiocarbamate and methyl iso-butyl ketone following the method of Grasshoff et al. (1999). The total dissolved nickel concentrations in seawater samples were analysed by atomic absorption spectrometry (Varian SpectrAA Model 220FS). A standard solution of nickel (1000 mg L−1, Merck, Germany) was used as the standard. To validate the results, the known standard and nickel chloride hexahydrate recovery samples were measured between the samples.
Towards the end of 30 days of chronic exposure, live test mullets were sacrificed and stored in Ultra freezer (−80°C) until further analysis. Frozen samples were thawed over ice, wiped with clean tissue paper and washed with ice cold phosphate buffer solution (0.1 M PBS pH 7.4). The samples were homogenized with liquid nitrogen followed by ice-cold extraction buffer using mortar and pestle in an ice bath. After homogenization, a clear supernatant was obtained through centrifugation repeated for 3 times, each for 10 min (12,000 rpm/10 min/4°C), which served as the enzyme source. The protein content of the extracts was estimated by the method described by Bradford (1976) using bovine serum albumin as the protein standard. Native PAGE (PolyAcrylamide Gel Electrophoresis) technique was carried out at 25 ± 1°C using Tris–glycine as running buffer (pH 8.3) under non-denaturing and non-reducing conditions (Laemmli 1970). A uniform amount of protein (100 μg) was loaded to each lane along with native sample buffer (devoid of sodium dodecyl sulphate and b-mercaptoethanol). Samples were then subjected to electrophoresis at 50 V through the stacking gel (4 %) and at 100 V through the resolving gel (8 %). Activity staining for SOD, MDH and EST on the gel was performed by following the method of Beauchamp and Fridovoch (1971) and Wendel and Weeden (1989). Activity staining for Esterase (EST): Activity staining for esterase was performed by placing the gel in a staining solution containing 100 mL of 100 mM phosphate buffer (pH 6.2), 50 mg alpha-napthyl acetate, 50 mg beta-napthyl acetate and 100 mg fast blue RR salt and incubated at room temperature (25°C) for 1 h to develop black, red or magenta colour bands. Both the substrates (alpha-napthyl acetate and beta-napthyl acetate) were dissolved quickly in 1 mL of acetone and mixed with the staining solution just before transferring of gel (Wendel and Weeden 1989). Activity staining for Superoxide dismutase (SOD): Activity staining of total superoxide dismutase on the gel band was performed by following the method of Beauchamp and Fridovich (1971). The gel was soaked in the staining solution containing 50 mL of 50 mM Tris–Cl (pH 8.00), 10 mg nitroblue tetrazolium chloride (NBT), 1 mg ethylene diamine tetra acetic acid, and 2 mg riboflavin for 30 min in the dark at room temperature (25°C) and then illuminated in a light box with white fluorescent light for 30 min or until achromatic bands appeared. Activity staining for Malate dehydrogenase (MDH): Activity of malate dehydrogenase was detected by soaking the gel in 100 mL of Tris–Cl (50 mM, pH 8.5) containing 300 mg of malic acid, 20 mg of nicotinamide adenine dinucleotide (NAD), 20 mg of NBT (Hi-Media, India) and 4 mg of phenazine methosulphate (Hi Media, India). The gel was incubated at 25°C until the blue or indigo bands appeared (Wendel and Weeden 1989). Fish tissue samples such as gills and retina of eye were dissected freshly at the end of 30 days chronic exposure and fixed in Bouin’s solution. After fixation, the tissues were dehydrated through a graded alcohol series, cleared in xylene and embedded in paraffin wax (58–60°C). Tissue sections of 5 μM thickness were made and stained with hematoxylin and eosin and observed under light microscope to characterize the cellular changes.
Both acute and chronic toxicity values were calculated based on the measured dissolved Ni concentration in the treatment seawater. The 96 h LC50 and 95 % confidence limits were calculated based on the mortality against number of the test organisms exposed in each treatment concentration by using Probit Analysis software tool (Finney 1971). From the chronic test, NOEC, LOEC and chronic values were derived based on the survival of the test organisms at the end of 30-day of the experiment. One-way analysis of variance in conjunction with Dunnett’s (1964) test was used to determine whether the treatments were significantly different from the control group (p ≤ 0.05). The data were arc sine transformed. The enzyme profiles were analyzed by software provided with the gel documentation system (Biovis Gel 2012F, India).
Results and Discussion
In the present study, the temperature, DO, pH, salinity and Ni in experimental sea water were estimated to be 28 ± 1.16°C, 5.1 ± 1.46 mg L−1, 7.9 ± 0.2, 32.74 ± 1.64 psu and 6.5 ± 0.76 µg L−1 respectively. The nominal test concentrations were measured for dissolved Ni concentration in all the test chambers at 24 h intervals for acute and 10 days intervals for chronic toxicity tests respectively. For all toxicity tests, the nominal and measured concentrations of Ni were compared to evaluate the stability of Ni concentration in the test chamber and to determine the accuracy of analytical method. The mean dissolved concentration and percent recovery of Ni between nominal and measured concentrations are summarized in Table 1 and the acute and chronic toxicity values were calculated based on the dissolved and recoverable Ni concentration in the test medium. The average 96 h LC50 value derived in M. cephalus was found to be 42.2 ± 3.9 (39.6–45.1) mg L−1 Ni based on the mean values of three repetitive flows through experiments (Table 2). There was no mortality initially from exposure to different concentrations of Ni, the mortality increased with longer exposure time and elevated metal levels. Previous authors reported the 96 h LC50 for Ni was 7.96 mg L−1 in Menidia menidia (Cardin 1985); 10.0 mg L−1 in Danio rerio (PAN database 2012); 21.0 mg L−1 in Morone saxatills (Palawski et al. 1985); 26.56 mg L−1 in Atherinops affinis (Hunt et al. 2002); 38.0 mg L−1 in Menidia peninsulae (Hansen 1983) and 70.0 mg L−1 in Leiostomus xanthurus (Hansen 1983) and such reports concluded that the survival of the species depends on their life stage and tolerance limit and factors like salinity, temperature and hardness of the test medium used. The percentage survival of exposed organisms at the end of chronic exposure, the NOEC, LOEC, and chronic values are given in Table 3. From the results, it was ascertained that the survival of mullet decreased with increased exposure levels during the chronic toxicity test. Minimum percent (60 %) survival of organisms was observed at the highest concentration of Ni (22.6 mg L−1) in Chronic I test and 55 % of survival at 21.9 mg L−1 Ni observed in Chronic II test after 30 days exposure. The average chronic value of 3.69 ± 0.14 mg L−1 was derived based on the geometric mean of the NOEC (2.9 ± 0.14) and LOEC (4.7 ± 0.14) respectively.
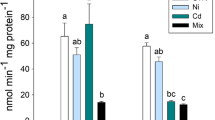
Sub lethal toxicity of Ni for 30 days in mullets caused an induction of EST, SOD and MDH activities (Fig. 1). Three isoforms of esterase activities were observed. The isoform 1 (Rm 0.212) of esterase activity was observed both at control and NOEC (3.0 mg L−1) and there is no esterase activity in different concentrations as evidenced with no band formations. There is no variation in esterase activity levels of isoforms 2 and 3 (Rm 0.258; 0.341 values). SOD1 isoform activity levels (Rm 0.296) was decreased and SOD2 isoform activity levels (Rm 0.169) was increased compared to control level. MDH activity levels (Rm 0.258) was increased when compared to control level with the exception of absence of gel band formation at LOEC (4.8 mg L−1) concentration. Enzymes have the ability to stabilize the effect of Ni toxicity and have their own specificity to respond and neutralize the reactive oxygen species formation. When the SOD activity was induced, it would be beneficial for the process of dismutation when there is an excess production of superoxide anion and to protect the cells from oxidative damages. Exposure to sodium hypochlorite in M. galloprovincialis reported inhibited acetylcholine esterase activity in gills (Lopez-Galindo et al. 2010). Padmini et al. (2009) studied low SOD activity in M. cephalus from Ennore estuary, in India. SOD activity increased significantly in zebrafish exposed to atrazine (Jin et al. 2010). Expression of MnSod was slightly increased when compared to control level but Cu/ZnSod expressions remain unchanged in the liver of Danio rerio exposed to sodium fluoride (Mukhopadhyay and Chattopadhyay 2014). Low SOD activity has been reported in the gill of M. cephalus from Bizerte lagoon, Tuniria (Ameur et al. 2015). Livingstone (2001) reported that the responses of antioxidant enzymes to pollution vary for different species, enzymes, and single or mixed contaminants, and even greater variability is found in field situations. Elevated ROS level in tissues leads to cellular damage when the rate of its generation surpasses the rate of its decomposition by antioxidant defense systems.
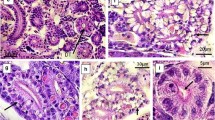
Further, gills are considered a prime target to contaminants and significant route for uptake, bioconcentration and excretion of toxicants, due to the broad surface area reduce distance between internal and external medium and direct contact with the environment. Structurally the gills of fish showed the primary lamellae arranged in double rows, projecting towards the lateral side with a series of alternatively arranged secondary lamellae (respiratory lamellae). Each lamella was made up of single layer of cells (Fig. 2a). Towards the end of exposure no change was observed in the secondary lamellae of fish gill exposed to 3 mg L−1 Ni (NOEC) (Fig. 2b), however exposure to 4.8 mg L−1 (LOEC) and 7.8 mg L−1 Ni resulted in changes such as hyperplasia at the ends of secondary lamellae (Fig. 2c, d). Similarly, hypertrophy and fusion at the ends of secondary lamellae and irregular inter-lamellar space were observed in the gills of fish exposed to 13.8 mg L−1 Ni (Fig. 2e). The fusion of secondary lamellae, lamellar swelling, and reduced inter-lamellar space (Fig. 2f) were also observed in the gills of fish exposed to 22.6 mg L−1 Ni. Hence in the present study, the effect of Ni on the gills was particularly severe since they serve as a major organ for gaseous exchange, osmotic and ionic regulation, acid-base balance and nitrogenous waste. Epithelial hypertrophy and hyperplasia, necrosis of secondary lamellae, lamellar swelling, fusion of adjacent lamellae and reduced inter-lamellar space were observed in the gills of mullets after long term exposure treatment to Ni under chronic stress (Fig. 2e, f). Vacuoles in branchial lamellae, necrosis and hypertrophy of epithelial cells in fish exposed to Fe(II) were reported (Zhihao et al. 2012). Ameur et al. (2015) studied thickening of primary lamellae, cellular hyperplasia, aneurism, curving, shortening and fusion of secondary lamellae in M. cephalus from Bizerte lagoon, Tuniria.
Section through the gill of M. cephalus exposed to different concentration of Ni (mg L−1) for 30 days stained with heamatoxylin and eosin (400X); a control fish showing normal arrangement of primary lamellae (PL) and secondary lamellae (SL); b 3 mg L−1 Ni (NOEC) exposed fish gill showing normal arrangement of gill filament (GF) and secondary lamellae (SL); c 4.8 mg L−1 Ni (LOEC) exposed fish gill showing hyperplasia at the ends of secondary lamellae (SL); d 7.8 mg L−1 Ni exposed fish gill showing hyperplasia of secondary lamellae (SL); e 13.8 mg L−1 Ni exposed fish gill showing hypertrophy and fusion at the ends of secondary lamellae (SL) and irregular interlamellar space; f 22.6 mg L−1 Ni exposed fish gill showing fusion, hypertrophy and necrosis of secondary lamellae; scale bar 10 µM
The retina of eye of control fish showed normal arrangement of the eight specific layers, viz., pigment epithelium layer (PEL), visual layer (cone and rod receptor cells) (VL), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer composed of a narrow chain of granular spherical cells surrounded by a fine connective tissue network (GCL) and nerve fibre layer (NFL) (Fig. 3a). No changes were observed in the retina of fish exposed to 3.0 mg L−1 Ni (NOEC) (Fig. 3b), while fish exposed to 4.8 mg L−1 (LOEC) and 7.8 mg L−1 Ni showed reduced number of granular spherical cells in ganglion cell layer (Fig. 3c, d). However, reduced number of granular spherical cells in ganglion cell layer and detachment of bipolar cells from photoreceptor cells at 13.8 mg L−1 (Fig. 3e) and 22.6 mg L−1 Ni (Fig. 3f) exposed to fish were observed. The detachment of bipolar cells from photoreceptor cells in the retina of the fish eye exposed to Ni for long term exposure under chronic stress was also observed, which affects the primary function of retina by converting light energy into nerve impulses which are transferred to the brain via the optic nerve consequences in loss of vision or poor vision. Choudhury et al. (2015) reported that accumulation of selenium in the eye lens may be the cause of ocular impairments in exposed fish. Cellular anomalies like epithelial hypertrophy and hyperplasia, necrosis of secondary lamellae, lamellar swelling in gills and detachment of bipolar cells from photoreceptor cells in the retina of eye of mullet are the net result of adverse physiological and biochemical changes inside the cells of organism. These changes indicated toxic effects of nickel on cellular functioning. A practical advantage of using fish histopathology is that multiple organs can be examined; and this increases the sensitivity at which pollution impacts can be detected. The acute and chronic toxicity values are important for environmental monitoring since the presence of Ni has been identified as an indicator of pollution. Further, exposure of fish to Ni in particular induces a number of biochemical alterations reflecting the imbalance of antioxidant related parameters which lead to onset of oxidative stress.
Section through the retina of eye of M. cephalus exposed to different concentration of Ni (mg L−1) for 30 days stained with heamatoxylin and eosin (400X); a retina of fish eye showing normal arrangement of pigment epithelium layer (PEL), visual layer (VL), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL) and nerve fibre layer (NFL); b 3 mg L−1 Ni (NOEC) exposed fish retina of eye showing normal arrangement of eight specific layers; c 4.8 mg L−1 Ni (LOEC) exposed fish retina of eye showing reduced number of granular spherical cells in ganglion cell layer (GL); d 7.8 mg L−1 Ni exposed fish retina of eye showing reduced number of granular spherical cells in ganglion cell layer (GL); e 13.8 mg L−1 of Ni exposed fish eye retina showing reduced number of granular spherical cells in ganglion cell layer and detachment of bipolar cells from photoreceptor cells (DBPC); f 22.6 mg L−1 Ni exposed fish eye retina showing reduced number of granular spherical cells in ganglion cell layer and detachment of bipolar cells from photoreceptor cells (DBPC); scale bar 10 µM<\fig>
References
Adil AW, Sikdar-Bar M, Borana K, Khan HA, Andrabi SSM, Pervaiz PA (2011) Histo-pathological alterations induced in gill epithelium of African catfish, Clarias gariepinus exposed to copper sulphate. Asian J Exp Biol Sci 2:278–282.
Ameur WB, Megdiche YEl, Lapuente Jde, Barhoumi B, Trabelsi S, Ennaceur S, Camps L, Serret J, Ramos-López D, Gonzalez-Linares J, Touil S, Driss MR, Borràs M (2015) Oxidative stress, genotoxicity and histopathology biomarker responses in Mugil cephalus and Dicentrarchus labrax gill exposed to persistent pollutants. A field study in the Bizerte Lagoon: Tunisia. Chemosphere. doi:10.1016/j.chemosphere.2015.02.050
APHA (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, New York
Au DWT, Wu RSS, Zhou BS, Lam PKS (1999) Relationship between ultrastructural changes and EROD activities in liver of fish exposed to Benzo[a]pyrene. Environ Pollut 104(2):235–247
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem Rev 44:276–287
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cardin JA (1985) Results of acute toxicity tests conducted with Nickel at ERL, Narragansett. U.S.EPA, Narragansett, RI: 1
Choudhury S, Thomas JK, Sylvain NJ, Ponomarenko O, Gordon RA, Heald SM, Janz DM, Krone PH, Coulthard I, George GN, Pickering IJ (2015) Selenium preferentially accumulates in the eye lens following embryonic exposure: a confocal X-ray fluorescence imaging study. Environ Sci Technol. doi:10.1021/es503848s
Davies KJ (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61:1–31
Dunnett CW (1964) New tables for multiple comparisons with a control. Biometrics 20:482–491
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge
Gernhofer M, Pawert M, Schramm M, Muller E, Triebskorn R (2001) Ultra-structural biomarkers as tool to characterize the health status of fish in contaminated streams. J Aquat Ecol 8:241–260
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of sea water analysis, Wiley-VCH Verlag GmbH, Weinheim, pp. 419–600
Hansen DJ (1983) Section on Acute Toxicity Tests to be Inserted in the April 1983 Report on Site Specific FAV’s. U.S. EPA, Narragansett, RI: 7.
Hunt JW, Anderson BS, Phillips BM, Tjeerderma RS, Puckett HM, Stephenson M, Tucker DW, Watson D (2002) Acute and chronic toxicity of nickel to marine organisms: implications for water quality criteria. Environ Toxicol Chem 21(11):2423–2430
Jin Y, Zhang X, Shu L, Chen L, Sun L, Qian H, Liu W, Fu Z (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852. doi:10.1016/jchemosphere.2009.11.044
Joseph KO, Srivastava JP (1993) Mercury in the Ennore estuary and in fish from Madras coastal waters. J Environ Biol 14:55–62
Kannan KS, Lee KJ, Krishnamoorthy R, Purusothaman A, Shanthi K, Rao R (2007) Aerobic chromium reducingBacillus cereusisolated from the heavy metal contaminated Ennore Creek sediment, North of Chennai, Tamilnadu, South East India. Res J Microbiol 2(2):130–140
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Lopez-Galindo C, Vargas-Chacoff L, Nebot E, Casanueva JF, Rubio D, Mancera JM, Sole M (2010) Sublethal responses of the common mussel (Mytilus galloprovincialis) exposed to sodium hypochlorite and Mexel 432 used as antifoulants. Ecotoxicol Environ Safety. doi:10.1016/j.ecoenv.2010.01.001
Mukhopadhyay D, Chattopadhyay A (2014) Induction of oxidative stress and related transcriptional effects of sodium fluoride in female Zebrafish liver. Bull Environ Contam Toxicol. doi:10.1007/s00128-014-1271-0
Padmini E, Usha Rani M, Vijaya Geetha B (2009) Studies on antioxidant status in Mugil cephalus in response to heavy metal pollution at Ennore estuary. Environ Monit Assess. doi:10.1007/s10661-008-0430-3
Palawski D, Hunn JB, Dwyer FJ (1985) Sensitivity of young striped bass to oranic and inorganic contaminants in fresh and saline water. Trans Am Fish Soc 114:748–753
PAN (Pesticide Network) Pesticides database (2012) Chemical toxicity studies on Aquatic Organisms. Toxicity studies for Nickel on All Organism Groups. http://www.pesticideinfo.org/.
Ridgway J, Shimmield G (2002) Estuaries as repositories of historical contamination and their impact on shelf Seas. Estuar Coast Shelf Sci. doi:10.1006/ecss.2002.1035
Seshan BRR, Usha N, Deepthi K (2011) Geochemical evidence of terrgenous influence in sediments of Buckingham canal, Ennore, South East Coast of India. Environ. Earth Sci. doi:10.1007/s12665-011-1258-7
Slaninova A, Smutna M, Modra H, Svobodova Z (2009) A review: oxidative stress in fish induced by pesticides. Neuroendocrinol Lett 30:2–12
Sprague JB (1973) The ABC’s of pollutant bioassay using fish. Biol Methods Assess Water Qual. doi:10.1520/STP34714S
Stephan CE, Mount DI, Hansen DJ, Gentile JH, Chapman GA, Brungs WA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. National Technical Information Service, Springfield, pp B85–B227049
Tu HT, Silvestre F, Wang N, Thome JP, Thanh N, Kestemont P (2010) A multi-biomarker approach to assess the impact of farming systems on black tiger shrimp (Peneaus monodon). Chemosphere. doi:10.1016/j.chemosphere.2010.09.039
US Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th ed. October. U.S. EPA, Washington. 4303T. EPA-821-R-02-012.
Wendel JF, Weeden NF (1989) Visualization and interpretation of plant isozymes. In: Soltis DEDE, Soltis PS (eds) Isozymes in plant biology. Dioscordes Press, Oregon, pp 5–45
Zhihao Wu, Feng You, Hongjun Liu, Mengxia Liu, Jun Li, Peijun Zhang (2012) Effects of waterborne Fe(II) on juvenile turbot Scophthalmus maximus: analysis of respiratory rate, hematology and gill histology. Chin J Oceanol Limnol. doi:10.1007/s00343-012-1065-3
Acknowledgments
The authors are thankful to the Ministry of Earth Sciences, Government of India to provide funds for implementing the project on ‘Marine Ecotoxicology’. Thanks are also due to the Project Director, Integrated Coastal and Marine Area Management for constant encouragement and facilities provided for carrying out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagarjuna, A., Mohan, D. Biochemical and Histopathological Changes Induced by Nickel in the Striped Mullet, Mugil cephalus (Linnaeus 1758). Bull Environ Contam Toxicol 98, 33–40 (2017). https://doi.org/10.1007/s00128-016-1961-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1961-x