Abstract
High temperature melting treatment and cement solidification are technologies currently used to reduce the leaching of heavy metals in municipal solid waste incinerator (MSWI) fly ash. In this paper, to ascertain the feasibility of melting MSWI fly ash with blast furnace (BF) slag, ultra-risk MSWI(U-MSWI) fly ash having high heavy metal (Zn, Pb, Cu, and Cr) contents were blended with BF slag, then melted and quenched into water to prepare reconstructed slag. The melting and solidification behaviors, phase composition and microstructure, and heavy metal leachability of reconstructed slag were studied. In addition, to study the further solidification and utilization of reconstructed slag in cement, the compressive strength and leaching concentration of cement composites with reconstructed slag were also investigated. The results indicate that the presence of heavy metals in the U-MSWI fly ash had a little influence on the microstructure and phase composition of reconstructed slag. The leaching concentration of heavy metals in the reconstructed slag increased with the increasing of U-MSWI fly ash content, and when the content of U-MSWI fly ash was less than 50 wt%, the reconstructed slag could meet the environmental requirements. The reconstructed slag further solidified by cement could be applied to landfill and construction materials. The technology of melting reconstruction treatment with cement solidification was a technical-economical choice for the industrial treatment of U-MSWI fly ash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste incinerator (MSWI) fly ash belongs to a kind of secondary pollutant generated by incineration of municipal solid waste in large volume, and it is hazardous for both human health and natural environment because of its saturation with harmful heavy metals (such as Zn, Pb, Cu, and Cr) (Mee 2016; Bie et al. 2016). Therefore, suitable treatment technologies of MSWI fly ash are required to ensure safe disposal to the environment.
Currently, there are many treatment methods have been done to reduce the MSWI fly ash harmful impact on human health and natural environment. Generally, the treatments are classified into three main categories (Phua et al. 2019): (i) separation processes, such as washing processes (Wang et al. 2009; Funari et al. 2017), leaching processes (Luo et al. 2019; Loginova et al. 2019), electrochemical processes (Pedersen et al. 2005), and thermal evaporation( Nowak et al. 2012); (ii) solidification/stabilization processes, such as chemical stabilization (Youcai et al. 2002; Sun et al. 2011), accelerated carbonation (Todorovic and Ecke 2006), and chemical fixation with cement binder (Aubert et al. 2007; Zhan et al. 2018); (iii) thermal processes, such as sintering (Wey et al. 2006; Huber et al. 2018a; Huber et al. 2018b), vitrification (Alhadj-Mallah et al. 2015), melting (Ni et al. 2012), and hydrothermal (Shi et al. 2017a; Shi et al. 2017b). Wherein, the separation processes are often operated as pretreatment methods to remove heavy metals from MSWI fly ash before the utilization or solidification/stabilization of MSWI fly ash. Solidification/stabilization is an effective technology to immobilize heavy metals via chemical and physical modification (Malviya and Chaudhary 2006), among them the method of solidification with cement binder is widely adopted due to the mature technology, simple process, low cost, and the main components (CaO, SiO2, and Al2O3) of MSWI fly ash which are similar to cement (Guo et al. 2014; Yang et al. 2018; Zhan et al. 2018). However, due to the low activity and high heavy metal content of MSWI fly ash, the effective solidification amount of MSWI fly ash in cementitious materials is greatly limited. Besides, the Cl in MSWI fly ash do great harm to the mechanical properties of cement products (Lederer et al. 2017). The thermal process of MSWI fly ash generally aims to produce a more stable product, which can be disposed or utilized. The leaching concentration of heavy metals in MSWI fly ash can be reduced during the melting process (Jiang et al. 2009). However, the main disadvantage of melting process is usually associated with high treatment cost and high energy consumption, and the obtained molten slag cannot be efficiently recycled due to its low activity. Therefore, both processes of solidification with cement binder and melting have their own limitations.
Currently, the research and development of MSWI fly ash treatment technology is mainly focused on combining the advantages of solidification with cement binder and melting processes and compensating for the shortcomings of both processes, which is significant for its further development and applicability. Therefore, reducing the energy consumption and cost of MSWI fly ash melting process, improving the activity of the molten slag, and increasing the effective solidification amount of MSWI fly ash in cement should be solved. Blast furnace (BF) slag, as the byproduct during the iron-making process, generated by melting of various fluxes along with the gangue minerals during smelting. Globally, over 2.10 million tons of BF slag is generated every year in China, and its discharged temperature is extremely high, about 1450–1550 °C, with the carried energy about 1700 MJ/tslag (Xie et al. 2019). Therefore, such an available high-temperature source may be as a free high-temperature carrier to melt U-MSWI fly ash, and can greatly reduce the energy consumption and cost of U-MSWI fly ash melting treatment. Moreover, this melting treatment process can mix BF slag with U-MSWI fly ash and may produce reconstructed slag with certain potential activity. In addition, the reconstructed slag generated can be used as an admixture for further solidification and application in cement, increasing the solidification effect of cement on heavy metals in U-MSWI fly ash and promoting the application of reconstructed slag in building materials. Therefore, the method of combing melting reconstruction treatment and cement solidification on U-MSWI fly ash is feasible.
In this study, the ultra-risk municipal solid waste incinerator (U-MSWI) fly ash formed by mixing heavy metal ions with MSWI fly ash was first prepared. Then, using blast furnace (BF) slag in high temperature molten state as a high-temperature carrier to melt U-MSWI fly ash, water quenched into reconstructed slag, and then mixed into cement at room temperature for re-solidification. The experimental method in this paper realized the double solidification of U-MSWI fly ash for combining melting reconstruction treatment and cement solidification. Through the analysis of heavy metal leachability and compressive strength, the final solidified product can meet the environmental requirements and can be used in landfills and construction materials. This study would lay a groundwork for the future safe and economical treatment and utilization of MSWI fly ash based on industrial processing.
Materials and methods
Materials
MSWI fly ash used in this study was collected from the cyclone of a mass-burning incinerator located in the western part of Zhengzhou, China. The MSWI fly ash samples were dried and then ground to a fine powder with surface area of 420 m2 kg−1. Blast furnace (BF) slags with a specific surface area of 355 m2 kg−1 were provided by an iron-making procedure from Steel Holding Group Co., Ltd. in Shanxi, China. P. O 42.5 type of Portland cement with a specific surface area of 345 m2 kg−1 was used from Cement Co., Ltd. in Zhengzhou. All raw materials were sieved (passing through a #200 sieve) and stored in desiccators throughout the duration of the study. ZnO, ZnCl2, Zn (NO3)2·6H2O, CuO, CuCl2·2H2O, Cu(NO3)2·3H2O, PbO, PbCl2, Pb(NO3)2, CrO3, Cr2O3, CrCl3·6H2O, and Cr(NO3)3·9H2O were of analytical grade from Sinopharm Chemical Reagent Co., Ltd, China, which were used as the heavy metal reagents. Deionized water was used in all experiments.
Sample preparation
Preparation of U-MSWI fly ash
To prepare U-MSWI fly ash, the sieved MSWI fly ash was mixed with heavy metal reagents at a mass ratio of 10:1. The heavy metal reagents were added according to a Zn:Cu:Pb:Cr mass ratio of 100:100:5:15. Among them, Zn was provided by ZnO, ZnCl2, and Zn(NO3)2·6H2O at a mass ratio of 1:1:1, Cu was provided by CuO, CuCl2·2H2O, and Cu(NO3)2·3H2O at a mass ratio of 1:1:1, Pb was provided by PbO, PbCl2, and Pb(NO3)2 at a mass ratio of 1:1:1, and Cr was provided by CrO3, Cr2O3, CrCl3·6H2O, and Cr(NO3)3·9H2O at a mass ratio of 1.5:1:1:1. The U-MSWI fly ash was ground into powders to pass through a #200 sieve with a specific surface of approximately 460 m2 kg−1.
Formulation of U-MSWI fly ash-BF slag mixtures
The as-prepared U-MSWI fly ash were mixed with sieve BF slags with varied contents ranging from 0 to 100 wt% to form U-MSWI fly ash-BF slag mixtures, then the mixtures were mixed evenly in a ZKF-WF Temperature controlled flip oscillator and pre-homogenized at 20 °C for 24 h to use. The initial melting temperature and time of U-MSWI fly ash-BF slag mixtures (~ 2 g) were tested by RT-TS1500. High temperature microscope and the basicity (CaO/SiO2 weight ratio) of mixtures are presented in Table 1.
Preparation of reconstructed slag
In the laboratory, in order to study the mechanism of high temperature melting and reconstruction features of U-MSWI fly ash-BF slag mixtures, the SLQ1600-30 Electrically Heated Furnace was used to carry out the melting reconstruction experiment of the U-MSWI fly ash-BF slag mixtures. The sample 0–8 (~ 150 g each) were melted in the electrically heated furnace according to the results from initial melting temperature and time determination of the U-MSWI fly ash-BF slag mixture were shown in Table 1. However, due to the increase in the amount of sample during the experiment, the inside of all samples could not reach to a molten state, and the samples had poor fluidity and were easy to stick to the crucible after being released from the furnace. Therefore, combining with the discharged temperature (1450–1550 °C) of BF slag in actual industry processing and further debugging of the complete melting temperature of each sample (0–8), the reconstructed slag was formed by melting at 1400 °C for 150 s and quenching into water. Then the reconstructed slag samples were all dried at 105 °C for 12 h and ground into powders to pass through a #200 sieve with a specific surface of approximately 450.7 m2 kg−1.
Moreover, in order to simulate the industrial preparation of reconstructed slag and prepare cement composites with reconstructed slag, KGPS-50KW-2500HZ Induction Heating Device was used. The specific simulation preparation steps of reconstructed slag are shown in Fig. 1. The core temperature of the completely molten U-MSWI fly ash-BF slag mixtures reached about 1400 °C through temperature measurement, and the reconstructed slag was formed by quenching into water. This is basically consistent with the results of experiments performed with the SLQ1600-30 Electrically Heated Furnace.
Preparation of cement composites
In this work, the cement composites with replaced materials of reconstructed slag with different content of U-MSWI fly ash were prepared. The designed proportion of these cement composites are listed in Table 2.
Sample analysis
Composition and microscopic structure tests
The chemical compositions of BF slag, MSWI fly ash and U-MSWI fly ash was determined by X-ray Fluorescence Spectrometry (XRF, Bruker, S4-Explore, Germany). The measured time was set for 100 s with the counting rate of 27,000 cps at 100 mA and 40 kV.
The phase compositions of BF slag, MSWI fly ash and reconstructed slag was identified by means of X-ray Diffraction (XRD, Panalytical, X’Pert PRO MPD, Holland), using CuKα radiation at 30 mA and 45 kV. Measurements were carried out in the 2θ angle range of 5–80°, with a step of 0.02° and counting time 2 s per step. Mineral identification was done in an X’Pert HighScore Plus application with PDF 2004.
The microstructure of reconstructed slag was examined using the Scanning Electron Microscope (SEM, JEOL, JSM-6700F, Japan) with an acceleration voltage of 20 kV for gold-coated, loose powder samples. Images were taken under secondary electron mode.
Absorption ATR spectra of reconstructed slag was measured using a FT-IR Spectrometer (FT-IR, ALPHA, FT/IR-300E, Germany) with a KBr beamsplitter. The spectrometer was equipped with attenuated total reflection (ATR) accessory with heated diamond crystal plate and temperature controller. ATR instrument during measurements was purged with dry air to remove water vapor. The reconstructed slag was put to the ATR cell immediately after being mixed and then sealed with Teflon tape. Spectra were recorded in the region of 4000–500 cm−1 at a resolution of 2 cm−1. The instrument was controlled by OPUS 5.5 software, and the baseline correction was carried out with its use.
The elements distribution on the surface of reconstructed slag was characterized by Electron Probe Microanalyzer (EPMA, JEOL, JXA-8230, Japan) equipped with Oxford Instruments Energy Dispersive Spectrometer (EDS, INCA, X-Act, England). The samples were coated with carbon before transferred for analysis.
Leaching behavior
The leaching behavior of MSWI fly ash, U-MSWI fly ash, reconstructed slag, and cement composites was evaluated by the Toxicity Characteristic Leaching Procedure (TCLP) test according to specification SW 846-1311 (US Environmental Protection Agency 1986). The leachates were analyzed by using inductively coupled plasma-atomic emission spectroscopy (ICP-AES, PLASMA300, America) to determine the concentrations of heavy metal Zn, Cu, Pb, and Cr according to the determination method of solid waste elements in the identification standards for hazardous wastes–identification for extraction toxicity (GB/T 5085.3-2007) (National Environmental Protection Agency 2007).
Leaching concentration (CL, mg L−1) was used to indicate the leaching toxicity of MSWI fly ash, U-MSWI fly ash, reconstructed slag, and cement composites. Fixation rate (RF) was used to indicate the content change of heavy metals, and leaching rate (RL) was used to indicate the leaching ratio of heavy metals:
where c0 and c1 (mg kg−1) are heavy metal content of U-MSWI fly ash-BF slag mixtures before and after melting, respectively ,m0 and m1 (kg) are the mass of U-MSWI fly ash-BF slag mixtures before and after melting, respectively, Cm(leaching) is the leaching content of heavy metals in unit mass samples, CM is the total content of heavy metals in unit mass samples.
Compressive strength test
Cement composites were prepared from water/binder with a mass ratio of 0.43~0.48 according to the cement standard consistency water consumption in the Chinese standard GB/T 1346-2001. The slurry was poured into a 40 mm × 40 mm × 40 mm cube model covered by the polythene sheet and left in the laboratory for 24 h. After then, the resulting material was demolded and then cured at 20 ± 2 °C and 95 ± 2% relative humidity prior for 3 and 28 days, respectively. The compressive strength of samples was measured in triplicate according to the Chinese standard GB/T 17671-1999.
Results and discussion
Characteristic of materials
The chemical compositions of BF slag, MSWI fly ash and U-MSWI fly ash are shown in Table 3. It can be obtained that SiO2, Al2O3, and CaO are the main components of BF slag, MSWI fly ash and U-MSWI fly ash. Wherein, MSWI fly ash and U-MSWI fly ash also contain a certain amount of ZnO, CuO, PbO, Cr2O3, and Cl.
Figure 2 shows the phase compositions of BF slag and MSWI fly ash. The X-ray diffraction pattern of BF slag shows an obviously broadened XRD pattern (a hump) between the 2θ of 25°and 35°, which is confirmed that the phase in the BF slag mainly exists in an amorphous form. From Fig. 2b, the phases in MSWI fly ash mainly contain quartz, anhydrite, calcite, and aluminosilicate, which reflect the resource utilization of MSWI fly ash, and there are also certain amount of NaCl and KCl. The X-ray diffraction pattern of U-MSWI fly ash is consistent with that of MSWI fly ash despite the addition of a certain proportion of heavy metals.
Heavy metal content and concentrations in MSWI fly ash and U-MSWI fly ash are given in Table 4; it is obtained that the leaching concentration of Zn, Cu, Pb, and Cr in MSWI fly ash did not exceeded the limit of Chinese standard GB/T 5085.3-2007, which may be due to the large-scale loss of heavy metal ions in MSWI fly ash caused by a long term stack. Correspondingly, the leaching concentrations of Zn, Cu, Pb, and Cr in U-MSWI fly ash all exceeded the limit of Chinese standard GB/T 5085.3-2007.
The melting features of U-MSWI fly ash-BF slag mixtures
The basicity (CaO/SiO2 weight ratio), initial melting temperature (T), and time (t) of U-MSWI fly ash-BF slag mixtures are shown in Table 1. The initial melting temperature and time of the samples were generally in accordance with a linear relationship, T = 10 t. This correlation between initial melting point and time was possibly due to a longer time was need when the mixture has a higher melting temperature. It can be obtained that the initial melting temperature (time) of mixtures increases from 1180 °C (118 s) to 1204 °C (145 s) by increasing the U-MSWI fly ash content from 0 to 30 wt%, and then decreases to 1170 °C (117 s) with further increasing to 100 wt%. This may be due to the formation of trace transition minerals with higher melting point when the basicity (CaO/SiO2 weight ratio) of mixtures was close to 1.0 (Li et al. 2004). Li et al. (2004) also pointed out the optimal immobilization effect of heavy metals was achieved when the basicity of mixtures was about 1.0 because the mixtures tended to form amorphous slag.
The reconstruction features of reconstructed slag
Microstructure analysis
Microstructures of the reconstructed slag (sample 0–8 were melted at the 1400 °C for 150 s) are presented in Fig. 3. Irregular block polyhedrons and small sheet particles with a particle size of less than 100 μm can be seen in all quenched slag samples. In general, small particles with sharp features and irregular angularity present in amorphous dispersion state (Yuan 1996). Compared with the original BF slag (sample 0), the reconstructed slag exhibited loose vitreous features and the particle size became larger (especially samples 5 and 6). Further observation indicates that the reconstructed slag possessed a certain number of interconnected pores, which demonstrates that some internal and surface defects were generated during water quenching process at high temperature. These characteristics in reconstructed slag may bring out the occurrence of hydration reaction after being activated by alkali, which is similar to BF slag. However, compared with BF slag, the level of potential hydration activity of reconstructed slag is not clear, and further testing is should be required through mechanical properties. Simultaneously, it is unable to determine whether there are specific changes of the structure of reconstructed slag samples compared with that of the original BF slag on morphology alone, so further analyses are needed. The specific changes of the reconstructed slag samples can be determined by the following XRD and FT-IR analysis.
Phase composition analysis
XRD patterns of the reconstructed slag samples are shown in Fig. 4. A large and broad hump was observed in the original BF slag (sample 0) and reconstructed slag (sample 1–8) , because after the U-MSWI fly ash with BF slag mixtures were melted at a high temperature and quenched into water, resulting in a change in the original crystal structure of U-MSWI fly ash to form an amorphous vitreous structure. No other obvious changes were found in these slag samples. The observation also suggests that the reconstructed slag possessed a certain hydration activity, which was similar to BF slag.
Chemical bond analysis
Figure 5 shows the FT-IR spectra of the original and reconstructed slag. The absorption valleys centered at 1628 (or 1630) cm−1, and 3429 (or 3435) cm−1 were respectively corresponding to bending and stretching vibration of water. Absorption valleys at 860–1175 cm−1 and around 700 cm−1 were ascribed to asymmetric silicon oxygen tetrahedron, aluminium oxygen tetrahedron, respectively. The strong and wide absorption peaks related to silicon oxygen tetrahedron of the slags indicates the different degree of polymerization (Wang et al. 2011). This difference in polymerization was caused by the fact that the slag comprises silicon oxygen tetrahedron monomer, dimer, and trimer. This observation suggests that the reconstructed slag could maintain a certain reactivity similar to the original BF slag, and can be used as construction materials. As shown in Fig. 5, compared with the original BF slag, the characteristic peaks at about 700 cm−1 related to the asymmetric aluminium oxygen tetrahedron of the reconstructed slags shift to higher wave numbers. This observation indicates that the adhesion strength of the aluminum of the reconstructed slag sample was higher.
The heavy metal leachability of reconstructed slag
The immobilization effect of heavy metal in reconstructed slag
Spot analyses with their related images for Zn, Cu, Pb, and Cr of reconstructed slag samples are shown in Fig. 6. The contents of Zn, Cu, Pb, and Cr in the slags were very low, probably due to the volatilization of these heavy metals during the melting process. EPMA of the samples indicates that the presence of relatively high amount of Cr than theirs Zn, Cu, and Pb contents, which also indicates that immobilization effect of Cr was probably much better than that of Zn, Cu, and Pb.
The heavy metal fixation rate and leaching concentration of reconstructed slag
Table 5 shows the heavy metal fixation rate and leaching concentration of reconstructed slag. The fixation rates of volatile heavy metals, i.e., Pb, Zn, and Cu were less than 51%, 27%, and 5%, respectively. The low fixation rates of Pb, Zn, and Cu were possibly due to the easy evaporation of these elements. Because of the high concentration of Cl in U-MSWI fly ash, the added heavy metal oxides or sulfates with high melting points formed low melting and boiling points chlorides (Xin et al. 2004). As a result, a large number of Zn, Cu, and Pb effectively partition into the vapor phase during melting at 1400 °C, leading to enhanced evaporation of Pb, Zn, and Cu. The influence of high Cl content on the volatilization of different heavy metals has been reported in previous work. It is found that high Cl content could effectively increase the volatilization ratio of Zn (Nowak et al. 2012). In comparison, the fixation rate of Cr is over > 93% after melting the U-MSWI fly ash-BF slag mixtures at 1400 °C. The results are in consistent with the conclusions from previous research (Chan et al. 2000; Chris and Donald 1999). Considering that Cr was a type of non-volatile heavy metal, and Cr-compounds in molten slag were CrCl3 (boiling point 1200–1500 °C) and Cr2O3 (melting point 2266 °C), the high fixation rates of Cr suggest that Cr was effectively fixed in the reconstructed slag.
Since the volatilization of heavy metals might increase the complexity of the flue gas purification process and make it more difficult to manage, the fixation rates of heavy metals should be raised to maximize the safety utilization of the reconstructed slag and resources recovery.
Samples melted in air atmosphere when the temperature reached 1400 °C, ZnO reacted with SiO2, and A12O3 formed non-volatile complexes easily, such as willemite (Zn2SiO4), and zinc aluminate gahnite (ZnAl2O4), et al, and then its volatilization rate decreased. Another explanation is that the existence of aluminosilicate suppressed the precipitation of volatile substances from the molten slag, which exerted a negative influence on the volatility of heavy metals (Hong et al. 2000). The fixation rate of Cr was very high. However, at melting temperature, Cr had low vapor pressure and very low volatilization rate. Thus, fixation rate was relatively high. Furthermore, the liquid phase in samples 1–7, which suppressed the volatilization of heavy metals from the molten slag, appeared earlier than that of sample 0 due to the fluxing action of Cu and Cr. Moreover, chemical duplex decomposition precipitation reactions occurred in microporosity of cured resin among heavy metal ions Zn2+, Cu2+, Pb2+, Cr3+, and OH- based on the alkaline condition offered by the reconstructed slag. At the same time, isomorphous replacement of heavy metal cations partial with Ca2+, Al3+, and Si4+ in layered silicate lattice also played a certain role in solidifying heavy metals. Heavy metals solidification effect of sample 5, with a basicity of 0.99, was the best, and the result is consistent with the conclusion of Li et al. (2004).
Although the fixation rates of Zn, Cu, Pb, and Cr were varied (Table 5), the leaching concentrations of Zn, Cu, Pb, and Cr from samples 1–5 were very low. The lowered leaching concentration of Zn, Cu, Pb, and Cr might be due to the substitution of Zn, Cu, Pb, and Cr with Ca2+ and Al3+ of silicate and subsequent dissolution in the reticular basal body during the melting process. Additionally, Si-O reticular structure was formed during melting; the heavy metals in the molten slag were encapsulated and solidified in the dense grid of glassed material. As a result, the heavy metal leaching concentrations of the reconstructed slag, especially Samples 1–5, were reduced.
All the heavy metal leaching concentrations of reconstructed slag samples 1–5 were below the corresponding limit defined in the Chinese standard GB/T 5085.3-2007. But the leaching concentrations of Cu in the reconstructed slag samples 6–8 were above the standard limit; the requirements of environmental emission of these reconstructed slags (samples 6–8) were not met. Hence, in order to ensure reconstructed slag can be safely landfilled, the content of U-MSWI fly ash in reconstructed slag should be less than 50 wt%.
Solidification and utilization of reconstructed slag in cement
As discussed in the above results, the reconstructed slag had certain resource utilization characteristics because its potential hydration activity was similar to BF slag. However, when the content of U-MSWI fly ash in reconstructed slag exceeded 50 wt%, the leaching concentration of heavy metals could not meet the requirement of GB/T 5085.3–2007, which limits the treatment and application of reconstructed slag. In order to comply with the safe disposal requirement and make use of its resource utilization characteristics, reconstructed slag needs to be further treated. Cement has several advantages in solidifying heavy metals and using pozzolanic materials (Bie et al. 2016; Lin and Lin 2006), so reconstructed slag can be further solidified and used in cement. Therefore, the compressive strength and leaching concentration of cement composites with reconstructed slag were investigated in this experiment, and the designed scheme is shown in Table 2.
Compressive strength of cement composites with reconstructed slag
In order to investigate the effect of U-MSWI fly ash content on the utilization of reconstructed slag in cement composites, reconstructed slags with different content of U-MSWI fly ash were admixed into cement as partial replacement of OPC, respectively (whose scheme is shown in Table 2), and then the mechanical properties of cement composites generated were studied. Figure 7 show the compressive strength of cement composite samples after curing for 3 and 28 days. These samples exhibited a compressive strength between 5.8 and 45.5 MPa. Compared with pure OPC (sample F0), the strength properties of cement composites with reconstructed slag added became worse, mainly because in the liquid phase environment of cement hydration; the potential pozzolanic properties of reconstructed slag could not be activated well.
For cement composites with reconstructed slag added, the increasing of U-MSWI fly ash content (from 0 to 100 wt%) generally reduced compressive strength: the 3-day and 28-day compressive strength decreased from 16.0 and 40.8 to 5.8 and 18.5 MPa, respectively. This possibly happened due to with the increase of U-MSWI fly ash content, the pozzolanic properties of reconstructed slag decreased, and the hydration rate of cement composites was reduced, less hydration products of Ca(OH)2 were generated and less compounds in reconstructed slag could be activated by alkali to participate in the hydration reaction during the curing of cement composites, thus the compressive strength of cement composites with reconstructed slag added gradually decreased with the increasing of U-MSWI fly ash content in the reconstructed slag. In addition, the chlorides in U-MSWI fly ash can also cause volume expansion problems (Chen et al. 2019), but this phenomenon was not observed in this experiment, which is mainly related to the volatilization and solidification of chlorides in the melt reconstruction process of U-MSWI fly ash. Moreover, when the content of U-MSWI fly ash was increased to 80 wt% and 100 wt% (samples F5 and F6), the compressive strength drastically decreased to 8.8–24.2 MPa and 5.8–18.5 MPa, respectively. It is mainly also due to the retarding effect of heavy metals (He et al. 2006; Garcia-Lodeiro et al. 2016). With the large increase of U-MSWI fly ash content, the concentration of leached heavy metals in the reconstructed slag increased sharply (see Table 6), which is more detrimental to the hydration and solidification of cement composites, resulting in a worse mechanical properties. However, in general, admixing reconstructed slags into cement could make the samples obtain a certain compressive strength and more compact, and it is confirmed that reconstructed slag can be applied to landfill and construction materials by the further solidification of cement.
Leaching concentrations of heavy metals in cement composites with reconstructed slag
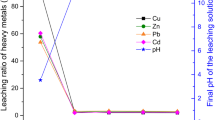
Table 6 shows the heavy metal leaching concentration of cement composites with reconstructed slag curing 3 days and 28 days. With the prolonging of curing time, the leaching concentration of heavy metals in all cement composite samples with reconstructed slag decreased significantly. It indicates that during the hydration reaction of cement slurry, heavy metals could be stabilized in the hydration products or participate in the hydration reaction to form new hydration products (Mangialardi et al. 1999; Bie et al. 2016). Therefore, the leaching concentration of heavy metals tended to decrease significantly. However, with the increase of U-MSWI fly ash content in reconstructed slag, the leaching concentration of heavy metals in cement composites with reconstructed slag increased, especially when the content of U-MSWI fly ash was increased to 80 wt% and 100 wt% (samples F5 and F6), the leaching concentration of Cu and Cr increased sharply. Which is due to the high leaching concentration of Cu and Cr in reconstructed slag with 80 wt% and 100 wt% U-MSWI fly ash added (samples 7 and 8) and the limited ability of cement to stabilize heavy metals. However, compared with the single melting reconstruction treatment process (see Table 5), the leaching concentrations of heavy metals after melting reconstruction treatment with cement solidification (Samples F2–F6) were all far below the corresponding limit defined in the Chinese standard GB/T 5085.3-2007 and meet the environmental requirement.
Analysis of U-MSWI fly ash treatment cost
In the experiment of this paper, the treatment process of U-MSWI fly ash mainly includes: raw materials preparation, high temperature melting treatment, mechanical grinding treatment, and cement solidification. The cost of U-MSWI fly ash in laboratory and industrial treatment processes are compared in Table 7. The treatment cost of U-MSWI fly ash was calculated by the formula:
The treatment cost of U-MSWI fly ash per unit ($/t) = unit cost of raw materials ($/t) × usage amount (t) + unit cost of energy consumption ($/kWh) × electricity consumption (kWh).
Among the raw materials used in this experiment, the price of BF slag is $ 43 per ton, and P.O42.5 Portland cement is $ 65 per ton. Energy consumption price is $ 0.17 per kWh.
In the laboratory treatment process of U-MSWI fly ash, the purchase of BF slag and the high temperature melting treatment process were charged to the cost. Wherein, when U-MSWI fly ash was treated by the single melting reconstruction treatment without cement solidification, according to the content of BF slag added (≥ 50 wt%) in reconstructed slag limited by the leaching concentration of heavy metals, melting temperature, and time, the lower treatment cost of U-MSWI fly ash per unit is $ 623–815. When U-MSWI fly ash was treated by the melting reconstruction treatment with cement solidification, the energy consumption of the mechanical grinding of reconstructed slag and the cost of cement needed to be calculated, but the usage amount of BF slag could be greatly reduced; therefore, the lower treatment cost of U-MSWI fly ash per unit is $ 485–526.
In the industrial treatment process of U-MSWI fly ash, the high temperature molten BF slag that has just been fired in the iron-making process could be directly used as the free melting reconstruction carrier of U-MSWI fly ash. This can not only make full use of the excess energy in industrial output, but also greatly reduce the cost of U-MSWI fly ash melt reconstruction. During the single melting reconstruction treatment without cement solidification, the energy consumption of U-MSWI fly ash dumped into the molten BF slag was only needed to be calculated, and the lower treatment cost of U-MSWI fly ash per unit is $ 16–28. When U-MSWI fly ash was treated by the melting reconstruction treatment with cement solidification process, the energy consumption of the mechanical grinding of reconstructed slag and the cost of cement are needed to be calculated, and the lower treatment cost of U-MSWI fly ash per unit is $ 53–65.
The laboratory treatment process of U-MSWI fly ash is mainly used for theoretical research, while the actual safety treatment of a large amount of U-MSWI fly ash needs to rely on industrial treatment process, and the industrial treatment process according to this experimental method can provide significant economic benefits for the treatment and recycling of U-MSWI fly ash. In addition, comparing the two methods (melting reconstruction treatment with and without cement solidification) based on the industrial treatment process, the cost of melting reconstruction treatment without cement solidification method was lower, but this method has limited ability to solidify heavy metals in U-MSWI fly ash. However, the addition of cement can not only improve the ability to solidify heavy metals in U-MSWI fly ash, but also give full play to the resource characteristics of reconstructed slag as cement admixture. Therefore, comprehensively considering the above factors, the melting reconstruction treatment with cement solidification method provides a technical-economical possibility for industrial treatment of U-MSWI fly ash, and can improve the safety of products as a landfill or construction material. Moreover, as shown in Table 7, compared with other cement admixtures such as slag and fly ash, the price of reconstructed slag produced in this experiment is relatively economical, so it has certain production and utilization value for construction.
## 1: melting reconstruction treatment without cement solidification method
## 2: melting reconstruction treatment with cement solidification method
Conclusions
Blast furnace (BF) slag in high temperature molten state from iron-making process can be used as a free high-temperature carrier to melt U-MSWI fly ash. The melting reconstruction features and heavy metals solidification behaviors of U-MSWI fly ash-BF slag mixtures were investigated according to phase composition, microstructure and heavy metals leachability. Moreover, the further solidification and utilization of reconstructed slag in cement were also studied. The conclusions can be drawn as following:
-
1.
The main chemical compositions of U-MSWI fly ash are SiO2 (22.39%), Al2O3 (10.68%), CaO (20.18%), and Cl (6.81%), and the major phases are SiO2, CaSO4, CaCO3, NaCl, KCl, and aluminosilicate. The leaching concentration of Zn, Cu, Pb, and Cr is 1686 mg/L, 321.85 mg/L, 10.17 mg/L, and 29.96 mg/L, respectively.
-
2.
The reconstructed slag formed by melting U-MSWI fly ash and BF slag, has a certain potential hydration activity similar to BF slag. However, when the content of U-MSWI fly ash exceeds 50 wt%, the leaching concentration of heavy metals in reconstructed slag cannot meet the environment requirement. Therefore, for the further solidification and utilization of reconstructed slag, the cement solidification process is strongly suggested.
-
3.
Adding cement to reconstructed slag can generate hydration products, which can further solidify heavy metals and ensure the safe treatment and application of reconstructed slag. The method of melting reconstruction treatment with cement solidification can provide a technical-economical possibility for industrial treatment of U-MSWI fly ash, and can improve the safety of products as a landfill or construction material.
Further work for studying the durability of the products generated by melting reconstruction treatment with cement solidification treatment of U-MSWI fly ash should be done, and the use of reconstructed slag to prepare geopolymers, and other further solidification and utilization methods should be also investigated.
References
Alhadj-Mallah M-M, Huang Q, Cai X, Chi Y, Yan J (2015) Vitrification of municipal solid waste incineration fly ash using biomass ash as additives. Environ Technol 36(5):654–660
Aubert JE, Husson B, Sarramone N (2007) Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 2: mechanical strength of mortars and environmental impact. J Hazard Mater 146(1-2):12–19
Bie R, Chen P, Song X, Ji X (2016) Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J Energy Inst 89:704–712
Chan CCY, Kirk DW, Marsh H (2000) The behaviour of Al in MSW incinerator fly ash during thermal treatment. J Hazard Mater 76(1):103–111
Chen ZL, Lu SY, Tang MH, Ding JM, Buekens A, Yang J, Qiu QL, Yan JH (2019) Mechanical activation of fly ash from MSWI for utilization in cementitious materials. Waste Manag 88:182–190
Chris CY, Donald WK (1999) Behavior of metals under the conditions of roasting MSW incinerator fly ash with chlorinating agents. J Hazard Mater B64(1):75–89
Funari V, Mäkinen J, Salminen J, Braga R, Dinelli E, Revitzer H (2017) Metal removal from municipal solid waste incineration fly ash: a comparison between chemical leaching and bioleaching. Waste Manag 60:397–406
Garcia-Lodeiro I, Carcelen-Taboada V, Fernández-Jiménez A, Palomo A (2016) Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Constr Build Mater 105:218–226
Guo X, Hu W, Shi H (2014) Microstructure and self-solidification/stabilization (S/S) of heavy metals of nano-modified CFA–MSWIFA composite geopolymers. Constr Build Mater 56:81–86
He X, Hou H, Zhang D (2006) Study on cement solidification of municipal solid waste incineration fly ash. Environ Poll Control 28:425–428
Hong KJ, Tokunaga S, Kajiuchi T (2000) Extraction of heavy metals from MSWI incinerator fly ashes by chelating agents. J Hazard Mater 75(1):57–73
Huber F, Herzel H, Adam C, Mallow O, Blasenbauer D, Fellner J (2018b) Combined disc pelletisation and thermal treatment ofMSWI fly ash. Waste Manag 73:381–391
Huber F, Laner D, Fellner J (2018a) Comparative life cycle assessment of MSWI fly ash treatment and disposal. Waste Manag 73:392–403
Jiang Y, Xi B, Li X, Zhang L, Wei Z (2009) Effect of water-extraction on characteristics of melting and solidification of fly ash from municipal solid waste incinerator. J Hazard Mater 161:871–877
Lederer J, Trinkel V, Fellner J (2017) Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: the case of Austria. Waste Manag 60:247–258
Li RD, Nie YF, Wang L et al (2004) Heavy metal migration during vitrifciation of municipal solid waste incinerator fly ash. J Tsinghua Univ (Sci and Technol) 44(9):1180–1183
Lin KL, Lin DF (2006) Hydration characteristics of municipal solid waste incinerator bottom ash slag as a pozzolanic material for use in cement. Cem Concr Compos 28(9):817–823
Loginova E, Proskurnin M, HJH B (2019) Municipal solid waste incineration (MSWI) fly ash composition analysis: a case study of combined chelatant-based washing treatment efficiency. J Environ Manag 235:480–488
Luo H, Cheng Y, He D, Yang E (2019) Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ 668:90–103
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: a review. J Hazard Mater 137(1):267–276
Mangialardi T, Paolini AE, Polettini A, Sirini P (1999) Optimization of the solidification/stabilization process of MSW fly ash in cementitious matrices. J Hazard Mater 70:53–70
MEE, 2016. National Catalogue of Hazardous Waste, Ministry of Ecology and Environment of the People's Republich of China
National Environmental Protection Agency (2007) GB5085.3-2007, Identification standard of hazardous waste: identification of leaching toxicity. Standards Press of China, Beijing
Ni G, Zhao P, Jiang Y, Meng Y (2012) Vitrification of MSWI fly ash by thermal plasma melting and fate of heavy metals. Plasma Sci Technol 14(9):813–818
Nowak B, Sandra FR, Aschenbrenner P, Rechberger H, Winter F (2012) Heavy metal removal from msw fly ash by means of chlorination and thermal treatment: influence of the chloride type. Chem Eng J 179(1):178–185
Pedersen AJ, Ottosen LM, Villumsen A (2005) Electrodialytic removal of heavy metals from municipal solid waste incineration fly ash using ammonium citrate as assisting agent. J Hazard Mater 122(1):103–109
Phua Z, Giannis A, Dong Z, Lisak G, Ng W (2019) Characteristics of incineration ash for sustainable treatment and reutilization. Environ Sci Pollut Res 26:16974–16997
Shi D, Hu C, Zhang J, Li P, Zhang C, Wang X, Ma H (2017a) Siliconaluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration. Fuel Process Technol 165:44–53
Shi D, Zhang J, Zhang C, Hu C, Li P (2017b) Seed-induced hydrothermal synthesis of tobermorite from municipal solid waste incinerator fly ash. J Residuals Sci Technol 14(S1):11–19
Sun Y, Zheng J, Zou L, Liu Q, Zhu P, Qian G (2011) Reducing volatilization of heavy metals in phosphate-pretreated municipal solid waste incineration fly ash by forming pyromorphite-like minerals. Waste Manag 31:325–330
Todorovic J, Ecke H (2006) Demobilisation of critical contaminants in four typical waste-to-energy ashes by carbonation. Waste Manag 26(4):430–441
US Environmental Protection Agency (1986) Method 1311 SW-846. The method for evaluation solid waste, physical/ chemical methods. Governmental Printing Office, Washington
Wang L, He Z, Zhang B, Cai XH (2011) Polymerization mechanism of C-S-H: identified by FTIR and NMR. J Build Mater 14(4):447–451
Wang Q, Yang J, Wang Q, Wu T (2009) Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. J Hazard Mater 162(2):812–818
Wey MY, Liu KY, Tsai TH, Chou JT (2006) Thermal treatment of the fly ash from municipal solid waste incinerator with rotary kiln. J Hazard Mater 137(2):981–989
Xie HQ, Li RQ, Wang ZY, Yao X, Yu QB (2019) Hydrogen production of bio-oil steam reforming combining heat recovery of blast furnace slag: thermodynamic analysis. Int J Hydrog Energy 44:25514–25523
Xin LJ, Huan YJ, Qi JY, Jiang NM (2004) Characteristic analysis of heavy metals in MSWI fly ash. J Zhejiang Univ 38(4):490–494 (in Chinese)
Yang Z, Ji R, Liu L, Wang X, Zhang Z (2018) Recycling of municipal solid waste incineration by-product for cement composites preparation. Constr Build Mater 162:794–801
Youcai Z, Lijie S, Guojian L (2002) Chemical stabilization of MSW incinerator fly ashes. J Hazard Mater 95(1):47–63
Yuan RZ (1996) Glue material science. Wuhan University of Technology, Wuhan
Zhan X, Wang L, Hu C, Gong J, Xu T, Li J, Yang L, Bai J, Zhong S (2018) Co-disposal of MSWI fly ash and electrolytic manganese residue based on geopolymeric system. Waste Manag 82:62–70
Funding
The financial supports provided by the National Natural Science Foundation of China (No.51202222) and the Open Project Foundation of State Key Laboratory of Solid Waste Reuse for Building Materials (No.SWR-2013-002) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Z., Chen, L., Zhang, M. et al. Analysis of melting reconstruction treatment and cement solidification on ultra-risk municipal solid waste incinerator fly ash–blast furnace slag mixtures. Environ Sci Pollut Res 27, 32139–32151 (2020). https://doi.org/10.1007/s11356-020-09395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09395-8