Abstract
Municipal solid waste incineration (MSWI) generates bottom ash, fly ash (FA), and air pollution control (APC) residues as by-products. FA and APC residues are considered hazardous due to the presence of soluble salts and a high concentration of heavy metals, and they should be appropriately treated before disposal. Physicochemical characterization using inductively coupled plasma mass spectroscopy (ICP-MS), X-ray diffraction (XRD), and X-ray fluorescence (XRF) have shown that FA and APC have potential for reuse after treatment as these contain CaO, SiO2, and Al2O3. Studies conducted on treatment of FA and APC are categorized into three groups: (i) separation processes, (ii) solidification/stabilization (S/S) processes, and (iii) thermal processes. Separation processes such as washing, leaching, and electrochemical treatment improve the quality and homogeneity of the ash. S/S processes such as chemical stabilization, accelerate carbonation, and cement solidification modify hazardous species into less toxic constituents. Thermal processes such as sintering, vitrification, and melting are effective at reducing volume and producing a more stable product. In this review paper, the treatment processes are analyzed in relation to ash characteristics. Issues concerning mixing FA and APC residues before treatment, true treatment costs, and challenges are also discussed to provide further insights on the implications and possibilities of utilizing FA and APC as secondary materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste incineration (MSWI) is a common waste management technique in many countries due to its capacity for high volume reduction and possibility of energy recovery (Kothari et al. 2010; Leckner 2015). However, the resulting bottom ashes and flue gas contain heavy metals and other hazardous pollutants, and controlling their emission is necessary. Typically, regulatory limits on incinerator emissions are strict, leading to many developments in air pollution control systems (Liu et al. 2015).
With the development of efficient air pollution control systems, the focus has now shifted from emission control to the treatment and disposal of fly ash (FA) and air pollution control (APC) residues collected by these systems. The fate of FA and APC residues depends principally on national policies. In most developed countries, treatment and reuse of these residues is possible because of environmental policies and possibly subsidies (Huang and Chuieh 2015), while in other countries, these ash residues are buried in landfills (Sun et al. 2016).
Despite the relatively high concentration of heavy metals, salts, and organic recalcitrant, which may limit utilization possibilities, the best strategy is still treatment followed by utilization as secondary resources. Proper characterization has to be conducted before treatment as the properties of these residues may differ greatly depending on the source of the municipal solid waste (MSW) (Quina et al. 2008a, 2008b; Ahmaruzzaman 2010; Fruergaard et al. 2010). Although extraction procedures are used to provide heavy metal speciation and leachability from metal-bearing ash particles, there are many arguments about their suitability in characterization of metal phases. Therefore, comprehensive characterization by analytical techniques has been implemented to reveal both qualitative and quantitative characterizations of FA and APC residues.
In a modern MSWI plant, there is the combustion chamber where the waste is burnt for volume reduction, the boiler system to recover energy, and the air pollution control system to reduce emissions. The air pollution control system removes fine particulates (with devices such as the electrostatic precipitator, fabric filter, or cyclone) and regulates harmful gases (with devices such as dry lime scrubbing or wet lime scrubbing).
It should be noted that different residues are generated at various stages in the MSWI plant (Fig. 1). In this review, only fly ash and APC residues collected at the air pollution control systems are considered. The definition of fly ash by the International Ash Working Group is “the particulate matter carried over from the combustion chamber and removed from the flue gas stream prior to addition of any type of sorbent material” (Chandler et al. 1997). Therefore, in this review, the ashes collected prior to the scrubbing process are classified as fly ash, while those generated during the scrubbing process are termed as air pollution control residues. In some incineration plants, there was no prior removal of the FA before the scrubbing process, and both FA and APC residues were collected together, so it is considered as a form of APC in this review paper by definition. It is important to classify the FA and APC residues properly as these have different properties, and inappropriate use of terms could cause misinterpretation (Quina et al. 2008a, 2008b). In addition, available research based on mixed APC and FA is not taken into account due to the large variance in the reported data.
Due to the presence of a substantial amount of chlorine in MSW, the utilization of FA and APC residues is difficult due to the accumulation of metal chlorides (Joseph et al. 2018). Despite the difficulties, many countries with land constraints have put in effort to reutilize FA and APC residues. With a combination of well-developed standards, strong enforcement of environmental regulations, and government support, most FA and APC residues can be reused as secondary raw materials in cement industry, in road pavement and soil amendment, and as a sludge-conditioning agent (Ahmaruzzaman 2010).
In case utilization standards are unavailable, countries have avoided the use of FA and APC residues but have instead enforced the treatment of these FA and APC residues before disposal into landfills. In less-developed countries without strict enforcement or disposal regulations, the FA and APC residues are usually mixed with bottom ash for dilution before disposal into a landfill in order to save cost.
The best management practice of FA and APC residues is no doubt reutilization followed by treatment before disposal. These materials should never be disposed without treatment. In addition, as MSWI is becoming a prevailing method for waste management, the amount of MSWI FA and APC residues is expected to increase. Therefore, there is a need for proper treatment so that these materials can be utilized as a resource instead of being wasted.
In this review paper, different treatment processes for FA and APC residues are evaluated in order to reutilize these ashes safely and economically. Due to the importance of proper characterization, this paper also reviewed the common characterization methods used for FA and APC. In addition, the paper provides new insights with regard to mixing FA and APC, true treatment cost, and treatment challenges.
Physicochemical characterization of FA and APC residues
The physicochemical properties of FA and APC residues are affected by factors such as MSW feedstock, incinerator design, and type of air pollution control units (Song et al. 2004; Moon et al. 2016). Therefore, characterization is important in order to determine the best strategy for subsequent ash management (Quina et al. 2008a, 2008b).
Physical properties such as particle size distribution, permeability, moisture content, and density generally affect heavy metal leaching and determine the environmental impacts after disposal (Fig. 2). The particle size distribution which is estimated by sieving is one of the most important physical properties as ash with smaller particle size tends to retain a higher concentration of heavy metals (Wan et al. 2018; Shi and Kan 2009) due to the larger surface area for condensation of volatile heavy metals. Other than dry sieving, the laser diffraction size analyzer has also been used to measure the size of particles in FA and APC residues (Aubert et al. 2006). Density is another important physical property of FA as this affects the uniformity and porosity of the ash particles (Chandler et al. 1997).
FA is generally very heterogeneous in nature. The total elemental composition via acid digestion followed by inductively coupled plasma mass spectroscopy (ICP-MS) is one of the most documented methods used to identify the amount of each element in the FA (Chang et al. 2009). In addition, other than elemental composition, the mineralogy and types of oxides affect the efficacy of treatment methods. Mineralogy and oxide phases have been studied using techniques such as X-ray diffraction (XRD) and X-ray fluorescence (XRF) (Wan et al. 2006; Haiying et al. 2010).
Other than inorganic elements, municipal solid waste incineration ash also contains toxic organic pollutants. Chlorinated organic compounds such as chlorobenzene, polychlorinated biphenyls (PCBs), polychlorinated dibenzodioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs) have been detected (Tabata et al. 2013). The presence of various forms of organic and inorganic chlorides results in the formation of PCDDs and PCDFs during the cooling process of the flue gas at around 450 °C (Kawabata et al. 2003). The toxicity of different PCDDs and PCDFs varies, and it is evaluated by their toxicity equivalence quantity (TEQ).
Particle size comparison
The particle size of FA and APC residues is in the micron scale. Size is influenced by the MSW feedstock and location that the particulates are collected. The average size in terms of % volume passing D50 of FA and APC is between 10–166.41 μm and 0.5–48.71 μm, respectively (Table 1). Other information such as the D10 and D90 indicating the smallest 10% and largest 10% fraction can also be used to illustrate the size distribution. Figure 3 shows the typical particle size distribution of FA and APC. FA is, in general, larger in size than APC and has a much larger spread in terms of size variation due to the difference in the point of collection and nature heterogeneity.
The particle size of FA is largely dependent on operating conditions of the incinerator such as flue gas velocity and temperature in the heat recovery system (boiler). In addition, among the different types of FA, the more downstream the point of collection is, the smaller the particle sizes are, as the larger FA particles are captured by the upstream systems (boiler, economizer).
The main constituent of APC is the lime additive added to neutralize the acidic flue gases. Therefore, the variations in particle size between different studies are due to the different types of scrubbing processes employed. In general, APC particles are smaller than their FA counterpart as most of the larger particulate matters are already being collected prior to the scrubbing process.
Comparison of elemental composition
The elemental composition of FA and APC is often studied by digesting the samples and followed by ICP-MS and/or inductively coupled plasma optical emission spectroscopy (ICP-OES). The outcome is classified as major elements (> 10,000 mg/kg), minor elements (< 10,000 mg/kg and > 1000 mg/kg), and trace elements (< 1000 mg/kg).
In Table 2, it can be seen that APC residues comprise mainly Ca, Cl, Si, and Al, while other major elements include K, Na, and S. The minor elements are usually Ba, Cu, Mn, Zn, Pb, Fe, and Mg. Trace elements include Hg, Ni, Cr, Ag, and As. Despite the trace elements are being a relatively small component, they have been studied extensively due to their high toxicity.
Although FA also consists of Ca, Cl, Si, and Al, these are in very different proportions compared to APC residues (Table 3). APC contains much higher Ca and Cl content, while FA has higher Si and Al. For air pollution control (scrubber unit), lime is added to the gas stream to neutralize the acidic gases such as HCl to form CaCl2. Another difference is that Mg, Ti, and Fe are considered major elements in FA, but minor in APC. This difference is due to the condensation or solidification of the less volatile metals and metal chlorides onto the FA during heat recovery where temperature is much lower than that in the combustion chamber. The amount of trace elements is relatively consistent in both APC and FA in most studies except for Hg. Since Hg has a relatively low boiling point, it exists in the gaseous state in the flue gas until finally captured in the APC unit.
Both APC and FA also contain a significant amount of Zn and Pb, which can easily leach out and harm the environment (if not managed properly). However, the total elemental composition is not fully representative of the actual leaching potential of each heavy metal due to the mineral speciation and other factors. Therefore, other characterizations such as XRF and XRD can provide more insights to the ecotoxicity of both APC and FA.
X-ray fluorescence
XRF is used to determine the elemental oxide composition in FA and APC residues, especially in preparation for ceramic and concrete applications. Although there are variations in the oxide composition reported by different studies, there is agreement that both FA and APC residues have high alkali content in the form of CaO.
In Table 4, it can be seen that APC contains a large amount of CaO (as high as 58.65%) and other oxides such as SiO2, Al2O3, Na2O, K2O, and SO3. High alkalinity is detected due to the calcium hydroxide added in excess to remove the acidic flue gases. Consequently, the pH and acid-neutralizing capacity (ANC) are usually very high. With pH around 12, APC residues can cause problems such as leaching of amphoteric metals (Zn and Pb), while the high ANC results in difficulty in lowering the pH to reduce leaching effect.
On the other hand, although FA contains a significant amount of alkaline oxides such as CaO, it has also a high amount of acidic SiO2 (Table 4). Because of that, there are differences in the chemical properties between FA and APC residues in terms of pH and ANC. Normally, FA has enough alkaline material to establish an alkaline pH of around 10 to 11 in water, but with low ANC. In some extreme cases that the alkalinity is not enough, FA results in a neutral or even slightly acidic pH.
Figure 4 presents a ternary diagram of CaO, Al2O3, and SiO2 for FA and APC as compared to ordinary Portland cement (OPC) and coal fly ash which are materials commonly used for construction. APC has composition very close to that of limestone and OPC since the major component is the lime additive, while FA also consists of relatively high Ca content. Some of the APC residues are, in fact, a mixture of FA and scrubbing residues due to the lack of prior filtering process; therefore, some of the APC samples tend to exhibit compositions relatively similar to FA.
X-ray powder diffraction
The mineral phases in FA and APC have also been studied using XRD. XRD analysis measures the intensity of different crystal peaks from each unique compound to estimate relative amounts within the sample. However, both FA and APC contain many different phases, which can cause overlapping and difficulties in identifying the phases. This problem is usually overcome by the use of software programs to simplify the matching process.
Due to the nature of FA and APC, XRD only provides information on the different types of minerals found without proper quantification (Tian et al. 2018). In Table 5, it can be seen that the main minerals in APC are CaClOH, NaCl, KCl, CaSO4, CaCO3, and Ca(OH)2, while those in FA consisted of many different heterogeneous phases such as calcium aluminosilicates, SiO2, and other more complex metallic compounds. In comparison, the composition of APC is more consistent than that of FA. A large proportion of APC is the reaction product from the acidic flue gas and the lime injected, while the content of FA consists of many different entrained metallic silicate particles carried over from the combustion chamber.
Treatment technologies
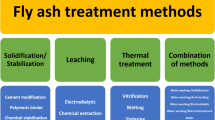
Due to the hazardous nature of FA and APC residues, treatment is required before their disposal into a landfill or reutilization. Currently, most of the treatment technologies are focused on reducing the environmental impacts, e.g., by separation to reduce the total pollutant concentration or stabilization to control the rate of leaching. The treatments are classified into three main groups: (i) separation processes, (ii) solidification/stabilization (S/S) processes, and (iii) thermal processes. Figure 5 presents the treatment process roadmap for FA and APC residues.
Depending on various factors such as disposal regulations, potential for utilization, and cost of treatment, different treatment strategies are adopted. Despite the variations in details, treatment typically comprises separation (e.g., washing) (Lam et al. 2010) to reduce the soluble fraction followed by either thermal treatment (Chou et al. 2009a, 2009b) or S/S (Mangialardi 2003; Zheng et al. 2011). A summary of such treatments has been compiled by Van der Sloot et al. (2001), while Sakai et al. (1996) have emphasized the importance of treatment cost.
In the assessment of the most appropriate treatment methods, both technical and economic factors are considered. Technical considerations include treatment outcomes and scaling up before proceeding to economic feasibility. The latter includes the operational cost and the value and amount of the final products generated. For instance, the costly thermal treatment usually generates products that can be utilized to reduce the overall cost, while S/S usually generates a larger volume of waste that has to be constantly monitored in a hazardous landfill and can increase the overall cost of treatment. Therefore, a complete analysis in terms of both technical and economic factors should be taken into consideration with the local context kept in sight.
Separation
Separation methods make use of the different properties in the ash components to separate them using water washing, solvent leaching, thermal evaporation, and electrodialysis (Table 6). The main objective of these methods is to improve the quality and homogeneity of the product for further treatment or utilization, but some methods can also extract valuable metals (De Boom and Degrez 2015; Nagib and Inoue 2000).
Washing processes
The washing process exploits the differences in solubility of salts in water. Water washing is commonly used as a pretreatment step prior to other treatment methods as the high salt content might be detrimental to the stability and quality of the final product (Mangialardi 2003; Chimenos et al. 2005; Chen et al. 2017). In the literature, various conditions such as the liquid-to-solid (L/S) ratio and duration have been studied and it is found that water washing can remove up to 90–95% of the chlorides and the final product is improved in quality for further utilization (Chen et al. 2016). Mangialardi (2003) recommended an L/S ratio of 25 with a 15-min duration, while Wang et al. (2009) used only an L/S ratio of 2 with a 1-h duration to reduce the amount of effluent.
However, some researchers have noted that the washing effluent contains a high content of heavy metals (Pb and Zn) (Wang et al. 2001) and possibly high pH (pH 12). Chimenos et al. (2005) varied the L/S ratio of 1 to 10 using a two-step washing process and found that the optimal L/S ratio is around 3. They also found that the use of MgSO4 to reduce the pH from 12.5 to 11 can reduce the leaching of Pb and Zn. Others have suggested chemical additives such as phosphate to reduce the solubility of heavy metals (Vavva et al. 2017; Ecke 2003; Shi and Kan 2009).
The extraction efficiency and leaching of undesirable metals are highly dependent on the pH of the ash, the L/S ratio, and the metal of concern. FA with pH lower than 11 and low alkalinity is suitable for water washing as a pretreatment step since the leaching of harmful amphoteric metals is limited, while for APC, the pH is too high and more precautions have to be taken due to the possibility of Pb, Zn, and Cr leaching into the effluent.
The selective recovery of heavy metals and salts based on particle size before washing has also been demonstrated (Funari et al. 2017); however, their recovery can only be beneficial of subsequent use. Another approach towards washing is the development of better agitation equipment (Ko et al. 2013) or recirculation of wastewater in various sequential washing steps to reduce the amount of wastewater generated (Chimenos et al. 2005).
Leaching
The leaching process aims to extract heavy metals from ashes via the use of an aggressive leaching agent. Both acidic and alkaline leaching agents for FA are proven to be effective in improving the extraction of heavy metals, while alkaline leaching agents are more suitable for APC residues because of the high acid-neutralizing capacity.
Nagib and Inoue (2000) applied NaOH and HCl leaching on FA at high temperature (90 °C) and long duration (up to 100 min) and found that it increases the extraction of Pb (45.7%) and Zn (29%), while Chiang et al. (2008) managed to extract 89% Cd, 60% Zn, and 32.5% Cu from APC by using a high L/S ratio of 40 and a duration of 2 h with 1 N HCl. In other studies, multiple leaching steps and different chelating agents have been deployed (Jiao et al. 2016), while Van der Bruggen et al. (1998) showed that Pb leaching could be enhanced by the use of EDTA and predicted the leaching by simulation using MINTEQ.
Other than physicochemical leaching, researchers have also explored the potential in making use of bacteria to enhance the leaching process of FA (Yang et al. 2009a; Fedje et al. 2010). Wang et al. (2009) used Aspergillus niger with 4% (w/v) FA in 100 mL sucrose to extract up to 96% Cd, 91% Mn, 73% Pb, 68% Zn, 35% Cr, and 30% Fe.
Despite the continuous growth in the use of bioleaching as an environmentally friendly option, chemical leaching is still more efficient and consistent in selective leaching and stabilization of heavy metals in FA and APC (Funari et al. 2017). Commercially available chemicals such as LIX860M-I and Cyanex 923 are found to have high selectivity and low extractability of other metals, while the extraction efficiency can be further enhanced with dilution in organic solvent such as kerosene (Tang and Steenari 2015).
Electrochemical processes
The electrochemical process applies a potential difference to separate and recover the heavy metals via redox reactions at the interface of the electrodes. The advantage of this method is that no additional chemicals are required and no residues are generated. Jensen et al. (2015) have investigated the potential of electrodialysis on three different types of fly ash. However, a major limitation is the low efficiency when a concentration of the metals becomes low. To improve the low efficiencies, Viader et al. (2017) have tried electrodialytic separation assisted by cationic membranes and achieved good removal efficiencies for Cr, Cu, Ni, Pb, and Zn. In another study, pretreatment steps (water washing and sieving) were used to improve the electrodialytic process (Chen et al. 2018).
Jensen et al. (2010) worked on the electrochemical extraction of APC and showed that the conductivity of the suspension decreases from 52 mS/cm to between 1.7 and 5 mS/cm indicating the removal of most charged species, while Kirkelund et al. (2010) also proved that despite negligible change in the pH after treatment, most metals in the final product meet the requirement except for Pb and Zn. Important operating parameters include the current density, pH, distance between electrodes, and dosage of assisting agents.
In another study, ultrasound-enhanced electrokinetic remediation was applied for the removal of Zn, Pb, Cu, and Cd from FA. It was demonstrated the synergetic effects of acidification and ultrasonication for enhancing the remedial efficiencies of heavy metals in the further electrokinetic technology. It was also demonstrated that the combined system reduced the environmental toxicity for the FA to the maximum extent (Huang et al. 2018).
Thermal evaporation
Thermal evaporation is a separation process that makes use of high temperature to separate volatile metals from the bulk ash (Yu et al. 2012). The bulk ash is heated to a certain temperature to vaporize volatile metals (Pb and Cd), and these are then recovered through condensation. Hg, which has low boiling point, is not condensed but remains in the gas phase, while other less volatile metals remain in the solid phase.
Important operating factors reported include duration, temperature, and additives. Jakob et al. (1995) have reported that higher temperature and longer duration generally could improve the removal efficiencies for FA, but they could be decreased if the operating temperature exceeded the melting range of the target species. Due to this limitation on the operating temperature, the removal efficiencies for other less volatile metals (Zn and Cu) are lower, and the most effective temperature for evaporating Zn, Pb, Cu, and Cd is 1300 °C in argon atmosphere.
To overcome the high-temperature conditions, many researchers investigated the use of chlorine additives to induce a reducing condition in order to increase the removal of other less volatile trace metals. Nowak et al. (2012) investigated the use of various chlorides such as MgCl2, CaCl2, and NaCl and found that MgCl2 was the best chloride additive for removal of up to 95% Pb and Cd at 1000 °C. The mechanism behind this was the formation of volatile metal chlorides, whereas another mechanism was that the presence of chlorides prevents the incorporation of heavy metal into the melted matrix. In general, this method is mostly carried out on FA due to the nature of the ash composition to form a more stable product for other utilization purpose.
Thermal evaporation process is well developed, and various lab-scale and commercial projects are available such as CT-Fluapur® (Jakob and Mörgeli 1999) and ASH DEC (Nowak et al. 2010). However, the treatment cost is still relatively high and has to be combined within the waste incinerator to reduce the cost.
Solidification and stabilization
S/S is a well-proven technology to immobilize heavy metals via chemical and physical modifications (Malviya and Chaudhary 2006). In general, solidification reduces the mobility of hazardous species by improving physical properties such as permeability and surface area, while stabilization chemically modifies the hazardous species by converting them into less soluble or less toxic constituents. Currently, S/S is focused on process optimization to improve the efficiencies and economic viability (Galiano et al. 2011). S/S includes chemical stabilization, accelerated carbonation, and Portland cement solidification (Table 7).
Chemical stabilization
Chemical stabilization makes use of chemical additives to produce thermodynamically favored solid phases in order to reduce the total availability for leaching. This treatment method consists of (i) dissolution of the waste residues, (ii) addition of chemicals for stabilization and pH adjustment, and (iii) filtration to separate the high salt filtrate from the stabilized residues.
The technology is quite established, and various additives such as soluble phosphates, sulfides, and activated carbon have been used at full scale (Nzihou and Sharrock 2002). Eighmy et al. (1997) demonstrated that 1.2 mol of H3PO4 per kg APC with an L/S ratio of 0.4 could reduce Pb leaching by 99.5%, while the pH of the final product was not affected by the H3PO4 added due to high alkalinity. Quina et al. (2010) suggested that H3PO4 could affect the neutralizing capacity since both Zn and Pb were stabilized, and there was increased leaching of Cr(VI). Recently, Wang et al. (2018) have utilized phosphate and other chelate agents to stabilize FA, while leaching effect was examined using citric acid.
Other additives such as ferrous sulfate, colloidal silica, Bayer red mud, and alumina oxides have also been investigated with good immobilizing potential (Hu 2005; Park 2009; Liu et al. 2018a). In general, APC is commonly treated via chemical stabilization due to the high alkalinity and high acid-neutralizing capacity.
Accelerated carbonation
Carbonation is a natural weathering process due to the dissolution of CO2 in water, which lowers the pH and eventually results in the formation of calcite and other new minerals capable of entrapping heavy metals. Ecke (2003) has reported decreased leaching of APC at 2 orders of magnitude for amphoteric metals such as Zn and Pb after carbonation but also highlighted increased release of other metals such as Cd, Cr, Ni, and Sb. Li et al. (2007) studied the effect of L/S ratio between 0.1 and 0.8 using accelerated carbonation and suggested the optimum L/S ratio is 0.3. The main factors affecting the rate of carbonation are diffusivity and reactivity of carbon dioxide, and these can be controlled by adjusting the pH, L/S ratio, humidity, temperature, and pressure (McCarthy et al. 2018).
Solidification and chemical fixation with cement binder
Solidification involves the mixing of FA or APC with a binder such as cement to form a more stable monolithic solid after the hydration reaction. The enhanced properties of the final product such as lower hydraulic conductivity and lower porosity limit the leachability of the heavy metals by reducing contact, while the increase in structural strength and durability ensures long-term stability and structural integrity (Bie et al. 2016). In addition, the formation of a crystalline structure during the pozzolanic reaction is capable of incorporating heavy metals into the matrix to provide potential long-term immobilization (Yakubu et al. 2018).
Although FA seems to delay the hydration process, the final strength is acceptable with high stabilization of Pb, Zn, Cr, Cd, Ni, and Hg. Aubert et al. (2007) pretreated the FA with a series of washing solutions and, afterwards, calcination at 600 °C, attaining pozzolanic effects which made it suitable to replace cement partially, while the formation of ettringite was able to entrap various heavy metals.
Solidification is generally carried out on pretreated FA to remove chlorides due to its pozzolanic properties, which enables it to partially replace cement, while APC is usually not favored as it contains excessively high Ca content (Shi et al. 2018). However, solidification using a binder results in an increase in the mass of the secondary waste which has to be disposed of. A new trend to resolve the increase in mass of the secondary product is to make use the combination of various wastes such as APC, FA, coal FA, and solidifying agents to increase the proportion of waste-to-binder ratio (Wang et al. 2015).
Thermal methods
Thermal processes generally aim to reduce the volume of the bulk ash and produce a more stable product, which can be disposed of or utilized. Based on the different operating conditions such as temperature and additives, thermal treatments can be classified into three types: (i) sintering, (ii) vitrification, and (iii) melting/fusion (Table 8) (Lindberg et al. 2015). Although thermal vaporization is considered a separation method in this review, it can also occur concurrently in all three of the thermal methods mentioned. The main disadvantage of thermal methods is usually associated with high treatment cost, but due to the advancement in technology, such methods are becoming increasingly commercially acceptable in countries such as Japan, China, and the USA.
Sintering
Sintering is the coalescence and densification of powdered substances into larger particles at elevated temperatures below their melting point. This process usually takes place at temperatures between 900 and 1100 °C and so is the lowest among all the thermal treatment processes, resulting to lower porosity and higher strength and density. Sintering is effective in reducing leaching of volatile metals (Cd, Pb, and Hg) due to vaporization during the heating process and of other heavy metals due to a reduction in the porosity. Sintering treatment mainly focuses on FA only due to the formation of the amorphous matrix to stabilize the different heavy metals.
The main parameters affecting sintering include pressure, temperature, and duration. Others have investigated the production of ceramics and construction materials using sintering of FA (Mangialardi 2001; Polettini et al. 2004; Wey et al. 2006; Haiying et al. 2007; Wang et al. 2017). In order to reduce treatment cost and improve final product quality, Wey et al. (2006) and Mangialardi (2001) have reported the use of water washing process prior to sintering. Above 960 °C, it is shown that sintering can produce ceramic tiles with low leachability and high mechanical properties (Haiying et al. 2007).
Co-sintering with various additives such as bottom ash and slag to achieve the optimal proportion of CaO and SiO2 ratio for better mechanical properties has been demonstrated (Hu et al. 2016). It is also found that Ca aluminosilicate formed during co-sintering is capable of suppressing ash agglomeration and favoring metal chloride recovery via volatilization.
Vitrification
Vitrification is the melting of ash residues with other glass-forming additives to produce a homogenous liquid phase between 1100 and 1500 °C followed by rapid cooling to form a rigid and amorphous glassy matrix without crystallization (Quina et al. 2008a, 2008b). Vitrified products are generally more consistent and chemically stable due to the incorporation of heavy metals into the glass matrix to form a homogenous solid phase and also the encapsulation effects provided by the outermost glassy layer (Ghouleh and Shao 2018).
Although vitrification is a technically feasible solution, the high energy consumption during the process increases the treatment cost and this has limited its potential due to economic issues. Therefore, the research has focused on using other additives such as biomass (Alhadj-Mallah et al. 2015) or make use of co-treatment with other wastes (Huber et al. 2016) to reduce the energy demand and make this process more economically viable. However, due to the heterogeneous nature of the FA and APC, the quality of the final product is affected, and the subsequent utilization can be limited.
Melting
Melting process operates at similar temperature as vitrification, but the main difference is that during melting, no additives are added. Also, the final product formed is homogenous and contains various major crystalline phases. As compared to vitrification, melting is more preferable as no additives are required and there is no need for rapid cooling of the final product. Melting systems are reported to be used on a commercial scale to treat ash especially in Japan where disposal at landfills is costly (Ecke et al. 2000). According to Sakai and Hiraoka (2000), the two main systems are (i) fuel-burning melting system and (ii) electric melting system. In general, the fuel-burning system is generally used at smaller incinerators with no power generation, while the electric melting system is preferred at larger incinerators with power generation (Carnogurska et al. 2015; Quina et al. 2008a, 2008b).
Hydrothermal
Hydrothermal process is a form of thermochemical of subcritical or supercritical water at relatively low temperature with the advantage of self-generated pressure. In the recent years, investigations of hydrothermal treatment on FA have become more popular due to the destruction of organic contaminants and improved stability of heavy metal leaching (Hu et al. 2015). It is found out that hydrothermal treatment under alkaline condition can result in crystallization of FA into more stable aluminosilicate (Murayama et al. 2002) which immobilize the leaching of metal ions. The silicon-aluminum additives were also found that can assist the hydrothermal process by the formation of aluminosilicate minerals (zeolites) and stabilization of heavy metals (Shi et al. 2017a, 2017b). One of the main products is the tobermorite which is capable of being an ion exchange for heavy metals such as Pb, Cd, and Zn (Coleman and Brassington 2003). Most of the studies revolves for coal fly ash mainly and not so much for FA and APC due to the high amount of Ca and lower amount of Si/Al, which is deemed to be the most important factor for tobermorite synthesis (Shi et al. 2017a, 2017b). However, the newly developed method microwave-assisted hydrothermal treatment with the Na2HPO4 additive has demonstrated the high efficiency for solidifying heavy metals in FA, the great energy savings, and low leaching toxicity (Qiu et al. 2017).
Comparison of treatment technologies
Table 9 summarizes the different treatment methods for FA and APC residues. Each treatment method is evaluated based the following factors:
-
Technical requirement
-
Treatment cost
-
Technology development
-
Volume change
-
Secondary pollutants
-
Destruction of organic pollutants
Technical requirement refers to the capital cost of the facilities and the skilled personnel required operating the facilities, which is directly related to the treatment cost. A simple treatment such as water washing and cement encapsulation tends to have low cost (45 € t−1), while simplified equipment is required. Leaching, chemical stabilization, and accelerated carbonation require a certain amount of chemical additives (cost 61–92 € t−1). Lastly, treatments such as electrodialysis and thermal processing tend to be rather costly (> 367 € t−1) because of the high energy demand and expensive start-up cost of the facilities (Ecke et al. 2000; Quina et al. 2008a, 2008b).
Technology development refers to the stage of the technology (lab, pilot, or industrial scale), and it is an important factor to consider when deciding on a treatment strategy. In general, most established technologies such as chemical stabilization and solidification have lower risks of implementation, but lesser room for research and development. Washing treatment is considered a pretreatment step in combination with other processes. New technologies such as electrodialysis and hydrothermal treatment are at the stage of lab scale to pilot scale due to scalability and economic issues. Thermal processes are more developed (especially in Japan and China) and exist in pilot to industrial scale, but not fully economically viable yet due to the high cost and energy demand (Lindberg et al. 2015). Leaching process such as FLUWA is an industrial-scale technology (Schlumberger et al. 2007) for recovery of metals via chemical leaching. Chemical stabilization is another widely studied and developed technology for many years with industrial-scale projects such as COSMOS (Bontempi et al. 2010), Ferrox (Bontempi et al. 2010), and WesPhix (Eighmy et al. 1997). Cement stabilization has also carried out in industrial scale for FA and APC residues and handles up to 15,000 tons of FA from WtE each year.
Volume change is another important factor when considering the treatment technology. Methods that make use of separation tend to experience a decrease in volume of the final product, but they generate a secondary effluent which is more concentrated and toxic and requires further treatment. Separation methods such as washing, leaching, and electrodialysis generate wastewater that contains soluble salts and heavy metals. High-temperature treatment such as vitrification produces a concentrated fly ash with highly volatile metal chlorides of Pb and Zn. On the other hand, sintering, cement binder, and chemical stabilization focus on the encapsulation and stabilization of the heavy metals and they do not produce secondary pollutants, but the volume of the final product tends to increase due to the various additives added to stabilize FA.
Lastly, the fate of organic pollutants such as PCDD/F emitted from waste incineration is of a major concern over the past few decades due to their high toxicity. These organic pollutants are usually found in boiler and filter ash (Vehlow et al. 2006). Although their leaching can be reduced via encapsulation and chemical fixation, the optimal solution is to destroy them using the thermal process. Thermal methods such as vitrification and melting which use extremely high temperatures (over 1000 °C) have shown over 99.9% destruction of organic compounds (Sakai and Hiraoka 2000; Nishida et al. 2001).
Utilization of FA and APC residues
The main objectives of waste incineration are volume reduction and energy recovery, whereby the residual ashes are considered as a form of secondary waste to be landfilled. In the recent years, due to the increasing amount of FA and APC generated, there is much controversy on the proper management of these ashes and residues. Even after proper treatment, the final product faces some limitations preventing utilization in many applications (Lam et al. 2010). Table 10 provides a summary of the utilization methods categorized by the ash types, development stage, and the limitations faced.
Metals and salt recovery
Recovery of metals and salt from FA and APC has been investigated widely, and the process is well established in large commercial scale (Schlumberger et al. 2007). The recovery process usually involves chemical leaching such as acid (Funari et al. 2015), commercial chelating agent with metal selectivity (Tang and Steenari 2015), or bioleaching for a more environmentally friendly and low-cost process (Ramanathan and Ting 2016).
Typically, volatile heavy metals, such as Pb, Zn, Cd, and other rare earth metals, in FA are being separated from the rest of the residues for detoxification and recovery, while APC does not undergo such treatment due to high acid-neutralizing capacity and low metallic contents. However, the recovery of rare earth metals is not economical due to the low amount of those metals in FA (Funari et al. 2015). After the selective removal of metals, a large amount of soluble salt remains within the extracting liquid. These salts are usually not recovered, and instead, the extracting liquid is required for further treatment before disposal (Colangelo et al. 2012). An exception is the HALOSEP® process developed to recover chloride brine from FA and APC, which can be used in winter to de-ice the road (Quina et al. 2018).
Cement production and cement replacement
Cement is the most commonly used building material in the world, and as FA and APC have very similar composition, they are used as partial replacement to reduce the amount of energy required and CO2 generated during cement production. There is huge interest in using FA and APC in both production of cement and as a cement replacement (Siddique 2010).
The application of FA in various studies has shown positive results in the hydration behavior for up to 30% replacement as alkali metal enhances the hydration, while Zn, Pb, and Cd retard the hydration (Lederer et al. 2017). The presence of high chlorides and sulfate content can also be detrimental to the early strength due to slowing hydration. Although chlorides and sulfate can be easily removed by a simple washing method, the washed FA experiences a loss of pozzolanic activity after washing (Keppert et al. 2012). Also, the metallic Al and Zn present in FA generates a significant amount of H2 which results in expansion and voids within the concrete and affects the compressive strength (Aubert et al. 2004). Despite these limitations, FA as a cement replacement is still a viable option as it has the added advantage of stabilizing the heavy metals, while it also possesses pozzolanic properties that can help improve the long-term strength of the concrete (Joseph et al. 2018).
APC, on the other hand, is not directly used as a cement replacement due to the high Ca content (Shi et al. 2018), but it is commonly co-processed as a raw material in cement production in rotary kiln (Joseph et al. 2018). Major advantage of using APC is that it contains lesser carbon phases than limestone, and thus, it helps to reduce the emissions during cement production. In addition, the high temperature of the rotary kiln can concurrently destroy the organic pollutants in APC (Bogush et al. 2015). It is suggested that the use of APC in clinker production results in a cement with lower workability and lesser setting time, so gypsum should be added to delay the setting (Kikuchi 2001). The presence of high chloride content in APC is also a concern as it might cause clogging and corrosion problems. As such, it is of high importance that the APC undergoes proper pretreatment before being used as raw material in cement production (Saikia et al. 2007).
Use as pavement and embankment
The construction of roads and embankments tends to use a large amount of natural aggregates. The use of FA in replacement of these natural aggregates can help to conserve the natural aggregates for better applications (Francois and Pierson 2009). The majority of the studies make use of bottom ash as the base layer for road construction, while lesser studies use FA and APC due to the higher leaching and lower durability.
The leaching issue, however, can be resolved by washing pretreatment of FA and APC to meet the leaching standards before utilization (Åberg et al. 2006). A FA melting plant for stone production in Japan also successfully demonstrated a commercially viable use of FA pavement blocks in a park (Nishida et al. 2001).
Zeolite synthesis
Zeolites are crystalline aluminosilicates that can be used as sorbents, catalysts, and ion exchange applications. Despite the low Al and Si content in FA and APC due to the significant portion of Ca, the hydrothermal process under alkaline conditions has proven to be effective in synthesizing zeolites (Wałek et al. 2008). Although the typical cation exchange capacity (CEC) of FA and APC zeolites is only 90 mEq/100 g as compared to commercial zeolites at 200–300 mEq/100 g, the feasibility of using waste-derived zeolite is still of high interest. Research on the modification of the FA-derived zeolite improved the CEC to be comparable to other sorbents (Miyake et al. 2002). To deliver calcium-containing aluminosilicate zeolites such as tobermorite, the addition of a 30% mass of composite additive (including CFA and diatomite) and a 3% mass of tobermorite seed (reaction temperature 200 °C) was applied for seed-assisted hydrothermal solidification of MSWI fly ash (Shi et al. 2016). The developed technology increased the kinetic formation of tobermorite, while inhibited the hibschite generated from tobermorite.
Glass ceramics and lightweight aggregates
FA and APC contain a high amount of SiO2, Al2O3, and CaO and are considered suitable to replace clay for the production of glass ceramics after thermal treatment such as sintering and vitrification (Andreola et al. 2001). Despite the use of raw FA which resulted in detrimental effects on the mechanical properties of the ceramic due to excessive chloride content, Haiying et al. (2007) managed to achieve high-strength ceramic tiles with low water adsorption rate with 20% FA. Successful synthesis of lightweight aggregate was achieved by heating APC with clay at an elevated temperature of 1170 °C (Quina et al. 2014). Such lightweight aggregates have dense ceramic outer shell with good mechanical properties with up to 50% lower density (due to honeycombed internal non-interconnected pores).
Perspectives
Mixing of FA and APC
In some countries, FA and APC are usually collected as a combined waste (del Valle-Zermeño et al. 2013). However, others suggested that FA and APC should be managed separately due to their differences in the composition and, hence, chemical properties (De Boom and Degrez 2012).
In this review, APC and FA are compared in terms of physicochemical characteristics and treatment methods, and the literature has suggested separate collection of APC and FA, and treatment as two different materials. The compositions of FA and APC are sufficiently different, and their properties could be used in different applications. For example, FA which has a higher content of Al2O3 and SiO2 is favorable for zeolite production (Yoo and Jo 2015), while APC which contains excess lime content is useful for cement clinker production (Stegemann 2014). When the two are mixed, the resultant waste is less favorable for both applications.
Mixing of FA and APC could also result in some undesirable outcomes such as explosion due to the release of hydrogen gas (Mizutani et al. 2000). The release and buildup of hydrogen gas can be due to the presence of aluminum under high pH conditions (incompatible waste) (Zhang et al. 2009; Hu et al. 2011). The authors argue separation of FA and APC is desirable to prevent FA (which has a high aluminum content) contacting APC (which has high alkalinity) because it can cause production of hydrogen gas.
Lastly, when FA and APC are mixed, the pH of the combined residue is still high (10 to 12) and this might result in the enhanced leaching of amphoteric metals from the FA as reported by Zhang et al. (2016). In addition to the increase in pH, the ANC of the combined waste is also relatively high, and this would make it harder to adjust the pH for other processes.
True cost of treatment
Treatment cost has always been a concern. However, treatment cost should not be evaluated on the basis of only the treatment process. In addition to the cost of the treatment process, there are also the cost of risks and environmental impacts of the resulting material, the value of the final products, and the cost of maintenance/monitoring after disposal.
As mentioned previously, different treatment methods yield different types of treated products with various qualities. Therefore, it is important to consider the cost in a more holistic manner. Low-cost treatment methods such as stabilization and solidification usually generate a larger volume of final product, which might not be suitable for utilization, and it requires to be sent to the landfill for disposal. In this case, the cost of maintaining the landfill for a larger amount of residue should be added on to the true cost of treatment, whereas in using the thermal treatment, the final product is usually of higher quality and can be utilized in various applications. In this case, the value of these final products can be used to offset the higher cost of treatment (Luo et al. 2018).
One important aspect that has been undervalued is the risk of monetization by evaluation of environmental risks into dollar terms for quantification. It is very difficult to translate the value of long life, good health, and clean air into dollar as there is the fundamental flaw that each individual values each factor very differently. Therefore, there is a need for environmental and government agencies to evaluate each factor and incorporate subsidies and tax to promote proper treatment for FA and APC residues.
Challenges and opportunities
Despite the numerous reports in the literature, there are few case studies treating FA and APC residues at full scale for utilization purposes. The challenges and opportunities for further utilization of these secondary resources are identified in this review paper.
Firstly, one of the main issues with FA and APC is the variation in terms of mineralogy, morphology, and chemical properties due to the different operating conditions at the various locations. It is, therefore, difficult to propose a single method to treat FA and APC. Proper characterization before treatment in each case is essential before deciding on the most appropriate method to use. In addition, the heterogeneous nature of the treated products makes these less valuable and less applicable. Consequently, researchers have focused on stabilizing the FA and APC just enough for disposal in landfill. The development of a treatment method, which can convert FA and APC into a homogenous product, is a challenge.
Next, many researchers have focussed on the heavy metal content before and after treatment and have neglected the effect of treatment on organics such as dioxins due to the difficulties associated with analysis of such compounds. Development in analytical methods for such compounds is needed.
Lastly, the treated products could still create problems in the longer term because of leachable contents and such concern would reduce public acceptance on utilization of treated FA and APC. More studies on the long-term effects of treated FA and APC should be conducted to increase knowledge and public confidence in utilizing these resources.
Conclusions
As solid waste management increasingly shifts towards incineration for energy recovery, more by-products such as FA and APC residues are generated. These are considered hazardous due to the presence of heavy metals and soluble salts and must be treated before disposal. However, it is evident that FA and APC residues have potential in many applications such as zeolite production, road pavement, construction material, and ceramics. In order to utilize or safely dispose of FA and APC residues, treatment must be applied to improve quality and reduce the negative environmental implications. In general, the three treatment types are separation, thermal, and solidification/stabilization processes. Both thermal processes and S/S processes may be found at full scale with separation processes often serving as a pretreatment. Increasing amounts of FA and APC are being produced, and there is a need to explore the potential application of the treated material instead of just disposal. This is necessary for a more sustainable approach to waste management.
References
Åberg A, Kumpiene J, Ecke H (2006) Evaluation and prediction of emissions from a road built with bottom ash from municipal solid waste incineration (MSWI). Sci Total Environ 355(1-3):1–12
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energ Combust 36(3):327–363
Alhadj-Mallah M-M, Huang Q, Cai X, Chi Y, Yan J (2015) Vitrification of municipal solid waste incineration fly ash using biomass ash as additives. Environ Technol 36(5):654–660
Andreola F, Barbieri L, Corradi A, Lancellotti I, Manfredini T (2001) The possibility to recycle solid residues of the municipal waste incineration into a ceramic tile body. J Mater Sci 36(20):4869–4873
Aubert J, Husson B, Vaquier A (2004) Use of municipal solid waste incineration fly ash in concrete. Cem Concr Res 34(6):957–963
Aubert J, Husson B, Sarramone N (2006) Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 1: processing and characterization of MSWI fly ash. J Hazard Mater 136(3):624–631
Aubert JE, Husson B, Sarramone N (2007) Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 2: mechanical strength of mortars and environmental impact. J Hazard Mater 146(1-2):12–19
Baciocchi R, Costa G, Di Bartolomeo E, Polettini A, Pomi R (2009) The effects of accelerated carbonation on CO2 uptake and metal release from incineration APC residues. Waste Manag 29(12):2994–3003
Bertos MF, Li X, Simons S, Hills C, Carey P (2004) Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2. Green Chem 6(8):428–436
Bie R, Chen P, Song X, Ji X (2016) Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J Energy Inst 89:704–712
Bogush A, Stegemann JA, Wood I, Roy A (2015) Element composition and mineralogical characterisation of air pollution control residue from UK energy-from-waste facilities. Waste Manag 36:119–129
Bontempi E, Zacco A, Borgese L, Gianoncelli A, Ardesi R, Depero L (2010) A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J Environ Monit 2(11):2093–2099
Carignan J, Libourel G, Cloquet C, Le Forestier L (2005) Lead isotopic composition of fly ash and flue gas residues from municipal solid waste combustors in France: implications for atmospheric lead source tracing. Environ Sci Technol 39(7):2018–2024
Carnogurska M, Lazar M, Puskar M, Lengyelova M, Vaclav J, Sirillova L (2015) Measurement and evaluation of properties of MSW fly ash treated by plasma. Measurement 62:155–161
Chandler AJ, Eighmy T, Hjelmar O, Kosson D, Sawell S, Vehlow J, Van der Sloot H, Hartlén J (1997) Municipal solid waste incinerator residues, Elsevier. https://www.elsevier.com/books/municipal-solid-waste-incinerator-residues/chandler/978-0-444-82563-6
Chang CY, Wang CF, Mui D, Chiang HL (2009) Application of methods (sequential extraction procedures and high-pressure digestion method) to fly ash particles to determine the element constituents: a case study for BCR 176. J Hazard Mater 163(2):578–587
Chen X, Bi Y, Zhang H, Wang J (2016) Chlorides removal and control through water-washing process on MSWI fly ash. Procedia Environ Sci 31:560–566
Chen W, Kirkelund GM, Jensen PE, Ottosen LM (2017) Comparison of different MSWI fly ash treatment processes on the thermal behavior of As, Cr, Pb and Zn in the ash. Waste Manag 68:240–251
Chen W, Kirkelund GM, Jensen PE, Ottosen LM (2018) Electrodialytic extraction of Cr from water-washed MSWI fly ash by changing pH and redox conditions. Waste Manag 71:215–223
Chiang KY, Jih JC, Chien MD (2008) The acid extraction of metals from municipal solid waste incinerator products. Hydrometallurgy 93(1):16–22
Chimenos J, Fernandez A, Cervantes A, Miralles L, Fernández M, Espiell F (2005) Optimizing the APC residue washing process to minimize the release of chloride and heavy metals. Waste Manag 25(7):686–693
Chou SY, Lo SL, Hsieh CH, Chen CL (2009a) Sintering of MSWI fly ash by microwave energy. J Hazard Mater 163(1):357–362
Chou JD, Wey MY, Chang SH (2009b) Evaluation of the distribution patterns of Pb, Cu and Cd from MSWI fly ash during thermal treatment by sequential extraction procedure. J Hazard Mater 162(2):1000–1006
Colangelo F, Cioffi R, Montagnaro F, Santoro L (2012) Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag 32(6):1179–1185
Coleman NJ, Brassington DS (2003) Synthesis of Al-substituted 11 Å tobermorite from newsprint recycling residue: a feasibility study. Mater Res Bull 38(3):485–497
De Boom A, Degrez M (2012) Belgian MSWI fly ashes and APC residues: a characterisation study. Waste Manag 32(6):1163–1170
De Boom A, Degrez M (2015) Combining sieving and washing, a way to treat MSWI boiler fly ash. Waste Manag 39:179–188
De Boom A, Aubert JE, Degrez M (2014) Carbonation of municipal solid waste incineration electrostatic precipitator fly ashes in solution. Waste Manage Res 0734242X14527637. https://www.ncbi.nlm.nih.gov/pubmed/24718362
del Valle-Zermeño R, Formosa J, Chimenos J, Martínez M, Fernández A (2013) Aggregate material formulated with MSWI bottom ash and APC fly ash for use as secondary building material. Waste Manag 33(3):621–627
Deng Y, Gong B, Chao Y, Dong T, Yang W, Hong M, Shi X, Wang G, Jin Y, Chen ZG (2018) Sustainable utilization of municipal solid waste incineration fly ash for ceramic bricks with eco-friendly biosafety. Mater Today Sustain 1-2:32–38
Ecke H (2003) Sequestration of metals in carbonated municipal solid waste incineration (MSWI) fly ash. Waste Manag 23(7):631–640
Ecke H, Sakanakura H, Matsuto T, Tanaka N, Lagerkvist A (2000) State-of-the-art treatment processes for municipal solid waste incineration residues in Japan. Waste Manag Res 18(1):41–51
Ecke H, Menad N, Lagerkvist A (2003) Carbonation of municipal solid waste incineration fly ash and the impact on metal mobility. J Environ Eng 129(5):435–440
Eighmy T, Domingo D, Krzanowski J, Stämpfli D, Eusden D (1993) The speciation of elements in incineration residues. Proceedings Municipal Waste Combustion Conference, Williamsburg, Virginia. Air and Waste Management Association, Pittsburgh, Penn. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=ORD&dirEntryID=49317
Eighmy TT, Eusden JD, Krzanowski JE, Domingo DS, Staempfli D, Martin JR, Erickson PM (1995) Comprehensive approach toward understanding element speciation and leaching behavior in municipal solid waste incineration electrostatic precipitator ash. Environ Sci Technol 29(3):629–646
Eighmy TT, Crannell BS, Butler LG, Cartledge FK, Emery EF, Oblas D, Krzanowski JE, Eusden JD, Shaw EL, Francis CA (1997) Heavy metal stabilization in municipal solid waste combustion dry scrubber residue using soluble phosphate. Environ Sci Technol 31(11):3330–3338
Fedje KK, Ekberg C, Skarnemark G, Steenari BM (2010) Removal of hazardous metals from MSW fly ash—an evaluation of ash leaching methods. J Hazard Mater 173(1):310–317
Florea M, Quaas L, Brouwers H, Schmidt W, Msinjili N (2016) MSWI by-products and immobilisates as concrete constituents. Advances in Cement and Concrete Technology in Africa: 2nd International Conference, Dar es Salaam, Tanzania
Francois D, Pierson K (2009) Environmental assessment of a road site built with MSWI residue. Sci Total Environ 407:5949–5960
Fruergaard T, Hyks J, Astrup T (2010) Life-cycle assessment of selected management options for air pollution control residues from waste incineration. Sci Total Environ 408(20):4672–4680
Funari V, Braga R, Bokhari SNH, Dinelli E, Meisel T (2015) Solid residues from Italian municipal solid waste incinerators: a source for “critical” raw materials. Waste Manag 45:206–216
Funari V, Mäkinen J, Salminen J, Braga R, Dinelli E, Revitzer H (2017) Metal removal from municipal solid waste incineration fly ash: a comparison between chemical leaching and bioleaching. Waste Manag 60:397–406
Galiano YL, Pereira CF, Vale J (2011) Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J Hazard Mater 185(1):373–381
Ghouleh Z, Shao Y (2018) Turning municipal solid waste incineration into a cleaner cement production. J Clean Prod 195:268–279
González I, Vázquez MA, Romero-Baena AJ, Barba-Brioso C (2017) Stabilization of fly ash using cementing bacteria. Assessment of cementation and trace element mobilization. J Hazard Mater 321:316–325
Haiying Z, Youcai Z, Jingyu Q (2007) Study on use of MSWI fly ash in ceramic tile. J Hazard Mater 141(1):106–114
Haiying Z, Youcai Z, Jingyu Q (2010) Characterization of heavy metals in fly ash from municipal solid waste incinerators in Shanghai. Process Saf Environ 88(2):114–124
Hartmann S, Koval L, Škrobánková H, Matýsek D, Winter F, Purgar A (2015) Possibilities of municipal solid waste incinerator fly ash utilisation. Waste Manag Res 33(8):740–747
Heuss-Aßbichler S, Magel G, Fehr KT (2010) Abiotic hydrogen production in fresh and altered MSWI-residues: texture and microstructure investigation. Waste Manag 30(10):1871–1880
Hu SH (2005) Stabilization of heavy metals in municipal solid waste incineration ash using mixed ferrous/ferric sulfate solution. J Hazard Mater 123(1):158–164
Hu Y, Bakker M, De Heij R (2011) Recovery and distribution of incinerated aluminum packaging waste. Waste Manag 31(12):2422–2430
Hu Y, Zhang P, Li J, Chen D (2015) Stabilization and separation of heavy metals in incineration fly ash during the hydrothermal treatment process. J Hazard Mater 299:149–157
Hu H-Y, Liu H, Zhang Q, Zhang P-A, Li A-J, Yao H, Naruse I (2016) Sintering characteristics of CaO-rich municipal solid waste incineration fly ash through the addition of Si/Al-rich ash residues. J Mater Cycles Waste 18(2):340–347
Huang TY, Chuieh PT (2015) Life cycle assessment of reusing fly ash from municipal solid waste incineration. Procedia Eng 118:984–991
Huang T, Zhou L, Liu L, Xia M (2018) Ultrasound-enhanced electrokinetic remediation for removal of Zn, Pb, Cu and Cd in municipal solid waste incineration fly ashes. Waste Manag 75:226–235
Huang T, Zhang S, Liu L (2019) Immobilization of trace heavy metals in the electrokinetics-processed municipal solid waste incineration fly ashes and its characterizations and mechanisms. J Environ Manag 232:207–218
Huber F, Blasenbauer D, Mallow O, Lederer J, Winter F, Fellner J (2016) Thermal co-treatment of combustible hazardous waste and waste incineration fly ash in a rotary kiln. Waste Manag 58:181–190
Huber F, Laner D, Fellner J (2018a) Comparative life cycle assessment of MSWI fly ash treatment and disposal. Waste Manag 73:392–403
Huber F, Herzel H, Adam C, Mallow O, Blasenbauer D, Fellner J (2018b) Combined disc pelletisation and thermal treatment of MSWI fly ash. Waste Manag 73:381–391
Jakob A, Mörgeli R. (1999) Detoxification of municipal solid waste incinerator fly ash: the CT-FLUAPUR process. REWAS’99: Global Symposium on Recycling, Waste Treatment and Clean Technology. https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200902124360625918&rel=0
Jakob A, Stucki S, Kuhn P (1995) Evaporation of heavy metals during the heat treatment of municipal solid waste incinerator fly ash. Environ Sci Technol 29(9):2429–2436
Jakob A, Stucki S, Struis RPWJ (1996) Complete heavy metal removal from fly ash by heat treatment: influence of chlorides on evaporation rates. Environ Sci Technol 30(11):3275–3283
Jensen PE, Ferreira CM, Hansen HK, Rype JU, Ottosen LM, Villumsen A (2010) Electroremediation of air pollution control residues in a continuous reactor. J Appl Electrochem 40(6):1173–1181
Jensen PE, Kirkelund GM, Pedersen KB, Dias-Ferreira C, Ottosen LM (2015) Electrodialytic upgrading of three different municipal solid waste incineration residue types with focus on Cr, Pb, Zn, Mn, Mo, Sb, Se, V, Cl and SO4. Electrochim Acta 181:167–178
Jiao F, Zhang L, Dong Z, Namioka T, Yamada N, Ninomiya Y (2016) Study on the species of heavy metals in MSW incineration fly ash and their leaching behaviour. Fuel Process Technol 152:108–115
Jin M, Zheng Z, Sun Y, Chen L, Jin Z (2016) Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J Non-Cryst Solids 450:116–122
Joseph AM, Snellings R, Van den Heede P, Matthys S, De Belie N (2018) The use of municipal solid waste incineration ash in various building materials: a Belgian point of view. Materials 11:141
Jung C, Matsuto T, Tanaka N, Okada T (2004) Metal distribution in incineration residues of municipal solid waste (MSW) in Japan. Waste Manag 24(4):381–391
Karkfeldt K, Steenari B-M (2007) Assessment of metal mobility in MSW incineration ashes using water as the reagent. Fuel 86(12-13):1983–1993
Kawabata H, Usui T, Marukawa K, Hara S, Tanaka T, Ono-Nakazato H (2003) Mechanism of dioxins/furans formation at high temperature in combustion processes. ISIJ Int 43(3):461–467
Keppert M, Pavlík Z, Tydlitat V, Volfová P, Švarcová S, Šyc M, Černý R (2012) Properties of municipal solid waste incineration ashes with respect to their separation temperature. Waste Manag Res 30(10):1041–1048
Kikuchi R (2001) Recycling of municipal solid waste for cement production: pilot-scale test for transforming incineration ash of solid waste into cement clinker. Resour Conserv Recycl 31(2):137–147
Kirkelund GM, Jensen PE, Villumsen A, Ottosen LM (2010) Test of electrodialytic upgrading of MSWI APC residue in pilot scale: focus on reduced metal and salt leaching. J Appl Electrochem 40(6):1049–1060
Ko M-S, Chen Y-L, Wei P-S (2013) Recycling of municipal solid waste incinerator fly ash by using hydrocyclone separation. Waste Manag 33(3):615–620
Kothari R, Tyagi V, Pathak A (2010) Waste-to-energy: a way from renewable energy sources to sustainable development. Renew Sust Energ Rev 14(9):3164–3170
Lam CH, Ip AW, Barford JP, McKay G (2010) Use of incineration MSW ash: a review. Sustainability 2(7):1943–1968
Le Forestier L, Libourel G (1998) Characterization of flue gas residues from municipal solid waste combustors. Environ Sci Technol 32(15):2250–2256
Leckner B (2015) Process aspects in combustion and gasification waste-to-energy (WtE) units. Waste Manag 37:13–25
Lederer J, Trinkel V, Fellner J (2017) Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: the case of Austria. Waste Manag 60:247–258
Li CT, Huang YJ, Huang KL, Lee WJ (2003) Characterization of slags and ingots from the vitrification of municipal solid waste incineration ashes. Ind Eng Chem Res 42(11):2306–2313
Li X, Bertos MF, Hills CD, Carey PJ, Simon S (2007) Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Manag 27(9):1200–1206
Li H, Liu G, Cao Y (2015) Levels and environmental impact of PAHs and trace element in fly ash from a miscellaneous solid waste by rotary kiln incinerator, China. Nat Hazards 76(2):811–822
Li H, Muhammad F, Yan Y, Zhang M, Jiao B, Yu L, Li D (2018) Electrokinetic remediation of heavy metals from municipal solid waste incineration fly ash pretreated by nitric acid. R Soc Open Sci 5(8):180372
Lin X, Chen Z, Lu S, Zhang S, Zhang M, Li X, Yan J (2018) Emission characteristics of polychlorinated dibenzo-p-dioxins and dibenzofurans from the co-combustion of municipal solid waste in a lab-scale drop-tube furnace. Energy Fuel 32(4):5396–5404
Lindberg D, Molin C, Hupa M (2015) Thermal treatment of solid residues from WtE units: a review. Waste Manag 37:82–94
Liu Y, Zheng L, Li X, Xie S (2009) SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures. J Hazard Mater 162(1):161–173
Liu A, Ren F, Lin WY, Wang JY (2015) A review of municipal solid waste environmental standards with a focus on incinerator residues. Int J Sust Built Environ 4:165–188
Liu S, Li Z, Li Y, Cao W (2018a) Strength properties of Bayer red mud stabilized by lime-fly ash using orthogonal experiments. Constr Build Mater 166:554–563
Liu X, Zhao X, Yin H, Chen J, Zhang N (2018b) Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: hydration characteristics and heavy metals solidification behavior. J Hazard Mater 349:262–271
Loginova E, Proskurnin M, Brouwers HJH (2019) Municipal solid waste incineration (MSWI) fly ash composition analysis: a case study of combined chelatant-based washing treatment efficiency. J Environ Manag 235:480–488
Luo Y, Ma S, Zheng S, Liu C, Han D, Wang X (2018) Mullite-based ceramic tiles produced solely from high-alumina fly ash: preparation and sintering mechanism. J Alloys Compd 732:828–837
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: a review. J Hazard Mater 137(1):267–276
Mangialardi T (2001) Sintering of MSW fly ash for reuse as a concrete aggregate. J Hazard Mater 87(1):225–239
Mangialardi T (2003) Disposal of MSWI fly ash through a combined washing-immobilisation process. J Hazard Mater 98(1):225–240
Massardier V, Moszkowicz P, Taha M (1997) Fly ash stabilization-solidification using polymer-concrete double matrices. Eur Polym J 33(7):1081–1086
McCarthy MJ, Zheng L, Dhir RK, Tella G (2018) Dry-processing of long-term wet-stored fly ash for use as an addition in concrete. Cement Concrete Comp 92:205–215
Miyake M, Tamura C, Matsuda M (2002) Resource recovery of waste incineration fly ash: synthesis of zeolites A and P. J Am Ceram Soc 85(7):1873–1875
Mizutani S, Sakai SI, Takatsuki H (2000) Investigation of hydrogen generation from municipal solid waste incineration fly ash. J Mater Cycles Waste 2(1):16–23
Moon GD, Oh S, Choi YC (2016) Effects of the physicochemical properties of fly ash on the compressive strength of high-volume fly ash mortar. Constr Build Mater 124:1072–1080
Murayama N, Yamamoto H, Shibata J (2002) Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int J Miner Process 64(1):1–17
Nagib S, Inoue K (2000) Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy 56(3):269–292
Ni G, Zhao P, Jiang Y, Meng Y (2012) Vitrification of MSWI fly ash by thermal plasma melting and fate of heavy metals. Plasma Sci Technol 14(9):813–818
Nishida K, Nagayoshi Y, Ota H, Nagasawa H (2001) Melting and stone production using MSW incinerated ash. Waste Manag 21(5):443–449
Nowak B, Pessl A, Aschenbrenner P, Szentannai P, Mattenberger H, Rechberger H, Hermann L, Winter F (2010) Heavy metal removal from municipal solid waste fly ash by chlorination and thermal treatment. J Hazard Mater 179(1-3):323–331
Nowak B, Rocha SF, Aschenbrenner P, Rechberger H, Winter F (2012) Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: influence of the chloride type. Chem Eng J 179:178–185
Nzihou A, Sharrock P (2002) Calcium phosphate stabilization of fly ash with chloride extraction. Waste Manag 22(2):235–239
Park YJ (2009) Stabilization of a chlorine-rich fly ash by colloidal silica solution. J Hazard Mater 162(2):819–822
Pedersen AJ, Ottosen LM, Villumsen A (2005) Electrodialytic removal of heavy metals from municipal solid waste incineration fly ash using ammonium citrate as assisting agent. J Hazard Mater 122(1):103–109
Polettini A, Pomi R, Sirini P, Testa F (2001) Properties of Portland cement-stabilised MSWI fly ashes. J Hazard Mater 88(1):123–138
Polettini A, Pomi R, Trinci L, Muntoni A, Mastro SL (2004) Engineering and environmental properties of thermally treated mixtures containing MSWI fly ash and low-cost additives. Chemosphere 56(10):901–910
Qiu Q, Jiang X, Chen Z, Lu S, Ni M (2017) Microwave-assisted hydrothermal treatment with soluble phosphate added for heavy metals solidification in MSWI fly ash. Energy Fuel 31(5):5222–5232
Quina MJ, Bordado JC, Quinta-Ferreira RM (2008a) Treatment and use of air pollution control residues from MSW incineration: an overview. Waste Manag 28(11):2097–2121
Quina MJ, Santos RC, Bordado JC, Quinta-Ferreira RM (2008b) Characterization of air pollution control residues produced in a municipal solid waste incinerator in Portugal. J Hazard Mater 152(2):853–869
Quina MJ, Bordado JC, Quinta-Ferreira RM (2009) The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manag 29(9):2483–2493
Quina MJ, Bordado JC, Quinta-Ferreira RM (2010) Chemical stabilization of air pollution control residues from municipal solid waste incineration. J Hazard Mater 179(1):382–392
Quina MJ, Almeida MA, Santos R, Bordado JM, Quinta-Ferreira RM (2014) Compatibility analysis of municipal solid waste incineration residues and clay for producing lightweight aggregates. Appl Clay Sci 102:71–80
Quina MJ, Bontempi E, Bogush A, Schlumberger S, Weibel G, Braga R, Lederer J (2018) Technologies for the management of MSW incineration ashes from gas cleaning: new perspectives on recovery of secondary raw materials and circular economy. Sci Total Environ 635:526–542
Ramanathan T, Ting Y-P (2016) Alkaline bioleaching of municipal solid waste incineration fly ash by autochthonous extremophiles. Chemosphere 160:54–61
Saikia N, Kato S, Kojima T (2007) Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag 27(9):1178–1189
Sakai SI, Hiraoka M (2000) Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag 20(2):249–258
Sakai SI, Sawell S, Chandler A, Eighmy T, Kosson D, Vehlow J, Van der Sloot H, Hartlen J, Hjelmar O (1996) World trends in municipal solid waste management. Waste Manag 16(5):341–350
Schlumberger S, Schuster M, Ringmann S, Koralewska R (2007) Recovery of high purity zinc from filter ash produced during the thermal treatment of waste and inerting of residual materials. Waste Manag Res 25(6):547–555
Shi HS, Kan LL (2009) Leaching behavior of heavy metals from municipal solid wastes incineration (MSWI) fly ash used in concrete. J Hazard Mater 164(2):750–754
Shi D, Zhang C, Zhang J, Li P, Wei Y (2016) Seed-assisted hydrothermal treatment with composite silicon–aluminum additive for solidification of heavy metals in municipal solid waste incineration fly ash. Energy Fuel 30(12):10661–10670
Shi D, Hu C, Zhang J, Li P, Zhang C, Wang X, Ma H (2017a) Silicon-aluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration. Fuel Process Technol 165:44–53
Shi D, Zhang J, Zhang C, Hu C, Li P (2017b) Seed-induced hydrothermal synthesis of tobermorite from municipal solid waste incinerator fly ash. J Residuals Sci Tech 14(S1):11–19
Shi D, Wang P, Xu X, Gu L, Li L, Ma H, Hu C (2018) Effect of source-classified collection of municipal solid waste on heavy metals and pozzolanic properties of incineration residues. Int J Environ Res 12(5):661–670
Siddique R (2010) Use of municipal solid waste ash in concrete. Resour Conserv Recycl 55(2):83–91
Song GJ, Kim KH, Seo YC, Kim SC (2004) Characteristics of ashes from different locations at the MSW incinerator equipped with various air pollution control devices. Waste Manag 24(1):99–106
Stegemann JA (2014) The potential role of energy-from-waste air pollution control residues in the industrial ecology of cement. J Sust Cement-Based Mater 3(2):111–127
Sun J, Bertos MF, Simons SJR (2008) Kinetic study of accelerated carbonation of municipal solid waste incinerator air pollution control residues for sequestration of flue gas CO2. Energy Environ Sci 1(3):370–377
Sun X, Li J, Zhao X, Zhu B, Zhang G (2016) A review on the management of municipal solid waste fly ash in American. Procedia Environ Sci 31:535–540
Tabata M, Ghaffar A, Shono A, Notomi K (2013) Hydrodechlorination/detoxification of PCDDs, PCDFs, and co-PCBs in fly ash by using calcium polysulfide. Waste Manag 33:356–362
Tang J, Steenari B-M (2015) Solvent extraction separation of copper and zinc from MSWI fly ash leachates. Waste Manag 44:147–154
Tian S, Zhu Y, Meng B, Guan J, Nie Z, Die Q, Xu W, Yu M, Huang Q (2018) Chemical speciation of lead in secondary fly ash using X-ray absorption spectroscopy. Chemosphere 197:362–366
Todorovic J, Ecke H (2006) Demobilisation of critical contaminants in four typical waste-to-energy ashes by carbonation. Waste Manag 26(4):430–441
Van der Bruggen B, Vogels G, Van Herck P, Vandecasteele C (1998) Simulation of acid washing of municipal solid waste incineration fly ashes in order to remove heavy metals. J Hazard Mater 57(1):127–144
Van der Sloot H, Kosson D, Hjelmar O (2001) Characteristics, treatment and utilization of residues from municipal waste incineration. Waste Manag 21(8):753–765
Vavva C, Voutsas E, Magoulas K (2017) Process development for chemical stabilization of fly ash from municipal solid waste incineration. Chem Eng Res Des 125:57–71
Vehlow J, Bergfeldt B, Hunsinger H (2006) PCDD/F and related compounds in solid residues from municipal solid waste incineration—a literature review. Waste Manag Res 24(5):404–420
Viader RP, Jensen PE, Ottosen LM (2017) Electrodialytic remediation of municipal solid waste incineration residues using different membranes. Chemosphere 169:62–68
Wałek TT, Saito F, Zhang Q (2008) The effect of low solid/liquid ratio on hydrothermal synthesis of zeolites from fly ash. Fuel 87(15-16):3194–3199
Wan X, Wang W, Ye T, Guo Y, Gao X (2006) A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J Hazard Mater 134(1):197–201
Wan S, Zhou X, Zhou M, Han Y, Chen Y, Geng J, Wang T, Xu S, Qiu ZD, Hou H (2018) Hydration characteristics and modeling of ternary system of municipal solid wastes incineration fly ash-blast furnace slag-cement. Constr Build Mater 180:154–166
Wang KS, Chiang KY, Lin KL, Sun CJ (2001) Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 62(2):73–81
Wang Q, Tian S, Wang Q, Huang Q, Yang J (2008) Melting characteristics during the vitrification of MSWI fly ash with a pilot-scale diesel oil furnace. J Hazard Mater 160(2):376–381
Wang Q, Yang J, Wang Q, Wu T (2009) Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. J Hazard Mater 162(2):812–818
Wang F-H, Zhang F, Chen Y-J, Gao J, Zhao B (2015) A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater 300:451–458
Wang H, Zhu M, Sun Y, Ji R, Liu L, Wang X (2017) Synthesis of a ceramic tile base based on high-alumina fly ash. Constr Build Mater 155:930–938
Wang H, Fan X, Wang YN, Li W, Sun Y, Zhan M, Wu G (2018) Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J Enviro Manage 208:15–23
Wey MY, Liu KY, Tsai TH, Chou JT (2006) Thermal treatment of the fly ash from municipal solid waste incinerator with rotary kiln. J Hazard Mater 137(2):981–989
Yan D, Peng Z, Yu L, Sun Y, Yong R, Helge Karstensen K (2018) Characterization of heavy metals and PCDD/Fs from water-washing pretreatment and a cement kiln co-processing municipal solid waste incinerator fly ash. Waste Manag 76:106–116
Yang J, Wang Q, Luo Q, Wang Q, Wu T (2009a) Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger. Biochem Eng J 46(3):294–299
Yang GCC, Yang T-Y (1998) Synthesis of zeolites from municipal incinerator fly ash. Journal of Hazardous Materials 62(1):75–89
Yang J, Xiao B, Boccaccini AR (2009b) Preparation of low melting temperature glass-ceramics from municipal waste incineration fly ash. Fuel 88(7):1275–1280
Yoo YS, Jo JH (2015) Characteristics of MSWI ash and its application to zeolite synthesis. Materials Science Forum, Trans Tech Publ
Youcai Z, Lijie S, Guojian L (2002) Chemical stabilization of MSW incinerator fly ashes. J Hazard Mater 95(1):47–63
Yu J, Sun L, Xiang J, Hu S, Su S, Qiu J (2012) Vaporization of heavy metals during thermal treatment of model solid waste in a fluidized bed incinerator. Chemosphere 86(11):1122–1126
Yu J, Qiao Y, Jin L, Ma C, Paterson N, Sun L (2015) Removal of toxic and alkali/alkaline earth metals during co-thermal treatment of two types of MSWI fly ashes in China. Waste Manag 46:287–297
Zhan X, Wang L, Hu C, Gong J, Xu T, Li J, Yang L, Bai J, Zhong S (2018) Co-disposal of MSWI fly ash and electrolytic manganese residue based on geopolymeric system. Waste Manag 82:62–70
Zhang J, Klasky M, Letellier BC (2009) The aluminum chemistry and corrosion in alkaline solutions. J Nucl Mater 384(2):175–189
Zhang Y, Cetin B, Likos WJ, Edil TB (2016) Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 184:815–825
Zheng L, Wang C, Wang W, Shi Y, Gao X (2011) Immobilization of MSWI fly ash through geopolymerization: effects of water-wash. Waste Manag 31(2):311–317
Funding
The author, Zhenghui Phua, is thankful to the Interdisciplinary Graduate School (IGS) and Nanyang Environment and Water Research Institute (NEWRI), Nanyang Technological University (NTU) (Singapore), for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phua, Z., Giannis, A., Dong, ZL. et al. Characteristics of incineration ash for sustainable treatment and reutilization. Environ Sci Pollut Res 26, 16974–16997 (2019). https://doi.org/10.1007/s11356-019-05217-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05217-8