Abstract
Metal oxide nanoparticles have wide applications, which have elevated serious alarms about their impacts on the environment. Therefore, we investigated the potential adsorptive capacity of rice husk toward Fe2O3 and Al2O3 nanoparticles to reduce their genotoxic effects. Fish were subjected to 10 mg/l of Fe2O3 and Al2O3 nanoparticles in single and combined doses with and without rice husk water treatment for 7 days. The genotoxic effects were evaluated using the micronucleus test in the peripheral blood and comet assay in liver tissues. Significant elevation of micronuclei induction in addition to eight nuclear and cytoplasmic abnormalities (P < 0.05) was observed in all fish groups compared to the control groups. Fish that exposed to Fe2O3 nanoparticle showed the maximum induction of all recorded anomalies. Moreover, two indices of DNA damage were evaluated by the comet assay (comet score and % tail DNA) in liver tissues. The scoring of comet cells indicated that the highest frequencies of stage 0 (undamaged DNA) were in control and Al2O3 exposed groups, while stage 4 (extensive DNA damage) was significantly elevated in Fe2O3 exposed fish. The % of DNA damage was maximized in the Fe2O3 nanoparticles exposed fish and minimized in Al2O3 nanoparticles exposed fish. Based on the frequencies of nuclear anomalies, degree, and percentage of DNA damage, all rice husk treated groups showed a marked reduction in the genotoxic damage compared with untreated groups. Finally, both nanoparticles showed genotoxic potential and the rice husk had an efficient absorptive capacity for both of them individually or combined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rising exploration of nanotechnology has resulted in the identification of many useful properties of metallic nanoparticles (MNPs) such as enhanced magnetic, optical, and catalytic properties compared to their micro-sized counterparts (Rekulapally et al. 2019). Therefore, aquatic organisms have a greater occasion to be exposed to MNPs via the occupational environment and waste product discharges (Abdel-Khalek et al. 2016). Nanoparticles of Fe2O3 have an important role in many biomedical industries, such as magnetic resonance imaging contrast enhancement, delivery of many drugs, and hyperthermic destruction of tumor cells (Thorek et al. 2006; Cherukuri et al. 2010). While, Al2O3 NPs have been used up in many clinical applications in addition to the electronics, ceramics, and cosmetics industries (Lewis et al. 2010). The nano-toxicity is a problematic issue because the small size with a relatively large surface area of NPs resulted in high toxic properties compared to their micro-sized counterparts. However, it is not clear enough whether the high toxic properties are a specific feature of all nanoparticles with different chemical compositions or not (Jones and Grainger 2009; Landsiedel et al. 2009). Although the developing nano-technology, the information about the biological impacts of metal oxide NPs is still scarce especially those dealing with NPs-DNA interaction (Sayed 2016). The nano-size of different NPs facilitated their entrance mechanism to the biological system and consequently many vital tissues have been affected at the cellular and molecular levels (Abdel-Khalek 2016). As the blood is the main path for any foreign substance that enters the fish, the present work evaluated the induction of micronucleus (MN) and other nuclear anomalies (NAs) in the erythrocytes of the studied fish. The generation of MN was used as a sensitive genotoxicity test to detect clastogenic and aneugenic chromosomal alterations (Heddle et al. 1991 and Abdel-Khalek 2015). In addition, comet assay is the most common genotoxic biomarker that deals with strand breaks before the occurring of DNA repair systems (Kaygisiz and Ciğerci 2017). The comet assay is a useful technique to assess the severity of DNA damage in nucleated cells exposed to nano-genotoxic metals. Several studies revealed that MNPs could increase the production of reactive oxygen species (ROS) and/or interact with nucleic acid causes indirect and/or direct DNA damage (Abdel-Khalek 2016; Zhu et al. 2019). Thus, reducing NPs concentrations before their discharge into the aquatic ecosystem is necessary. Cheng et al. (2014) described the bio-adsorption as an effective method to remove metals in their ordinary size. Moreover, the dependence on renewable and biodegradable lingo-cellulosic agricultural or industrial by-products have been used in water purification and bioremediation processes (Krishnani and Ayyappan 2006). Although a large number of reports on the toxicological impacts of NPs have been published, there is very limited data about the treatment of those NPs. Therefore, the purpose of this study is to do the first trial to investigate the rice husk (RH) adsorptive capacity to Fe2O3 and Al2O3 NPs (in single and combined doses) with a focus on genotoxicity endpoints.
2 Materials and Methods
2.1 Preparation and Characterization of Nanoparticles
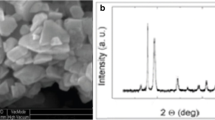
Both Fe2O3 and Al2O3 nano-powders were purchased from Sigma-Aldrich, St. Louis, MO, USA with sizes less than 50 nm (confirmed by Field Emission Transmission Electron Microscopy -FETEM, JEM-2100F, JEOL Inc., Japan at accelerating voltage of 200 kV). The molecular weight of Fe2O3 NPs was 159.69 with a surface area from 50 to 245 m2/g while the molecular weight of Al2O3 NPs was 101.96 with surface area > 40 m2/g (information provided by the manufacturer). The selected sub-lethal concentration (10 mg/l) was selected according to Saravanan et al. (2015); Murali et al. (2017); Canli et al. (2018). The NPs were prepared by dissolving the metal oxides nano-powders in the dechlorinated water (pH 7.4) and the quality assurance methods of preparation, handling, and preservation of samples were in accordance with USEPA procedures. Before use, samples were ultrasonicated about 1 h (100 W, 40 kHz) using an ultrasonic homogenizer (BioLogics, Inc., Manassas, VA, USA) to increase the dispersion of NPs.
2.2 Acclimatization of the Experimental Fish
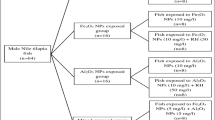
From an uncontaminated fish farm in El-Ismailia governorate (Egypt), sixty-four male fish were obtained with total length from 10.5–13 cm and 30–39.2 g bodyweight. With a good aeration condition, all fish were transported in a large plastic container to the ecology laboratory, Faculty of Science, Cairo University. Fish were maintained for 2 w in glass aquaria (40x70x26 cm) with 40 l of aerated, dechlorinated tap water with 8 fish per aquarium. The temperature of the water was kept at 25 ± 1 °C, while the dissolved oxygen and pH were 6.5–7.8 mg l−1 and 7.1–7.3, respectively. After 2 days of the transportation process, fish were started eating once per day with commercial pellet food (20% crude protein, 4% crude fat, 5% crude fiber, 12% crude ash, and 10% crude moisture). All procedures performed in this study involving animals (fish) were approved and were in accordance with the ethical standards of Faculty of Science, Cairo University, Institutional Animal Care and Use Committee (IACUC) at which the studies were conducted.
2.3 Preparation of Rice Husk (RH)
The RH obtained from rice mills, Kafr El-Sheikh, was cut into tiny parts, washed several times by deionized water and left to dry at 80 °C for 1 day. After that, the RH was minced and passed through a 70-mesh sieve (< 210 μm) to be ready for use. The used rice husk concentration was 50 mg/l (5 times the NPs concentration). In the aquaria, RH was isolated by a porous mesh that allows the passage of NPs to RH without escaping of RH to the water to avoid eating of RH by fish.
2.4 Experimental Design
Fish were divided into eight groups (for 7 days) as shown in Fig. 1. The conditions of the experiment were as the acclimatization period.
2.5 Fish Sampling
After the end of the experiment, blood samples from the caudal vein were withdrawn using heparin as an anticoagulant, and then the liver tissues were isolated for the comet assay.
2.6 Micronucleus Test
The blood samples were smeared on well dried microscopic slides. After air-drying of the blood smears (1 h at room temperature), each slide was fixed for 10 min in absolute methanol and left to air dry at room temperature for 30 min. In 5% Giemsa stain each blood smear was stained for 20 min and dipped in deionized water to remove any excess stain. All slides were coded and blind scoring of MN and other NAs were done according to the criteria described by Fenech et al. (2003). The examination process was done using ZEISS Primo Star light microscopy and photographed by Tucsen IS 1000, 10.0 MP Camera. For each group, 16 slides were prepared (2 slides/fish) to reduce the individual variation among erythrocytes. Totally 16,000 cells were scored for each group (2000 cells/fish) under oil immersion at a final magnification of 1000 × .
2.7 Comet Assay
According to Tice et al. (2000) and Recio et al. (2010), the alkaline (pH > 13) comet assay was performed. A small piece of liver tissues (1 cm2) was placed in 1 ml cold Hank’s balanced salt solution (HBSS; Ca++and Mg++ free) containing 20 mM EDTA and 10% DMSO and minced into fine pieces. After the precipitation of large pieces, 10 μl aliquot of cell suspension containing approximately 10.000 cells was mixed with 80 μl of 0.5% low melting point agarose (Sigma) and spread on a fully frosted slide pre-dipped in normal melting agarose (1%). After solidification (on a cold surface), the slides were placed in cold lysis buffer (2.5 M NaCl, 100 mM EDTA and 10 mM Tris, pH 10, with freshly added 10% DMSO and 1% Triton X-100) for 24 h at 4 °C in dark conditions. The slides were incubated for 20 min in a fresh alkaline buffer (300 mM NaOH and 1 mM EDTA, pH > 13) then electrophoresis was done for 20 min at 300 mA and 25 V (0.90 V/cm) for unwinding DNA. To neutralize the alkali, slides were immersed in an excess amount of 0.4 M Trizma base (pH 7.5). Then slides were fixed in 100% cold ethanol, air-dried and finally stored at room temperature in a desiccator until the scoring process. The slides were stained with ethidium bromide (2 μg/ml) then the extent of migration among cells for each sample was recorded by simultaneous image capture and examined at ×400 magnification power using Komet 5 image analysis software developed by Kinetic Imaging, Ltd. (Liverpool, UK). The DNA damage was evaluated and compared among the different groups using two DNA damage indices. First, the comets were classified into 5 stages (stages 0–4) in relation to the intensity and the length of the comet tail according to Liao et al. (2009). Four hundred comets per group were randomly selected (4 different slides from each group; 100 cell/slide), and the comets in each stage were assessed. Second, the percentage of the DNA in the comet tail (% tail DNA) in each cell (200 cells/group) were estimated. The % tail DNA is the intensity of all tail pixels/the total intensity of all pixels in the comet and expressed as a percentage.
2.8 Statistical Analysis
All statistical analyses were done using Statistical Processor Systems Support, SPSS software, version 16.0, IBM, Chicago, IL, USA. The results were expressed as the mean (mean%) ± SE. Data were statistically analyzed with analyses of variance F-test and Duncan’s multiple ranges to assess the comparability between the different experimental groups as indicated by different letters in the descending order at p < 0.05 as a significance level. Data were also statistically analyzed using the Student t test to estimate the significant difference between RH treated and untreated groups at the same significant level.
3 Results
3.1 Micronucleus Test
To evaluate the genotoxic and clastogenic effects of the studied metal oxides NPs, frequencies of MN in addition to other 8 nuclear and cytoplasmic deformities have been observed and scored in the peripheral erythrocytes of the studied fish. As shown in Table 1, the different metal oxide NPs exposed groups showed a remarkable increase (p < 0.05) in all recorded abnormalities compared to the control groups (Duncan’s test capital letters). Comparing the frequencies of MN of RH treated and untreated water groups that exposed to the same metal oxide NPs showed that the recorded MN was started to decrease significantly after the RH treatment (Duncan’s test small letters). The other NAs had the same trend as MN whereas the induction of most studied anomalies showed a significant decrease after the RH water treatment. Generally, the clastogenic effects of studied treatments were represented by the formation of micronuclei in the following order: Control groups < Al2O3 NPs exposed groups < mixture exposed groups < Fe2O3 NPs exposed groups.
All recorded nuclear and cytoplasmic abnormalities were represented in Fig. 2.
Representative nuclear and cytoplasmic alterations in erythrocytes of O. niloticus exposed to Fe2O3 and Al2O3 NPs (in single and combined doses) after and before rice husk (RH) water treatment. Estimated means of the recorded nuclear and cytoplasmic abnormalities with and without RH water treatment. A, micro-nucleated erythrocyte; B, notched nucleus, C, bi-nucleated erythrocyte; D, nuclear bud; E, cell with two MN; F, karyolysis; G, fragmented nucleus; H, polynucleated erythrocyte; I, vacuolated cytoplasm. ×1000 magnification.
3.2 Comet Assay
According to Liao et al. (2009), the comets were classified into 5 stages (Fig. 3) in relation to the intensity and the length of the comet tail. The first class was stage 0 (no DNA damage), the second class was stage 1 (mild DNA damage), the third class was stage 2 (moderate DNA damage), the fourth class was stage 3 (high DNA damage),, and the fifth class was stage 4 (extensive DNA damage). As shown in Table 2, the scored liver cells of the control groups displayed the maximum % of stage 0 and the absence of stage 3 and stage 4. The liver cells of the Fe2O3 NPs exposed groups showed the highest-scoring values for stage 3 and stage 4 followed by mixture exposed groups. The recorded % of stage 3 and stage 4 was significantly decreased in all exposed groups after RH treatment except for Al2O3 NPs exposed groups. Based on % tail DNA, the DNA damage was increased significantly in all exposed fish (Fig. 4) compared to the control groups. The liver tissues of Fe2O3 NPs exposed fish showed maximum DNA damage followed by mixture exposed groups then Al2O3 NPs exposed groups. The decreased % tail DNA in all RH treated groups was observed in comparison to RH untreated groups.
The percentage of DNA damage in liver tissues of O. niloticus exposed to Fe2O3 and Al2O3 NPs (in single and combined doses) after and before rice husk (RH) water treatment. aData are represented as means of four samples ± SE.bThe small letters represent the Duncan’s test (p < 0.05) between rice husk treated and untreated groups of the same group. Columns with the same letters are not significantly different; otherwise, they do.cThe capital letters represent Duncan’s test (p < 0.05) between different NPs exposed groups compared with control groups. Columns with the same letters are not significantly different; otherwise, they do
4 Discussion
Metallic nanoparticles have provided a key role in the development of many biomedical, bioengineering, electronics, and cosmetic industries (Thorek et al. 2006; Cherukuri et al. 2010). The nano-genotoxicity has major environmental concerns due to the ability of NPs to invade the nucleus and trigger chromosomal break, mitotic spindle malfunction and/or DNA damage through different mechanisms. The nano-genotoxicity needs to be sensibly evaluated to reduce various genetic disorders that could be spread among generations. Compared with other animals, fish have a very slow repairing mechanism, therefore, they might be used as an ideal species to check the presence of genotoxic substances in water (Mahboob et al. 2014). Among many genotoxic techniques, recording the induction rates of MN and other NAs is considered a sensitive sign of genotoxic exposure (Abdel-Khalek 2015, 2016). Based on the recoded nuclear deformities, Fe2O3 NPs showed the highest clastogenic effects on the studied fish compared with weak clastogenic effects of Al2O3 NPs. This result agreed with Song et al. (2012) who revealed the high genotoxic impacts of iron NPs and their high ability to induce MNs to the generation of oxidative stress via the Fenton reaction and their physical interaction with the genetic materials. While several studies reported that Al2O3 NPs did not cause genotoxic effects using various methods (Sadiq et al. 2015; Rajiv et al. 2016). Size, surface properties, and aggregation power are some of the major characteristics that classify the toxicity of different NPs. The high genotoxicity of Fe2O3 NPs compared to Al2O3 NPs with the same concentration may be due to the high adhesion tendency of iron nanoparticles to the surfaces of the gills (observed as reddish-brown aggregates) which could cause physical injury to the epithelial cell and enable the entry of Fe2O3 NPs into the bloodstream. This observation was confirmed by Chen et al. (2012) who reported high aggregation potency and toxic effects of nanoscale iron in medaka fish. The invading of NPs to the peripheral erythrocytes may induce spindle imperfections during the segregation progression in anaphase and leading to the formation of chromosomal fragments that can not join with the daughter nuclei (Fenech et al. 2011). Besides the MN there are other nuclear abnormalities that indicated the malfunctions in segregating tangled or gene amplification mechanisms during the amplified DNA elimination process (Khan et al. 2017). The recorded cytoplasmic vacuolation revealed a disruption in ionic balance and sometimes irreversible vacuolization gives an early sign of cell death (Aki et al. 2012).
To give a complete picture of the genotoxic effects of the studied NPs, comet assay was applied to the liver tissues which represents the major organ for metal detoxification in fish (Abdel-Khalek et al. 2020). As previously confirmed by Nabiev et al. (2007), particles with size < 30 nm could enter the nucleus through the nuclear pore and directly might interact with the DNA in a nano-specific mechanism. The actual size ranges of Fe2O3 and Al2O3 NPs was17.3–34.8 nm 21.4–44.5 nm respectively as confirmed by Field Emission Transmission Electron Microscopy therefore both NPs have a high probability to invade the nucleus and cause DNA damage. Numerous valuable results have been obtained using the comet assay to evaluate nano-genotoxicity of different NPs (Kazimirova et al. 2019). In the present study, two indices of DNA damage were evaluated in the liver by the comet assay (comet score and % tail DNA) indicated the high genotoxic potency of Fe2O3 NPs compared by Al2O3 NPs. These results may be due to the smaller size of Fe2O3 NPs and the tendency of the liver to store the excess Fe in the form of heme protein and ferritin for various metabolic activities which increase the hepatic iron content and consequently the DNA damage (Javed et al. 2016). The exposure to genotoxic NPs could induce DNA breakdown and relaxing the DNA supercoiling, therefore, negatively charged DNA fragments are enabled to travel through agarose gel under the effect of an electric field. The degree of DNA migration directly proportions with the level of DNA damage giving the image as comet-like form (Kazimirova et al. 2019). The scoring of comet cells indicated that the highest frequencies of stage 0 (undamaged DNA) were in control and Al2O3 NPs groups while stage 4 (extensive DNA damage) was markedly elevated in Fe2O3 NPs exposed fish. In addition, the values of % tail DNA confirmed the potent genotoxic ability of the Fe2O3 NPs compared to Al2O3 NPs. The high genotoxic potency of Fe2O3 NPs (< 50 nm) was also reported by Kaygisiz and Ciğerci (2017) who revealed this strong genotoxicity to their ability to induce DNA breaks and oxidative DNA damage, i.e., Fe2O3 NPs could induce both direct and indirect DNA damage. Moreover, the results of the present work showed that the combination of both NPs caused an intermediate level of genotoxicity because the co-exposure could alter the bioavailability and aggregation tendency of the NPs affecting their properties (Mottola et al. 2019).
As an efficient method, the adsorption process has verified to be the most effective method for the removal of metals from metal-polluted water (Acharya et al. 2009). In this context, the present study was the first approach that tested the efficiency of RH to decrease the toxic properties of metal oxide NPs. The results revealed that RH untreated groups showed higher frequencies of nuclear abnormalities and higher % of DNA damage than RH treated groups. This goes with the hypothesis of the present study and proved that RH has probable biosorption capacity (removal of the substance by biological material) toward the studied metal oxides NPs. The removal efficiency of RH is due to the high adsorptive capacity due to its high cellulose, hemicellulose, lignin, and silica content (Srivastava et al. 2006). Therefore, more studies are needed to improve the RH removal efficiency towards other NPs.
5 Conclusions
It can be concluded that both Fe2O3 NPs and Al2O3 NPs in single and combined doses could induce genotoxic damage to Oreochromis niloticus. Based on the studied genotoxic endpoints such as MN and comet assay, the Fe2O3 NPs had higher genotoxic potency than Al2O3 NPs, while the combined dose of both metal NPs showed an intermediate genotoxic effect. The recorded results confirmed that RH had a good adsorptive capacity for both metal oxides NPs and could reduce their genotoxic effects in fish.
References
Abdel-Khalek, A. A. (2015). Antioxidant responses and nuclear deformations in freshwater fish, Oreochromis niloticus, facing degraded environmental conditions. Bulletin of Environmental Contamination and Toxicology, 94(6), 701–708.
Abdel-Khalek, A. A. (2016). Comparative evaluation of genotoxic effects induced by CuO bulk and nano-particles in Nile Tilapia, Oreochromis niloticus. Water, Air and Soil Pollution, 227, 35.
Abdel-Khalek, A. A., Badran, S. R., & Marie, M.-A. S. (2020). The efficient role of rice husk in reducing the toxicity of iron and aluminum oxides nanoparticles in Oreochromis niloticus: Hematological, bioaccumulation, and histological endpoints. Water, Air and Soil Pollution, 231, 53.
Abdel-Khalek, A. A., Hamed, A., & Marie, M.-A. S. (2016). The accumulation potency of bulk and nano zinc metal and their impacts on the hematological and histological perturbations of Oreochromis niloticus. Water, Air and Soil Pollution, 227, 206.
Acharya, J., Sahu, J., Mohanty, C., & Meikap, B. (2009). Removal of lead (II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chemical Engineering Journal, 149, 249–262.
Aki, T., Nara, A., & Uemura, K. (2012). Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biology and Toxicology, 28, 125–131.
Canli, E. G., Dogan, A., & Canli, M. (2018). Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environmental Toxicology and Pharmacology, 62, 181–187.
Chen, P. J., Tan, S. W., & Wu, W. L. (2012). Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in medaka fish. Environmental Science and Technology, 46(15), 8431–8439.
Cheng, C., Wang, J., Yang, X., Li, A., & Philippe, C. (2014). Adsorption of Ni (II) and cd (II) from water by novel chelating sponge and the effect of alkali-earth metal ions on the adsorption. Journal of Hazardous Materials, 264, 332–341.
Cherukuri, P., Glazer, E. S., & Curley, S. A. (2010). Targeted hyperthermia using metal nanoparticles. Advanced Drug Delivery Reviews, 62, 339–345.
Fenech, M., Chang, W. P., Kirsch-Volders, M., Holland, N., Bonassi, S., & Zeiger, E. (2003). HUMN project: Detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutation Research, 534(1–2), 65–75.
Fenech, M., Kirsch-Volders, M., Natarajan, A. T., Surralles, J., Crott, J. W., Parry, J., Norppa, H., Eastmond, D. A., Tucker, J. D., & Thomas, P. (2011). Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis, 26(1), 125–132.
Heddle, J. A., Cimino, M. C., Hayashi, M., Romagna, F., Shelby, M. D., Tucker, J. D., Vanparys, P., & MacGregor, J. T. (1991). Micronuclei as an index of cytogenetic damage: Past, present, and future. Environmental and Molecular Mutagenesis, 18, 277–291.
Javed, M., Ahmad, I., Usmani, N., & Ahmad, M. (2016). Bioaccumulation, oxidative stress and genotoxicity in fish (Channa punctatus) exposed to a thermal power plant effluent. Ecotoxicology and Environmental Safety, 127, 163–169.
Jones, C. F., & Grainger, D. W. (2009). In vitro assessments of nanomaterial toxicity. Advanced Drug Delivery Reviews, 61, 438–456.
Kaygisiz, S. Y., & Ciğerci, İ. H. (2017). Genotoxic evaluation of different sizes of iron oxide nanoparticles and ionic form by SMART, Allium and comet assay. Toxicology and Industrial Health, 33(10), 802–809.
Kazimirova, A., Baranokova, M., Staruchova, M., Drlickova, M., Volkovova, K., & Dusinska, M. (2019). Titanium dioxide nanoparticles tested for genotoxicity with the comet and micronucleus assays in vitro, ex vivo and in vivo. Mutation Research, 843, 57–65.
Khan, M. S., Qureshi, N. A., & Jabeen, F. (2017). Assessment of toxicity in freshwater fish Labeo rohita treated with silver nanoparticles. Applied Nanoscience, 7, 167–179.
Krishnani, K. K., & Ayyappan, S. (2006). Heavy metals remediation of water using plants and lignocellulosic agrowastes. Reviews of Environmental Contamination and Toxicology, 188, 59–84.
Landsiedel, R., Kapp, M. D., Schulz, M., Wiench, K., & Oesch, F. (2009). Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations—Many questions, some answers. Mutation Research, 681, 241–258.
Lewis, W. K., Harruff, B. A., Gord, J. R., Rosenberger, A. T., Sexton, T. M., Guliants, E. A., & Bunker, C. E. (2010). Chemical dynamics of aluminum nanoparticles in ammonium nitrate and ammonium perchlorate matrices: Enhanced reactivity of organically capped aluminum. Journal of Physical Chemistry C, 115, 70–77.
Liao, W., McNutt, M. A., & Zhu, W. (2009). The comet assay: A sensitive method for detecting DNA damage in individual cells. Methods, 48, 46–53.
Mahboob, S., Al-Balwai, H. F. A., Al-Misned, F., & Ahmad, Z. (2014). Investigation on the genotoxicity of mercuric chloride to freshwater Clarias gariepinus. Pakistan Veterinary Journal, 34, 100–103.
Mottola, F., Iovine, C., Santonastaso, M., Romeo, M. L., Pacifico, S., Cobellis, L., & Rocco, L. (2019). NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials, 9(11), 1511.
Murali, M., Suganthi, P., Athif, P., Bukhari, A. S., Mohamed, S. H. E., Basu, H., & Singhal, R. K. (2017). Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. Journal of Trace Elements in Medicine and Biology, 44, 125–131.
Nabiev, I., Mitchell, S., Davies, A., Williams, Y., Kelleher, D., Moore, R., Gunko, Y. K., Byrne, S., Rakovish, Y. P., Donegan, J. G., Sukhanova, A., Conroy, J., Cottell, D., Gaponik, N., Rogach, A., & Volkov, Y. (2007). Non-functionalized nanocrystals can exploit a cell’s active transport machinery delivering them to specific nuclear and cytoplasmic compartments. Nano Letters, 7(11), 3452–3461.
Rajiv, S., Jerobin, J., Saranya, V., Nainawat, M., Sharma, A., Makwana, P., Gayathri, C., Bharath, L., Singh, M., Kumar, M., Mukherjee, A., & Chandrasekaran, N. (2016). Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Human and Experimental Toxicology, 35(2), 170–183.
Recio, L., Hobbs, C., Caspary, W., & Witt, K. L. (2010). Dose-response assessment of four genotoxic chemicals in a combined mouse and rat micronucleus (MN) and comet assay protocol. The Journal of Toxicological Sciences, 35(2), 149–162.
Rekulapally, R., Murthy Chavali, L. N., Idris, M. M., & Singh, S. (2019). Toxicity of TiO2, SiO2, ZnO, CuO, au and Ag engineered nanoparticles on hatching and early nauplii of Artemia sp. Peer Journal, 6, 1–16.
Sadiq, R., Khan, Q. M., Mobeen, A., & Hashmat, A. J. (2015). In vitro toxicological assessment of iron oxide, aluminum oxide and copper nanoparticles in prokaryotic and eukaryotic cell types. Drug and Chemical Toxicology, 38(2), 152–161.
Saravanan, M., Suganya, R., Ramesh, M., Poopal, R. K., Gopalan, N., & Ponpandian, N. (2015). Iron oxide nanoparticles induced alterations in haematological, biochemical and ionoregulatory responses of an Indian major carp Labeo rohita. Journal of Nanoparticle Research, 17, 274–285.
Sayed, A. E. D. H. (2016). Genotoxicity detection following exposure to silver nanoparticles in African catfish (Clarias gariepinus). International Journal of Nanoparticles, 9(1), 41–53.
Song, M. F., Li, Y. S., Kasai, H., & Kawai, K. (2012). Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. Journal of Clinical Biochemistry and Nutrition, 50(3), 211–216.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2006). Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. Journal of Hazardous Materials, 134(1–3), 257–267.
Thorek, D. L., Chen, A. K., Czupryna, J., & Tsourkas, A. (2006). Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Annals of Biomedical Engineering, 34, 23–38.
Tice, R. R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Miyamae, Y., Rojas, E., Ryu, J.-C., & Sasaki, Y. F. (2000). Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis, 35, 206–221.
Zhu, B., He, W., Hu, S., Kong, R., & Yang, L. (2019). The fate and oxidative stress of different sized SiO2 nanoparticles in zebrafish (Danio rerio) larvae. Chemosphere, 225, 705–712.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through research group project under grant number (R.G.P.1–56–40) and to the Faculty of Science, Cairo University, Egypt for supporting the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript complies with the ethical rules applicable for this journal.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Dajem, S.B. & Morsy, K. The Potential Use of Rice Husk for Reducing the Genotoxic Effects of Iron and Aluminum Oxides Nanoparticles in Oreochromis niloticus. Water Air Soil Pollut 231, 139 (2020). https://doi.org/10.1007/s11270-020-04495-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04495-0