Abstract
In this study, a novel, simple, and highly sensitive analytical procedure for the quantitative evaluation of oxygenated and nitrated polycyclic aromatic hydrocarbons in volcanic ash samples based on dispersive solid–liquid microextraction (DSLME) coupled to ultra high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) was developed. Diverse chemometric tools were applied to optimize DSLME working conditions. Thus, a linear calibration curve for all the target analytes in the concentration range from 0.01 to 100 μg g−1 (r2 > 0.994) was obtained. The limits of detection for all the compounds were between 14.6 and 56.0 pg g−1, with high reproducibility (relative standard deviation (RSD) was below 8.1% for all the analytes). Additionally, recoveries ranged from 94.2 to 100%. The applicability of the method was evaluated and the feasibility of the existence of nitrated and oxygenated-PAHs in volcanic ashes at ultra-trace levels was demonstrated, which reveals an unknown source of distribution of these pollutants to the environment.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volcanic activity is a natural source of pollution, which contributes considerable amounts of pollutants, mainly to the atmosphere. The impact of volcano eruptions on the environment and the atmosphere chemistry has been informed (Ivy et al. 2017; von Glasow et al. 2009). Several interesting works have been published focusing on the impact of a spectrum of volcano-induced pollutants on the environment (Cabré et al. 2016; Geyer et al. 2017; Ilyinskaya et al. 2017; Ivy et al. 2017). Also, the risks this activity represents for ecosystems and human populations that are located near volcanic sites have been documented (Cabré et al. 2016; Ilyinskaya et al. 2017; Vigneri et al. 2017). In addition to the effects, particles smaller than 2 μm in diameter form the plume that can travel long distances downwind and generate the characteristic ash falls (Geyer et al. 2017; Vigneri et al. 2017). Particulate matter (PM) weathering has been investigated (Ilyinskaya et al. 2017), but only a limited amount of information is available regarding the organic compounds present in volcanic ash (Kozak et al. 2017; Tomašek et al. 2018).
Polycyclic aromatic hydrocarbons (PAHs) and nitrated (nitro-PAHs) and oxygenated (oxy-PAHs) derivative forms are persistent environmental contaminants and can be also transported, resulting in exposure from multimedia pathways, such as air, soil, sediment, water, and plants. Thus, their monitoring in these samples is of concern because of their potential bioaccumulation and toxicity, including carcinogenicity and mutagenicity (Humans 2010; Lam et al. 2018; Tang et al. 2019; Tiwari et al. 2019).
As known, PAHs complex mixtures and derivatives are produced by incomplete combustion of fossil fuels (Wang et al. 2016), biomass (de Oliveira Galvão et al. 2018), forest fires (Campo et al. 2017), volcanic eruptions (Kozak et al. 2017), among others. A previous investigation demonstrated that by heating a mixture composed by H2O/CO2/NH3, simulating the composition of volcanic gas in a reactor containing volcanic ash at 340–1000 °C, PAHs are formed together with nitrogen-containing organic compounds (Stracquadanio et al. 2003). Although a recent study reported PAH concentrations in the order of ng g−1 levels in volcanic ashes (Kozak et al. 2017; Stracquadanio et al. 2003), the presence and amounts of nitrated and oxygenated PAH derivatives in this type of samples have not yet been reported.
Nitrated and oxygenated PAHs are considered as pollutants that require scientists to design analytical methodologies to separate and to determine them with high sensitivity (Lundstedt et al. 2007; Tang et al. 2019). Measurements of nitro-PAHs and oxy-PAHs in complex environmental matrices tend to involve difficult analytical chemistry procedures. Generally, the extraction of these compounds in solid samples (soils, food, plant material, fish, sediments, airborne particulate matter) involves the use of large volumes of solvents, such as dichloromethane (DCM), methanol (MeOH), acetone, and solvent mixtures, as well as Soxhlet-based procedures, ultrasonic extraction, pressurized liquid extraction (also called accelerated solvent extraction), microwave extraction, automated shaking, supercritical fluid extraction, among others alternatives (Bandowe and Meusel 2017; Lundstedt et al. 2007).
Currently, substantial efforts have been made to adjust traditional extraction methods and to develop new strategies to save time, labor, and materials. In this sense, since its introduction in 2006 (Rezaee et al. 2006), the dispersive liquid microextraction (DLLME) has undergone a rise in popularity due to its capability to practically, instantaneously, allow the extraction. It has been applied mainly for the removal of numerous compound in water samples (Canales et al. 2017; Chormey et al. 2018; Kaw et al. 2018; Leong et al. 2014; Lu et al. 2019; Rezaee et al. 2010). In the case of solid matrices, a previous transference of the analytes to an aqueous solution is needed (Leong et al. 2014; Rezaee et al. 2010). For optimization, several extraction variables, among them nature and volume of extraction and dispersive solvents, ionic strength, pH, mixing assistance (manual-, vortex-, and ultrasonic-assisted modes), and the time required should also be studied (Leong et al. 2014; Rezaee et al. 2006; Rezaee et al. 2010). The “one-variable-at-a-time” optimization procedure does not guarantee the attainment of a true optimum working condition (Mas et al. 2010). It is well known that the use of chemometric tools helps the analyst to perform a better optimization of the experimental development, saving time and materials. In general, from any chemometric perspective, a screening step, where many factors are studied to identify those which are significant and, the optimization itself to obtain the best experimental conditions should be considered for optimization (Myers et al. 2016; Vera Candioti et al. 2014). Thus, a central composite uses quantitative data to fit a second-order response surface (Vera Candioti et al. 2014).
The goal of this work was based on the development and optimization of methodology for the sensitive quantification of nitrated and oxygenated PAHs in volcanic ashes. An original approach was developed as an alternative to performing DLLME, which is based on “dispersive solid–liquid microextraction (DSLME)” (Guiñez et al. 2020). Relevant microextraction factors were optimized by applying both experimental design and response surface methodology, respectively. Thus, DSLME was applied to the quantitative extraction of nitrated and oxygenated-PAHs from volcanic ash samples. Afterward, trace level determination by chromatographic separation coupled to atmospheric pressure ionization and tandem mass spectrometry (UHPLC-APCI-MS/MS) was performed.
Material and methods
Reagents and instrumentation
Chemical standards of nitrated and oxygenated PAH derivatives, 1-nitropyrene (1-NPYR), 2-nitrofluorene (2-NFLU), 3-nitrofluoranthene (3-NFLUANTH), 9-nitroanthracene (9-NANTHR), 5,12-naphthacenedione (5,12-NAPHTONE), 9,10-anthracenedione (9,10-ANTHRONE), and 2-fluorenecarboxaldehyde (2-FLUCHO), were purchased from Sigma Chemical (St. Louis, MO, USA). Acetonitrile (ACN), methanol (MeOH), and water Optima® LC-MS grade and acetone, cyclohexane, toluene, and n-hexane HPLC® grade obtained from Fisher Scientific (Fair Lawn, New Jersey). Formic acid was purchased from Fisher Scientific (Loughborough, UK). Ultrapure water (18 MΩ cm) from a Milli-Q water purification system from EASY pure (RF Barnstead, IA, USA) was used.
Acetonitrile-based working standard solutions were prepared by stepwise dilution from 100.0 mg L−1 stock standard solutions of each compound. The daily standard working solutions at different concentration levels were obtained by diluting the stock solutions. All solutions prepared were stored in dark containers at 4 °C.
The laboratory apparatus used included an ultrasonic water bath (Testlab TB-10TA., Argentina), a vortex (Arcano HX-2000-1, Argentina), and a centrifuge (U-320R-BOECO, Germany).
Instrument and analytical conditions
An Acquity™ Ultra High Performance LC system (Waters, Milford) equipped with autosampler injection and pump systems (Waters, Milford) was used for nitro-PAH and oxy-PAH separation. The autosampler vial tray was maintained at 4 °C. The separation was performed by injecting 10-μL sample onto an ACQUITY UPLC® BEH Phenyl (Waters, Milford, USA) analytical column with 2.1 mm internal diameter, 100 mm length, and 1.7 μm particle size. The binary mobile phases consisted of water with 0.1% (v/v) of formic acid (A) and acetonitrile with 0.1% (v/v) of formic acid (B).
Mass spectrometry analyses were performed on a Quattro PremierTM XE Micromass MS Technologies, triple quadrupole mass spectrometer with a ZSprayTM equipped with an APCI interface (Waters, Milford, USA) configured in positive ion mode. Quantitative determination was performed in multiple reaction monitoring (MRM) mode of selected. The MRM conditions were further optimized for each analyte to obtain maximum sensitivity and the most sensitive transitions for each compound were selected for quantification. The chromatographic conditions and mass spectrometric instrumental parameters were the same as in our previous work (Guiñez et al. 2018).
Sampling and sample preparation
The volcanic ash fall samples of Puyehue, Copahue, and Calbuco volcanoes (Chile) were collected from touristic places located in the Neuquén Province (Argentina) as shown in Fig. 1. Volcanic ash particulate material was collected from sites: (I) San Martín de Los Andes (ashes from the Puyehue volcano), between June and July (winter in the Southern Hemisphere), 2011; (II) Cutral Co (ashes from the Copahue volcano), between December and January (summer in the Southern Hemisphere), 2012–2013; and (III) Villa La Angostura (ashes from the Calbuco volcano), between March and April (autumn in the Southern Hemisphere), 2015. Polyethylene bags were used for sample collection. These containers were wrapped in aluminum foil and stored in a dry and dark place at room temperature. Spiked samples were prepared by the addition of specific volumes of standard solutions to 0.5-g samples. The mixtures were stirred for 1 min and then incubated at 35 °C for 3 h before analysis.
Volcanoes locations and samples collecting areas (I–III). Volcanoes are located in the border between Chile and Argentina. Distances between volcanoes and sampling sites are shown. Source: Data from the Google Maps (https://maps.google.com/)
Conditions for DSLME
The schematic diagram of the DSLME is shown in Fig. 2. A 0.5 g of the dry sample was placed into a screw cap glass test tube and spiked with 20 ng g−1 of nitro-PAH and oxy-PAH standard mixture. After complete mixing of the sample and evaporation of the solvent, 7.5 mL of water at 35 °C was directly added into the sample and the glass tube was vigorously vortexed for a few seconds. In this step, a suspension of volcanic ash in water (“slurry sampling” (Resano et al. 2008)) was formed. Immediately, a mixture of 0.7 mL of acetone (dispersive solvent) and 0.8 mL of n-hexane (extraction solvent) was introduced and vortex-mixed for 1 min. Consequently, a cloudy solid-liquid suspension (consisting of volcanic ash, water, acetone, and n-hexane) was observed, which resulted from the dispersion of fine n-hexane droplets in the suspension formed in the test tube. Then, this mixture was centrifuged for 5 min at 3500 rpm (1291.3g) to favor phase separation. The resulting pellet was moved into a vial and dried under a N2 stream, then 100 μL of acetonitrile was added, and the vial was vortexed (30 s) for subsequent analysis.
Statistical methods for optimization
A two-level full factorial (24) approach was used for screening to obtain the most significant variables that affect the microextraction in volcanic ashes. Subsequently, experimental parameters showing significant effects such as water, extractant, and dispersive solvent volume were studied in a CCD design, which consisted of 20 experiments. Finally, the desirability function (D) was used to fully optimize the multiple-response analysis. This function is a value between 0 and 1 and increases as the corresponding response value becomes more desirable. The Design-Expert statistical package software version 7.0.5 (Minneapolis, USA) was employed to analyze the obtained data.
Results and discussion
Multivariate optimization of DSLME conditions
Factorial design
An adequate extraction solvent in the dispersive-based liquid microextraction procedure should have properties such as lower density and solubility in water and good extraction capability of the analytes (Guiñez et al. 2017; Leong et al. 2014; Ming-Jie et al. 2015). In this regards, in previous studies, different solvents including cyclohexane (δ: 0.78 g mL−1), n-hexane (δ: 0.66 g mL−1), and toluene (δ: 0.87 g mL−1) as extraction solvents and acetone, acetonitrile and ethanol as dispersive solvents based on their extraction efficiency of the compounds under study. From the obtained results, n-hexane and acetone were adopted for further experiments, because they showed higher extraction efficiency for all nitrated and oxygenated PAHs studied.
The purpose of DSLME optimization was to select the appropriate main variables affecting the extraction performance of the compounds in the volcanic ash samples. Thus, a factorial experimental strategy was chosen because it reduces the number of experiments and no loss of significant information occurs. Although the design considered does not determine the exact quantity, it can provide some important information about each variable by relatively few experiments (Myers et al. 2016; Vera Candioti et al. 2014). Moreover, this approach involves three central points to estimate the experimental error (pure error). In this work, the analyzed variables were evaluated at two levels, which were selected according to previous experiments (Canales et al. 2017; Guiñez et al. 2018; Guiñez et al. 2020; Guiñez et al. 2017). They were (A) water volume (5 mL to 10 mL), (B) n-hexane volume (0.5 mL to 1 mL), (C) acetone volume (0.5 mL to 1 mL), and (D) vortex agitation time (1 min to 5 min). The experimental responses were calculated as the chromatographic peak area obtained for each nitro- and oxy-PAHs. The design matrix used in the 24 full factorial design, with the responses for each analyte, is shown in Table S1-Supplementary material. All experiments were conducted under random order and homogeneous conditions to minimize the effects of uncontrolled factors. Different tools were used to analyze the effects of the factors: Pareto chart and analysis of variance (ANOVA). The ANOVA test according to Fisher’s statistical analysis was applied to the experimental data to obtain an estimate of standard errors in the coefficients. The variables were shown to be significant (p value < 0.05), with positive and negative effects (Table 1). Similar to previous work for PAHs (Fernández et al. 2015), different results were observed for nitro-PAHs and oxy-PAHs, probably because of the different physicochemical characteristics of these compounds. Pareto charts were also examined to define the influential factors (Fig. S1-Supplementary material). The effects exceeding the statistical limit of Bonferroni and the t value limit were considered significant.
As a general conclusion of this analysis, water volume (A), n-hexane volume (B), and acetone volume (C) factors and the double-interaction (AB, AC, and BC) demonstrated to have significant effects on the efficiency extraction. Also, a significant high triple-interaction effect (ABC) was observed for all nitro-PAHs and oxy-PAHs under study. These results are usually observed for dispersive liquid microextraction methodologies, given that both extraction and dispersive solvent volumes are extremely important variables that must be optimized in the development of this type of strategy (Rezaee et al. 2010). On the other hand, in techniques of “slurry sampling” (suspension of the solid sample), the volume of water (A) is also a fundamental variable to be studied (Resano et al. 2008). The analysis of the effects of the variables on the responses allowed to conclude that the vortex time (D) was the only factor with no significant influence on the extraction method and it showed no interaction with the others (see Fig. S1-Supplementary material), probably because the large surface area created between aqueous solution and extraction solvent (Leong et al. 2014; Rezaee et al. 2010). Therefore, the transition of the analytes between these phases formed is very fast and agitation times greater than 1 min do not affect the efficiency extraction. Consequently, the vortex time is the factor that remains fixed (1 min), while the other factors were included in the following optimization process.
Response surface design
The second step was to optimize the previously observed affecting variables for the proposed DSLME technique. Thus, water, extraction, and dispersive solvent volumes were studied using a central composite design. The CCD consisted of 20 experiments in the following ranges: (A) water volume (5–10 mL), (B) extraction solvent volume (0.5–1 mL), and (C) dispersive volume (0.5–1 mL). Thus, a single block rotatable design (α = 2.0), with six central points, was built. Table S2-Supplementary material summarizes the experimental design matrix altogether with the responses obtained (peak area) for each nitro-PAHs and oxy-PAHs studied.
To determine a critical point, it is necessary for the polynomial function to contain a quadratic model (Table 2). The results obtained, p value at 95% confidence level, demonstrated that the water volume (A) affects the extraction (peak area) of all of the compounds. On the other hand, except for 9-NANTHR and 5,12-NAPTHONE, all responses were affected by the extractant volume, n-hexane (B). Finally, the acetone volume (C) only affected the responses of 1-NPYR and 9,10-ANTHRONE. Besides, the two interactions (A2, B2, and C2) were statistically significant for all the compounds’ responses.
ANOVA and regression analysis were used to assess the significance of the variables (Table 3). As can be seen, quadratic models (with p value < 0.05) are those that better explain the extraction efficiency for all the compounds under study. The model coefficients were calculated by multiple regression and validated by ANOVA (Table S3-Supplementary material). The coefficient of variation ranged between 1.01 and 28.87%. The model presented high determination coefficients (between 0.854 and 0.999) for all cases. These values indicated a good agreement with the experimental data and a good fitting ability for the model. Thus, the procedure demonstrated the capability of the model to work as a predictive tool. The large adjusted R2 (between 0.806 and 0.999) values indicated an adequate relationship between the experimental data and the fitted model.
Actual and predicted values were scattered in close proximity with the line, which indicates a good correlation between predicted and actual responses and, in turn, the good fit of the proposed quadratic models for all nitro-PAHs and oxy-PAHs. Tests of the normality of the residuals of experimental data were also analyzed. The errors were normally distributed, and there were almost no critical violations of the assumptions that underlie the analysis. Thus, the normality hypotheses could be approved: the predictive regression model accounted for all information available from the experimental data.
Finally, the response criterion was successfully applied to optimize the DSLME extraction of nitro-PAHs and oxy-PAHs. The criterion followed to simultaneously optimize the seven responses was to maximize the peak area, giving more importance to the analytes with the smallest area (2-NFLU and 9-NANTHR) and, in descending order, of importance for the rest of the analytes (Table S4-Supplementary material). Besides, some boundaries were considered to use the minimum volume of acetone and n-hexane, following the principles of green chemistry.
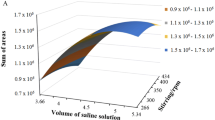
After optimization, the experimental conditions (maximum in the desirability function (D = 0.682, which is considered satisfactory for a multi-analyte optimization strategy) were water volume: 7.5 mL, n-hexane volume: 0.83 mL, and acetone volume: 0.69 mL. Then, to simplify the experimental procedure, the extracting solvent volume was chosen to be 0.80 mL and the dispersive solvent volume to be 0.7 mL. As was observed in Fig. 3, these conditions do not produce any significant variation in the D value. The results suggested through the optimization procedure were experimentally corroborated with a close agreement and used for sample analyses.
Effect of mode and agitation time
In any dispersive microextraction, the mass transfer between phases is improved as the emulsion is formed. It would be also anticipated that agitating the system after the addition of the extractant solvent could have some influence in the extraction efficiency; thus, manual shaking, vortex, magnetic stirring, or ultrasonic agitation, among others, has been used (Mansour and Danielson 2017). Similarly, in this work, the attempts were primarily centered on optimizing different types of emulsification strategies: manual shaking, ultrasound application, and vortex agitation. The results revealed the highest recovered for vortex agitation in comparison with the other tested emulsification strategies (Fig. 4a). The effect of the vortex agitation time was assessed in the range of 0.25 (15 s) to 5 min. The results of this study showed that the extraction recovery of both nitro-PAHs and oxy-PAHs increased as the vortex time increased up to 1 min and, then, leveled off at longer vortexing times (Fig. 4b). Thus, a 1-min vortex agitation period was selected as optimum for extraction.
Effect of sample mass
In general, a microextraction technique should minimize the use of organic solvents. However, the reduction of volumes can lead to some practical difficulties related to phase collection, extraction, and extraction times. In order to improve the detection of nitrated and oxygenated PAHs in volcanic ashes, different sample masses (0.5–1.5 g) were studied, while the remaining optimized conditions extracting solvent (800 μL of n-hexane), dispersive solvent (700 μL of acetone), and water volume (7.5 mL) were kept constant.
The obtained results (Fig. 5) showed that there were no significant differences in recovery values by increasing the sample mass from 0.5 to 1 g. However, for sample mass greater than 1 g, a marked decrease in recovery (R) was observed for most of the nitrated and oxygenated PAH derivative compounds. Thus, a mass of 0.5 g was used for further experiments.
Effect of sample mass on the recovery (%) of the nitrated and oxygenated PAHs. Concentration of mixture PAH derivative standard solution: 15 ng g−1; water volume: 7.5 mL; acetone (dispersive solvent) volume: 0.7 mL; n-hexane (extracting solvent volume): 0.8 mL; vortex time: 1 min; centrifugation: 5 min at 3500 rpm
Method validation
To evaluate the developed method, analytical parameters such as linear ranges (LR), limits of detection (LODs) and quantification (LOQs), recoveries, and inter-day and intra-day precisions were calculated (Table 4).
The linearity was obtained from analyzing a mix of samples spiked with a standard solution of nitro-PAHs and oxy-PAHs; the linear concentration level was from 0.05 to 100.0 ng g−1. Thus, correlation coefficients (r) from 0.994 to 0.998 were obtained, showing satisfactory linearity between the concentration and the peak area of each analyte in the calibration curves. Detection limits and quantification limits were calculated as recommended by the IUPAC (Uhrovčík 2014). Thus, LODs and LOQs obtained were in the range of 0.015 to 0.056 ng g−1 and 0.046 to 0.170 ng g−1, respectively. Repeatability (intra-day) and reproducibility (inter-day) were evaluated through five replicate extractions during a day (intra-day) and five replicates at five subsequent days (inter-day) samples, with three concentration levels (5, 15, and 30 ng g−1) for the nitrated and oxygenated PAH compounds studied. The results showed an RSD% lower than 8.1% for all the analytes. Additionally, accuracy was also evaluated by addition/recovery tests. The repeatability of the method proposed was studied for five replicate extractions using a mix of volcanic ash mix samples spiked at the 5, 15, and 30 ng g−1 levels. The results (Table S5-Supplementary material) showed relative recovery average ranging from 95 to 100% and acceptable repeatability as indicated by relative standard deviations (RSDs) of ≤ 5.7%. Comparison of slopes (b) of the calibration curves of standards in both pure solvent (ACN) and spiked samples allowed the estimation of the matrix effect. The extent of the ion suppression or signal enhancement was calculated as 100 − (b spiked / b solvent × 100). No significant matrix effect was observed.
Analysis of real ash volcanic samples
To demonstrate the feasibility and applicability of the method for the extraction and determination of nitro-PAHs and oxy-PAHs in volcanic ashes, samples collected as described in “Sampling and sample preparation”were analyzed. The results are outlined in Table 5. Different contribution sources could affect the presence of the PAH derivatives under study. Thus, forest fires, contaminated air masses, as well as, local and regional emissions, natural processes forming crude oil, biosynthesis by microorganisms, adsorption and photochemical processes, temperature, vapor, and atmospheric pressures, among others (Stracquadanio et al. 2003), might increase their concentration levels in the environment. Additionally, Kozak et al. (2017) determined a considerable increase in the concentration of PAHs in the environment (from air and surface water samples) that correlated with periods of volcanic eruption and proposed that natural sources of emission are not insignificant compared to anthropogenic emissions. However, the concentrations of nitrated and oxygenated PAHs cannot easily be predicted from the intensity of anthropogenic or natural activity or easily related to those of PAHs because emission source strengths of nitro-PAHs oxy-PAHs can be different. In this sense, PAH derivatives have additional sources which can be ascribed to different meteorological and climatic conditions affecting its atmospheric formation due to photochemical conversion or radical reactions.
In this particular study, the volcanic ash samples from Puyehue (I), Copahue (II), and Calvuco (III) volcanoes contained nitro-PAHs and oxy-PAHs at concentration ranges from 3.48 to 15.90 ng g−1 and 1.89 to 20.66 ng g−1, respectively. The nitro-PAHs: 2-NFLU and 3-NFLUANTH were not detected in the volcanic ash of the Puyehue volcano. Additionally, 9-NANTHR was not detected either in Puyehue nor in Copahue volcanoes and, compared to the other compounds, exhibited the lowest concentration (0.98 ng g−1) in the Calvuco volcano samples. This compound was reported as highly photolysable in the atmosphere (Albinet et al. 2007; Fujiwara et al. 2014), which could explain the low levels found. On the other hand, for the oxy-PAHs, 2-FLUCHO was detected and quantified in variable concentrations, ranging from 1.89 to 13.97 ng g−1, and 5,12-NAPHTONE and 9,10-ANTRHONE were the most abundant compounds and exhibiting similar concentrations, in the range from 12.55 to 20.66 ng g−1.
In terms of comparison with existing reports on this topic, no literature references have been found for the presence of mentioned nitrated and oxygenated PAH derivatives in volcanic ashes, which represent a significant contribution and an important aspect linked to environmental monitoring. In summary, the presence of nitro-PAHs and oxy-PAHs in volcanic ashes is reported as well as an analytical tool for its determination at trace levels. As mentioned, due to the lack of information in this topic, further investigations are needed to correlate its concentrations to the precursors or to establish comparisons among the pollutants.
Conclusions
This work constitutes the first evidence of the existence of seven nitrated and oxygenated PAH compounds in volcanic ash samples at concentration levels that might represent a health risk for people’s health. For their determination, a novel and efficient DSLME approach coupled to UHPLC-MS/MS separation/detection was developed. The simple sample treatment developed represents a new and simple contribution to the liquid microextraction of solid samples. In this context, many advantages such as excellent performance, simplicity, stability, operability, low cost, speed, and minimum consumption of organic solvents, agreeing with the current demands of Green Chemistry, were attained.
References
Albinet A, Leoz-Garziandia E, Budzinski H, ViIlenave E (2007) Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (south of France): concentrations and sources. Sci Total Environ 384:280–292
Bandowe BAM, Meusel H (2017) Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment–a review. Sci Total Environ 581–582:237–257
Cabré J, Aulinas M, Rejas M, Fernandez-Turiel JL (2016) Volcanic ash leaching as a means of tracing the environmental impact of the 2011 Grímsvötn eruption. Iceland Environ Sci Poll Res 23:14338–14353
Campo J, Lorenzo M, Cammeraat ELH, Picó Y, Andreu V (2017) Emerging contaminants related to the occurrence of forest fires in the Spanish Mediterranean. Sci Total Environ 603-604:330–339
Canales R, Guiñez M, Bazán C, Reta M, Cerutti S (2017) Determining heterocyclic aromatic amines in aqueous samples: a novel dispersive liquid-liquid micro-extraction method based on solidification of floating organic drop and ultrasound assisted back extraction followed by UPLC-MS/MS. Talanta 174:548–555
Chormey DS, Er EÖ, Erarpat S, Özzeybek G, Arı B, Bakirdere S (2018) A novel analytical approach for the determination of parathion methyl in water: quadrupole isotope dilution mass spectrometry-dispersive liquid–liquid microextraction using multivariate optimization. Analyst 143:1141–1146
de Oliveira Galvão MF, de Oliveira Alves N, Ferreira PA, Caumo S, de Castro Vasconcellos P, Artaxo P, de Souza Hacon S, Roubicek DA, Batistuzzo de Medeiros SR (2018) Biomass burning particles in the Brazilian Amazon region: mutagenic effects of nitro and oxy-PAHs and assessment of health risks. Environ Pollut 233:960–970
Fernández M, Clavijo S, Forteza R, Cerdà V (2015) Determination of polycyclic aromatic hydrocarbons using lab on valve dispersive liquid–liquid microextraction coupled to high performance chromatography. Talanta 138:190–195
Fujiwara F, Guiñez M, Cerutti S, Smichowski P (2014) UHPLC-(+)APCI-MS/MS determination of oxygenated and nitrated polycyclic aromatic hydrocarbons in airborne particulate matter and tree barks collected in Buenos Aires city. Microchem J 116:118–124
Geyer A, Marti A, Giralt S, Folch A (2017) Potential ash impact from Antarctic volcanoes: insights from Deception Island’s most recent eruption. Sci Rep 7:16534. https://doi.org/10.1038/s41598-017-16630-9
Guiñez M, Martinez LD, Fernandez L, Cerutti S (2017) Dispersive liquid–liquid microextraction based on solidification of floating organic drop and fluorescence detection for the determination of nitrated polycyclic aromatic hydrocarbons in aqueous samples. Microchem J 131:1–8
Guiñez M, Bazan C, Martinez LD, Cerutti S (2018) Determination of nitrated and oxygenated polycyclic aromatic hydrocarbons in water samples by a liquid–liquid phase microextraction procedure based on the solidification of a floating organic drop followed by solvent assisted back-extraction and liquid chromatography–tandem mass spectrometry. Microchem J 139:164–173
Guiñez M, Canales R, Talio C, Gómez D, Smichowski P (2020) Determination of heterocyclic aromatic amines in ashes from biomass burning by UHPLC-MS/MS after ultrasound-assisted dispersive solid-liquid microextraction. Talanta 206:120182
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 92:1
Ilyinskaya E et al (2017) Understanding the environmental impacts of large fissure eruptions: aerosol and gas emissions from the 2014–2015 Holuhraun eruption (Iceland). Earth Planet Sci Lett 472:309–322
Ivy DJ, Solomon S, Kinnison D, Mills MJ, Schmidt A, Neely RR (2017) The influence of the Calbuco eruption on the 2015 Antarctic ozone hole in a fully coupled chemistry-climate model. Geophys Res Lett 44:2556–2561
Kaw HY, Li J, Jin X, Wang Z, Cong L, He M, Li D (2018) Ultrasound-assisted liquid–liquid spray extraction for the determination of multi-class trace organic compounds in high-volume water samples. Analyst 143:4575–4584
Kozak K, Ruman M, Kosek K, Karasiński G, Stachnik Ł, Polkowska Ż (2017) Impact of volcanic eruptions on the occurrence of PAHs compounds in the aquatic ecosystem of the southern part of West Spitsbergen (Hornsund Fjord, Svalbard). Water 9:42
Lam MM, Bülow R, Engwall M, Giesy JP, Larsson M (2018) Methylated PACs are more potent than their parent compounds: a study of aryl hydrocarbon receptor–mediated activity, degradability, and mixture interactions in the H4IIE-luc assay, Environmental Toxicology and Chemistry. 37:1409–1419
Leong M-I, Fuh M-R, Huang S-D (2014) Beyond dispersive liquid–liquid microextraction. J Chromatogr A 1335:2–14
Lu D, Liu C, Deng J, Zhou X, Shi G, Zhou T (2019) Rational design of an ionic liquid dispersive liquid–liquid micro-extraction method for the detection of organophosphorus pesticides. Analyst 144:2166–2172
Lundstedt S et al (2007) Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. AMBIO: A Journal of the Human Environment 36:475–485
Mansour FR, Danielson ND (2017) Solidification of floating organic droplet in dispersive liquid-liquid microextraction as a green analytical tool. Talanta 170:22–35
Mas S, de Juan A, Tauler R, Olivieri AC, Escandar GM (2010) Application of chemometric methods to environmental analysis of organic pollutants: a review. Talanta 80:1052–1067
Ming-Jie L, Zhang H-Y, Xiao-Zhe L, Chun-Yan C, Zhi-Hong S (2015) Progress of extraction solvent dispersion strategies for dispersive liquid-liquid microextraction. Chin J Anal Chem 43:1231–1240
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. John Wiley & Sons
Resano M, Vanhaecke F, de Loos-Vollebregt M (2008) Electrothermal vaporization for sample introduction in atomic absorption, atomic emission and plasma mass spectrometry—a critical review with focus on solid sampling and slurry analysis. J Anal At Spectrom 23:1450–1475
Rezaee M, Assadi Y, Hosseini M-RM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A 1217:2342–2357
Stracquadanio M, Dinelli E, Trombini C (2003) Role of volcanic dust in the atmospheric transport and deposition of polycyclic aromatic hydrocarbons and mercury. J Environ Monit 5:984–988
Tang Y, Yamamoto S, Imasaka T (2019) Determination of nitrated polycyclic aromatic hydrocarbons in particulate matter 2.5 by laser ionization mass spectrometry using an on-line chemical-reduction system. Analyst 144:2909–2913
Tiwari J, Tarale P, Sivanesan S, Bafana A (2019) Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environ Sci Pollut Res 26:28650–28667 1–18
Tomašek I et al (2018) Respiratory hazard assessment of combined exposure to complete gasoline exhaust and respirable volcanic ash in a multicellular human lung model at the air-liquid interface. Environ Pollut 238:977–987
Uhrovčík J (2014) Strategy for determination of LOD and LOQ values–some basic aspects. Talanta 119:178–180
Vera Candioti L, De Zan MM, Cámara MS, Goicoechea HC (2014) Experimental design and multiple response optimization Using the desirability function in analytical methods development. Talanta 124:123–138
Vigneri R, Malandrino P, Gianì F, Russo M, Vigneri P (2017) Heavy metals in the volcanic environment and thyroid cancer. Mol Cell Endocrinol 457:73–80
von Glasow R, Bobrowski N, Kern C (2009) The effects of volcanic eruptions on atmospheric chemistry. Chem Geol 263:131–142
Wang R, Yousaf B, Sun R, Zhang H, Zhang J, Liu G (2016) Emission characterization and δ13C values of parent PAHs and nitro-PAHs in size-segregated particulate matters from coal-fired power plants. J Hazard Mater 318:487–496
Acknowledgments
Dr. Valeria Rivero Osimani (Universidad Nacional del Comahue, Neuquén, Argentina) is kindly acknowledged for providing the volcanic ash samples.
Funding
The sources of financial support are Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica, and Instituto de Química de San Luis (INQUISAL, UNSL-CONICET).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Constantini Samara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 212 kb)
Rights and permissions
About this article
Cite this article
Guiñez, M., Escudero, L., Mandelli, A. et al. Volcanic ashes as a source for nitrated and oxygenated polycyclic aromatic hydrocarbon pollution. Environ Sci Pollut Res 27, 16972–16982 (2020). https://doi.org/10.1007/s11356-020-08130-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08130-7