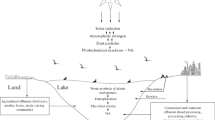
Abstract
Nitroaromatic compounds (NACs) are extensively used in different industries and are synthesized in large quantity due to their heavy demand worldwide. The broad use of NACs poses a serious pollution threat. The treatment processes used for the removal of NACs are not effective and sustainable, leading to their release into the environment. The nitro group attached to benzene ring makes the compounds recalcitrant due to which they persist in the environment. Being hazardous to human as well as other living organisms, NACs are listed in the USEPA’s priority pollutant group. This review provides updated information on the sources of NACs, prevalence in different environmental matrices, and recent developments in methods of their detection, with emphasis on current trends as well as future prospects. The harmful effects of NACs due to exposure through different routes are also highlighted. Further, the technologies reported for the treatment of NACs, including physico-chemical and biological methods, and the challenges faced for their effective implementation are discussed. Thus, the review discusses relevant issues in detail making suitable recommendations, which can be helpful in guiding further research in this subject.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitroaromatic compounds (NACs) are aromatic compounds with one or more nitro groups. The commonly used NACs are depicted in Fig. 1. Nitro group has certain unique properties which make NACs useful as raw materials in chemical synthesis of a variety of compounds like drugs, dyes, cosmetics, herbicides, pesticides, fungicides, explosives, paints, preservatives, antioxidants, gasoline additives, corrosion inhibitors, and other industrial chemicals (Sinha et al. 2006; Ju and Parales 2010; Singh and Ramanathan 2013). The release of these NACs from manufacturing industries, shipping, warehousing, and other activities coupled with improper waste disposal creates serious environmental pollution. NACs are continuously discharged into the environment, and contaminate water and soil as well as air, which present hazard to humans and other living organisms (Khan and Anjaneyulu 2005; Cai et al. 2007). NACs are of primary concern for being mutagenic and carcinogenic as well as toxic to living organisms (Toropov et al. 2007). Hence, NACs are listed in priority pollutants by the United States Environmental Protection Agency (Fares et al. 2008).
A number of physical, chemical, and biological methods have been established for the treatment of NAC-contaminated wastewater and soil. Several of these methods are effective in degradation and mineralization of NACs. AOPs (advanced oxidation processes) can degrade recalcitrant NACs into biodegradable products, whereas biological methods employ microorganisms, which metabolize NACs into less harmful products or completely mineralize them (Rodgers and Bunce 2001; Oller et al. 2011). While these technologies have been successfully demonstrated at laboratory scale, treatment of NACs is still not implemented adequately at industries due to reasons like excessive cost and infeasibility at large scale (Khan and Anjaneyulu 2005; Cai et al. 2007). Hence, the NACs are continuously disposed off in water bodies, and there is need to develop economical and feasible NAC remediation technologies for the industries.
Over the last two decades, review papers pertaining to toxicity and biodegradation of NACs, including chloro-nitroaromatics and nitro-explosives, have been published (Spain 1995; Purohit and Basu 2000; Ju and Parales 2010; Kovacic and Somanathan 2014). However, the information about prevalence of NACs in different environments and their effect has not been documented, compared, and reviewed to date. The information about presence and concentration of NACs in different environments is essential to quantify their health effects and devise appropriate prevention and mitigation strategies. Therefore, this review paper focuses on the sources, distribution, and effects of NACs in different environments and methods for their detection in the environmental matrices. Besides, latest developments in remediation of NAC-contaminated wastes are also discussed.
Sources of NAC contamination
Natural sources
Naturally occurring NACs are rare, but few are present in the environment. Some microbes, fungi, parasites, and plants produce NACs as secondary metabolites in stress conditions or as pheromones to attach to the host. For example, western skunk cabbage is reported to produce 1-nitro-2-(4-methoxyphenyl)ethane under stress. Some fungi, like Coniothyrium and certain marine fungi, also produce NACs. Few bacteria, such as Streptomyces eurocidicus, Nocardia mesenterica, and Pseudomonas fluorescens, have been found to produce the antibiotic 2-nitroimidazole (Parry et al. 2011).
Anthropogenic sources
The presence of the nitro group imparts versatile applications to NACs. NACs are used as starting material, precursor, or intermediates in different manufacturing industries (Arora et al. 2012; Tiwari et al. 2017). NACs are also extensively used in explosives. The nitro group provides available oxygen to oxidize the aromatic ring, which creates self-oxidant. This characteristic imparts explosive properties to NACs like TNT, TNP, HMX, and dinitrophenol (Upson and Burns 2006; Ju and Parales 2010). Certain drugs containing nitro groups are used for the treatment of diseases like Parkinson’s disease. Similarly, NACs like nitazoxanide, metronidazole, and nifurtimox are used as drugs to treat parasitic infections. Pentachloronitrobenzene (PCNB) is used to control many plant diseases (Zhou et al. 2012; McNeil et al. 2013). The nitroaromatic parathion is used as a pesticide, while NB is used as an intermediate for the production of fungicides and linear alkyl benzene detergents (Wu et al. 2006). Several NACs like Martius yellow are used as dyestuff. The nitro-chromophore contains nitrogen with two oxygen atoms bonded with equal strength. The electrons are delocalized between the nitrogen and the oxygen atoms, which imparts the dye color. NACs like 2-methyl-4-nitroaniline are used as intermediates in the preparation of hair dyes and paints (Pinheiro et al. 2004; Zhao et al. 2011).
Due to their diverse applications, NACs are continuously discharged into the environment through agricultural runoffs, industrial discharges, improper waste disposal practices, and activities like packaging, loading, and transportation. This results in pollution of the neighboring water and soil. Contamination by explosives in and around ammunition plants causes the locality to suffer from direct contact with soil or polluted drinking water (Psillakis et al. 2004). The spray of nitroaromatic pesticides contaminates the soil, which spreads further by agricultural run-off. Consumption of crops contaminated with nitro-pesticides, herbicides, and fungicides is toxic to human as well as animals (Wu et al. 2006).
Thus, anthropogenic sources result in considerable release of NACs into the environment. The data on the distribution of NACs in different environments are important to reflect the contamination level, which will further aid in quantifying the exposure, risk, and dose-adverse effect relationship.
Methods for detection of NACs in the environment
NACs are abundant in the environment and several methods have been tested for their monitoring. Due to the importance of detection of NAC explosives for military and security concerns, a lot of works has been carried out towards their examination. A variety of methods namely, electrochemical, chemical, biosensors, spectrophotometric, and analytical techniques, including gas chromatography (GC), liquid chromatography, and capillary electrophoresis (CE), have been described for the detection of NACs (Woltman et al. 2000; Pon Saravanan et al. 2006; Singh 2007; Ganranoo et al. 2010; Wang et al. 2018).
UV-visible spectrophotometer presents a simple and rapid tool for quantitative measurement of certain NACs at UV or visible range (200–760 nm). 3-Dinitrobenzene, TNB, and 3-NP react with lithium aluminum hydride to form azo dyes which can be detected at visible wavelengths (Tiwari and Pande 1972). However, the method is highly non-specific and prone to interferences. The sensitivity is also very low and it requires high analyte concentrations. Further, the technique is not suitable for all NACs.
Accurate identification and quantification of NACs require pre-processing such as removal of interferences, extraction, concentration, and separation of NACs from the sample matrix. Analytical separation methods like HPLC (high-performance liquid chromatography), GC, and CE have been widely used in laboratories for analyte separation in detection of NACs. HPLC is normally coupled to UV absorbance detectors. However, UV detection is not suitable for all NACs. Sensitivity and co-elution from the column also affect the results from UV detector. It is reported that HPLC-UV is suitable for 12 highly polar NACs (aminonitrotoluenes, nitrotoluenes, NB, etc.). The reported detection limit was 14–40 mg/L (Schmidt et al. 2006). Woltman et al. (2000) used HPLC followed by post-column reduction of NACs. The reduced NACs subsequently transferred electrons to specific dye resulting in photoluminescence. This resulted in detection of NACs in nanomolar range. Use of MS (mass spectrometer) detectors in LC-MS or LC-MS/MS provides improved detection and identification. Dinitrophenols, dinitrotoluenes, and amino-dinitrotoluenes were detected by LC-MS/MS, but it was found to be unsuitable for the detection of some complex NACs like dinitrobenzoic acids, TNB, and TNT (Schmidt et al. 2006). LC-MS is generally used for analytes with high molecular weight, low volatility, and temperature sensitivity. GC, on the other hand, is mostly used for volatile and semi-volatile NACs. The most commonly used GC detectors are FID (flame ionization detector), ECD (electron capture detector), NPD (nitrogen phosphorous detector), TEA (thermal energy analyzer), and MS. TEA is a preferred detector for NACs due to its specificity for nitro group. However, MS and MS/MS detectors achieve lower detection limit (Kirchner et al. 2007). CE is another technique that has been used for the detection of different NACs (Oehrle 1996). It offers many advantages such as low sample concentration, great separation speed, and lower analysis time. When combined with additional detectors, it can be a promising technique for the accurate and sensitive detection of NACs. Nie et al. (2010) applied CE with electrochemical detector for the analysis of dinitrotoluenes, dinitrobenzenes, and TNT with the detection limit 3–4.7 μg/L. Electrochemical detection methods include amperometric, conductometric, coulometric, potentiometric, or voltammetric measurements. In this technique, samples are detected on the basis of their redox activity. This method is very useful for non-UV absorbing analytes. However, electrode fouling and air bubble limit its sensitivity.
The analytical methods described above require skilled personnel, sophisticated analytical facility, high run time, and extraction and concentration of samples for the detection of NACs from the environment (Wang et al. 2018). Besides, hazardous solvents are used during laborious sample preparation steps, and issues like matrix effect, co-elution, and co-extraction of interferences are unavoidable in some cases. In this regard, sensor-based detection methods present attractive alternative for on-site detection. They are also reported to be more sensitive and selective, less time-consuming, and portable on the field (Sun et al. 2018).
Chemosensors for NACs are based on their electron-deficient nature, which leads to fluorescence quenching in electron-rich moieties. Li et al. (2018) synthesized metal organic frameworks for sensitive detection of NACs, particularly TNT. Similarly, molecular imprinted polymer based–sensors are reported for the detection of 2,4-DNT and TNT with detection limit of 30–40 μM (Stringer et al. 2010). Electrochemical detectors are also proposed for fast and economic field detection of NACs. Mesoporous SiO2 of MCM-41 type exhibited large surface area with strong adsorption of NACs, making it useful for electrochemical detection of NACs down to nanomolar levels (Zhang et al. 2006). Although the chemical and electrochemical sensors offer great sensitivity, the preparation and functionalization of sensor materials is difficult and costly. Also, the sensor materials, in many cases, are non-biodegradable and toxic, resulting in secondary pollution (Sun et al. 2018). Biosensors present an ecofriendly alternative to the above sensors. Different biosensors based on antibodies, NAC-binding proteins and nucleic acids, and enzymes like nitroreductase, have been reported for the detection of NACs (Charles et al. 2001). Gingras et al. (2013) reported detection of NACs including TNT by fluorescence quenching of endogenous tryptophan residues of certain proteins. While biosensors have high specificity and sensitivity, there are issues like instability and susceptibility to harsh environmental conditions, restricted substrate range, stringent storage requirements, and high cost. In recent years, nanosensors have been frequently studied for the detection of environmental pollutants including NACs at trace level. Sangili et al. (2018) successfully used cerium oxide nanoparticles for the detection of NB. Nanosensors, such as nanofibers, nanotubes, nanowires, and nanomaterials, are fairly stable and can be functionalized cost-effectively. They have potential for real-time monitoring, high sensitivity with no cost of sample preparations. In spite of these applications, nanomaterials have been reported to be ecotoxic and their risk assessment in the environment must be investigated (Soni et al. 2017).
In all, a variety of sensors have been demonstrated for NAC detection with potential for field application. Now the focus should be on sealing the lacunae like improvement of specificity and reproducibility, robustness to tolerate interferences from sample matrix and contaminants, easy fabrication, and cost reduction.
Environmental contamination by NACs
The extensive use of explosives and pesticides has contaminated the water and land, making NACs ubiquitous contaminants of the environment. The air also gets adulterated with NACs by natural or man-made activities like volcanic discharge, forest fires, burning of garbage, fuels, plant material and tobacco, and volatilization (Park et al. 2001). NACs are produced in thousand tons per year for satisfying their heavy demand. In India, it is reported that 84.16 thousand metric tons per annum of nitrotoluene is produced, and the production is rising at an average rate of 7% per annum (Singh et al. 2015; DoCP 2017). In the USA, about 230417 pounds NB was released from organic chemical industries during the year 2000 (Doble and Kumar 2005).
Atmospheric contamination
Incomplete burning of fossil fuel releases hydrocarbons. These hydrocarbons undergo photo-oxidation in the atmosphere, where they react with the atmospheric NO2 and generate NACs. The liberation of NACs into aerosols destroys the quality of ambient air. The presence of NACs has been demonstrated in the atmosphere at several locations globally (Table 1) Nitrophenolic compounds were detected in rain water and also in diesel exhaust particles by Mori et al. (2003). Shen et al. (2013) estimated emission factor of nine different NPAHs (nitro-PAH) to be 0.14–297 mg/kg of wood. In Rome, 6 different types of nitrophenols were detected in the air in the range of 1–20 ng/m3 (Cecinato et al. 2005). Predominant nitrogenous compounds present in the gas phase and particle phase were 2-NP (10.4 ng/m3)and 4-NP (17.8 ng/m3) respectively. In Europe, 4-NP was detected in the range of 0.1–27 ng/m3 while methyl-NP was detected in the range of 0.11–12.3 ng/m3. Further, 0.004–4.5 ng/m3 of mono- and dinitrophenols was detected in China (Teich et al. 2017). Kitanovski et al. (2012) proposed the theory of seasonal variation in the concentration of NACs. They observed that the aerosol concentration of 4-NP and methyl-NP in winter season was 8–15 times higher than that in summer. This was attributed to higher biomass burning in winter and higher evaporative loss of NACs from aerosols in summer.
Terrestrial contamination
Table 1 shows the reports on soil contamination with nitro compounds. In a study, around 87 g/kg NACs were detected in contaminated soils from Texas and Kansas (Meyers et al. 2007). Pichtel (2012) reviewed and highlighted severe contamination of soil with explosives, including NACs, in USA and Canada due to manufacturing and military activities. Bausinger et al. (2007) reported nitro explosive compounds and their metabolites at a concentration of 11 mg/kg soil at an ammunition burning site in France, while Podlipná et al. (2015) reported 9 mg/kg of 2,4-DNT near an ammunition plant in Europe. NAC-based explosives are also used in mines due to which the miners are highly affected with these pollutants. Activities like coal burning, engine emissions, and photo-oxidation also give rise to NAC contamination in urban areas. Fifteen different nitroaromatics were detected in the range of 28–128 ng/g in soil from Jiaxing City of China (Zhang et al. 2014). Pham et al. (2015) compared the distribution of NPAH and PAH between soil and air. The NPAH strongly accumulated in soil. Thus, there is higher possibility of the presence of NACs in soil irrigated with pesticide-contaminated effluent or leachate. Certain NACs are hydrophobic in nature and easily adsorb to soil. High-molecular-weight NACs in the atmosphere also tend to accumulate in land as dry or wet deposition.
Aquatic contamination
An accidental explosion in petrochemical plant followed by release of NB and other pollutants in China resulted in 8 deaths and 60 injuries along with contamination of the Armer and Songhua river in 2005 (Fu et al. 2008). Extensive studies and remediation efforts have been undertaken to effectively clean the river. In another report, 4-NP was detected in the agricultural runoff in the range of 832–913 ng/ml in Punjab, India (Saini et al. 2017). Landfill of industrial and domestic solid wastes is another important source of NACs. The landfill leachate contains heavy metals and other harmful compounds. The NACs can also leach out from the landfill into the ground water. Nitromusks, which are used in perfumery industry, were detected in landfill leachate in India (Ghosh et al. 2014). In another study, 13 different types of nitrophenols and 20 different nitrobenzoic acids were identified in ground water and leachate in Germany (Preiss et al. 2007). Different studies on aquatic NAC contamination are summarized in Table 1.
Thus, a variety of NACs have been detected in different geographical regions demonstrating their widespread application. In general, higher concentration of NACs was reported in urban settings due to vehicular emission around traffic areas, incomplete burning, volatilization, etc. Nitrotoluenes were commonly detected in soil (0.143–18.7 mg/kg soil) at several places such as Chicago, France, India, and UK. Use of nitrotoluenes in explosives may explain their widespread presence across many countries. Nitrophenols were also very common in the environment in different matrices such as air, water, and soil. Nitrophenols constitute the most predominant NAC contaminants due to their wide range of applications and pose a major concern due to their toxic nature. Release of nitrophenols may be attributed mainly to discharge of wastewater from manufacturing units (Singh and Srivastava 2011). Literature review also indicated that data on the presence of nitroaromatics in the environment is fragmented due to lack of systematic monitoring efforts. Hence, their prevalence in the environment may be more common than that reported so far, due to their wide applications. Hence, it is important to generate more data on the occurrence of NACs and other pollutants co-existing with them to estimate the exposure and co-exposure of NACs, respectively. This will help in estimation of accurate environment-dependent and dose-dependent health and environmental risk of NACs.
Adverse effects of NACs
Widespread NAC contamination is of serious concern due to their toxicity and persistence. The recalcitrance of these compounds results in their accumulation in the environment. Inhalation, ingestion, or direct contact of these NACs cause acute and chronic toxicity. Therefore, the discharge of these compounds into the environment is a potential source of toxicity and carcinogenicity.
Effects in atmospheric environment
The presence of NACs in the air is a major concern since they are mutagens and carcinogens. The emission of NACs into the atmosphere by different sources like diesel exhaust or cigarette smoke and their subsequent inhalation can decrease the lung function. In a study, NACs were reported to be immune suppressive as well as tumor inducers in a human bronchial epithelial cell. NPAHs pose severe threat to lung function and there is a strong correlation between the exposure time and chances of lung cancer (Mori et al. 2003; Landvik et al. 2007). The higher the exposure period and concentration, the higher the adverse effect on the lung. Exposure to NPAH is also reported to induce pulmonary inflammation. Nitropyrene induces chemokines and cytokines, which are responsible for inflammation. Some induced cytokines are also responsible for tumor growth. The exposure of NPAH present in diesel exhaust particles causes chromosomal damage. Nitropyrene and 3-nitrobenzathrone are reported to damage the DNA causing genotoxicity (Øvrevik et al. 2010).
Effects in terrestrial environment
The persistence of NACs in soil affects the associated biota, including plants, insects, and microbes. It has been observed that 2,4-dinitroanisole decreases the growth of perennial rye-grass (Lolium perenne) (Dodard et al. 2013), and is also lethal to earthworms (Kennedy et al. 2015). NACs exert reproductive toxicity in crickets (Acheta domesticus) (Karnjanapiboonwong et al. 2009). Nitrophenols exhibit estrogenic activity and are toxic to yeast cells. Reduced growth rate was reported in salamander (Ambystoma tigrinum), and hemolytic anemia and increased spleen weight were reported in mice and rat models upon exposure of technical DNT (Brüning et al. 2002).
Effects in aquatic environment
NACs are toxic to aquatic animals and plants and their toxicity is extensively studied in fishes. 3-Trifluoromethyl-4-nitrophenol uncouples oxidative phosphorylation, which, in turn, impairs mitochondrial ATP production in fishes (Birceanu et al. 2014). Similarly, some of the nitrophenols like 2,4-dichloro-6-nitrophenol have been shown to interfere with hormone synthesis and transport pathways, disrupting the endocrine system of fishes (Jugan et al. 2010; Chen et al. 2016, 2017). Substituted NB and aniline are lethal to fishes such as carp (Cyprinus carpio) and tilapia (Tilapia zilli). NACs are also reported to be lethal to some algae like Chlorella vulgaris, and flea-like Daphnia pulex (Yen et al. 2002). 2,4-DNT is known to be highly toxic to aquatic plants like duckweed (Roberts et al. 2007).
Toxicity in animals including humans
NACs are hepatotoxic, carcinogens, and potent mutagens. Epidemiological data validate the carcinogenicity of NACs like NB, nitrotoluenes, and nitrophenols. Chronic exposure to nitrotoluenes results in cancer in mice and rats. In humans, dinitrotoluenes are reported to cause cancer, liver cytotoxicity, anemia, leukocytosis, cyanosis, dark urine, diarrhea, headache, insomnia, labored breathing, nausea, orange feces, prostrate posture, red discharge around the nose, squinting, and weakness (Tchounwou et al. 2003; Coe et al. 2007; Lent et al. 2012). Exposure to DNT is reported to be nephrotoxic and cancerous to the urinary tract. DNT exposure also causes methemoglobinemia as well as discoloration of hair and skin in human. Certain NACs affect the reproductive system which results in sperm count reduction, sperm morphology alteration, and aspermatogenesis. NACs have been established to be genotoxic in various human cell lines (Pinheiro et al. 2004; Karnjanapiboonwong et al. 2009). Several NACs can interrupt the nervous system by inhibiting the enzyme acetylcholinesterase. They are also known to exert endocrine disrupting activity and cytotoxicity (Rodgers and Bunce 2001). The health effects of different NACs are listed in Table 2.
Several reports on acute NAC poisoning in humans have been published, although the frequency has reduced over the years. Bharadiya et al. (2014) reported symptoms like methemoglobinemia, epigastric discomfort, and nausea following ingestion of NB by a 17-year-old patient. Chronic health effects of NACs following occupational exposure are well reported in old literature. The workers in a DNT manufacturing plant were found to show symptoms like unpleasant taste, weakness, headache, loss of appetite, nausea, insomnia, vomiting, cyanosis, and anemia (McGee et al. 1942). Although carcinogenicity of some NACs like NB in humans is uncertain, others like TNT are well-established carcinogens. In a retrospective case-control study, Kolb et al. (1993) identified higher prevalence of acute and chronic myelogenous leukemia in both males and females due to chronic exposure to TNT-contaminated soil. In another epidemiological study on poisoning in India, Srivastava et al. (2005) compiled 3-year (1999–2002) data of telephonic enquiries in the National Poisons Information Centre (NPIC). About 1.03% of poisoning cases were reported to be due to NB.
Mechanism of toxicity of NACs
The toxicity of NACs arises partly due to their metabolism in the body. The metabolism of NACs is well studied (Fig. 2). NACs are absorbed in the respiratory tract, the skin, and the gastrointestinal tract, and then excreted in urine. Traces of metabolites of NACs have been detected in the urine of workers, who are daily exposed to NACs. NACs can be metabolized in the liver either through oxidative or through reductive pathways (Purohit and Basu 2000). Different microsomal oxidative enzymes convert NACs to their phenolic derivatives, but the reaction proceeds slowly as compared to the reductive pathway. In the reductive pathway, NACs are reduced by nitroreductases to form nitroso compounds, hydroxylamines, and then amines (Yamazaki et al. 2000). These intermediates can be metabolically activated through hydroxylation, acetylation, and sulfate conjugation by mixed-function oxidases, acetyltransferases, and sulfotransferases. The resulting conjugates eventually give rise to highly reactive nitrenium ions that react with nucleic acids causing genotoxicity. Further, one-electron reduction of NACs results in the formation of radicals that are scavenged with oxygen giving rise to superoxide radical and subsequent oxidative stress. Studies on rabbit and rat have showed that exposure of NB leads to the formation of aniline. Similarly, TNT is reduced in the liver to metabolites like 2-amino-4,6-DNT and 4-amino-2,6-DNT by nitroreductases. Aromatic amines can produce hypersensitivity and carcinogenicity. Dihydroxyl amine, a metabolite of TNT and NB, causes mutagenic and carcinogenic effect (Homma-Takeda et al. 2002). The toxicity of NACs further depends on the number, position, and electronic state of the nitro group relative to the aromatic ring. For example, the toxicity of TNB is higher than NB, as nitro group from TNB reduces easily as compared to NB (Toropov et al. 2007).
Some NACs are known to activate the aryl hydrocarbon receptor (AhR). When NACs bind to these receptors, the AhR-regulated genes are upregulated, resulting in metabolic activation that triggers cell death. Some of the NACs, particularly 1-nitropyrene and benzo-pyrene, induce chemokine and cytokine responses, which trigger inflammatory response that in turn produces oxidative stress causing DNA damage (Øvrevik et al. 2010; Kovacic and Somanathan 2014). Mechanisms of toxicity of NACs are briefly depicted in Fig. 2.
Based on reported data, it can be concluded that the NACs are present ubiquitously in the environment and these pollutants, as well as their transformation products, are toxic and carcinogenic (Hawari et al. 1999). However, more work is needed to determine the effect of environmental matrix, prevalent dose, bioavailability, and co-existence of other pollutants for realistic estimation of risk from NAC exposure and co-exposure. Also, due to the hazardous and persistent nature of NACs, there is an urgent need of their remediation from the environment.
Remediation methods for NACs
A holistic approach is required to degrade, detoxify, or mineralize these harmful nitroaromatics. Nitro group imparts electrophilic properties to the benzene ring making NACs resistant to oxidative degradation. Presence of multiple nitro groups, or nitro group along with other electron withdrawing groups, makes benzene ring further electron deficient and recalcitrant. Therefore, most of the NACs are included under the category of recalcitrant pollutant (Nishino et al. 2000). Several remediation methods categorized as biological, physical, and chemical methods are studied and published for the removal of NACs.
Physico-chemical methods
The physical methods which are used to remove NACs include absorption, adsorption, extraction, ultrafiltration, incineration, photo-oxidation, and volatilization, while major chemical methods are hydrolysis and AOPs. Physical methods generally separate and concentrate the NACs from water and soil, but do not degrade them. Thus, they can clean the polluted site, but the resulting concentrated waste has to be further treated before disposal. In chemical methods, the NACs are degraded or mineralized to smaller molecules, which are generally nontoxic. Adsorption and AOPs are the most generally applied physico-chemical methods, and are described in greater detail below.
Adsorption
Adsorption method concentrates NACs from the environmental matrix. Table 3 highlights the different types of adsorbents used for the adsorption of NACs. The advantage of this method is that the adsorbents can be regenerated and reused several times. Chen and Chen (2015) investigated adsorption of NB, 1,3-DNB, and 4-NT on graphene oxide and graphene nanosheets. Amezquita-Garcia et al. (2013) demonstrated simultaneous adsorption and chemical reduction of 3-chloronitrobenzene and 4-NP by using by activated carbon fibers as redox mediator. However, such adsorbents adsorb not only NACs, but also other organic compounds. Also, the adsorbed pollutants need further treatment after completion of each cycle of adsorption. The final treatment involves incineration, landfill, etc. to safely dispose the hazardous NACs. Therefore, the non-specificity and residual toxicity are some of the major drawbacks of this method. To make adsorption technique a viable option, there is a need to find out cheaper adsorption methods which not only physically remove the hazardous NACs but also degrade or detoxify them. Adsorption technique can be coupled with other methods like biological treatment to enhance the remediation efficiency and avoid non-ecofriendly incineration/landfill treatments.
Advanced oxidation processes
AOPs apply strong oxidizing agents or photocatalysts for the degradation of nitroaromatic pollutants. Fenton’s reagent, H2O2, and ozonation are used as oxidizing agents, whereas Fe, Mn, TiO2, CdS, and Zn are reported as photocatalysts. These oxidizing agents and photocatalysts generate radicals (OH− and SO42−) which open the aromatic ring and degrade it into smaller molecules, inorganic salts, water, and CO2 (Rodgers and Bunce 2001; Deng and Zhao 2015). Generally, individual AOPs like ozonation are limited to lab-scale study only. However, combining two or more AOPs, like H2O2/UV, Fenton and UV/ultrasound, and ozonation along with UV, helps in rapid and effective degradation of nitroaromatic pollutants. Table 4 summarizes the studies regarding application of AOPs to NAC treatment.
Though different physico-chemical methods are effective and can be operated in the field, there are very few successful industrial applications reported to date. Further, the effectiveness of AOPs is pollutant specific and, hence, the choice of AOP method depends on the specific NAC pollutant. Other major drawback of AOPs is that they are expensive and are not economically applicable on a large scale (Oller et al. 2011). On the other hand, biological methods are economically feasible and ecofriendly. They can completely mineralize and detoxify the NACs.
Biodegradation
Many microorganisms have the capacity to degrade/detoxify majority of natural and man-made NACs. The NAC pollutants in the environment are degraded by algae, fungi, and bacteria as well as yeasts. In the last few decades, many bacterial degradation studies have been reported, as bacteria can be effortlessly cultured, are easier to handle, and can be genetically modified to improve the performance. Biodegradation of NACs proceeds either through aerobic or anaerobic route. Aerobic degradation is initiated by oxygenation through monooxygenase or dioxygenase catalyzed reactions. Though certain NACs are amenable to aerobic degradation, many of them resist the initial oxidative attack by bacteria. Highly electronegative NACs preferably undergo reductive metabolism where nitro group is first reduced to amino group. Sometimes, NACs are only partially reduced by the bacteria. Complete mineralization of few NACs is challenging with the addition of hydride ions to nitroaromatic ring leading to the establishment of a highly stable hydride-Meisenheimer compound, which is a dead-end complex; i.e., it cannot be metabolized further (Spain 1995). In some cases, co-substrates, such as surfactants like Tween 80, carbon sources like molasses, glucose, citric acid, sodium succinate, and inorganic salts like calcium, copper, zinc, sulfide, are added to achieve complete mineralization of NACs. Microbes also differ in their potential to degrade a specific NAC. 2-Chloro-4-nitrophenol degradation has been reported only in a few species like Cupriavidus, Arthrobacter, Rhodococcus, and Burkholderia (Arora and Jain 2011; Min et al. 2016; Min et al. 2017; Tiwari et al. 2017). Hence, isolation and selection of suitable microorganisms are of paramount importance. Some of the microorganisms reported for the biodegradation of NACs are listed in Table 5.
It may be concluded that microbial degradation is a crucial component in the NAC remediation strategy. However, further study is required in the field of active and enhanced bioremediation to reduce the treatment time, and retro-fitting the microbial treatment in existing treatment infrastructure setup to save cost. A major issue with biodegradation and even other remediation methods is their inability to treat high concentration of NACs. Sometimes, the NAC contamination in the environment is more than the remediation potential of available methods. In an army ammunition plant in the USA, the contamination of DNT in soil was found to be around 18.5 g/kg (Zhang et al. 2000). AOP using Fe (II) can degrade DNT only up to 25 ppm in 50 min, while bacteria are reported to degrade the same up to 300 ppm (Nishino et al. 2000). Therefore, the biodegradation capability may be limited as compared to the concentration of NACs in the environment. The use of integrated biological and physico-chemical methods may present an effective method to meet the challenges present in the environment in terms of pollutant concentration. However, additional studies are required to establish feasibility of such methods.
Future scope
NACs are abundantly present in the environment due to their wide range of applications. They are toxic to humans, animals, and plants as well as to the environment. Though some countries have banned the use of certain NACs like nitro-pesticides, they are frequently detected in environment samples. There is a need of implementation of firm policies for the control of NACs. Regular monitoring of NAC contaminants is also very important. The early and accurate detection of NACs in the environment can greatly help in the timely implementation of preventive measures. Several efforts have been made for the development and improvement of high-throughput sensors for NACs, but very few sensors are commercially available so far. This is because of practical and technical concerns, such as matrix effects, reliable quantification, reproducibility, reliability of calibration in field, and sensor bulkiness. More efforts are needed to overcome these limitations.
Efforts to control sources of NAC pollution can significantly reduce their release in the environment. Several physico-chemical methods are reported for the removal of NACs, although only a few have been demonstrated successfully at field scale. The physico-chemical methods are not economical at large scale, and hence, biological methods are preferred owing to their cheaper, safer, and effective nature. Many microbes, especially bacteria, have been listed for degradation of several NAC pollutants. However, studies related to biodegradation of complex NACs (di- and trinitro group containing compounds) are still at the preliminary stage. Very low concentration of certain complex NACs can be biodegraded, and more efforts are required to improve performance at high concentrations. Understanding of the underlying molecular and enzymatic mechanism will help to develop a more effective biodegradation technique for NACs. Integrated methods involving physical, chemical, and biological steps present tremendous potential for NAC treatment. However, very few studies have addressed such approach, especially at large scale. Authors propose the following treatment regime for NAC degradation. An initial physical technique such as adsorption will collect and concentrate nitroaromatic pollutants from the source, after which suitable AOP treatment (e.g., exposure of TiO2-UV) will degrade the pollutants to more biodegradable forms. The resulting intermediates can then be subjected to biological treatment methods to achieve complete mineralization and toxicity reduction. The combined treatment approach will fetch a quantum leap towards the development of economical, sustainable, and ecofriendly system for the removal of NACs from the environment. The combination of regular monitoring, sustainable remediation techniques, public awareness, and regulations will aid to solve the curse of NAC pollutants.
Abbreviations
- 1,3,8-TNN:
-
1,3,8-Trinitronaphthaline
- TNB:
-
2,4,6-Trinitrobenzene
- TNP:
-
2,4,6-Trinitrophenol
- TNT:
-
2,4,6-Trinitrotoluene
- DNB:
-
Dinitrobenzene
- DNN:
-
Dinitronaphthalene
- DNP:
-
Dinitrophenol
- DNT:
-
Dinitrotoluene
- RDX:
-
Hexahydro-1,3,5-trinitro-1,3,5-triazine
- NA:
-
Nitroaniline
- NACs:
-
Nitroaromatic compounds
- NB:
-
Nitrobenzene
- NC:
-
Nitrocatechol
- NP:
-
Nitrophenol
- NSA:
-
Nitrosalicylic acid
- NT:
-
Nitrotoluene
- NPAH:
-
Nitro-polyaromatic hydrocarbons
- HMX:
-
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
- PCNB:
-
Pentachloronitrobenzene
- PAHs:
-
Polyaromatic hydrocarbons
References
Alber T, Cassidy M, Zablotowicz R, Lee J (2000) Degradation of p-nitrophenol and pentachlorophenol mixtures by Sphingomonas sp. UG30 in soil perfusion bioreactors. J Indust Microbiol Biotechnol 25:93–99. https://doi.org/10.1038/sj.jim.7000040
Amezquita-Garcia T, Razo-Flores E, Cervantes F, Rangel-Mendez J (2013) Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbo 55:276–284. https://doi.org/10.1016/j.carbon.2012.12.062
Arora P, Jain R (2011) Pathway for degradation of 2-Chloro-4-Nitrophenol in Arthrobacter sp. SJCon Curr Microbiol 63:568–573. https://doi.org/10.1007/s00284-011-0022-2
Arora P, Sharma A, Mehta R, Shenoy B, Srivastava A, Singh V (2012) Metabolism of 4-chloro-2-nitrophenol in a Gram-positive bacterium Exiguobacterium sp. PMA. Microb Cell Fact 11:150. https://doi.org/10.1186/1475-2859-11-150
Bausinger T, Preuss J (2009) Stability of nitroaromatic specialty explosives in reversed-phase liquid chromatographic systems. J Haz Mat 162:1578–1582. https://doi.org/10.1016/j.jhazmat.2008.05.139
Bausinger T, Bonnaire E, Preub J (2007) Exposure assessment of a burning ground for chemical ammunition on the Great War battlefields of Verdun. Sci Total Environ 382:259–271. https://doi.org/10.1016/j.scitotenv.2007.04.029
Belloli R, Barletta B, Bolzacchini E, Meinardi S, Orlandi M, Rindon B (1999) Determination of toxic nitrophenols in the atmosphere by high-performance liquid chromatography. J Chromatography A. 846:277–281. https://doi.org/10.1016/S0021-9673(99)00030-8
Bergauer P, Fonteyne P-A, Nolard N, Schinner F, Margesin R (2005) Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 59:909–918. https://doi.org/10.1016/j.chemosphere.2004.11.011
Bharadiya AA, Lakhotia AN, Patange A, Jaju JB, Choraria K (2014) Nitrobenzene poisoning presenting as methemoglobinemia. Muller Journal of Medical Sciences and Research 5:185–187
Bhushan B, Chauhan A, Samanta S, Jain R (2000) Kinetics of biodegradation of p-nitrophenol by different bacteria. Biochem Biophysical Res Comm 274:626–630. https://doi.org/10.1006/bbrc.2000.3193
Birceanu O, Sorensen L, Henry M, McClelland G, Wang Y, Wilkie M (2014) The effects of the lampricide 3-trifluoromethyl-4-nitrophenol (TFM) on fuel stores and ion balance in a non-target fish, the rainbow trout (Oncorhynchus mykiss). Comparative Biochem Physiol Part C 160:30–41. https://doi.org/10.1016/j.cbpc.2013.10.002
Boopathy R, Melancon E (2004) Metabolism of compounds with nitro-functions by Klebsiella pnuemoniae isolated from a regional wetland. International Biodeterioration and Biodegrad 54:269–275. https://doi.org/10.1016/j.ibiod.2004.03.004
Braeckevelt M, Mirschel G, Wiessner A, Rueckert M, Reiche N, Vogt C, Chultz A, Paschke H, Kuschk P, Kaestner M (2008) Treatment of chlorobenzene-contaminated groundwater in a pilot-scale constructed wetland. Ecol Eng 33:45–53. https://doi.org/10.1016/j.ecoleng.2008.02.002
Brüning T, Their R, Bolt H (2002) Nephrotoxicity and nephro carcinogenicity of dinitrotoluene: new aspects to be considered. Rev Environ Health 17:163–172. https://doi.org/10.1515/REVEH.2002.17.3.163
Cai Q-Y, Mo C-H, Wu Q-T, Zeng Q-Y, Katsoyiannis A (2007) Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere 68:1751–1762. https://doi.org/10.1016/j.chemosphere.2007.03.041
Cecinato A, Palo V, Pomata D, Sciano M, Possanzini M (2005) Measurement of phase-distributed nitrophenols in Rome ambient air. Chemosphere 59:679–683. https://doi.org/10.1016/j.chemosphere.2004.10.045
Charles PT, Dingle BM, Van Bergen S, Gauger PR, Patterson CH, Kusterbeck AW (2001) Enhanced biosensor performance for on-site field analysis of explosives in water using solid-phase extraction membranes. Field Analyt Chem Technol 5:272–280. https://doi.org/10.1002/fact.10007
Chavan A, Mukharji S (2008) Treatment of hydrocarbon-rich wastewater using oil degrading bacteria and phototrophic microorganisms in rotating biological contactor: effect of N: P ratio. J Hazard Mater 154:63–72. https://doi.org/10.1016/j.jhazmat.2007.09.106
Chen X, Chen B (2015) Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ Sci Technol 49:6181–6189. https://doi.org/10.1021/es5054946
Chen W, Chiang W, Lai C (2007a) Recovery of nitrotoluenes in wastewater by solvent extraction. J Haz Mat 145:23–29. https://doi.org/10.1016/j.jhazmat.2006.12.066
Chen W, Duan L, Zhu D (2007b) Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ Sci Technol 41:8295–8300. https://doi.org/10.1021/es071230h
Chen W, Juan C, Wei K (2007c) Decomposition of dinitrotoluene isomers and 2,4,6-trinitrotoluene in spent acid from toluene nitration process by ozonation and photo-ozonation. J Haz Mat 147:97–104. https://doi.org/10.1016/j.jhazmat.2006.10.072
Chen R, Liu C, Yuan L, Zha J, Wang Z (2016) 2, 4-Dichloro-6-nitrophenol, a photonitration product of 2, 4- dichlorophenol, caused anti-androgenic potency in Chinese rare minnows (Gobiocypris rarus). Environ Pollut 1:8–598. https://doi.org/10.1016/j.envpol.2016.06.016
Chen R, Yuan L, Zha J, Wang Z (2017) Developmental toxicity and thyroid hormone-disrupting effects of 2,4-dichloro-6-nitrophenol in Chinese rare minnow (Gobiocypris rarus). Aquatic Toxicology 185:40–47. https://doi.org/10.1016/j.aquatox.2017.02.005
Chunli Z, Jiti Z, Jing W, Baocheng Q (2008) Isolation and characterization of a nitrobenzene degrading yeast strain from activated sludge. J Hazard Mat. 160:194–199. https://doi.org/10.1016/j.jhazmat.2008.02.101
Coe K, Jia Y, Ho H, Rademacher P, Bammler T, Beyer R, Farin F, Woodke L, Plymate S, Fausto N, Nelson S (2007) Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening. Chem Res Toxicol 20:1277–1290. https://doi.org/10.1021/tx7001349
Contreras S, Rodriguez M, Chamarro E, Esplugas S (2001) UV- and UV/Fe(III)-enhanced ozonation of nitrobenzene in aqueous solution. J Photochem Photobiol A Chemistry 142:79–83. https://doi.org/10.1016/S1010-6030(01)00460-9
Deng Y, Zhao R (2015) Advanced oxidation processes (AOPs) in wastewater treatment. Curr Pollut Reports 1:167–176. https://doi.org/10.1007/s40726-015-0015-z
Doble M, Kumar A (2005) Treatment of waste from organic chemical industries. In: Doble M, Kumar A (eds) Biotreatment of Industrial Effluents. Elsevier, Amsterdam, pp 55–64
DoCP (Department of Chemicals and Petrochemicals) (2017) Chemical and petrochemical statistics at a glance. Ministry of Chemicals and Fertilizers. Government of India, New Delhi
Dodard S, Sarrazina M, Hawari J, Paquet L, Ampleman G, Thiboutot S, Sunahara G (2013) Ecotoxicological assessment of a high energetic and insensitive munitions compound: 2,4-Dinitroanisole (DNAN). J Haz Mat 262:143–150. https://doi.org/10.1016/j.jhazmat.2013.08.043
Du D, Wang M, Zhang J, Cai J, Tu H, Zhang A (2008) Application of multiwalled carbon nanotubes for solid-phase extraction of organophosphate. Electrochem Comm 10:85–89. https://doi.org/10.1016/j.elecom.2007.11.005
Essam T, Amin M, Tayeb O, Mattiasson B, Guieysse B (2007) Solar-based detoxification of phenol and p-nitrophenol by sequential TiO2 photocatalysis and photosynthetically aerated biological treatment. Water Res 41:1697–1704. https://doi.org/10.1016/j.watres.2007.01.015
Fares M, Moayyed S, Ahmed S, Al-Shannag (2008) Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds. J Environ Sci 147:97–104. https://doi.org/10.1016/S1001-0742(08)62112-9
Fritsche W, Hofrichter M (2000) Aerobic degradation by microorganisms. In: Rehm H-J, Reed G (eds) Biotechnology, 2nd edn, vol 11b, Environmental processes II. Wiley, Weinheim, pp 145–155
Fritsche W, Scheibne K, Harrre A, Hofrichter A (2000) Fungal degradation of explosives: TNT and related nitroaromatic compounds. In: Spain JC, Hughes JB, Knackmuss H-J (eds) Biodegradation of Nitroaromatic Compounds and Explosives. CRC Press, Florida, pp 213–238
Fu W, Fu H, Skott K, Yang M (2008) Modeling the spill in the Songhua River after the explosion in the petrochemical plant in Jilin. Environ Sci Pollut Res 15:178–181. https://doi.org/10.1065/espr2007.11.457
Ganranoo L, Mishra S, Azad A, Shigihara A, Dasgupta P, Breitbach Z, Armstrong D, Grudpanand K, Rappenglueck B (2010) Measurement of nitrophenols in rain and air by two-dimensional liquid chromatography-chemically active liquid core waveguide spectrometry. Anal Chem 82:5838–5843. https://doi.org/10.1021/ac101015y
García Einschlag F, Carlos L, Capparelli A, Braun A, Oliveros E (2002) Degradation of nitroaromatic compounds by the UV–H2O2 processusing polychromatic radiation sources. Photochem Photobiol Sci 1:520–525. https://doi.org/10.1039/b203152c
Gehrke M, Kapila S, Hambacker K, Flanigan V (2000) Design of an automated rapid vapor concentrator and its application in nitroaromatic vapor sampling: detection and remediation technologies for mines and mine like targets V. SPIE Vol. 4038. https://doi.org/10.1117/12.396219
Gharbani P, Mehrizad A (2014) Heterogeneous catalytic ozonation process for removal of 4-chloro-2-nitrophenol from aqueous solutions. J Saudi Chem Soc 18:601–605. https://doi.org/10.1016/j.jscs.2012.07.013
Ghosh A, Khurana M, Chauhan A, Takeo M, Chakraborti A, Jain R (2010) Degradation of 4-nitrophenol, 2-chloro-4-nitrophenol, and 2,4-dinitrophenol by Rhodococcus imtechensis strain RKJ300. Environ Sci Technol 44:1069–1077. https://doi.org/10.1007/s11356-014-2802-2
Ghosh P, Das M, Thakur I (2014) Mammalian Cell-line based bioassays for toxicological evaluation of landfill leachate trated by Pseudomonas sp. ISTDF1. Environ. Sci Pollut Res 21:8084–8094. https://doi.org/10.1007/s11356-014-2802-2
Gingras A, Sarette J, Shawler E, Lee T, Freund S, Holwitt E, Hicks BW (2013) Fluorescent proteins as biosensors by quenching resonance energy transfer from endogenous tryptophan: detection of nitroaromatic explosives. Biosens Bioelectron 48:251–257. https://doi.org/10.1016/j.bios.2013.03.076
Grundlingh J, Paul I, El-Zanfaly M, Wood D (2011) 2,4-Dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol 7:205–212. https://doi.org/10.1007/s13181-011-0162-6
Haderlein S, Weissmahr K, Schwarzenbach R (1996) Specific adsorption of nitroaromatic explosives and pesticides to clay minerals. Environ Sci Technol 30:612–622. https://doi.org/10.1021/es9503701
Hawari J, Halasz A, Beaudet S, Ampleman G, Thiboutot S (1999) Biotransformation of 2,4,6-trinitrotoluene with Phanerochaete chrysosporium in agitated cultures at pH 4.5. Appl Environ Microbiol 65:2977–2986
Homma-Takeda S, Hiraku Y, Ohkuma Y, Oikawa S, Murata M, Ogawa K, Iwamuro T, Li S, Sun GF, Kumagai Y, Shimojo N, Kawanishi S (2002) 2,4,6-trinitrotoluene-induced reproductive toxicity via oxidative DNA damage by its metabolite. Free Radic Res 36:555–566. https://doi.org/10.1080/10715760290025933
Hrapovic S, Majid E, Liu Y, Male K, Luong L (2006) Metallic nanoparticle−carbon nanotube composites for electrochemical determination of explosive nitroaromatic compounds. Analytical Chemistry 78:5504–5512. https://doi.org/10.1021/ac060435q
Ju K-S, Parales R (2010) Nitroaromatic compounds from synthesis to biodegradation. Microbiol Mol Bio Reviews 74:250–272
Jugan M, Levi Y, Blondeau J (2010) Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol 79:939–947. https://doi.org/10.1016/j.bcp.2009.11.006
Karim K, Gupta S (2001) Biotransformation of nitrophenols in upflow anaerobic sludge blanket reactors. Bioresource Technol 8:179–186. https://doi.org/10.1016/S0960-8524(01)00092-X
Karnjanapiboonwong A, Zhang B, Freitag C, Dobrovolny M, Salice C, Smith P, Kendall R, Anderson T (2009) Reproductive toxicity of nitroaromatics to the cricket, Acheta domesticus. Sci Total Environ 407:5046–5049. https://doi.org/10.1016/j.scitotenv.2009.05.048
Kennedy A, Laird J, Lounds C, Gong P, Barker N, Brasfield S, Russell A, Johnson M (2015) Inter- and intraspecies chemical sensitivity: a case study using 2,4-Dinitroanisole. Environ Toxicol Chem 34:402–411. https://doi.org/10.1002/etc.2819
Khan Z, Anjaneyulu Y (2005) Influence of soil components on adsorption –desorption of hazardous organic-development of low cost technology for reclamation of hazardous waste dump sites. J Hazard Mater 118:161–169. https://doi.org/10.1016/j.jhazmat.2004.10.010
Kirchner M, Matisova E, Hrouzkova S, Huskova R (2007) Fast GC and GC-MS analysis of explosives. Petroleum & Coal 49:72–79
Kitanovski Z, Grgić I, Vermeylen R, Claeys M, Maenhaut W (2012) Liquid chromatography tandem mass spectrometry method for characterization of monoaromatic nitro-compounds in atmospheric particulate matter. J Chromatography A 1268:35–43. https://doi.org/10.1016/j.chroma.2012.10.021
Kolb G, Becker N, Scheller S, Zugmaier G, Pralle H, Wahrendorf J, Havemann K (1993) Increased risk of acute myelogenous leukemia (AML) and chronic myelogenous leukemia (CML) in a county of Hesse, Germany. Soz Praventivmed 38:190–195
Kovacic P, Somanathan R (2014) Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. Appl Toxicol J 34:810–824. https://doi.org/10.1002/jat.2980
Labana S, Pandey G, Paul D, Sharma N, Basu A, Jain R (2005) Pot and field studies on bioremediation of p-nitrophenol contaminated soil using Arthrobacter protophormiae RKJ100. Environ Sci Technol 39:3330–3337. https://doi.org/10.1021/es0489801
Landvik N, Gorria M, Arlt V, Asare N, Solhaug A, Lagadic-Gossmann D, Holme J (2007) Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: possible implications for their mutagenic and carcinogenic effects. Toxicol 231:159–174. https://doi.org/10.1016/j.tox.2006.12.009
Lent E, Crouse L, Quinn M, Wallace S (2012) Assessment of the in vivo genotoxicity of isomers of dinitrotoluene using the alkaline Comet and peripheral blood micronucleus assays. Mut Res 742:54–60. https://doi.org/10.1016/j.mrgentox.2011.11.013
Lessner D, Johnson G, Parales R, Spain J, Gibson D (2002) Molecular characterization and substrate specificity of nitrobenzene dioxygenase from Comamonas sp. strain JS765. Appl Environ Microb 68:634-641. https://doi.org/10.1128/AEM.68.2.634-641.2002
Leungsakul T, Keenan B, Yin H, Smets B, Wood T (2005) Saturation mutagenesis of 2,4-DNT Dioxygenase of Burkholderia sp. Strain DNT for enhanced dinitrotoluene degradation. Biotechnol Bioeng 92:416–426. https://doi.org/10.1002/bit.20602
Li Y, Hsieh W, Mahmudov R, Wei X, Huang C (2013) Combined ultrasound and Fenton (US-Fenton) process for the treatment of ammunition wastewater. J Haz Mat 244–245:403–411. https://doi.org/10.1016/j.jhazmat.2012.11.022
Li T, Deng X, Wang J, Chen Y, He L, Sun Y, Song C, Zhou Z (2014) Biodegradation of nitrobenzene in a lysogeny broth medium by a novel halophilic bacterium Bacillus licheniformis. Marine Pollut Bulletin 89:384–389. https://doi.org/10.1016/j.marpolbul.2014.09.028
Li J, Luo X, Zhou Y, Zhang L, Huo Q, Liu Y (2018) Two metal–organic frameworks with structural varieties derived from cis–trans isomerism nodes and effective detection of nitroaromatic explosives. Cryst Growth Des 18:1857–1863. https://doi.org/10.1021/acs.cgd.7b01726
Lima S, Castro P, Morais R (2003) Biodegradation of p-nitrophenol by microalgae. J Appl Phycol 15:137–142. https://doi.org/10.1023/A:1023877420364
Liou M, Lu M, Chen J (2003) Oxidation of explosives by Fenton and photo-Fenton processes. Water Res 37:3172–3179. https://doi.org/10.1016/S0043-1354(03)00158-1
Liu H, Wang S, Zhou N (2005) A new isolate of Pseudomonas stutzeri that degrades 2-chloronitrobenzene. Biotechnology Letters 27:275–278. https://doi.org/10.1007/s10529-004-8293-3
Liu Z, Yang C, Qiao C (2007) Biodegradation of p-nitrophenol and 4-chlorophenol by Stenotrophomonas sp. FEMS Microbial Letters 277:150–156. https://doi.org/10.1111/j.1574-6968.2007.00940.x
Lotharius J, O’Malley K (2000) The Parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Bio Chem 275:38581–38588. https://doi.org/10.1074/jbc.M005385200
Ma F, Yuan G, Meng L, Oda Y, Hu J (2012) Contributions of flumequine and nitroarenes to the genotoxicity of river and ground waters. Chemosphere 88:476–483. https://doi.org/10.1016/j.chemosphere.2012.02.080
Mahan K, Penrod J, Ju K, Kass N, Tan W, Truong R, Parales J, Parale R (2015) Selection for growth on 3-nitrotoluene by 2-nitrotoluene-utilizing Acidovorax sp. strain JS42 identifies nitroarenedioxygenases with altered specificities. Appl Environ Biol 81:309–319. https://doi.org/10.1128/AEM.02772-14
McGee LM, McCausland A, Plume CA, Mariett NC (1942) Metabolic disturbances in workers exposed to dinitrotoluene. Am J Dìgest Dìs 9:329–332
McNeil E, Ritchie A, Melton D (2013) The toxicity of nitrofuran compounds on melanoma and neuroblastoma cells is enhanced by Olaparib and ameliorated by melanin pigment. DNA Repair 12:1000–1006. https://doi.org/10.1016/j.dnarep.2013.08.017
Meyers S, Deng S, Basta N, Clarkson W, Wilber G (2007) Long-term explosive contamination in soil: effects on soil microbial community and bioremediation. Soil Sediment Contam 16:61–77. https://doi.org/10.1080/15320380601077859
Min J, Zhang J, Zhou N (2016) A two-component para-nitrophenol monooxygenase initiates a novel 2-chloro-4-nitrophenol catabolism pathway in Rhodococcus imtechensis RKJ300. Appl Environ Microbiol 82:714–723. https://doi.org/10.1186/s13568-018-0574-7
Min J, Wang B, Hu X (2017) Effect of inoculation of Burkholderia sp. strain SJ98 on bacterial community dynamics and para-nitrophenol, 3-methyl-4-nitrophenol, and 2-chloro-4-nitrophenol degradation in soil. Sci Rep 7:5983–5994. https://doi.org/10.1186/s13568-018-0574-7
Mori Y, Amata K, Oda N, Ayashi H, Eki K, Aneda K, Oshino S, Akushima A, Akata A, Suzuki A (2003) Isolation of nitrophenols from diesel exhaust particles (DEP) as vasodilatation compounds. Biol Pharm Bull 26:394–395. https://doi.org/10.1248/bpb.26.394
Mulla S, Hoskeri R, Shouche Y, Ninnekar H (2011) Biodegradation of 2-nitrotoluene by Micrococcus sp. strain SMN-1. Biodegradation 22:95–102. https://doi.org/10.1007/s10532-010-9379-3
Nie D, Li P, Zhang D, Zhou T, Liang Y, Shi G (2010) Simultaneous determination of nitroaromatic compounds in water using capillary electrophoresis with amperometric detection on an electrode modified with a mesoporous nano-structured carbon material. Electrophoresis 31:2981–2988. https://doi.org/10.1002/elps.201000275
Nishino S, Paoli G, Spain J (2000) Aerobic degradation of dinitrotoluenes and the pathway for bacterial degradation of 2,6-dinitrotoluene. Appl Environ Microbiol 66:2139–2147. https://doi.org/10.1128/AEM.66.5.2139-2147.2000
Oehrle S (1996) Analysis of nitramine and nitroaromatic explosives by capillary electrophoresis. J Chromat A 745:233–237. https://doi.org/10.1016/0021-9673(96)00388-3
Oh S, Kang S, Chiu P (2010) Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci Total Environ 408:3464–3468. https://doi.org/10.1016/j.scitotenv.2010.04.032
Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166. https://doi.org/10.1016/j.scitotenv.2010.08.061
Øvrevik AV, Øya E, Nagy E, Mollerup S, Phillips D, Låg M, Holme J (2010) Differential effects of nitro-PAHs and amino-PAHs on cytokine and chemokine responses in human bronchial epithelial BEAS-2B cells. Toxicol Appl Pharmacol 242:270–280. https://doi.org/10.1016/j.taap.2009.10.017
Pandey J, Heipieper H, Chauhan A, Arora P, Prakash D, Takeo M, Jain R (2011) Reductive dehalogenation mediated initiation of aerobic degradation of 2-chloro-4-nitrophenol (2C4NP) by Burkholderia sp. strain SJ98. Appl Microbiol Biotechnol 92:597–607. https://doi.org/10.1007/s00253-011-3254-y
Park J, Wade T, Sweet S (2001) Atmospheric distribution of polycyclic aromatic hydrocarbons and deposition to Galveston Bay, Texas USA. Atmos Environ 35:3241–3249. https://doi.org/10.1016/S1352-2310(01)00080-2
Parry R, Nishino S, Spain J (2011) Naturally-occurring nitro compounds. Nat Prod Rep 28:152–167. https://doi.org/10.1039/c0np00024h
Pavlostathis S, Jackson G (2002) Biotransformation of 2,4,6-trinitrotoluene in a continuous-flow Anabaena sp. system. Water Res 36:1699–1706. https://doi.org/10.1016/S0043-1354(01)00382-7
Pham C, Tang N, Toriba A, Hayakawa K (2015) Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in atmospheric particles and soil at a traffic site in Hanoi, Vietnam. Polycyc Aromat Compd 35:355–371. https://doi.org/10.1080/10406638.2014.903284
Pichtel J (2012) Distribution and fate of military explosives and propellants in soil: a review. App Environ Soil Sci 617236:1–33. https://doi.org/10.1155/2012/617236
Pinheiro H, Touraud E, Thomas O (2004) Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigm 61:121–139. https://doi.org/10.1016/j.dyepig.2003.10.009
Podlipná R, Pospíilová B, Vanek T (2015) Biodegradation of 2,4-dinitrotoluene by different plant species. Ecotoxicol Environ safety 122:54–59. https://doi.org/10.1016/j.ecoenv.2014.07.026
Pon Saravanan N, Venugopalan S, Kumar S, Santhosh P, Kavita B, Gurumallesh Prabu H (2006) Voltammetric determination of nitroaromatic and nitramine explosives contamination in soil. Talanta 69:656–662. https://doi.org/10.1016/j.talanta.2005.10.041
Prakash D, Kumar R, Jain R, Tiwary R (2011) Novel pathway for the degradation of 2-chloro-4-nitrobenzoic acid by Acinetobacter sp. strain RKJ12. Appl Environ Microbiol 77:6606–6613. https://doi.org/10.1128/AEM.00685-11
Preiss A, Elend M, Gerling S, Berger-Preiss E, Steinbach K (2007) Identification of highly polar nitroaromatic compounds in leachate and ground water samples from a TNT-contaminated waste site by LC-MS, LC-NMR, and off-line NMR and MS investigations. Anal Bioanal Chem 389:1979–1988. https://doi.org/10.1007/s00216-007-1573-8
Psillakis E, Mantzavinos D, Kalogerakis N (2004) Development of a hollow fibre liquid phase microextraction method to monitor the sonochemical degradation of explosives in water. Anal Chimica Acta 501:3–10. https://doi.org/10.1016/j.aca.2003.09.015
Purohit V, Basu K (2000) Mutagenicity of nitroaromatic compounds. Chemical Res Toxicol. 13:673–692. https://doi.org/10.1021/tx000002x
Rabaaoui N, Moussaoui Y, Allagui S, Ahmed B, Elaloui E (2013) Anodic oxidation of nitrobenzene on BDD electrode:variable effects and mechanisms of degradation. Separ Purificat Technol 107:318–323. https://doi.org/10.1016/j.seppur.2013.01.047
Roberts M, Rugh C, Li H, Teppen B, Boyd S (2007) Reducing bioavailability and phytotoxicity of 2,4-dinitrotoluene by sorption on K-smectite clay. Environ Toxicol Chem 26:358–360. https://doi.org/10.1897/06-254R1
Rodgers J, Bunce N (2001) Treatment methods for the remediation of nitroaromatic explosives. Water Res 35:2101–2111. https://doi.org/10.1016/S0043-1354(00)00505-4
Rodrigues M, Silva F, Paiva T (2009) Combined zero-valent iron and fenton processes for the treatment of Brazilian TNT industry wastewater. J Haz Mat 165:1224–1228. https://doi.org/10.1016/j.jhazmat.2008.09.120
Saini S, Copello G, Rao A (2017) HPLC-UV platform for trace analysis of three isomeric mononitrophenols in water with chitin based solid phase extraction. Anal Methods 9:4143–4150. https://doi.org/10.1039/C7AY01000A
Sanchez C, Carlsson H, Colmsjo A, Crescenzi C, Batlle R (2003) Determination of nitroaromatic compounds in air samples at femtogram level using C18 membrane sampling and on-line extraction with LC-MS. Anal Chem 75:4639–4645. https://doi.org/10.1021/ac034278w
Sander M, Pignatello J (2005) Characterization of charcoal adsorption sites for aromatic compounds: insights drawn from single-solute and bi-solute competitive experiments. Environ Sci Technol 39:1606–1615. https://doi.org/10.1021/es049135l
Sangili A, Annalakshami M, Chen S-M, Chen T-W, Kumaravel S, Govindasami M (2018) A Facile synthesis of ultra-small cerium oxide nanoparticles for enhanced electrochemical detection of nitrobenzene in water samples. Int J Electrochem Sci 13:6135–6143. https://doi.org/10.20964/2018.06.118
Schenzle A, Lenke H, Spain J, Knackmuss H (1999) Chemoselective nitro group reduction and reductive dechlorination initiate degradation of 2-Chloro-5-nitrophenol by Ralstonia eutropha JMP134. Appl Environ Microb 65:2317–2323
Schmidt A, Niehus B, Matysik F (2006) Identification and quantification of polar nitroaromatic compounds in explosive-contaminated waters by means of HPLC-ESI-MS-MS and HPLC-UV. Chromatographia 63(2):1–11. https://doi.org/10.1365/s10337-005-0703-8
Shen X, Shan X, Dong D, Hua X, Owens G (2009) Kinetics and thermodynamics of sorption of nitroaromatic compounds to as-grown and oxidized multiwalled carbon nanotubes. J Colloid Interface Sci 330:1–8. https://doi.org/10.1016/j.jcis.2008.10.023
Shen G, Tao S, Wei S, Chen Y, Zang Y, Shen H, Haung Y, Zhu D, Yuan C, Wang H, Wang Y, Pei L, Liao Y, Duan Y, Wang B, Wang R, Lv Y, Li W, Wang X, Zheng X (2013) Field measurement of emission factors of PM, EC, OC, parent, nitro- and oxy- polycyclic aromatic hydrocarbons for residential briquette, coal cake, and wood in Rural Shanxi. China. Environ Sci Technol. 47:2998–3005. https://doi.org/10.1021/es304599g
Shi X, Ji L, Zhu D (2010) Investigating roles of organic and inorganic soil components in sorption of polar and nonpolar aromatic compounds. Environ Pollut 158:319–324. https://doi.org/10.1016/j.envpol.2009.06.036
Singh S (2007) Sensors- an effective approach for the detection of explosives. Journal of Hazard Mater 144:15–28. https://doi.org/10.1016/j.jhazmat.2007.02.018
Singh D, Ramanathan G (2013) Biomineralization of 3-nitrotoluene by Diaphorobacter species. Biodegradation 24:645–655. https://doi.org/10.1007/s10532-012-9612-3
Singh M, Srivastava R (2011) Sequencing batch reactor technology for biological wastewater treatment: a review. Asia-Pac J Chem Eng 6:3–13. https://doi.org/10.1002/apj.490
Singh D, Mishra K, Ramanathan G (2015) Bioremediation of nitroaromatic compounds. In: Samer M (ed) Wastewater Treatment Engineering. IntechOpen, London, pp 51–83
Sinha S, Kulkarni P, Shah S, Desai N, Patel G, Mansuri M, Saiyed H (2006) Environmental monitoring of benzene and toluene produced in indoor air due to combustion of solid biomass fuel. Sci Tot Environ 357:280–287. https://doi.org/10.1016/j.scitotenv.2005.08.011
Smida H, Jamoussi J (2012) Degradation of nitroaromatic pollutant by titanium dioxide/zinc phthalocyanine: study of the influencing factors. J Appl Chem 2:2278–5736. https://doi.org/10.9790/5736-0231117
Soni D, Gandhi D, Tarale P, Bafana A, Pandey RA, Sivanesan S (2017) Oxidative stress and genotoxicity of zinc oxide nanoparticles to Pseudomonas species, human promyelocytic leukemic (HL-60), and blood cells. Biol Trace Elem Res 178:218–227
Soojhawon I, Lokhande P, Kodam K, Gawai K (2005) Biotransformation of nitro-aromatics and their effects on mixed function oxidase system. Enz Microb Technol 37:527–533. https://doi.org/10.1016/j.enzmictec.2005.03.011
Spain J (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49:523–555. https://doi.org/10.1146/annurev.mi.49.100195.002515
Srivastava A, Peshin S, Kaleekal T, Gupta S (2005) An epidemiological study of poisoning cases reported to the National Poisons Information Centre, All India Institute of Medical Sciences, New Delhi. Human & Experiment Toxicol 24:279–285. https://doi.org/10.1191/0960327105ht527oa
Stringer R, Gangopadhyay S, Grant S (2010) Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal Chem 82:4015–4019. https://doi.org/10.1021/ac902838c
Sun R, Huo X, Lu H, Feng S, Wang D, Liu H (2018) Recyclable fluorescent paper sensor for visual detection of nitroaromatic explosives. Sensors and Actuators B: Chemical. 265:476–487. https://doi.org/10.1016/j.snb.2018.03.072
Takagi K, Iwasaki A, Kamei I, Satsuma K, Yoshioka Y, Harada N (2009) Aerobic mineralization of hexachlorobenzene by newly isolated pentachloronitrobenzene-degrading Nocardioides sp. strain PD653. Appl Environ Microbiol 75:4452–4458. https://doi.org/10.1128/AEM.02329-08
Tchounwou P, Newsome C, Glass K, Centeno J, Leszczynski J, Bryant J, Okoh J, Ishaque A, Brower M (2003) Environ toxicology and health effects associated with dinitrotoluene exposure. Rev Environ Health 18:203–229. https://doi.org/10.1515/REVEH.2003.18.3.203
Teich M, Pinxteren D, Wang M, Kecorius S, Wang Z, Müller T, Mocnik G, Herrmann H (2017) Contributions of nitrated aromatic compounds to the light absorption of water-soluble and particulate brown carbon in different atmospheric environments in Germany and China. Atmos Chem Phys 17:1653–1672. https://doi.org/10.5194/acp-17-1653-201
Tian H, Guo Y, Pan B, Gu C, Li H, Boyd S (2015) Enhanced photoreduction of nitro-aromatic compounds by hydrated electrons derived from indole on natural montmorillonite. Environ Sci Technol 49:7784–7792. https://doi.org/10.1021/acs.est.5b01026
Tiwari R, Pande U (1972) Spectrophotometric determination of some aromatic nitro compounds in microgram quantities. Microchem J 17:476–479. https://doi.org/10.1016/0026-265X(72)90118-X
Tiwari J, Naoghare P, Sivanesan S, Bafana A (2017) Biodegradation and detoxification of chloronitroaromatic pollutant by Cupriavidus. Bioresource Technol 223:184–191. https://doi.org/10.1016/j.biortech.2016.10.043
Toropov A, Rasulev B, Leszczynski J (2007) QSAR modeling of acute toxicity for nitrobenzene derivatives towards rats: comparative analysis by MLRA and optimal descriptors. QSAR Comb Sci 26:686–693. https://doi.org/10.1002/qsar.200610135
Trapido M, Kallas J (2010) Advanced oxidation processes for the degradation and detoxification of 4-nitrophenol. Environ Technol 21:799–808. https://doi.org/10.1080/09593330.2000.9618966
Upson R, Burns S (2006) Sorption of nitroaromatic compounds to synthesized organoclays. J Colloid Interface Sci 297:70–76. https://doi.org/10.1016/j.jcis.2005.10.040
Vikram S, Pandey J, Kumar S, Raghava G (2013) Genes involved in degradation of para-nitrophenol are differentially arranged in form of non-contiguous gene clusters in Burkholderia sp. strain SJ98. Plosone 8: e84766. https://doi.org/10.1371/journal.pone.0084766
Walsh M, Walsh M, Ramsey C, Brochu S, Thiboutot S, Ampleman G (2013) Perchlorate contamination from the detonation of insensitive high-explosive rounds. J Haz Mat 262:228–233. https://doi.org/10.1016/j.jhazmat.2013.08.045
Wang K, Hsieh W, Chou M, Chang C (1999) Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution. Appl Catalysis B environ 21:1–8. https://doi.org/10.1016/S0926-3373(98)00116-7
Wang T, Zhang N, Bai R, Bao R (2018) Aggregation-enhanced FRET-active conjugated polymer nanoparticles for picric acid sensing in aqueous solution. J Mat Chem C 6:266–270. https://doi.org/10.1039/C7TC05015A
Woltman S, Even W, Sahlin E, Weber S (2000) Chromatographic detection of nitroaromatic and nitramine compounds by electrochemical reduction combined with photoluminescence following electron transfer. Anal Chem 72:4928–4933. https://doi.org/10.1021/ac000170u
Wu J, Jiang C, Wang B, Ma Y, Liu Z, Liu S (2006) Novel partial reductive pathway for 4-chloronitrobenzene and nitrobenzene degradation in Comamonas sp. strain CNB-1. Appl Environ Microbiol 73:1759–1765. https://doi.org/10.1128/AEM.72.3.1759-1765.2006
Yamazaki H, Hatanaka N, Kizu R, Hayakawa K, Shimada K, Guengerich F, Nakajima M, Yokoi T (2000) Bioactivation of diesel exhaust particle extracts and their major nitrated polycyclic aromatic hydrocarbon components, 1-nitropyrene and dinitropyrenes, by human cytochromes P450 1A1, 1A2, and 1B1. Mutat Res 472:129-138. https://doi.org/10.1016/S1383-5718(00)00138-8
Yen J, Lin K, Wang Y (2002) Acute lethal toxicity of environmental pollutants to aquatic organisms. Ecotoxicol. Environ Safety 52:113–116. https://doi.org/10.1006/eesa.2002.2167
Zhang C, Hughes J, Nishino S, Spain J (2000) Slurry-phase biological treatment of 2,4-dinitrotoluene and 2,6-dinitrotoluene: role of bioaugmentation and effects of high dinitrotoluene concentrations. Environ Sci Technol 34:2810–2816. https://doi.org/10.1021/es000878q
Zhang H-X, Cao A-M, Hu J-S, Wan L-J, Lee S-T (2006) Electrochemical sensor for detecting ultratrace nitroaromatic compounds using mesoporous SiO2-modified electrode. Anal Chem 78:1967–1971. https://doi.org/10.1021/ac051826s
Zhang Y, Wang J, Ge Z, Guo G, Gao S (2014) Survey of polycyclic aromatic hydrocarbons and nitrated polycyclic aromatic hydrocarbons in Jiaxing city, China. Environ Life Sci 71:1095–1103. https://doi.org/10.1007/s12665-013-2513-x
Zhao D, Liu C, Zhang Y, Liu Q (2011) Biodegradation of nitrobenzene by aerobic granular sludge in a sequencing batch reactor (SBR). Desalination 281:17–22. https://doi.org/10.1016/j.desal.2011.07.037
Zhen D, Liu H, Wang S, Zhang J, Zhao F, Zhou N (2006) Plasmid-mediated degradation of 4-chloronitrobenzene by newly isolated Pseudomonas putida strain ZWL73. Appl Microbial Cell Physiol 72:797–803. https://doi.org/10.1007/s00253-006-0345-2
Zheng Y, Xu S, Yuan Y, Li W, Zhong Y, Xong L, Liu D (2008) Biodegradation of p-nitrophenol by Pseudomonas aeruginosa HS-D38. 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai
Zhou L, Ishizaki Z, Spitzer M, Taylor L, Temperley D, Johnson L, Brear P, Gautier P, Zeng Z, Micthell A, Narayan V, McNeil EM, Melton DW, Smith TK, Tyers M, Westwood NJ, Patton EE (2012) ALDH2 mediates 5-Nitrofuran activity in multiple species. Chem Biol 19:883–892. https://doi.org/10.1016/j.chembiol.2012.05.017
Zhu D, Kwon S, Pignatello J (2005) Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions. Environ Sci Technol 39:3990–3998. https://doi.org/10.1021/es050129e
Zou W, Yang J, Yang T, Hu X, Lian H (2012) Magnetic-room temperature phosphorescent multifunctional nanocomposites as chemosensor for detection and photo-driven enzyme mimetics for degradation of 2,4,6-trinitrotoluene. J Mat Chem 22:4720–4727. https://doi.org/10.1039/C2JM15139A
Acknowledgments
Jyoti Tiwari is thankful to the Department of Science and Technology (DST), India, for the financial assistance in the form of DST-INSPIRE senior research fellowship (Reference number DST/INSPIRE/03/2014/001429).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Hongwen Sun
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• NACs are one of the most widespread and hazardous pollutants of today’s world.
• Present review summarizes prevalence of NACs in the environment across the globe.
• The basis of hazardous effects of NACs on the environment and health is discussed.
• The remediation prospects and challenges associated with NACs are listed.
• Integrated approach involving different treatments is required for remediation.
Rights and permissions
About this article
Cite this article
Tiwari, J., Tarale, P., Sivanesan, S. et al. Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environ Sci Pollut Res 26, 28650–28667 (2019). https://doi.org/10.1007/s11356-019-06043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06043-8