Abstract
This paper aimed to optimize a method for extraction of 26 polycyclic aromatic hydrocarbons (PAHs), which are petroleum markers, and their derivatives in surface sediment samples. The gas chromatography coupled to mass spectrometry detection with automated headspace solid phase microextraction (HS-SPME-GC/MS) was used in the studies. Extraction temperature, extraction time, stirring, sample mass, the volume of saline solution and sorption time were the factors optimized applying 24 complete factorial and 23 rotational central composed designs. The linear range of the calibration curve for each PAH was from 10 to 40 ng g−1 and the Pearson correlations for all the compounds were above 0.98. The detection and quantification limits values ranged from 0.13 to 0.46 ng g−1 dry weight and 0.42 to 1.52 ng g−1 dry weight, respectively. The mean recoveries of the spiked samples ranged from 74 ± 16% (acenaphthylene) to 98 ± 5% (fluoranthene) and 84 ± 8% (retene) to 119 ± 6% (9-methylanthracene) for the spiked blanks. Sediment samples show the concentration of PAHs ranged from 0.55 ± 0.04 (naphthalene) to 17.4 ± 0.5 (pyrene) ng g−1 based upon the dry weight.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polycyclic aromatic hydrocarbons (PAHs) are a group of ubiquitous organic pollutants that have natural sources (volcanic eruptions, biomass burning, and forest fires) and anthropogenic ones (burning of fossil fuels, runoff from paved roads, sewage and industrial waste) [1,2,3]. These compounds are resistant to degradation, being included in the class of persistent organic pollutants known as POPs [2, 4] and sixteen are harmful to the environment and to humans due to their mutagenic and carcinogenic properties [4, 5]. These characteristics have made them included in the list of priority pollutants by the United States Environmental Protection Agency [6].
The determination of these compounds in sediment samples have been performed by several methods that employ various steps in sample treatment, such as extraction, concentration, transfers and purification, generating losses of the compounds, decreasing accuracy and precision of the methods [2, 7, 8]. As Sampei et al. [9] that applied ultrasonic bath with dichloromethane and methanol (9:1) to extraction of PAHs in sediment cores from lakes Shinji and Nakaumi in Japan. Chen et al. [10] used accelerated solvent extraction with 100 ml dichloromethane to extract 16 priority PAHs in surfaces sediments from Bering Sea, Chukchi Sea, and Canadian Basin. The solid-phase-dispersive microextraction method based on magnetic carbon nanocomposites coupled with dispersive liquid–liquid microextraction and two miscible stripping solvents (MSPE–DLLME) accompanied of gas chromatography–mass spectrometry (GC–MS) was developed by Yazdanfar et al. [11] for 16 PAHs priority in water and soil samples. Furthermore, these methods use organochlorine solvents such as dichloromethane [7, 12], a possible human carcinogen [13,14,15].
In this perspective, the solid phase microextraction (SPME) has gained prominence. Since it is coupled to chromatographic techniques, it allows the automation of the sample treatment process, reducing sample preparation time and smaller sources of error. It results in an improvement of extraction efficiency [16,17,18,19].
SPME is a technique developed for the preparation of samples for analysis in gas chromatography. The sorption of the compounds is performed on a chemically modified silica fiber with subsequent thermal desorption of the compounds in a chromatograph [18, 20, 21]. Hence, the sample can be placed directly in a vial, eliminating steps of extraction with solvent, purification and pre-concentration [16, 18, 21,22,23]. Feng et al. [24] developed a solid-phase microextraction tube based on barium sulfate nanoparticle-coated basalt fibers and eight PAHs was determinate in water samples by HPLC. The method showed low limit of detection (0.10 μg L−1) and satisfactory recovery (79–121%) [24].
The two main modes of SPME sampling are direct and headspace (HS) extraction. In the direct extraction mode, the fiber is immersed in the sample and the compounds are transported from the sample to the extractor film [18, 20]. In headspace mode, the compounds are transported through the air barrier before they reach the fiber coating. In this mode of extraction, the fiber is protected by possible damages caused by interferes and suspended particles, being recommended the use for the analysis of semi-volatile and volatile compounds in liquid and solid samples [18, 20]. The fiber coating type is an important factor to SPME analysis. Recently, Tian et al. [25] employed the UiO-66 metal–organic framework (MOFs) with coating fiber to quantification of nine PAHs in water samples, obtained good extraction performance and detection limits (0.01–0.03 μg L−1).
Ghiasvand and collaborators increased performance in the HS-SPME technique by simultaneously heating the sample and cooling the fiber coating (cold-fiber headspace solid-phase microextraction—CF-SPME) for six PAHs in sediment samples with very good sensitivity and reasonable precision [26]. The CF-SPME technique was improved employing with the use of the pressure-balanced procedure to eight PAHs in sediments getting efficiency in the extraction and good precision in comparison to regular CF-SPME [27]. Vacuum-assisted headspace solid phase microextraction (Vac-HSSPME) was reported for five PAHs in solid matrix, which was constated that lowering the pressure, the extraction kinetics of low volatile analytes can be significantly improved [28].
SPME has been gained increasing attention in the optimization of sample preparation methods because it contribute to greener chemistry practices. Galuszka and collaborators reviewed the principles of green chemistry and proposed twelve green analytical chemistry practices [29]. These are: (1) the use of direct analytical techniques, (2) minimal sample amounts, (3) measurements in situ, (4) integration of analytical processes, (5) employ of automated and miniaturized methods, (6) the derivatization should be avoided, (7) generation of a large volume of analytical waste should be avoided, (8) multi-compound or multi-parameter methods are preferred, (9) the use of energy should be minimized, (10) reagents obtained from renewable source should be preferred, (11) toxic reagents should be eliminated or replaced and (12) the safety of the operator should be increased [29].
Therefore, the present work aimed to perform the optimization of a method to extract to 26 PAHs in surface river sediments, employing automated system by headspace solid phase microextraction with gas chromatography coupled mass spectrometry (HS-SPME-CG/MS).
Materials and Methods
Standards and Reagents
Sodium chloride was used in the experiments for extraction via headspace. A standard mixture containing 26 PAHs (naphthalene; 2-methylnaphthalene; 1-methylnaphthalene; biphenyl; 2-ethylnaphthalene; 1-ethylnaphthalene; 2,6-ethylnaphthalene and 2,7-ethylnaphthalene; 1,7-dimethylnaphthalene; 1,3-dimethylnaphthalene; 1,6-dimethylnaphthalene; 2,3-dimethylnaphthalene and 1,4-dimethylnaphthalene; 1,5-dimethylnaphthalene; acenaphthylene; 1,2-dimethylnaphthalene; 1,8-dimethylnaphthalene; acenaphthene; fluorine; dibenzothiophene; phenanthrene; anthracene; 1-methylanthracene; 1-methylphenanthrene; 9-methylanthracene; fluoranthene; pyrene; 2-methylfluoranthene; retene) at 10 ng µL−1 and surrogates standard mixture containing naphthalene-d8, acenaphthene-d10 and phenanthrene-d10 at 10 ng µL−1 was prepared to the studies. Fluoranthene-d10 was employed with an internal standard at 10 ng µL−1. The individual standards were obtained commercially (Sigma-Aldrich).
Collection of Sediment Samples
Three sediment samples were collected using a Petersen dredger, in the city of Juiz de Fora. They were collected from the São Pedro (sample 1), João Penido (sample 2) and the Chapéu D’Uvas (sample 3) dams, in August of 2016. Initially, the samples were transferred to a previously decontaminated aluminum tray to remove litter and wood. The collected material was then stored in aluminum containers and transported to the laboratory. After collection, the sediment was dried in an oven at 38 °C for 72 h, and residues of branches, leaves, and trash were removed by priming [30]. Finally, the dried sample was sieved (3 mm sieve) to remove larger rocks over a mortar, in which the particle size reduction was carried out until a homogeneous granulometry. The treated material was stored in decontaminated glass bottles.
Instrumentation
PAHs analyses were performed on a gas chromatograph coupled mass spectrometer, GC 2010 Shimadzu, with RtX®-5 ms (30 m × 0.25 mm d.i. × 0.25 µm film; 5% diphenyl and 95% dimethylpolysiloxane) column. Equipped with AOC-5000 Autosampler with an incubator for headspace solid phase microextraction (HS-SPME-GC/MS). The gas chromatograph temperature was: 50 °C (2 min.), then 4 °C/min to 105 °C (2 min.), 2 °C/min to 120 °C, 4 °C/min to 145 °C (2 min), 2 °C/min to 160 °C, 4 °C/min to 200 °C (2 min), 4 °C/min to 250 °C and 20 °C/min to 290 °C (10 min). The mass spectrometer with quadrupole mass analyzer operated in the SIM mode, with the ionization source at 230 °C and electron beam at 70 eV. SPME Fiber Assembly Polydimethylsiloxane/Divinylbenzene (PDMS/DVB StableFlex—Supelco) of 65 μm thickness was used in studies.
Experimental Designs
Initially, a 24 complete factorial design with quintuplicates in the central point was performed as a preliminary study, with screening purposes. The factors evaluated were: sample mass (3 g and 5 g), solvent volume (4 mL and 5 mL), time (20 min and 60 min) and extraction temperature (70 °C and 90 °C). After this screening step, a 23 rotational central composite design (RCCD) with triplicate in the central point was performed.
The RCCD is usually composed by a factorial part, also known as cubic, an axial part or star and another with tests at the central point, with the axial point being α = 4√nfactorial [31]. The factors studied in this design were stirring, time and temperature of extraction, as presented in Table 1. In the 23 central composite design with triplicate in central point, seventeen experiments were performed in total, being fourteen trials in different conditions proposed by the cubic portion plus the axial portion and three tests in the central point for the evaluation of the experimental error. For all experiments, the analytical response was monitored through the sum of the area obtained for each compound studied. In the assays, the fortification was carried out with standard solutions of the 10 ng μL−1 PAHs and surrogates and the internal standard was 10 ng μL−1, adding 10 μL of each solution. The sample used in these studies was the sediment collected in São Pedro dam.
In the 20 mL flask, the sample mass required for the experiment was weighed. Then, the fortification of the sample was performed with the solution containing the PAHs and surrogate standards. Thereafter, the vial was sealed with foil and shaken for 5 min by hand to homogenize. After this process, the internal standard solution and the 30% (m/v) sodium chloride solution were added to the flask. Finally, the vial was sealed with a magnetic cap containing a 1.5 mm thick septum.
After optimizing the extraction conditions for the PAHs, a univariate study was conducted to optimize the sorption time of the compounds in the fiber. In this study, six flasks containing the fortified sample were subjected to the extraction conditions established, by headspace, and for each flask, different sorption times were applied. The sorption times studied were 0.5; 1; 2; 3; 4 and 5 min. All process was performed in triplicate.
Method Validation
For the evaluation and validation of the method, we performed a study of recovery employed samples and blank spiked following the procedures suggested by the International Union of Pure and Applied Chemistry (IUPAC) [32]. In this study, São Pedro dam sediment was used with matrix and for blank spiked was employed a sample of sediment, which had a negligible concentration of PAHs. The accuracy and precision of the method were evaluated by recovery studies of samples (n = 6) and blanks (n = 6) spiked. The spikes were performed in middle concentration level of calibration and the experiments were carried out using the conditions optimized.
Analytical curves for PAHs were constructed using internal standardization with sample overlap. The curves of each compound comprised the concentration levels of 0, 10, 20, 30 and 40 ng g−1 dry weight and were prepared from a standard mixture solution of PAHs and another one, containing surrogates and internal standards at 10 ng μL−1. The surrogates and internal standards were maintained at 20 ng g−1 in all experiments. Four replicates were performed in the central point, for further evaluation of the experimental error, regression parameters of the linear model and analysis of variance (ANOVA) to verify the fit of the regression model. Furthermore, the limits of detection and quantification were estimated based on the calibration curve obtained for each compound [33, 34]. The Microsoft Office® software, Excel 2013, was performed for all data handling and construction of the response surface methodology.
Results and Discussion
Calibration Curve
The calibration curves used for the quantification of PAHs in the sediment samples were constructed as described in Sect. “Method validation” and in Table 2 are shows the equations obtained, the regression parameters and the analysis of variance for each PAH.
The coefficients of Pearson obtained ranged from 0.9836 to 0.9960 indicating a good fit of the experimental points with the adjusted curve. In the study of the linearity of the analytical curve, it was observed that the regression of the model is significant for all the compounds (Fcalulated ≫ Ftabulated). Moreover, analysis of variance (ANOVA) for the linear regression models indicated that there is no significant evidence of non-fitted models at 95% confidence level (Fcalculated ≪ Ftabulated).
Extraction by HS-SPME-GC/MS and Experimental Designs
In the headspace extraction, factors such as sorption time, fiber coating, temperature, stirring speed, sample mass, salt-water volume, extraction time, type of fiber are some of the parameters that can influence the signal obtained [18]. The headspace extraction system in this work is complex because four-phase system can be considered: the fiber coating, the gas phase or headspace, the salt-water and the solid sample. In this perspective, mass conservation can be expressed by Eq. 1:
where \(C_{0}\) is the analyte concentration in the sample;\(C_{\text{s}}^{\infty } ,C_{\text{w}}^{\infty } ,\)\(C_{\text{h}}^{\infty }\) and \(C_{\text{f}}^{\infty }\) are the equilibrium concentrations of the analyte in the sample, salt-water, headspace and fiber coating, respectively; and \(V_{\text{s}} ,\)\(V_{\text{w}} ,\)\(V_{\text{h}}\) and \(V_{\text{f}}\) are the volume of sample, salt water, headspace and fiber coating, respectively. If the definition of the coating/gas distribution constant as \(K_{\text{fh}} = C_{\text{f}}^{\infty } /C_{\text{h}}^{\infty }\), the gas/salt-water distribution constant as \(K_{\text{hw}} = C_{\text{h}}^{\infty } /C_{\text{w}}^{\infty }\) and water/sample matrix distribution constant as \(K_{\text{ws}} = C_{\text{w}}^{\infty } /C_{\text{s}}^{\infty }\), the mass of the analyte absorbed by the fiber coating (\(n = C_{\text{f}}^{\infty } V_{\text{f}}\)), can be expressed by Eq. 2:
As \(K_{\text{fs}} = \frac{{C_{\text{f}}^{\infty } }}{{C_{\text{s}}^{\infty } }} = K_{\text{fh}} K_{\text{hw}} K_{\text{ws}} ,\) Eq. 2 can be rewriting:
Based on Eq. 3, the concentration of analyte adsorbed by fiber coating is directly proportional to the concentration contained in the sample [18]. Thus, a complete 24 factorial design was used to estimate the behavior of the signal varying the conditions of extraction time, sample mass, volume of sodium chloride solution at 30% (m/v) and the extraction temperature. In Supplementary material-Table 1, the values obtained to the sum of areas for the 26 PAHs in each experiment and the surrogates employed to calibration are presented.
A quadratic prediction model (Eq. 4 in supplementary material) was constructed with these values, in which the coefficients of this model and their respective errors were calculated by the expressions β = (XTX)−1 × (XTY) and expression V(β) = (XTX)−1σ2, respectively [31].
Based on the values presented by the model, it can be observed that the factors extraction time and extraction temperature were the factors that were found to be significant at 95% confidence for the model. The other factors and their interactions were not significant (Table 2 in supplementary material).
In addition to the calculations of the model coefficients and their errors, a variance analysis (ANOVA) was performed to verify the fit of the model. A relative standard deviation of 9.95% was calculated through the quintuplicates of the central point and indicates a good precision since the experimental error allowed for the PAHs in the concentration of ng g−1 is about 35% [35]. The value of calculated F (0.096) was lower than that of tabulated F (7.700) using a confidence of 95%, indicating that there is no evidence of lack of adjustment of the response model (Table 2 in supplementary material). For the evaluation of the behavior of the prediction model in front of the studied variables, were constructed responses surfaces with the two factors (time and temperature of extraction) that were significant for the model. Figure 1 shows the response surface, which is presenting the sum of areas predicted as a function of temperature, and extraction time when mass and volume are set to zero.
Other surfaces were constructed taking into account the combination of levels for each factor. However, they presented the same response profile, indicating that there is a higher response when extraction temperature and extraction time are in the high level (+1). Based on the results presented, the sample mass and the volume of sodium chloride solution were not significant factors, which suggests the use of its lower levels for the extraction process of PAHs from the headspace sediment. However, the highest sample mass level was chosen to be used in the extractions, since the concentration of PAHs in the samples is very low, in the order of ng g−1 and Eq. 3 shown directly relationship between the fiber coating and the concentration of analyte in the sample.
The effect of extraction temperature on the model is stronger, being almost three times greater than the effect of extraction time. This behavior of the temperature effect on the model may be overlapping with the extraction time effect and for this reason, the response surface shows a signal growth for a longer extraction time. Thus, to try to visualize an extraction condition in which the signal is more stable, a 23 central rotational composite design with triplicate at the central point was performed. One of the advantages of using composite central design is its versatility of work. Since it consists of a complete 23 factorial design, 6 axial points and central points, it can be converted to a complete factorial design if the generated quadratic model is not satisfactory [31]. In the 23 central composite design with triplicate in the central point the factors extraction time, volume of 30% (w/v) sodium chloride solution and stirring speed were studied, as shown in Table 1.
To try to decrease the extraction time proposed by the previous design, the stirring speed factor was inserted, because until now it was set at 300 rpm and a higher stirring speed can help in the homogenization of the system temperature and consequent decrease of the time to reach the time of balance [18]. In addition, the volume of the sodium chloride solution was investigated again because at a higher stirring speed the sediment can stay exposed.
Although the previous model indicates that at 90 °C better signals are obtained, it was observed that at the edge of the extraction flask water was occurring condensation, an undesirable phenomenon, since the water can drag impurities into the coating fiber. Thus, in the new experimental design, was setting the extraction temperature at 80 °C, which is not observed condensation of water in any region of the extraction flask.
Observing the date of experimental design (Table 3 in supplemental material), the experiment 14 showed the highest sum of areas for PAHs, using a lower extraction time, stirring speed at 400 rpm and a volume of 4.5 mL. The relative standard deviation obtained for this study was 10%, below the limit of 35% [35]. The factors and their interactions did not present evidence of significance for the model using a confidence of 95%. It suggests that the conditions to be applied for extracting the PAHs in the sediment samples have the lowest levels applied to factors of experimental design. The factors and their interactions did not present evidence of significance for the model at 95% of confidence (see Table 4 in supplemental material). It suggests that the conditions to be applied for extracting the PAHs in the sediment samples have the lowest levels applied to factors of experimental design.
Based on the model obtained, the response surfaces were constructed to evaluate the signal profile in the function of the factors studied. Observing the response surface shown in Fig. 2a, when the extraction time is set at the highest level (+1.69), the sum of the areas of PAH as a function of the stirring speed and volume of the saline solution is larger, indicated in Fig. 2a in the region of the grey color of the graph.
In the response surface generated when the stirring speed is 300 rpm, shown in Fig. 2b, it is observed that for a volume of 5 mL of NaCl solution, the extraction time has no variation for the analytical response, suggesting that this region has reached the chemical equilibrium of compounds with the fiber. Thus, the conditions chosen for extracting the PAHs in the sediment samples using the headspace were an extraction time of 53.2 min, extraction temperature of 80 °C, the volume of 5 mL saline solution, desorption time of 1 min, stirring at 300 rpm and 5 g sample.
After the optimization of these parameters, a univariate study was carried out to determine the sorption time of the compounds in the fiber as described in item 3.5. The results are shown in Fig. 3. It can be seen from the graph in Fig. 3 that the ideal sorption time was 5 min because from this moment on there was no significant response gain, which indicates that the equilibrium of analytes between the fiber and the headspace in the flask was reached. Thus, this time was defined for the extraction of PAHs in the sediment samples. Once the studies of extraction conditions optimization to the PAHs by headspace were carried out, the tests were done to verify the accuracy and precision of the optimized method.
Validation of the Method
The accuracy and precision studies were performed as reported in item 2.5 and the results are presented in Table 3. Mean recovery values for the spiked samples varied from 74 ± 16% (acenaphthylene) to 98 ± 5.0% (fluoranthene) and for spiked blanks the values varied from 84 ± 8.0% (retene) to 119 ± 6.0% (9-methylanthracene). The values of relative standard deviations are within the stipulated for the concentration level of the compounds, between 3.0% (pyrene) to 16% (acenaphthylene).
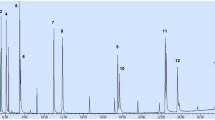
The surrogate standards presented excellent recovery values ranging from 78 ± 12% (phenanthrene-d10) to 87 ± 13% (naphthalene-d8) in spiked samples and 63 ± 15% (phenanthrene-d10) to 81 ± 4% (naphthalene-d8) in spiked blanks. Figure 4a, b shows the chromatogram obtained for a spiked sample and spiked blank, respectively. Since the calibration was performed by matrix overlap and under the same analysis conditions of the samples. Good accuracy for the method is to be expected because any effect of the matrix on the chemical equilibrium of analytes between the sample and fiber will also be produced on the calibration. However, the relative standard deviation values obtained for the spiked samples and blanks show the good accuracy of the method and its robustness over different matrices of sediments.
Chromatograms obtained in SIM mode by HS-SPME-GC/MS being (a) for São Penido spiked sample at 20 ng g−1 dry weight, b for spiked blank at 20 ng g−1 dry weight and c for São Pedro dam sediment sample. PAHs: 1: naphthalene-d8, 2: naphthalene, 3: 2-methylnaphthalene, 4: 1-methylnaphthalene, 5: biphenyl, 6: 2-ethylnaphthalene, 7: 1-ethylnaphthalene, 8: 2,6-ethylnaphthalene and 2,7-ethylnaphthalene, 9: 1,7-dimethylnaphthalene e 1,3-dimethylnaphthalene, 10: 1,6-dimethylnaphthalene, 11: 2,3-dimethylnaphthalene and 1,4-dimethylnaphthalene, 12: 1,5-dimethylnaphthalene, 13: acenaphthylene, 14: 1,2-dimethylnaphthalene, 15: 1,8-dimethylnaphthalene, 16: acenaphthene-d10, 17: acenaphthene, 18 : fluorene, 19: dibenzothiophene, 20: phenanthrene-d10, 21: phenanthrene, 22: anthracene, 23: 1-methylanthracene, 24: 1-methylphenanthrene, 25: 9-methylanthracene, 26: fluoranthene-d10, 27: fluoranthene, 28: pyrene, 29: 2-methylfluoranthene, 30: retene
The detection and quantification limits for PAHs were estimated based on the analytical curve. The LOD values varied from 0.13 to 0.46 ng g −1 dry weight and LOQ varied from 0.42 to 1.52 ng g −1 dry weight. Table 3 shows the detection and quantification limits of the method and the samples result from dams obtained for each compound.
In addition to the good values of accuracy and precision, the method may be used to perform a lot of analysis in less time. It shown to be a greener analytical practice because the method includes many principles of a greener chemistry [19, 29]. Among the principles, the method contemplates being automated, integration of processes, waste reduction, security for the analyst, eliminates toxic reagent and it is a multi-compound.
Samples Results
The concentration of PAHs in the dams sediment samples ranged from 0.55 ± 0.04 (naphthalene) to 17.4 ± 0.5 (pyrene) ng g−1 dry weight, being the highest total concentration found in sample 1. This sample was collected in São Pedro dam, which is located in the urban area of the city, with rapid transit routes for large vehicles and dwelling. In Fig. 4c is presented the chromatogram obtained for the sediment sample from São Pedro dam in Juiz de Fora, Brazil.
However, samples 2 and 3 (João Penido and Chapéu D’Uvas) are located in regions further away from the urban center and shows low values of polycyclic aromatic hydrocarbons. The origin of these compounds is diversified and can be introduced into the environment by both natural and anthropic processes. The most significant contributing source is the burning of fossil fuels and biomass, which result in the release of PAHs into the atmosphere [1, 3]. In general, the presence of lower molecular weight PAHs (2 and 3 aromatic rings) indicates contamination by petroleum and its derivatives. On the other hand, contamination of pyrolytic origin is characterized by the predominance of higher molecular weight PAHs [36,37,38].
The samples of surface sediments collected showing a contribution of PAHs by pyrolytic sources and anthropic sources. This was already expected, since the region of Juiz de Fora is characterized by the occurrence of many forest fires, mainly from May to September with colder period and with lower precipitations [39], which may be inserted by atmospheric deposition near the place of origin and by aqueous transport or via fine atmospheric particulate material. Moreover, the city is located in a region of Brazil that is considered an important route of land transportation, presenting in its surroundings important federal and state highways.
The introduction of PAHs through the combustion of fossil fuels is observed for the samples collected since that the naphthalene was quantified in all them. Juiz de Fora has a large vehicular fleet, and consequently, intense traffic of vehicles that contribute to this type of introduction. The levels of PAH concentrations found in the dam sediments at Juiz de Fora were similar to or lower than those found by Nagy and collaborators when determining PAHs in sediment samples from the Danube River in Hungary (8.3 a 1202.5 ng g−1) [40]. Nevertheless, we must consider the differences between PAHs used by each author to obtain the sum of PAHs and the differences between aquatic ecosystems.
Conclusions
The determination of polycyclic aromatic hydrocarbons in sediments was optimized using HS-SPME-GC/MS. The extraction consisted of using an extraction time of 53.2 min, extraction temperature of 80 °C, 5 mL of saline solution, desorption time of 1 min, sorption time of 5 min, stirring at 300 rpm and 5 g of sample.
The method was validated and exhibit good recovery values, accuracy, and precision. Moreover, the method does not use organic solvents, which reduced analyses time when compared to several other methodologies that were employed for similar purposes. It contributes to the generation of less toxic residues, a safer process for the analyst and a lower cost in terms of energy. Furthermore, the method is a good practice to a greener analytical chemistry.
References
Werres F, Balsaa P, Schmidt TC (2009) Total concentration analysis of polycylic aromatic hydrocarbons in aqueous samples with high suspended particulate matter content. J Chromatogr A 1216:2235–2240. https://doi.org/10.1016/j.chroma.2009.01.021
Li J, Dong H, Zhang D et al (2015) Sources and ecological risk assessment of PAHs in surface sediments from Bohai Sea and northern part of the Yellow Sea, China. Mar Pollut Bull 96:485–490. https://doi.org/10.1016/j.marpolbul.2015.05.002
Cao Z, Wang Y, Ma Y et al (2005) Occurrence and distribution of polycyclic aromatic hydrocarbons in reclaimed water and surface water of Tianjin, China. J Hazard Mater 122:51–59. https://doi.org/10.1016/j.jhazmat.2005.04.003
Huang S, He S, Xu H et al (2015) Monitoring of persistent organic pollutants in seawater of the Pearl River Estuary with rapid on-site active SPME sampling technique. Environ Pollut 200:149–158. https://doi.org/10.1016/j.envpol.2015.02.016
Li GL, Lang YH, Gao MS et al (2014) Carcinogenic and mutagenic potencies for different PAHs sources in coastal sediments of Shandong Peninsula. Mar Pollut Bull 84:418–423. https://doi.org/10.1016/j.marpolbul.2014.04.039
USEPA (2003) Priority Pollutants. In: Code Fed. Regul. (CFR).Title 40. Chapter I.Part 423 Append. A to Part 423. https://www3.epa.gov/region1/npdes/permits/generic/prioritypollutants.pdf. Accessed 29 Aug 2018
Santos MDR, Cerqueira MRF, de Oliveira MAL et al (2014) Box-Behnken design applied to ultrasound-assisted extraction for the determination of polycyclic aromatic hydrocarbons in river sediments by gas chromatography/mass spectrometry. Anal Methods 6:1650. https://doi.org/10.1039/c3ay41857j
Net S, Dumoulin D, El-Osmani R et al (2014) Experimental design approach to the optimisation of hydrocarbons extraction from the sediment: method development and application. Appl Geochem 40:126–134. https://doi.org/10.1016/j.apgeochem.2013.11.009
Sampei Y, Uraoka S, Ono T, Dettman DL (2019) Estuarine, Coastal and Shelf Science Polycyclic aromatic hydrocarbons (PAHs) in sediment cores from lakes Shinji and Nakaumi, SW Japan : a proxy of recent fi re events in the watershed. Estuar Coast Shelf Sci 226:106269. https://doi.org/10.1016/j.ecss.2019.106269
Chen F, Lin Y, Cai M et al (2018) Occurrence and risk assessment of PAHs in surface sediments from Western Arctic and Subarctic Oceans. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15040734
Yazdanfar N, Shamsipur M, Ghambarian M, Esrafili A (2018) A highly sensitive dispersive microextraction method with magnetic carbon nanocomposites coupled with dispersive liquid-liquid microextraction and two miscible stripping solvents followed by GC–MS for quantification of 16 PAHs in environmental samples. Chromatographia 81:487–499. https://doi.org/10.1007/s10337-018-3469-5
Ontiveros-Cuadras JF, Ruiz-Fernández AC, Sanchez-Cabeza JA et al (2019) Recent history of persistent organic pollutants (PAHs, PCBs, PBDEs) in sediments from a large tropical lake. J Hazard Mater 368:264–273. https://doi.org/10.1016/j.jhazmat.2018.11.010
IARC (1999) IARC monographs on the evaluation of carcinogenic risks to humans. Re-eval Some Org Chem Hydrazine Hydrog Peroxide 71:319–335
Araujo FM, Santos MDR, de Oliveira MAL et al (2017) Box-Behnken design applied to optimize the ultrasound-assisted extraction of petroleum biomarkers in river sediment samples using green analytical chemistry. Anal Methods 9:5859–5867. https://doi.org/10.1039/C7AY01470H
Poli D, Manini P, Andreoli R et al (2005) Determination of dichloromethane, trichloroethylene and perchloroethylene in urine samples by headspace solid phase microextraction gas chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 820:95–102. https://doi.org/10.1016/j.jchromb.2005.03.009
Arrebola FJ, Frenich AG, González Rodríguez MJ et al (2006) Determination of polycyclic aromatic hydrocarbons in olive oil by a completely automated headspace technique coupled to gas chromatography-mass spectrometry. J Mass Spectrom 41:822–829. https://doi.org/10.1002/jms.1040
Ishizaki A, Saito K, Hanioka N et al (2010) Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line in-tube solid-phase microextraction coupled with high-performance liquid chromatography-fluorescence detection. J Chromatogr A 1217:5555–5563. https://doi.org/10.1016/j.chroma.2010.06.068
Pawliszyn J (2012) Development of SPME devices and coatings
Arthur LC, Pawliszyn J (1990) Solid Phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a019
LANÇAS, F. M (2004) Extração em Fase Sólida (SPE), 4o. RiMa
Afshar Mogaddam MR, Mohebbi A, Pazhohan A et al (2019) Headspace mode of liquid phase microextraction: a review. TrAC—Trends Anal Chem 110:8–14. https://doi.org/10.1016/j.trac.2018.10.021
Valente ALP, Augusto F (2000) MICROEXTRAÇÃO POR FASE SÓLIDA. Quim Nova 23:523
Vas G, Vékey K (2004) Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J Mass Spectrom 39:233–254. https://doi.org/10.1002/jms.606
Feng J, Sun M, Wang X et al (2018) Barium sulfate nanoparticles as a coating for solid-phase microextraction of polycyclic aromatic hydrocarbons in aqueous samples. Chromatographia 81:1287–1292. https://doi.org/10.1007/s10337-018-3568-3
Tian Y, Sun M, Wang X et al (2018) A nanospherical metal-organic framework UiO-66 for solid-phase microextraction of polycyclic aromatic hydrocarbons. Chromatographia 81:1053–1061. https://doi.org/10.1007/s10337-018-3524-2
Ghiasvand AR, Hosseinzadeh S, Pawliszyn J (2006) New cold-fiber headspace solid-phase microextraction device for quantitative extraction of polycyclic aromatic hydrocarbons in sediment. J Chromatogr A 1124:35–42. https://doi.org/10.1016/j.chroma.2006.04.088
Xu S, Shuai Q, Pawliszyn J (2016) Determination of polycyclic aromatic hydrocarbons in sediment by pressure-balanced cold fiber solid phase microextraction. Anal Chem 88:8936–8941. https://doi.org/10.1021/acs.analchem.6b01944
Yiantzi E, Kalogerakis N, Psillakis E (2015) Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples. Anal Chim Acta 890:108–116. https://doi.org/10.1016/j.aca.2015.05.047
Gałuszka A, Migaszewski Z, Namieśnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal Chem 50:78–84. https://doi.org/10.1016/j.trac.2013.04.010
Sun F, Littlejohn D, David Gibson M (1998) Ultrasonication extraction and solid phase extraction clean-up for determination of US EPA 16 priority pollutant polycyclic aromatic hydrocarbons in soils by reversed-phase liquid chromatography with ultraviolet absorption detection. Anal Chim Acta 364:1–11. https://doi.org/10.1016/S0003-2670(98)00186-X
BARROS NETO B., SCARMINIO IS., BRUNS RE (2001) Como fazer experimentos: Pesquisa e desenvolvimento na ciência e na indústria
Thompson M, Ellison SLR, Wood R (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem 74:835–855. https://doi.org/10.1351/pac200274050835
Ribani M, Grespan Bottoli CB, Collins CH et al (2004) VALIDAÇÃO EM MÉTODOS CROMATOGRÁFICOS E ELETROFORÉTICOS. Quim Nova 27:771–780. https://doi.org/10.1590/S0100-40422004000500017
Grob RoL, Barry EF (2004) Modern practice of gas chromatography modern practice of gas chromatography
Boyer KW, Horwitz W, Albert R (1985) Interlaboratory variability in trace element analysis. Anal Chem 57:454–459. https://doi.org/10.1021/ac50001a031
De Luca G, Furesi A, Micera G et al (2005) Nature, distribution and origin of polycyclic aromatic hydrocarbons (PAHs) in the sediments of Olbia harbor (Northern Sardinia, Italy). Mar Pollut Bull 50:1223–1232. https://doi.org/10.1016/j.marpolbul.2005.04.021
Heemken OP, Stachel B, Theobald N, Wenclawiak BW (2000) Temporal variability of organic micropollutants in suspended particulate matter of the River Elbe at Hamburg and the River Mulde at Dessau, Germany. Arch Environ Contam Toxicol 38:11–31. https://doi.org/10.1007/s002449910003
Figueira RCL, Lourenço RA, Bícego MC et al (2009) Historical record of polycyclic aromatic hydrocarbons (PAHs) and spheroidal carbonaceous particles (SCPs) in marine sediment cores from Admiralty Bay, King George Island, Antarctica. Environ Pollut 158:192–200. https://doi.org/10.1016/j.envpol.2009.07.025
Torres FTP (2006) Relações entre fatores climáticos e ocorrências de incêndios florestais na cidade de Juiz de Fora (MG). Caminhos Geogr 7:162–171
Nagy AS, Simon G, Szabó J, Vass I (2013) Polycyclic aromatic hydrocarbons in surface water and bed sediments of the Hungarian upper section of the Danube River. Environ Monit Assess 185:4619–4631. https://doi.org/10.1007/s10661-012-2892-6
Acknowledgements
The authors are grateful for the financial support from Brazilian Foundations CAPES, CNPq, and State Foundation FAPEMIG. This research was supported by FAPEMIG (Research Support Foundation of the State of Minas Gerais) (process: APQ-00197-18), CNPq (National Council for Scientific and Technological Development), CAPES (Coordination for the Improvement of Higher Education Personnel) and PROPESQ/UFJF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors confirm that there are no known conflicts of interest associated with this publication and this article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Araujo, F.M., Azevedo, G.C., Nogueira, F.d.S. et al. Eco-friendly Method for the Determination of Polycyclic Aromatic Hydrocarbons in Sediments by HS-SPME-GC/MS. Chromatographia 83, 261–271 (2020). https://doi.org/10.1007/s10337-019-03825-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03825-7