Abstract
Lake Hamana is a semi-enclosed brackish lake amid agricultural and residential land. Monthly vertical profiles of nutrients, total phosphorus (TP), and total nitrogen (TN) at twelve sampling stations in the lake were obtained from 1995 to 2016. Freshwater samples were also obtained from five stations in the river flowing into the lake. Significant decreases were seen in phosphate, TP, and TN concentrations at most lake and all river stations. Decrease in phosphate concentration reflects reduced organic matter and nutrient load into the lake due to increased sewage coverage. Nitrate concentration significantly increased at four stations, whereas ammonium and TN concentrations significantly decreased. This could be due to inefficient nitrification/denitrification of wastewater. At all stations, the nitrogen to phosphate ratio in surface water was higher than 16 and increased significantly. Therefore, phosphate limitation could be strengthened by the decrease in phosphate and increase in nitrate concentrations in the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nitrogen and phosphorus load into coastal waters have increased by anthropogenic contamination through the past half century (e.g., Nixon 1995; Amin et al. 2017; Lefcheck et al. 2018). Cultural eutrophication is progressing in coastal waters resulting in elevated phytoplankton production rates and induces bottom hypoxia due to high organic matter production and degradation. The extent of hypoxia has increased dramatically around the world (Diaz and Rosenberg 2008; Jenny et al. 2016). Currently, many coastal ecosystems heavily stressed by eutrophication and hypoxia are threatened by the loss of biodiversity and alteration of food webs (e.g., Liu et al. 2015; Ding et al. 2016; Schmidt et al. 2017).
Nutrient concentrations in the highly urbanized coastal waters have decreased significantly in recent years because of the implementation of advanced wastewater treatment techniques (Staehr et al. 2017; Tong et al. 2017; Kubo et al. 2019). Therefore, there is the potential to greatly change the nutrient cycling in coastal waters surrounded by residential and agricultural land with increasing sewage coverage because advanced treatment processes directly reduce nutrient concentrations in wastewater. At sewage treatment plants (STPs), the process of removing contaminants from municipal wastewater is classified in three general ways. In primary treatment, sewage flows through large tanks which are used to settle suspended solids. Secondary treatment generally consists of microbial removal of the particulate and dissolved organic matter. Moreover, advanced treatment directly removes nutrients; this includes nitrification/denitrification (ammonia and nitrate removal) and/or phosphate removal processes (Kadlec and Wallace 2008). Although sewage coverage has improved worldwide, only a very small fraction undergoes advanced treatment (WWAP 2017). Since there is limited research on the relationship between the amount of treated wastewater and the nutrient concentrations in lakes, estuaries, and coastal waters, available data for predicting the nutrient cycling change in coastal waters when advanced treatment is increased are scarce globally.
Lake Hamana, located in central Japan, has agricultural land to the west, residential areas to the east, and forested land to the north. It is a brackish lake connected to the Pacific Ocean, with an area of approximately 70.4 km2. River discharge from the northeastern part of the lake accounts for 70% of the total river discharge. Water residence time in the lake is about 30 days (Mazda 1999). The ammonium, nitrate, and phosphate concentrations significantly increased between 1970 and 1990, then remained constant until 2000 (Inoue et al. 2005). In the watershed of Lake Hamana, sewage coverage increased significantly from 57 to 78% between 1995 and 2010, and most STPs conducted advanced treatment (Japan Sewage Works Association 2017). Consequently, nutrient concentrations in the lake may have decreased, and altered nutrient loads into the lake.
Materials and methods
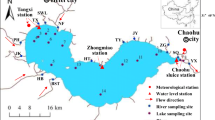
Observations were conducted monthly at twelve stations in Lake Hamana (Stations 1–12) and five stations in the river flowing into the lake (Stations A–E) from January 1995 to March 2016 (Fig. 1). Lake water samples were collected using a bucket or water bottle sampler (Rigo-B transparent water bottle, Rigo Co. Ltd., Japan) on the R/V Hamana of the Shizuoka Fisheries Experimental Station Hamanako Branch. Vertical water samples were collected at 2-m intervals (mean water depth 4.3 m). The maximum depth was approximately 12 m and was at Station 10.
Samples for nutrient analysis (NH4+, NO3−, PO43−) were filtered through a 0.8-μm membrane filter (25CS080AN, Toyo Roshi Kaisha Ltd., Japan). Nutrient concentrations were determined following the methods of Solórzano (1964), Strickland and Parsons (1972), and Murphy and Riley (1962) for ammonium, nitrate, and phosphate, respectively. Detection limits were 0.01, 0.01, and 0.005 mg L−1 for ammonium, nitrate, and phosphate, respectively. Total nitrogen (TN) and total phosphorous (TP) were determined using persulfate oxidation. Chemical oxygen demand (COD) in river waters was measured by titration with potassium permanganate. The detection limits for TN, TP, and COD were 0.01, 0.005, and 0.5 mg L−1, respectively. The concentration of dissolved oxygen (DO) was measured using the Winkler technique.
Quantities of secondary and advanced treatment discharge into Lake Hamana were obtained from statistical data of Japan Sewage Works Association (2017).
The seasonal Mann–Kendall test, a non-parametric statistical trend test, is used to detect monotonic trends in hydrological time series data. To eliminate the effect of serial correlation on the Mann–Kendall trend test, a further trend test was conducted following the method of Yue and Wang (2004). To estimate increasing/decreasing rate, Sen’s method (Gilbert 1987) was used for significant trend data (α < 0.05). The Mann–Kendall trend test and Sen’s slope were calculated using XLSTAT-Forecast (version 2015).
Results
The Mann–Kendall trend analyses for temperature, salinity, nutrients, TN, TP, and DO concentrations in surface lake waters are summarized in Table 1. Surface water temperature did not change significantly from 1995 to 2016, except at Stations 9 and 11 (northwestern part of the lake). Salinity of the surface water decreased significantly at six stations, which were predominantly in the western part of the lake (Table 1). The ammonium concentrations decreased at the southern part of the lake (Stations 1–5) from 1995 to 2016. In contrast, nitrate concentration showed a significant increase at four stations, predominantly in the eastern part of the lake (Stations 3, 5, 6, and 11). Phosphate concentration in the surface water, and in most of the bottom waters, decreased significantly at all stations (Tables 1 and S1). The phosphate concentrations were below the detection limit (< 0.005 mg L−1) at the surface waters of the lake throughout the year, after 2007. TN and TP concentrations decreased significantly at all stations, except for TN concentration at two stations (Stations 7 and 11). In contrast, DO concentration increased significantly at all stations. Time series of TN, TP, and DO concentrations in the surface water at Stations 5 (eastern part of the lake) and 10 (central part of the lake) are presented in Figs. 2 and 3 respectively. The annual mean values of ammonium and phosphate concentrations in the surface water at Station 5 decreased from 61.2 and 26.9 μg L−1, in 1995, to 4.2 and 2.1 μg L−1 in 2015, respectively. In contrast, nitrate concentrations increased from 55.0 μg L−1, in 1995, to 67.5 μg L−1 in 2015. The annual mean values of TN and TP concentrations in the surface water at Station 5 decreased from 0.35 and 0.056 mg L−1, in 1995, to 0.27 and 0.025 mg L−1 in 2015, respectively. The concentrations at Station 10 were lower than those at Station 5. The annual mean values of ammonium, nitrate, and phosphate concentrations in the surface water at Station 10 were 11.3, 13.7, and 6.5 μg L−1 in 1995, and 19.2, 30.0, and 6.1 μg L−1 in 2015, respectively. The annual mean values of TN and TP concentrations in the surface water at Station 10 decreased from 0.31 and 0.037 mg L−1, in 1995, to 0.20 and 0.016 mg L−1 in 2015, respectively, which were aligned with the results of Sen’s slope (Table 2).
The moving average of the 12-month data for dissolved inorganic nitrogen (DIN) and phosphate ratios (DIN/phosphate; μmol μmol−1) at Stations 5 and 10 ranged from 8 to 393 and from 11 to 398 respectively (Fig. 4). These ratios are much higher than 16 (Redfield et al. 1963) at all stations and for all years, except 1996. The DIN/phosphate ratio increased significantly from 1995 to 2015 at most stations (Stations 5–7, 9, 11–12; p < 0.001).
The Mann–Kendall trend analyses for TN, TP, COD, and DO concentrations in the river water flowing into the lake are summarized in Table 3. During the 22-year analysis period, TN, TP, and COD concentrations decreased consistently at all stations. The exception was the COD concentration at Station D. Time series of TN, TP, COD, and DO concentrations in the surface river water at Station C are presented in Fig. 5. The annual mean concentrations of TN, TP, and COD in the surface water at Station C decreased from 8.3, 0.18, and 4.6 mg L−1, in 1995, to 6.2, 0.12, and 2.9 mg L−1 in 2015, respectively (Fig. 5), which were aligned with the results of Sen’s slope (Table 3). There was a large reduction in TN and TP concentrations in the river that was greater than reductions in concentrations in Lake Hamana. Even now, the concentrations in the river water are several times higher than those in the lake (Figs. 2, 3, and 5; Tables 1 and 2).
Discussion
As the installation of STPs was increasing, decrease in phosphate concentrations was likely because of the implementation of advanced wastewater treatment at watershed of Lake Hamana. The total amount of wastewater treated water increased from 2.1 × 106 to 4.0 × 106 m3 year−1 between 1995 and 2016. The amount of advanced treated water also increased from 2.1 × 106 to 3.4 × 106 m3 year−1 (Japan Sewage Works Association 2017). The amount of total and advanced wastewater treated water within the catchment area increased significantly (p < 0.001; Fig. 6). In the surface water, there is a strong negative correlation between the phosphate concentrations and amount of advanced treated wastewater (p < 0.001; Fig. 7), suggesting that the adoption of advanced treatment at STPs is an important factor in phosphate decline in the lake. Because the advanced STPs effluent increased by about 5.8 × 104 m3 year−1, the phosphate reduction rates of − 0.63 and − 0.23 μg L−1 year−1 could be obtained at Stations 5 and 10, respectively, using the regression line (Fig. 7). These values almost agree with the Sen’s slope value for surface phosphate (Table 2). As shown using the trend test, riverine TP concentrations have decreased significantly from 1995 to 2016 (Table 3). Therefore, decreasing phosphate concentration reflects the reduction of nutrient loads into the lake by introducing advanced wastewater treatment technology.
In contrast, there is no correlation between the DIN concentrations in the surface water and the amount of advanced treated wastewater in the Lake Hamana watershed (p > 0.1; Fig. 7). This could be explained by the increase in nitrate concentrations. Nitrate concentration increased significantly in the eastern part of the lake, whereas ammonium and TN concentration decreased significantly. This may be an effect of insufficient wastewater treatment efficiency with respect to the nitrification/denitrification process. Low nitrate removal efficiency has been reported at some treatment plants in the world because of insufficient oxygen control. In addition, TN removal rates greatly decrease when the COD/TN ratio of the sewage influent is below 4.5. This is because denitrification is limited by insufficient organic carbon concentrations (Hashimoto 1989). The ratio is low for the STP influents to the lake basin (3.1 ± 1.4; Japan Sewage Works Association 2017). As a result, the removal rate of nitrification/denitrification may be reduced. The TN removal rate of STPs in the lake basin was 93.6 ± 2.7% in 2013, which is lower than the COD (95.8 ± 1.4%) and TP (97.1 ± 1.8%) removal rates of that year (Japan Sewage Works Association 2017). TN concentrations at the two stations did not decrease significantly (Table 1). The sewage coverage is still less than 80% in the lake basin, and there is a possibility that the inflow of domestic wastewater may continue in some areas.
The lake is adjoined by agricultural land in the west. Currently, the area of agricultural land in the lake basin is decreasing (p < 0.001); it was 104 km2 in 1995 and 58 km2 in 2015 (Ministry of Agriculture, Forestry, and Fisheries 2016). Therefore, the supply of nutrients derived from chemical fertilizers and manure from the catchment area is decreasing, unless the amount of chemical fertilizer and manure outflow per unit area has changed. To clarify the findings obtained from this study in more detail, it is necessary to observe the isotope ratios and determine the quantitative contribution of each source, because isotope analysis enables the identification of nitrate sources (sewage, chemical fertilizers, mineralized soil organic matter, nitrification, and atmospheric nitrate deposition) (e.g., Mayer et al. 2002; Chen et al. 2009; Lee et al. 2016; Zhang et al. 2018).
Decrease in nutrient concentrations in the lake was possibly due to an increase in primary production (Tanaka et al. 2012). Unfortunately, we did not have the data of primary production rate and chlorophyll a concentration (chl a). There is the possibility that photosynthesis has become active and nutrients have decreased due to increased dissolved oxygen concentrations at all stations (Table 1). However, the COD concentrations in the lake and river are also decreasing (Tables 1 and 3). Therefore, the amount of labile organic matter inflow has decreased, and the amount of oxygen consumption may have decreased accordingly. Monthly averaged chl a concentrations from January 1989 to December 1990 were approximately 5 and 40 μg L−1 at Stations 1 and 6, respectively (Okamoto 1995). In contrast, monthly averaged chl a concentrations from October 2017 to October 2018 were about 5 and 25 μg L−1 at Stations 1 and 6, respectively (Kubo unpublished data). Although the data of chl a in the lake is very limited, there is a high likelihood of a slight decreasing trend or no change. Hence, active nutrient consumption may be partly attributed to primary production increase; however, the scale is not significantly large enough to explain nutrients reduction at the lake.
In coastal waters, changing water residence time is also possibly attributing to declining nutrient concentrations (Boyer et al. 1999). Salinity decreased significantly at six stations (western part of the lake) and did not change significantly at another station. As the freshwater inflow amount increases, the water residence time of the lake becomes shorter and the utilization of nutrients may decrease. However, the only increased nutrients were nitrate concentrations. In addition, the stations with increasing of nitrate concentrations differed from the stations with decreasing salinity (Table 1). Concentrations of phosphate, TN, and TP decreased significantly at most stations. In addition, ammonium concentrations in the surface decreased at five stations and did not change significantly at others. Accordingly, the changing water flow into the lake was not significantly large enough to explain the nutrient reduction in the lake.
As stated above, decreased loads of phosphate and organic matter into Lake Hamana due to increased sewage coverage and implementation of advanced wastewater treatment techniques has probably contributed to phosphate concentrations decline in the lake.
The high DIN to phosphate ratio in Lake Hamana from 1995 to 2016 gives rise to the implication that phosphorus is an important element for maintaining primary production (Fig. 4). In most surface waters, the ratio increased significantly (Table 1, Fig. 4). This limitation is further enhanced by the increase in nitrate concentrations and of inputs from high DIN/phosphate STP effluent (> 25; Kubo et al. 2015). In addition, phosphate concentrations decreased and DO concentrations increased significantly in the benthic water at Station 10 (Fig. 8, Tables S1 and S2).
During the stratification season from June to September, the bottom water usually creates hypoxic conditions in the lake. As a result, phosphate was released from the lake sediment to the bottom water because of chemical iron reduction and dissolution of iron-phosphate complex (e.g., Mortimer 1942). However, at the bottom water of Station 10, phosphate concentration decreased significantly because of decreasing phosphate efflux with reduced volume of hypoxic water. Consequently, in the autumn, the supply of phosphate to the surface layers also decreased when surface layers mix with the bottom layer. This phenomenon further causes a decrease in the phosphate concentration and leads to limitation of primary production at the lake.
Conclusions
Decreases in phosphate, TN, and TP in the water column most likely reflect the reduced loading of nutrients and organic matter into the lake (Fig. 9). Phosphate concentrations have decreased significantly with the installation of advanced treatment at STPs. In contrast, nitrate concentration increased significantly in the eastern part of the lake, whereas ammonium and TN concentration decreased significantly. This may be an effect of the insufficient wastewater treatment efficiency of the nitrification/denitrification process. Stoichiometric evidence implies that primary production in the lake is limited by phosphorus.
Schematic diagram of nutrients and organic matter in Lake Hamana. The phosphate, TN, and TP concentrations decreased significantly in Lake Hamana due to increasing sewage coverage and implementation of advanced wastewater treatment. The nitrate concentrations in the western and eastern parts of the lake, which are surrounded by agricultural and residential land respectively, increased significantly due to insufficient wastewater treatment efficiency in the nitrification/denitrification process. In addition, the phosphate concentration decreased and DO concentration increased in the bottom waters of the central part of the lake due to the reduced volume of hypoxic water there
References
Amin MN, Kroeze C, Strokal M (2017) Human waste: an underestimated source of nutrient pollution in coastal seas of Bangladesh, India and Pakistan. Mar Pollut Bull 118:131–140
Boyer JN, Fourqurean JW, Jones RD (1999) Seasonal and long-term trends in the water quality of Florida Bay (1989–1997). Estuaries 22:417–430
Chen F, Jia G, Chen J (2009) Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, South China. Biogeochemistry 94:163–174
Diaz R, Rosenberg R (1995) Marine benthic hypoxia: a review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanogr Mar Biol 33:245–303
Ding Y, Rong N, Shan B (2016) Impact of extreme oxygen consumption by pollutants on macroinvertebrate assemblages in plain rivers of the Ziya River Basin, North China. Environ Sci Pollut Res 23:14147–14156
Gilbert RO (1987) Statistical methods for environmental pollution monitoring Willey New York p. 320
Hashimoto S (1989) High function activated sludge method utilizing biotechnology Gihodo Tokyo 276p (in Japanese)
Inoue K, Takeda S, Okamoto K, Furuya K (2005) Long-term changes in riverine nutrient loadings and bottom-water characteristics in the main basin of Hamana Bay. Bulletin on Coastal Oceanography (in Japanese with English abstract) 42:1141-1150
Japan Sewage Works Association (2017) Sewage statistics. Japan Sewage Works Association, Tokyo (in Japanese)
Jenny JP, Normandeau A, Frabcus P, Taranu E, Gregory-Eaves I, Lapointe F, Jautzy J, Ojala AE, Dorioz JM, Schimmelmann A, Zolitschka B (2016) Urban point sources of nutrients were the leading cause for the historical spread of hypoxia across European lakes. Proc Natl Acad Sci 113:12655–12660
Kadlec RH, Wallace SD (2008) Treatment wetlands, 2nd edn. CRC Press, Boca Raton, p 1000
Kubo A, Yamamoto-Kawai M, Kanda J (2015) Seasonal variations in concentration and lability of dissolved organic carbon in Tokyo Bay. Biogeosciences 12:269–279
Kubo A, Hashihama F, Kanda J, Horimoto-Miyazaki N, Ishimaru T (2019) Long-term variability of nutrient and dissolved organic matter concentrations in Tokyo Bay between 1989 and 2015. Limnol Oceanogr 64:S209–S222
Lee J, Park T, Kim M, Kim J, Lee S, Lee SK, Lee YS, Yu S, Rhew D (2016) Stable isotope on the evaluation of water quality in the presence of WWTPs in rivers. Environ Sci Pollut Res 23:18175–18182
Lefcheck JS, Orth RJ, Dennison WC, Wilcox DJ, Murphy RR, Keisman J, Gurbisz C, Hannam M, Landry JB, Moore KA, Patrick CJ, Testa J, Weller DE, Batiuk RA (2018) Long-term nutrient reductions lead to the unprecedented recovery of a temperate coastal region. Proc Natl Acad Sci 115:3658–3662
Liu C, Wang Q, Zou C, Hayashi Y, Yasunari T (2015) Recent trend in nitrogen flows with urbanization in the Shanghai megacity and the effects on the water environment. Environ Sci Pollut Res 22:3431–3440
Mazda Y (1999) Mystery of Lake Hamana: nature of inland water and seawater movement. Shizuoka Shimbun, Shizuoka (in Japanese)
Mayer B, Boyer E, Goodale C, Jaworski NA, Van Breemen N, Howarth RW, Seitzinger S, Billen G, Lajtha K, Nadelhoffer K, Van Dam D, Hetling LJ, Nosal M, Paustian K (2002) Sources of nitrate in rivers draining sixteen watersheds in the northeastern U.S.: isotopic constrains. Biogeochemistry 57(58):171–197
Ministry of Agriculture, Forestry, and Fisheries (2016) The census of agriculture and forestry (https://www.e-stat.go.jp/). Accessed 2019/8/12
Mortimer CH (1942) The exchange of dissolved substances between mud and water in lakes. J Ecol 30:147–201
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–210
Okamoto K (1995) Changes in macrofouling community in Shonai inlet, the Hamana Bay during recent 15 years. Marine Fouling 11:1–7
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. Ed. Hill M.N. In The Sea. Interscience, New York, pp 26–77
Schmidt S, Bernard C, Escalier JM, Etcheber H, Lamouroux M (2017) Assessing and managing the risks of hypoxia in transitional waters: a case study in the tidal Garonne River (South-West France). Environ Sci Pollut Res 24:3251–3259
Solórzano L (1964) Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:799–801
Staehr P, Testa J, Carstensen J (2017) Decadal changes in water quality and net productivity of a shallow Danish estuary following significant nutrient reductions. Estuar Coasts 40:63–79
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Board Canada 167:1–310
Tanaka Y, Kanno A, Shinohara R (2012) Effects of global brightening on primary production and hypoxic water in Ise Bay. J JSCE 68:1603–1608
Tong Y, Zhang W, Wang X, Couture RM, Larssen T, Zhao Y, Li J, Liang H, Liu X, Bu X, He W, Zhang Q, Lin Y (2017) Decline in Chinese lake phosphorus concentration accompanied by shift in sources since 2006. Nat Geosci 10:507–511
WWAP (United Nations World Water Assessment Programme) (2017) Wastewater: the untapped resource. The United Nations World Water Development Report 2017; UNESCO
Yue S, Wang C (2004) The Mann-Kendall test modified by effective sample size to detect trend in serially correlated hydrological series. Water Resour Manag 18:201–218
Zhang Y, Shi P, Li F, Wei A, Song J, Ma J (2018) Quantification of nitrate sources and fates in rivers in an irrigated agricultural area using environmental isotopes and a Bayesian isotope mixing model. Chemosphere 208:493–501
Acknowledgments
We thank the scientists and captain on board the R/V Hamana and acknowledge the members of the Shizuoka Fisheries Experimental Station Hamanako Branch and Shizuoka Institute of Environment and Hygiene for the sample measurement. We also thank Shino Yamauchi for making the original drawing of Fig. 9. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was supported by the River Fund of The River Foundation, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Boqiang Qin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Kubo, A., Imaizumi, R. & Yamauchi, S. Lake water phosphate reduction with advanced wastewater treatment in watershed, at Lake Hamana, Shizuoka Prefecture, Japan, from 1995 to 2016. Environ Sci Pollut Res 27, 2120–2130 (2020). https://doi.org/10.1007/s11356-019-06947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06947-5