Abstract
Phosphorus (P), an irreplaceable nutrient for all living organisms, is facing scarcity via phosphate resources. In this research, the effect of suitable additives and temperature on P and heavy metals speciation during sewage sludge (SS) thermochemical treatment was investigated. The results demonstrated that additives (CaO and MgO) could promote the conversion of non-apatite inorganic phosphorus (NAIP) to apatite phosphorus (AP). X-ray diffraction measurements indicated that the phosphorus mineral phase in sewage sludge ash (SSA) mainly was AP, with addition of MgO and CaO. Moreover, orthogonal testing revealed that the optimal molar ratio of Mg:Ca:P for P recovery as AP was 1:3.5:1 at 750 °C. Risk index results implied that the heavy metals in the phosphorus-enriched SSA have low potential ecological risk. Thermodynamic equilibrium calculations revealed that P reacted with the other metal ions was in the following order: Ca2+ > Mg2+ > Al3+ > Fe3+ > Zn2+ > K+.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
P is a limited resource that cannot be synthesized nor substituted in living organisms as essential nutrient. Some researchers have predicted that phosphate rock, which is used for phosphate fertilizer production, can be exploited for a few generations (Childers et al. 2011; Adam et al. 2009). SS, a by-product from wastewater treatment plants that riches in many nutrients, could be regarded as renewable resources (Wang et al. 2018; Zhang et al. 2017). The nutritional components of SS can be regarded as a manufactured soil conditioner or special agricultural fertilizer if the clean products could be obtained by new clean technologies (Kacprzak et al. 2017; Walter et al. 2006). However, contaminants in SS limit its direct application (Siebielska 2014; Stefaniuk and Oleszczuk 2016). SSA is a by-product from SS incineration plant which riches high concentration phosphate (Schwitalla et al. 2018). Therefore, SSA has significant potential for use as a renewable source of phosphate for production of fertilizers.
P can be classified into five fractions: total phosphorus (TP), organic phosphorus (OP), inorganic phosphorus (IP), none-apatite inorganic phosphorus (NAIP), and apatite phosphorus (AP); the relationship of the different P fractions could be described by the equation TP = IP + OP and IP = NAIP + AP (Pardo et al. 2003). AP (Ca/Mg–P) has higher bioavailability and NIAP (Fe/Al/Mn–P) has low bioavailability. P recovery from SSA is generally divided into two approaches: wet chemical recovery and thermal chemical recovery. Wet chemical recovery refers to the use of acid or alkalis to digest SSA, and then P is recovered using a precipitant agent. Many studies had focused on P recovery via acid leaching. Sulfuric acid (H2SO4) and hydrochloric (HCl) are widely used because of their low cost and wide availability, and 60–80% release of P from SSA through leaching (Donatello and Cheeseman 2013). Some researchers also reported that P could be recovered by phosphate precipitation (Xu et al. 2012; Ahmad and Idris 2014). Kong et al. (2018) also reported that Ca plays key role in sorbing P from aqueous solution. Thermal chemical recovery refers to the use of SS incineration processes to enrich P and to promote NAIP conversion to AP to improve P bioavailability. Han et al. (2009) reported that CaO could promote heavy metals and P stabilization in the SSA and high temperatures also could promote conversion between NAIP and AP to form Ca–P (Li et al. 2015; Qian and Jiang 2014; Wang et al. 2013), which has higher bioavailability.
One crucial problem for SS application lies in the content and transformation of heavy metals (Chen et al. 2014; Huang and Yuan 2016). During SS incineration, heavy metal pollutions have caused environmental and regulatory concern because of its toxicity and bioaccumulation (Lia et al. 2016). Most researches on heavy metals in SS thermal treatment are based on heavy metals stabilization or the removal of heavy metals to decrease potential risks to ecosystems (He et al. 2016a, b). Cl donors were beneficial in promoting the heavy metal vaporization during the SS thermal treatment processing (Li et al. 2015), whereas CaO/MgO and incineration conditions have a positive impact on heavy metal stabilization by promoting heavy metal conversion to aluminosilicate or silicate (Xu et al. 2013). Bairq et al. (2018) also reported that chloride additives, temperature, and residence time could enhance heavy metal removal during SS thermal treatment.
Furthermore, most studies on P recovery are mainly based on wet chemical P recovery from SSA and lack investigation of P fixation, transformation, and bioavailability. The novelty of our research was to convert SSA to phosphate fertilizer directly during SS incineration processes and to avoid using of acids compared with wet chemical P recovery. In addition, some researchers have presented the heavy metal pollution problems but seldom focused on the effect of suitable additives and temperatures on P and heavy metal speciation during SS incineration processes. The purpose of our research was to convert P into a raw material for phosphate fertilizer via suitable additives during SS incineration. An in-depth study on the fractions of heavy metals was conducted to reveal the potential environmental risk from heavy metals of P recovery during SS incineration and the order of P reacted with other metal ions was also investigated.
Materials and methods
Sewage sludge samples
SS samples used in this research were collected from Wastewater Treatment Plant in the city of Dalian, China. SS samples were dried at 105 °C for 2 days within a drum wind drying oven, then crushed by a grinder, sieved to less than 150 μm, and stored in a desiccator.
Incineration experiments
A muffle furnace (air gas atmosphere) was used to investigate the effect of suitable additives and temperature on P and heavy metal speciation in the temperature range 450 to 950 °C. In each test, CaO (3, 5, 7 wt%) and MgO (1, 2, 3 wt%) (analytical reagent, Tianjin Kemiou Chemical Reagent Co. Ltd., China) were added to dried SS and homogeneously mixed. Then, the SS samples were burned at setting temperature for 120 min. The SSA was collected, cooled to room temperature in a desiccator, weighed, grinded, and then stored in zip-lock bags for analysis. A 3 × 3 orthogonal test was used to investigate the joint effects of temperature, Mg:P and Ca:P on the P conversion to AP. SSA, with 2 wt% MgO and incinerated at 850 °C, was extracted via alkaline dissolution to attain a residue.
Risk assessment
Potential ecological risk index (RI) and potential ecological risk factor (\( {E}_i^r \)) were used to assess the ecological risks of heavy metals in SSA (Hakanson 1980). The ecological risk of single heavy metal was assessed by \( {E}_i^r \) and the ecological risk of multi heavy metals was assessed by RI. The relationship and calculating methods of RI and \( {E}_i^r \) are expressed in the following equations (Hakanson 1980).
\( {E}_i^r \): the single potential ecological risk factor. \( {T}_i^i \): the toxic response factor of heavy metals. \( {C}_D^i \): concentration of single heavy metal in SSA; \( {C}_R^i \): the reference value for heavy metals. The reference values for heavy metals are in the order of Zn = 80 > Cr = 60 > Ni = 40 > Cu = 30 > As = 15 (He et al. 2016a, b). RI: potential ecological risk index.
Transformation and interaction behaviors of P and heavy metals
Thermodynamic equilibrium calculations were performed in FactSage 6.4 to validate the transformation behaviors of P and the interaction behaviors of P and heavy metals during the SS incineration processes. The materials were input in the form of oxide and were calculated at 750, 850, and 950 °C, respectively.
Analytical methods
In this research, SMT protocol was applied to study the P fractions using a visible spectrophotometer (WFJ2100, UNICO, Shanghai, China) at 882 nm by molybdenum blue method (Kidd et al. 2007) (Fig. 1). The fractions of heavy metals in the SSA was extracted by BCR sequential extraction procedures (Gao et al. 2010). The detailed BCR operation steps could be found in our previous article (Li et al. 2015). Suspensions from BCR sequential extraction procedures were filtered through 0.45-μm membrane by vacuum filter and then stored in polyethylene bottles at 4 °C. The concentration of heavy metals was determined via inductively coupled plasma-atomic emission spectrometry (ICP-OES, PerkinElmer Analyst 8300, USA). The chemical compositions and ultimate analysis of the SS (as shown in Table 1) were examined via X-ray fluorescence (XRF) spectrometer (ZSX100e, MINIFLEX, Japan) and EuroVector Elemental Analyser (EURO ER300, Italy). X-ray diffraction (XRD) was used to identify information on the crystallized phases of the SSA via a PANalytical X’Pert diffractometer (Netherlands). Alkaline dissolution (1 M NaOH) was used to dissolve the non-apatite inorganic phosphorus in SSA. The remained residue was analyzed by XRD to identify the apatite phosphorus.
Results and discussion
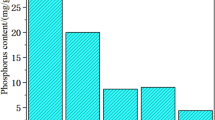
A summary of primary characteristics of SS is listed in Table 1. The basic data provided was used to understand the SS properties. As seen in Table 1, SS had high volatile matter content (62.5%). It indicated SS could be ignited easily and could be treated by incinerated to decrease its volume and quality. The TP content in SS was 20.41 ± 1.12 mg g−1. IP, the main fraction of P in SS. The percentage of IP in TP was 75.7%. The percentage of NAIP in IP was 70.5%.
Phosphorus fractions in the SSA obtained at different incineration temperatures
Figure 2 presents the contents of P fractions in the SSA at different incineration temperatures. As shown in Fig. 2, the IP content increased as temperature increased between 450 and 950 °C, reaching its maximum at 850 °C. TP had a similar tendency. AP increased with decreasing NAIP, from 550 to 850 °C, and obviously increased from 25.95 ± 1.37 to 37.77 ± 1.28 mg g−1, from 750 to 850 °C. This indicated the aluminum phosphate (Al–P) belonged to NAIP reacted with the magnesium salt or calcium salt to form the magnesium or calcium phosphate (Mg/Ca–P). Notably, the P fractions decreased in the temperature range of 850 to 950 °C. This phenomenon may be caused by the phase transformation of NAIP and unstable AP. Temperature played an important role in stability of P in SSA, higher temperature led to the phase transformation of some soluble ortho-P and pryo-P which are combined with Al/Fe-compounds (Qian and Jiang 2014).
Phosphorus fractions in the SSA with additives at different incineration temperatures
TP contents in the SSA with additives at different incineration temperatures are presented in Fig. 3. TP did not increase with the presence of additives or with temperature increase. In the contrary, the increasing of additives quality promoted the release of P at the same temperature. The TP content in SSA has the same result with no additives that is obviously decreased at 950 °C. The distributions of P fractions in the SSA are shown in Fig. 4 and indicate different additives had different effects on P transformation. CaO was better than MgO in promoting NAIP conversion to AP (Fig. 4a, b, respectively), as the capacity of P to bind Ca2+ is better than bind Mg2+, thus forming stable compounds. Gorazda et al. (2012) also reported that calcium could promote the formation of calcium phosphate during SS incineration processing. Han et al. (2009) also reported the similar results. It can be seen that AP did not increase when the Mg:P or Ca:P ratio was greater than or equal to 1. Figure 4 c shows that temperature was the dominant factor for the transformation to AP. The same figure also shows that the content of AP had no direct relationship with the additives at 950 °C. These results illustrated that the additives quality affected the AP at the same temperature and temperature affected the AP at a certain additive quality.
P recovery was based on the formation of AP. To investigate the joint effects of the optimal parameters for P recovery as AP, an orthogonal experiment was conducted (Table 2). Based on the results of the orthogonal texts, the optimal molar ratio of Mg:Ca:P for the precipitation of P as AP was 1:3.5:1 at 750 °C, for a large amount of TP and possible formation of AP. The highest total content of TP+AP reached 126.427 ± 0.936 mg g−1 under these conditions, and it can be seen that the parameters influencing the AP formation follows in the order of Mg:P > Ca:P > Temperature.
XRD analyses of the SSA under different conditions
XRD measurements were used to detect the mineral phases, as shown in Fig. 5. Based on the SMT protocol, IP is classified as NAIP and AP. AP (P was bonded with Ca or Al) is the apatite phosphorus which has high bioavailability such as hydroxyapatite, calcium phosphate, and magnesium phosphate. NAIP (P was bonded with Al or Fe) is the non-apatite inorganic phosphorus which has low bioavailability such as aluminum phosphate and iron phosphate. Al–P was detected (Fig. 5a), and Al-P dissipated with emerging Mg–P and Ca–P with MgO and CaO addition, respectively (Fig. 5a–c). The results demonstrated that MgO and CaO promoted NAIP conversion to AP during SS incineration processes. In addition, the kinds of additives influenced AP formation.
Comparing Fig. 5 b and a, a significant peak corresponding to Ca–P (2θ = 45°) appeared. From the XRD analyses shown in Fig. 5a, c, the peak of Ca–P (2θ = 35°) decreased with addition of MgO. With MgO addition, the peaks of Al–P, Na–P, and Mg–P appeared. These changes indicated that MgO destroyed the construction of Ca–P and formed a newly crystalline phosphate. Adding additives during SS thermal processing was found to be more advantageous for P transformation, it was more possible to obtain more Ca–P or Mg–P which has higher bioavailability that was significantly more than SS without CaO or MgO. Comparing Fig. 5 c and d, the Al–P disappeared completely after leaching through alkaline dissolution, and then Ca–P and Mg–P existed in the post-leaching residue. This contradictory finding could be interpreted by assuming that Al–P, a kind of NAIP, was dissolved by the aqueous alkali. The chemical equation for such may be as follows:
Table 3 shows the main P-containing crystal phases in the ash and post-leaching residues via XRD measurements. It can be found that the newly formed Ca–P is amorphous. Based on these results, CaO or MgO could promote the transformation of aluminum phosphate or iron phosphate to calcium phosphate hydroxide, calcium phosphate, calcium hydrogen phosphate, and finally existed in the SSA in the form of apatite phosphorus. The results presented in this section could provide a basis for P recovery from SSA.
Heavy metal speciation
Figure 5 shows the heavy metal speciation fractions under different incineration conditions obtained via BCR sequential extraction. Based on BCR, heavy metals can be divided into an acid soluble (ACE) fraction, a reducible (RED) fraction, an oxidizable (OXI) fraction, and a residue (RES) fraction. As shown in Fig. 6, temperature obviously influenced heavy metal fractions, inducing strong migration behavior of the metals. The percentage of the RED fraction was low enough to be considered negligible, except for that of As. At 850 °C, the main fraction of Cr, Cu, Ni, and Zn is the RES fraction, which respectively accounted for 97.3%, 81.2%, 93.5%, and 97.6%, exhibiting low biotoxicity. At 950 °C, for Cu, Ni, and Zn, the percentage of the RES fraction obviously decreased with the percentage of its ACE and RED fractions increased, especially for Ni, whose percentage of RED fraction increased by 90.6%. This may be due to Ni being at non-volatile heavy metal, as higher temperature increased its activity for easy reaction with other ions. As keeps its unique mobility characteristic, higher temperature cannot increase RES fraction of As, the sum of ACE and RED fractions is over 92.9%. Due to ACE and RED, fractions have a high mobility and high biotoxicity (Shi et al. 2013; Li et al. 2015), it directly limits the SSA application as phosphate fertilizer.
As shown in Fig. 6, the results show that CaO and MgO positively affected the mobility behavior of heavy metals. Additives are helpful for heavy metal migration to stable fractions, especially for As. The percentage of RES fractions of As and Cu increased with MgO addition, reaching to 51.5% and 80.1%, respectively, at 950 °C with 3% MgO. The sum percentage of the ACE and RED fractions of As, Cr, Cu, Ni, and Zn showed decreasing tendencies as additives increased, decreasing to 48.5%, 2.2%, 20.9%, 13.5%, and 5.8%, respectively, at 950 °C with 3% MgO. For Cu, Ni, and Zn, CaO also had similar effects on the migration characteristics of the heavy metals as MgO did; most of heavy metal fractions were changed to stable fractions by adding CaO. Han et al. (2009) also reported that CaO could stabilize heavy metals and the percentage of heavy metals in SSA increased with increasing CaO ratio. These results indicate that MgO/CaO could increase the stable fractions of heavy metals.
Potential ecological risk index assessment analyses
The values and corresponding contamination degrees of risk indices and potential ecological risk index of heavy metals in the SSA are listed in Table 4. The \( {E}_i^r \) of the heavy metals in the SSA were in the order of Cu > As>Zn > Ni > Cr. The \( {E}_i^r \) value for Ni without additives at 950 °C was 199.0, indicating considerable risk. The \( {E}_i^r \) values for As with 0, 1%, and 2% MgO at 750 °C; 0 and 3% MgO at 850 °C; and 2% and 3% MgO at 950 °C were 59.9, 64.5, 40.4, 46.4, 45.1, 43.8, and 45.3 respectively. The \( {E}_i^r \) values for Cu under all conditions, except at 950 °C with 5% CaO, were over 40, revealing moderate risk. These results mean high risk to ecosystems, which should be paid more attention, to evaluate the overall potential ecological risk of the heavy metals in the SSA under different incineration conditions. The RI of heavy metals in the SSA at 950 °C was as high as 311.2, revealing considerable risk. The RI values of heavy metals in the SSA under other incineration conditions were lower than 150, except for the conditions for 0 and 1% MgO at 750 °C (moderate risk), meaning low risk. Similarly, the RI values were also analyzed via clustering methods using R Software and organized in a dendrogram to identify groups that were similar. The results are shown in Fig. 7. The RI values could be grouped into five clusters, with considerable risk cluster with 1 sample, moderate risk cluster with 2 samples, and low risk cluster with 6, 4, and 8 samples. Base on hierarchical clustering analyses of RI values, the SSA obtained with 0% and 1% MgO at 750 °C or at 950 °C with no addition should be paid more attention and heavy metals in the SSA should be further removed if the SSAs were used as phosphate fertilizer. These results are helpful for applications of SSA as phosphate fertilizer. However, high concentrations of heavy metals are over the use limits for agricultural application. Therefore, new methods to decrease heavy metal concentrations should be developed.
Transformation and interaction behaviors of P and heavy metals
Thermodynamic equilibrium calculations were performed with FactSage 6.4 to reveal the transformation and interaction behaviors of P and heavy metals during SS incineration processing. As shown in Fig. 8, when the P content is 0, Cu was in the form of oxide with Fe2O3, Zn was mainly in the form of ZnFe2O4 and FeZn2O4, and Pb was in the form of Pb2Fe2Si2O9 and PbO(g), the percentage of PbO(g) increased with temperature increasing and was more than 90% at 950 °C; this is due to Zn was greatly influenced by temperature. When the P content increased to 5%, compared to 0% P content, the forms of Zn and Pb changed obviously. Zn was in the form of ZnAl2O4, with the percentage of ZnFe2O4 increasing as temperature increased. Pb was in the form of oxide with Al2O3. These results indicated that Ca2+ and Mg2+ firstly combined with P, Fe3+ need to combine with Si4+, so heavy metals had to react with Al3+ to form heavy metal aluminum compounds. When the P content increased to 10%, Cu was in the form of CuO and (CuO)(Fe2O3), Zn reacted with P to form Zn3(PO4)3, and Pb was mainly in gaseous form, indicating that excessive amounts of P could promote Pb volatilization.
Figure 9 shows the transformation of P for different percentages of P in the SSA at different temperatures. At 750 °C and 850 °C, first Ca5HP3O13 was generated, then when phosphorus content increased, the percentage of Ca5HP3O13 decreased, Mg3P2O8 and Ca3(PO4)2 were generated. When P content was greater than 5%, AlPO4 was generated and Ca5HP3O13 disappeared. With P content further increasing, FePO4 and Zn3(PO4)2 emerged, and K2HPO4 and CaP2O6 emerged lastly. At 950 °C, the percentage of Ca–P increased as Mg–P decreased. These results indicated that, at 750 °C and 850 °C, P reacting with other metal ions was in the following order: Ca2+ > Mg2+ > Al3+ > Fe3+ > Zn2+ > K+. In the actual experiments, NAIP in the SSA was transformed to AP during incineration at higher temperature. This demonstrated that the capacity of combining P and Ca2+/Mg2+ is stronger than that of combining P and Al3+/Fe3+, which is similar to the results obtained via thermodynamic equilibrium calculations.
Conclusions
In this research, P potential recovery with different additives during SS incineration was studied to provide a new method for P recovery. MgO and CaO could positively promote the conversion of NAIP to AP. The optimal condition for P recovery as AP was a Mg:Ca:P molar ratio of 1:3.5:1 at 750 °C. The main AP mineral phases in the SSA were Ca5(PO4)3OH, Ca4P2O5, Mg3(PO4)2, Mg3Ca3(PO4)4, and CaHPO4. The additives had a positive influence on heavy metal migration to stable fractions, which had lower biotoxicity. The RI results suggested heavy metals should be removed before sewage sludge incineration, though it showed low risk for the most part. P reacting with other metal ions was in the order: Ca2+ > Mg2+ > Al3+ > Fe3+ > Zn2+ > K+ during SS incineration processing.
Abbreviations
- SS:
-
sewage sludge
- SSA:
-
sewage sludge ash
- TP:
-
total phosphorus
- OP:
-
organic phosphorus
- IP:
-
inorganic phosphorus
- AP:
-
apatite phosphorus
- NAIP:
-
non-apatite inorganic phosphorus
- Ca/Mg–P:
-
P is associated with Ca or Mg
- Al/Fe–P:
-
P is associated with Al or Fe
- ACE:
-
acid soluble fraction
- RED:
-
reducible fraction
- OXI:
-
oxidizable fraction
- RES:
-
residue fraction
References
Adam C, Peplinski B, Michaelis M, Kley G, Simon FG (2009) Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag 29:1122–1128. https://doi.org/10.1016/j.wasman.2008.09.011
Ahmad AA, Idris A (2014) Release and recovery of phosphorus from wastewater treatment sludge via struvite precipitation. Desalin Water Treat 52:5695–5703. https://doi.org/10.1080/19443994.2013.813101
Bairq ZAS, Li RD, Li YL, Gao HX, Sema T, Teng WC, Kumar S, Liang ZW (2018) New advancement perspectives of chloride additives on enhanced heavy metals removal and phosphorus fixation during thermal processing of sewage sludge. J Clean Prod 188:185–194. https://doi.org/10.1016/j.jclepro.2018.03.276
Chen HC, Zhai YB, Xu BB, Xiang BB, Zhu L, Qiu L, Liu XT, Li CT, Zeng GM (2014) Fate and risk assessment of heavy metals in residue from co-liquefaction of Camellia oleifera cake and sewage sludge in supercritical ethanol. Bioresour Technol 167:578–581. https://doi.org/10.1016/j.biortech.2014.06.048
Childers DL, Corman J, Edwards M, Elser JJ (2011) Sustainability challenges of phosphorus and food: solutions from closing the human phosphorus cycle. Bioscience 61:117–124. https://doi.org/10.1525/bio.2011.61.2.6
Donatello S, Cheeseman CR (2013) Recycling and recovery routes for incinerated sewage sludge ash (ISSA): a review. Waste Manag 33:2328–2340. https://doi.org/10.1016/j.wasman.2013.05.024
Gao X, Chen CTA, Wang G, Xue QZ, Tang C, Chen SY (2010) Environmental status of Daya Bay surface sediments inferred from a sequential extraction technique. Estuar Coast Shelf Sci 86:369–378. https://doi.org/10.1016/j.ecss.2009.10.012
Gorazda K, Kowalski Z, Wzorek Z (2012) From sewage sludge ash to calcium phosphate fertilizers. Pol J Chem Technol 14:54–58. https://doi.org/10.2478/v10026-012-0084-3
Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Han J, Kanchanapiya P, Sakano T, Mikuni T, Furuuchi M, Wang G (2009) The behaviour of phosphorus and heavy metals in sewage sludge ashes. Int J Environ Pollut 37:357–368. https://doi.org/10.1504/ijep.2009.026054
He XW, Fang ZQ, Wang YX, Jia MY, Song JY, Cheng YJ (2016a) Pollution characteristics, potential ecological risk and health risk assessment of heavy metal in a sewage treatment plant in Beijing. Acta Sci Circumst 36:1092–1098 (In Chinese)
He X, Zhang YX, Shen MC, Zeng GM, Zhou MC, Li MR (2016b) Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour Technol 218:867–873. https://doi.org/10.1016/j.biortech.2016.07.045
Huang HJ, Yuan XZ (2016) The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour Technol 200:991–998. https://doi.org/10.1016/j.biortech.2015.10.099
Kacprzak M, Neczaj E, Figalkowski K, Grobelak A, Grosser A, Worwag M, Rorat A, Brattebo H, Almas A, Singh BR (2017) Sewage sludge disposal strategies for sustainable development. Environ Res 156:39–46. https://doi.org/10.1016/j.envres.2017.03.010
Kidd PS, Domínguez-Rodríguez MJ, Díez J, Monterroso C (2007) Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 66:1458–1467. https://doi.org/10.1016/j.chemosphere.2006.09.007
Kong LJ, Han MN, Shih K, Su MH, Diao ZH, Long JY, Chen DY, Hou LA, Peng Y (2018) Nano-rod Ca-decorated sludge derived carbon for removal of phosphorus. Environ Pollut 233:698–705. https://doi.org/10.1016/j.envpol.2017.10.099
Li RD, Zhao WW, Li YL, Wang WY, Zhu X (2015) Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J Hazard Mater 283:423–431. https://doi.org/10.1016/j.jhazmat.2014.09.052
Lia JP, Gan JH, Hu YJ (2016) Characteristics of heavy metal species transformation of Pb, Cu, Zn from Municipal sewage sludge by Thermal Drying. Procedia Environ Sci 31:961–969. https://doi.org/10.1016/j.proenv.2016.03.001
Pardo P, López-Sánchez JF, Rauret G (2003) Relationships between phosphorus fractionation and major components in sediments using the SMT harmonised extraction procedure. Anal Bioanal Chem 376:248–254. https://doi.org/10.1007/s00216-003-1897-y
Qian TT, Jiang H (2014) Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sustain Chem Eng 2:1411–1419. https://doi.org/10.1021/sc400476j
Schwitalla D, Reinmöller M, Forman C, Wolfersdorf C, Gootz M, Bai J, Guhl S, Neuroth M, Meyer B (2018) Ash and slag properties for co-gasification of sewage sludge and coal: an experimentally validated modeling approach. Fuel Process Technol 175:1–9. https://doi.org/10.1016/j.fuproc.2018.02.026
Shi WS, Liu CG, Ding DH, Lei ZF, Yang YN, Feng CP, Zhang ZY (2013) Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour Technol 137:18–24. https://doi.org/10.1016/j.biortech.2013.03.106
Siebielska I (2014) Comparison of changes in selected polycyclic aromatic hydrocarbons concentrations during the composting and anaerobic digestion processes of municipal waste and sewage sludge mixtures. Water Sci Technol 70:1617–1624. https://doi.org/10.2166/wst.2014.417
Stefaniuk M, Oleszczuk P (2016) Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge amended soil. Environ Pollut 218:242–251. https://doi.org/10.1016/j.envpol.2016.06.063
Walter I, Martínez F, Cala V (2006) Heavy metal speciation and phytotoxic effects of three representative sewage sludges for agricultural. Environ Pollut 139:507–514. https://doi.org/10.1016/j.envpol.2005.05.020
Wang L, Wang C, Ning P, Jiang M, Qin Y (2013) Phosphorus-fixation by hydrated lime in fluidized bed combustion of yellow phosphorus tail gas. J Cent South Univ (Sci and Technol) 44:835–842 (In Chinese)
Wang C, Geng YM, Cheng L, Mao YX (2018) Speciation, mass loading, and fate of phosphorus in the sewage sludge of China. Environ Sci Pollut Res 175:97–103. https://doi.org/10.1007/s11356-018-3520-y
Xu HH, He P, Gu WW, Wang GZ, Shao LM (2012) Recovery of phosphorus as struvite from sewage sludge ash. J Environ Sci-China 24:1533–1538. https://doi.org/10.5004/dwt.2018.22764
Xu GR, Liu MW, Li GB (2013) Stabilization of heavy metals in lightweight aggregate made from sewage sludge and rivers sediment. J Hazard Mater 260:74–81. https://doi.org/10.1016/j.jhazmat.2013.04.006
Zhang J, Yu T, Zhang J (2017) Release of phosphorus from sewage sludge during ozonation and removal by magnesium ammonium phosphate. Environ Sci Pollut Res 24:23794–23802. https://doi.org/10.1007/s11356-017-0037-8
Funding
This work was supported by the National Natural Science Foundation of China, China (No. 51276119 and No. 51576134).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, R., Teng, W., Li, Y. et al. Transformation of phosphorus and stabilization of heavy metals during sewage sludge incineration: the effect of suitable additives and temperatures. Environ Sci Pollut Res 26, 29917–29929 (2019). https://doi.org/10.1007/s11356-019-06146-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06146-2