Abstract
With the implementation of the “carbon neutrality” strategy, waste resource-utilization technologies have become the focus of future research. P recovery from excess sludge (ES) is of great significance. In this paper, P recovery in excess sludge ash (ESA) of different incineration temperatures was studied. The experiment results showed that the optimal incineration temperature of ESA was 750 °C, and its total phosphorus content was 90.7 mg/g, which were three times heavier than the original sludge. As the incineration temperature increased from 650 °C to 850 °C, the more crystals appeared to be agglomerated and there was melting phenomenon on the surface of ESA. Higher temperatures were conducive to the AIP formation. The new minerals such as Ca4(Mg.Fe)5(PO4)6 and (Ca.Mg)3(PO4)2 were produced in ESA of 800 °C and 850 °C. Under the optimal acid-leaching conditions that were leaching time of 90 min, liquid–solid ratio of 50:1 (mL/g), and sulfuric acid (H2SO4) concentration of 1 M, P leaching efficiencies could reach 100% in ESA of 700 °C and 750 °C, where P leaching contents were the most abundant and more suitable for P recovery. The research results provided theoretical basis and operational conditions for P recovery of excess sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential element for all living organisms and human beings [1]. It is a component of key biomolecules such as deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and adenosine triphosphate (ATP), etc. At the cellular level, phosphate bounds biosynthesis and hydrolysis are universal mechanisms for energy exchanges in all living beings’ metabolism [2]. At a more macroscopic level, P availability is often a limiting factor for plants growth in natural ecosystems [3]. It plays a significant role in industrial and agricultural development. However, phosphorus is a limited non-renewable resource that usually exists in nature in the form of organophosphorus compounds and phosphate [4]. Phosphate ore resources in the worldwide will be exhausted within 50–100 years, and the grade of phosphate ore is increasingly becoming low [5, 6]. Therefore, P has been listed as one of the 20 minerals that cannot meet the demand of Chinese economic growth [7]. The phosphate ore is mainly processed into fertilizers and subsequently flows through the food production and consumption system. However, more than 80% of the P-bearing fertilizers are not utilized and enter into wastewater [8].
Nowadays, most of the wastewater treatment plants (WWTPs) apply enhanced biologic phosphorus removal (EBPR) or chemical precipitation process to treat P-containing wastewater. More than 90% of P in raw wastewater will finally end up with excess sludge (ES). Therefore, it is of great significance to recover P from ES [9]. Generally, most of P in ES is compounds of phosphorus and Ca2+, Mg2+, Fe2+, Fe3+, Al3+ or other ions. Because it is rich in some toxic heavy metals, such as copper (Cu), lead (Pb), manganese (Mn), zinc (Zn), cadmium (Cd) and chromium (Cr), ES rich in P cannot be directly applied for agricultural use [10, 11].
Incineration is the most effective method of sludge disposal, which has many advantages including significant volume reduction (up to 80–90%), destruction of organic micro-pollutants and pathogens, and energy recovery [12, 13]. ES incineration has been widely used in the Netherlands, Switzerland, Japan and other countries [14, 15]. Due to the dramatic reduction of sludge volume, most of P is concentrated in excess sludge ash (ESA) and its content can vary from 10.0 to 25.7% (wt) as P2O5, which is comparable to the P content in phosphate ore (5–40%) [16]. Therefore, the recovery effect of phosphorus is better from ESA than ES. However, in addition to P, various metals are also concentrated in ESA, which not only contains Si, Al, Fe, P, and Ca elements, but also includes small amounts of heavy metals, such as Cu, Zn, Pb, Ni, As, Cr, etc. [17, 18].
Yang et al. [19] found when incinerated temperatures were between 600 °C and 800 °C, metal ions (Ca2+, Fe3+, etc.) in the sludge combine with phosphate to form orthophosphate. Qian et al. [20] also showed that various P species (orthophosphate monoesters, orthophosphate diesters, and pyrophosphate) in municipal sludge were all transformed into orthophosphate in incineration ash when combustion temperature was over 600 °C. Nanzer et al. [21] investigated four types of sludge-derived ash samples from mono-combustion plants and only observed the presence of orthophosphate. At present, there were few reports on the existing forms and leaching efficiencies of P in ESA under different incineration temperatures.
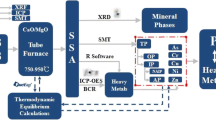
This paper aims to study the conversion mechanism and mineral components of P in ESA of different incineration temperatures, and further to compare their leaching efficiencies when sulfuric acid is used as the leaching agent. The research results provided theoretical basis and operational conditions for P recovery of excess sludge.
Materials and methods
ES samples preparation
ES used in this study were the dewatered sludge cake with 80% water content, which was obtained from Xi’an fifth wastewater treatment plant with A2/O activated sludge process in Xi’an, China. The collected sludge cake samples were first air-dried for 24 h and then dried to a constant weight in a constant temperature blast oven at 105 °C. Subsequently, after it was crushed and sifted through a 100 mesh (0.15 mm) screen mesh, the required sample (marked as 80ES) was obtained and stored in a self-sealing bag for later use.
ESA samples preparation
The 80ES samples were burned in a muffle furnace for 2 h at the temperatures of 650 °C, 700 °C, 750 °C, 800 °C, and 850 °C, respectively, to prepared five ESA samples. It was heated at a rate of 15 °C/min and was naturally cooled to room temperature in the furnace.
Experimental methods
Acid leaching methods of P in ESA
The different parameters of P leaching were set as the following: H2SO4 concentrations of 0.2 M, 0.5 M, 1 M, 2 M; liquid–solid ratios of 25:1, 50:1, 100:1, 150:1; and acid-leaching time of 30 min, 60 min, 90 min, 120 min, 150 min, respectively. The reaction process was in a constant temperature shaker at 220 rpm and 25 ± 1 °C. At the end of leaching, the mixture was separated by 0.45 μm filter membrane. P in the filtrate was determined by ammonium molybdate spectrophotometry, and the metal ion in the filtrate was determined by inductively coupled plasma mass spectrometry (ICP-MS).
The chemical equation formula of acid-leaching reaction was following [22]:
Calculation on leaching contents and leaching efficiencies of P
where P: P leaching contents (mg/g);
C: P leaching concentration (mg/L);
V: Sulfuric acid volume (L);
m: ESA (g), set 0.2 g
where Pi: P leaching recovery efficiency (%);
P: P leaching contents (mg/g);
PESA: Excess sludge ash contents (mg/g).
Characterization analysis of 80ES and ESA samples
The element contents of 80ES were determined by X-ray fluorescence spectrometer (XRF, AITM, USA). The standard procedure (SMT) [23] was used to analyze and determine the P morphology in 80ES and ESA samples. The surface properties of ESA as well as the elemental distribution were tested by SEM–EDS (ZEISS Sigma 300, Germany). The mineral phases of ESA were identified by X-ray diffractometer (XRD, XRD-7000, Shimadzu, Japan).
Results and discussion
Contents and components of elements in ES
The elements and their contents in the 80ES were shown in Table 1. The main components were Si, P, Ca, Al, Fe, Mg, and other elements in 80ES. It could be seen that P was abundant in 80ES. The top three elements in terms of contents were Fe, Si, P, whose contents were 23.38, 19.30, 16.24%, respectively. The highest content of Fe was because PFS was used as a coagulant in the sewage treatment process of the WWTP. In addition, some heavy metals such as Zn, Cu, and Cr existed in the sludge with relatively low contents.
The contents of P components in 80ES were shown in Fig. 1. The contents of total phosphorus (TP), inorganic phosphorus (IP) and organic phosphorus (OP) were 30.29 mg/g, 19.99 mg/g and 8.73 mg/g, respectively. The contents of Non-apatite Phosphorus (NAIP, Al/Fe/Mn-P) and Apatite Phosphorus (AIP, Ca/Mg-P) were 9.09 and 4.41 mg/g. IP is the main form of phosphorus in ES. But IP mainly existed in the form of NAIP, and the proportion of AIP was relatively low, indicating that the bioavailability of the sludge was low, because AIP was more favorable to plant growth [24].
Existing forms and contents of P in ESA of different incineration temperatures
The existing forms and contents of P in ESA of different incineration temperatures were shown in Fig. 2. It could be seen that when the incineration temperature was 650, 700, 750, 800, 850 °C, the TP contents were 84.8, 89.7, 90.7, 89.7, 85.8 mg/g, respectively. Variation trend of IP contents was similar to TP. When the incineration temperature rose from 650 °C to 750 °C, the contents of TP and IP increased. However, When the incineration temperature was further elevated from750 °C to 850 °C, the contents of TP and IP tended to decrease. The optimal incineration temperature was 750 °C, under the condition, the contents of TP and IP were 90.7 and 84.5 mg/g, respectively. TP enrichment content in ESA was up to three times heavier than it in 80ES. IP content in ESA was four times more than it in 80ES. It could be seen that sludge incineration not only reduced excess sludge weight but also promoted phosphorus enrichment, which was in favor of phosphorus recovery.
The contents of TP and IP tended to decrease between 750 °C and 850 °C, which was possibly due to partial volatilization of NAIP under the higher incineration temperatures [25]. When the incineration temperature was 650 °C, the content of OP was 3.4 mg/g, which decreased by 61.05% compared with 80ES. As the temperature rose from 650 °C to 850 °C, the contents of OP gradually declined from 3.4 mg/g to 0.8 mg/g because incineration destroyed the cell structure of biologic sludge and converted OP to IP. The content of NAIP was 41.6 mg/g when the temperature is 650 °C, which was more than three times heavier than it in 80ES. However, When the temperature rose to 850 °C, NAIP content dropped to 36.9 mg/g. With the elevation of incineration temperature, NAIP content gradually decreased, which was due to a part of NAIP converting to AIP. Because the ability of Ca and Mg ion binding P was better than that of Fe and Al ion, the stability of AIP was better than that of NAIP at higher temperature based on thermodynamic reaction [26]. Therefore, with the increase of the incineration temperature, the content of NAIP decreased, but the opposite content of AIP. The content of AIP was 21 and 37.7 mg/g at the temperature of 650 °C and 850 °C, respectively.
Physicochemical properties of ESA of different incineration temperatures
Surface characterization and element distribution in ESA samples
Through SEM–EDS analysis, the surface morphology and element distribution for ESA of different incineration temperatures were shown in Fig. 3. It could be seen from Fig. 3(a, b) that there were micropores on the surface of ESA of 650 °C with looser structure. Some crystals were attached to the surface of the ESA particles. Figure 3(d, e) showed that the more and smaller crystals aggregated on the surface for ESA of 850 °C, and there existed the silicate melting phenomenon, which led to micropores blockage and less micropores. It was due to the fact that under higher temperature alkaline earth metal oxide formed silicate with SiO2 with low melting point [27].
Figure 3(c) showed that at the incineration temperature of 650 °C, the contents of P, Fe, Al, Ca, Mg in ESA accounted for 12.61%, 10.64%, 13.28%, 5.92%, 2.66%, respectively. However, As the incineration temperature was 850 °C, the contents of P, Fe, Al, Ca, Mg in ESA were 16%, 6.47%, 13.11%, 8.08%, 2.94%, respectively (Fig. 3f). Compared with ISSA of 650 °C, the contents of Fe and Al in ESA of 850 °C decreased by 4.17% and 0.11%, while the Ca and Mg content in ESA of 850 °C increased by 2.16% and 0.28%. It indicated that an amount of NAIP turned into AIP with the increase of incineration temperature. In summary, when the incineration temperature was increased from 650 °C to 850 °C, the more crystals appeared to be agglomerated and there was melting phenomenon on the surface of ESA. Higher temperatures were conducive to the AIP formation.
Mineral speciation in 80ES and ESA samples
The mineral fractions in 80ES as well as ESA of different temperatures were shown in Fig. 4. The mineral components in 80ES were mainly quartz (SiO2). The main mineral components in ESA of 650–750 °C were quartz (SiO2) and aluminum phosphate (AlPO4). For ESA of 700 °C, there also existing ferrous phosphorite (FePO4) in addition to SiO2 and AlPO4. In ESA of 800 °C, the contents of AlPO4 and FePO4 decreased and a new mineral Ca4(Mg.Fe)5(PO4)6 appeared which was similar to the intermediate compound of Mg3(PO4)2-Ca3(PO4)2 [28]. As for ESA of 850 °C, the new characteristic peaks showed that the newly-produced compound was (Ca.Mg)3(PO4)2, called phosphor-calcite.
In conclusion, with the increase of sludge incineration temperature, FePO4 in ES was substituted by calcium– and magnesium–phosphorus compounds, which indicated that NAIP could be converted to AIP with the increase of sludge incineration temperature. Moreover, calcium ions in calcium phosphates (Ca3(PO4)2) could be also partially replaced by magnesium ions at higher incineration temperature [29].
Influencing factors of P leaching performance in ESA of different incineration temperatures
Acid leaching time
Under the acid-leaching conditions which was liquid–solid ratio of 50:1 (mL/g), and acid (H2SO4) concentration of 1 M, when the acid-leaching time was 30 min, 60 min, 90 min, 120 min, 150 min, respectively, the leaching efficiencies and leaching contents of P in ESA of different incineration temperatures were shown in Figs. 5 and 6.
For ESA of 650 °C, the P leaching content and leaching efficiency was 74.7 mg/g and 97.4% within 30 min, respectively, and leaching efficiency was almost 100% within 60 min. As for ESA of 700 °C and 750 °C, all the phosphorus in ESA could be released when the acid-leaching time was 90 min. However, as for ESA of 800 °C and 850 °C, the leaching efficiency was about 100% when the acid-leaching time was 150 min. Liang et al. [30] also found the same phenomenon that the leaching speed is decreased in the ash produced at higher incineration temperature even though the amount of acid-soluble Ca-phosphates in the ash increased. They thought it was the denser surface structure of ESA particles formed at higher incineration temperatures that inhibited acid penetration toward the core of the particles, thus decreasing the efficiency of P extraction. Luyckx et al. [31] also found the efficiency of P extraction with acids decreased in the temperature range 850–1100 °C, which is attributed to the incorporation of P into a poorly soluble silicate melt (agglomerate formation). It was consistent with our conclusions.
It could be seen from Fig. 6 that the contents of P leaching were almost equal in ESA of 700 °C and 750 °C at the time of 90 min, 120 min and 150 min. Moreover, under the same acid-leaching time, the P leaching contents were more in ESA of 700 °C and 750 °C than ones of other incineration temperatures. Therefore, the optimal leaching time for phosphorus release was 90 min.
liquid–solid ratio
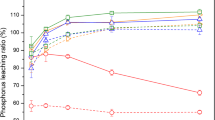
When the acid-leaching time was 90 min and acid (H2SO4) concentration of 1 M, for different liquid–solid ratios, the P leaching efficiencies and leaching contents in ESA of different incineration temperatures were shown in Figs. 7 and 8.
Figure 7 showed all P could be leached in ESA of 700 °C and 750 °C with leaching efficiencies near to 100% when liquid–solid ratio was 50:1 (mL/g). When the liquid–solid ratio was 100:1, the P leaching efficiencies in ESA of 650 °C, 700 °C and 750 °C are 97%, 97.7% and 97%, respectively. When the liquid–solid ratio was further increased to 150:1, the P leaching efficiencies in ESA of 650 °C, 700 °C and 750 °C were 91.8%, 88.1% and 91.7%, respectively. That is, with regard to ESA of 650 °C, 700 °C and 750 °C, the leaching efficiency of P tended to decrease when the liquid–solid ratios increased from 50:1 to 150:1. For ESA of 800 °C, the leaching efficiency of P also went up and down with the increase of liquid–solid ratio. However, the phosphorus leaching efficiency in ESA of 850 °C kept increasing with the increase of liquid–solid ratio. It might be because there were the more and smaller crystals aggregated on the surface in ESA of 850 °C, which contained acid-soluble compounds such as CaO, MgO and CaCO3 consuming excessive acid [32]. It could be seen from Fig. 8 that the P leaching contents in ESA of 700 °C and 750 °C were the most under the liquid–solid ratio of 50:1.
Acid concentration
When the acid-leaching time was 90 min and liquid–solid ratio of 50:1 (mL/g), The P leaching efficiencies and P leaching contents of ESA under the different acid concentrations were shown in Figs. 9 and 10.
Figure 9 showed when acid concentration was 1 M, the leaching efficiencies were the highest in ESA of 650 °C, 700 °C, 750 °C and 850 °C. When the acid concentration increased from 0.02 M to 2 M, the recovery efficiencies of phosphorus in ESA gradually increased. As all kinds of ESA in this study contained high phosphorus content, low acid concentration has low phosphorus recovery efficiency for ESA. As for ESA of 800 °C, the leaching efficiencies were the best when acid concentration was 0.5 M. However, it could be seen from Fig. 10 that when acid concentration was 1 M, the P leaching contents in ESA of 700 °C and 750 °C were the most. Although when the concentration of sulfuric acid is 0.5 M, the P leaching content in ESA of 800 °C was the best. But its leaching content was less than that in ESA of 700 °C and 750 °C. Therefore, the optimal acid concentration for phosphorus release is 1 M.
Phosphorous recovery effect in ESA of different incineration temperatures under the optimal leaching conditions
Under the optimal acid-leaching conditions which was leaching time of 90 min, liquid–solid ratio of 50:1 (mL/g), and acid (H2SO4) concentration of 1 M. P leaching efficiencies and leaching contents in ESA of different incineration temperatures were shown in Figs. 11, and 12, respectively.
Under the optimal leaching conditions, P leaching efficiencies were 99.3%, 100%, 100%, 91.4% and 96.3%, and P leaching contents were 76.2 mg/g, 84 mg/g, 84.5 mg/g, 74.7 mg/g, 77.6 mg/g, respectively in ESA of 650 °C, 700 °C, 750 °C, 800 °C and 850 °C. Therefore, P in ESA of 700 °C and 750 °C was the most abundant, whose resource-utilization value was better. ESA of this temperature was more suitable for P release and recovery.
P leaching was often accompanied by metal leaching from ESA solid phase. Under the optimal acid-leaching conditions, metal elements in the filtrate were shown in Fig. 13. Acid leaching of P in ESA of different incineration temperatures was often accompanied by dissolution of metal elements. With the increase of incineration temperature, Al, Ca and Mg elements all showed an increasing trend, while Fe element continued to decrease. This might be due to the formation of more complex iron compounds in higher temperature which were difficult to be dissolved by acid [33, 34].
Conclusions
In this study, dewatered excess sludge with 80% moisture from a WWTP was used to prepare ESA of different incineration temperatures for P recovery. The mineral speciation and leaching performance of P in various ESAs were studied. The experiment results showed that the optimal incineration temperature of ESA was 750 °C, and its total phosphorus content was 90.7 mg/g, which were three times heavier than the original sludge. As the incineration temperature increased from 650 °C to 850 °C, the more crystals appeared to be agglomerated and there was melting phenomenon on the surface of ESA. Higher temperatures were conducive to the AIP formation. The new minerals such as Ca4(Mg.Fe)5(PO4)6 and (Ca.Mg)3(PO4)2 were produced in ESA of 800 °C and 850 °C.
The optimal acid-leaching conditions were leaching time of 90 min, liquid–solid ratio of 50:1 (mL/g), and sulfuric acid (H2SO4) concentration of 1 M. Under the operational condition, the P leaching efficiencies could reach 100% in ESA of 700 °C and 750 °C, where P leaching contents were the most abundant and more suitable for P release and recovery with better resource-utilization value.
References
Gonzalez-Silva BM, Nair A, Fiksdal DB et al (2022) Enhancing nutrient recovery by optimizing phosphorus stripping of bio-P sludge: experimental analysis and modeling. J Water Process Eng 48:102857
Daneshgar S, Callegari A, Capodaglio AG, Vaccari D (2018) The potential phosphorus crisis: resource conservation and possible escape technologies: a review. Resources 7:37
Samreen S, Kausar S (2019) Phosphorus fertilizer: the original and commercial sources. Phosphorus recovery and recycling, Springer, Berlin
Sulieman S, Mühling KH (2021) Utilization of soil organic phosphorus as a strategic approach for sustainable agriculture. J Plant Nutr Soil Sci 184:311–319
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19(2):292–305
Li R, Zhao W, Li Y et al (2015) Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J Hazard Mater 283:423–431
Chen HD, Deng F, Zhang L (2022) Phosphorus recovery from sludge by rusty scrap iron enhanced anaerobic digestion and vivianite crystallization. J Water Process Eng 47:102697
Peng L, Dai H, Wu Y et al (2018) A comprehensive review of the available media and approaches for phosphorus recovery from wastewater. Water Air Soil Pollut 229:1–28
Sun D, Hale L, Kar G et al (2018) Phosphorus recovery and reuse by pyrolysis: applications for agriculture and environment. Chemosphere 194:682–691
Fang L, Li JS, Guo MZ et al (2018) Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 193:278–287
Benassi L, Zanolett A, Depero LE et al (2019) Sewage sludge ash recovery as valuable raw material for chemical stabilization of leachable heavy metals. J Environ Manage 245:464–470
Liu H, Hu GG, Alper BI et al (2021) Phosphrous recovery from municipal sluge-drived ash and hydrochar through wet-chemical technology: a review towards sustainable waste managment. Chem Eng J 417:129300
Mayer BK, Baker LA, Boyer TH et al (2016) Total value of phosphorus recovery. Environ Sci Technol 50(13):6606
Liang S, Yang L, Chen HM et al (2021) Phosphorus recovery from incinerated sewage sludge ash (ISSA) and reutilization of residues for sludge pretreated by different conditioners. Resour Conserv Recycl 169:105524
Lin H, Wang Y, Dong YB (2024) A review of methods, influencing factors and mechanisms for phosphorus recovery from sewage and sludge from municipal wastewater treatment plants. J Environ Chem Eng 12:111657
Wang QM, Li JS, Tang P et al (2018) Sustainable reclamation of phosphorus from incinerated sewage sludge ash as value-added struvite by chemical extraction, purification and crystallization. J Clean Prod 181:717–725
Li JS, Tsang DCW, Wang QM et al (2017) Fate of metals before and after chemical extraction of incinerated sewage sludge ash. Chemosphere 186:350–359
Xu G, Zhang Y, Shao H et al (2016) Pyrolysis temperature affects phosphorus transformation in biochar: chemical fractionation and 31p NMR analysis. Sci Total Environ 569:65–72
Yang F, Chen J, Yang M et al (2019) Phosphorus recovery from sewage sludge via incineration with chlorine-based additives. Waste Manage 95:644–651
Qian TT, Jiang H (2014) Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sustain Chem Eng 2(6):1411–1419
Nanzer S, Oberson A, Huthwelker T et al (2014) The Molecular environment of phosphorus in sewage sludge ash: implications for bioavailability. J Environ Qual 43(3):1050–1060
Atienza–Martínez M, Gea G, Arauzo J et al (2014) Phosphorus recovery from sewage sludge char ash. Biomass Bioenergy 65(1):42–50
Shiba NC, Ntuli F (2017) Extraction and precipitation of phosphorus from sewage sludge. Waste Manage 60:191–200
Ji SS, Huang J (2021) Research on the distribution of phosphorus forms and phosphorus migration in sludge incineration engineering. China Water Wastewater 37(5):26–30
Xu Y, Chen J, Yang F et al (2021) Transformation of phosphorus by MgCl2 and CaCl2 during sewage sludge incineration. Environ Sci Pollut Res 28(42):68–75
Chen M, Oshita K, Takaoka M et al (2022) Co-incineration effect of sewage sludge and municipal solid waste on the behavior of heavy metals by phosphorus. Waste Manage 152:112–117
Luyckx L, Sousa Correia DS et al (2021) Linking phosphorus extraction from different types of biomass incineration ash to ash mineralogy, ash composition and chemical characteristics of various types of extraction liquids. Waste Biomass Valoriz 12(9):5235–5248
Yu BH, Luo JH, Xie HH et al (2021) Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci Total Environ 786:147437
Li R, Teng W, Li Y et al (2017) Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. J Clean Prod 140:964–970
Liang S, Chen H, Zeng X et al (2019) A comparison between sulfuric acid and oxalic acid leaching with subsequent purification and precipitation for phosphorus recovery from sewage sludge incineration ash. Water Res 159:242–251
Luychx L, Van Caneghem J (2022) Recovery of phosphorus from sewage sludge ash: Influence of chemical addition prior to incineration on ash mineralogy and related phosphorus and heavy metal extraction. J Environ Chem Eng 10:108117
Huang HJ, Yuan XZ (2016) The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Biores Technol 200:991–998
Tang P, Zhao YC, Xia FY (2008) Thermal behaviors and heavy metal vaporization of phosphatized tannery sludge in incineration process. J Environ Sci 20:1146–1152
Galey B, Gautier M, Kim B et al (2022) Trace metal elements vaporization and phosphorus recovery during sewage sludge thermochemical treatment: a review. J Hazard Mater 424:127360
Acknowledgements
This work was financially supported by Xi'an Science and Technology Plan Project (Project No. 23GXFW0005), Natural Science Basic Research Program of Shaanxi Province (Program No. 2023-JC-YB-366) and Shijiazhuang County Special Industry Open Competition Mechanism Science and Technology Project (Project No. 237790722A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, C., Zhao, W., Han, Y. et al. Phosphorus recovery from municipal sludge-derived ash: influence of incineration temperature and heavy metal ion on ash mineralogy. J Mater Cycles Waste Manag 26, 3024–3033 (2024). https://doi.org/10.1007/s10163-024-02023-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-024-02023-4