Abstract
The release rule of phosphorus from sewage sludge during ozonation and removal by the magnesium ammonium phosphate (MAP) method were investigated. The results showed that the concentrations of total phosphorus in aqueous phase (TP(A)) and orthophosphate (PO4 3−-P) in ozonized sludge supernatant rose obviously with increasing ozone dose when ozone dose was below 61.2 mg O3/gSS then almost kept constant. The TP(A) and PO4 3−-P contents in the ozonized sludge supernatant were 70.9 and 63.3 mg/L when ozone dose was 61.2 mg O3/gSS, respectively. Total phosphorus in the sludge solid (TP(S)) was mostly distributed in inorganic phosphorus (IP) (more than 81.5% of TP(S)), and non-apatite inorganic phosphorus (NAIP) was the major component of IP in the sludge (more than 78.7% of IP) during ozonation. The release contribution (RC) of IP to TP(A) accounted for over 73.9%. The optimized conditions for the removal of phosphorus from ozonized sludge supernatant were set at an initial Mg2+/PO4 3−-P molar ratio of 1.8, pH 9.5, and reaction time of 5 min, under which the removal efficiencies of TP(A) and PO4 3−-P were 43.1 and 52.2%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge production has been continuously increasing, as a result of both the growing quantity of treated wastewaters and the application of progressively stricter standards for the effluent quality of wastewater treatment plants (Bertanza et al. 2014). Sewage sludge derived from sewage treatment plant, containing pathogenic bacterium, organic contaminants, and heavy metals, would bring huge damage to the environment and human health without appropriate treatment and disposal (Anjum et al. 2016). Once it was generated, the treatment and disposal of sewage sludge could occupy up to 60% of operating expenditure of sewage treatment plant (Zhang et al. 2016). Therefore, the sewage sludge reduction during the sewage treatment process is an ideal solution to this problem, instead of post-treatment of sewage sludge.
Lysis-cryptic growth through ozonation is a method to minimize the generation of sewage sludge during sewage treatment process (Wei et al. 2003). Ozone has a strong oxidizability, can disintegrate the sewage sludge flocs, and destroy microorganisms in sludge, releasing extracellular and intracellular materials into the supernatant, while a fraction of materials in the supernatant released from sewage sludge are mineralized to CO2 and H2O (Kamiya and Hirotsui 1998; Dytczak et al. 2007). The materials that were mineralized would lead to some degree of sewage sludge reduction, and this depends on ozone dose (Park et al. 2003; Manterola et al. 2008). The residual extracellular and intracellular materials in the supernatant released from sewage sludge can be reutilized in sewage treatment process and lead to the reduction of overall sewage sludge yield (Chu et al. 2009; Yang et al. 2012). It was reported that sewage sludge production could be reduced by 50–100% and no secondary pollution was released by ozonation (Zhang et al. 2016; Yan et al. 2009a).
It is worth pointing out that extracellular and intracellular materials released into the supernatant would increase the total phosphorus in aqueous phase (TP(A)) content in the supernatant during sewage sludge ozonation (He et al. 2006). Zhang et al. (2009) investigated the changes of the supernatant during ozonation, and the results indicated that TP(A) content in the supernatant increased from 2.8 to 69 mg/L after ozonation at 50 mg O3/gDS. Yang et al. (2012) also found that TP(A) content in the supernatant increased from 9.13 to 23.26 mg/L after ozonation at 0.15 g O3/gDS. These researches mentioned above mainly focused on the total contents of phosphorus released during ozonation, while little is known about the detailed phosphorus fractions in sludge solid and the release contribution of each fraction to the phosphorus in the supernatant during ozonation. Furthermore, the TP(A) released into the supernatant during ozonation can cause an extra burden for the wastewater treatment system and result in an increase of phosphorus concentration in the effluent, when it was returned to the wastewater biological treatment process (Chu et al. 2009; Yan et al. 2009b). Thus, the TP(A) in the supernatant must be removed to some extent before it is returned into wastewater biological treatment process. To reduce the phosphorus content in the supernatant, the most common methods are biological treatment, chemical precipitation, and recovery (Bertanza et al. 2013). The removal of supernatant TP(A) during biological treatment process is called biological treatment. It is obvious that only the biological phosphorus removal cannot remove the TP(A) in the supernatant effectively (Chu et al. 2009; Yan et al. 2009b). Chemical precipitation is achieved through adding chemical reagents to the supernatant in order to form phosphorus-containing precipitation, which is then removed by sedimentation and/or filtration. However, the application of this technique is limited due to the high cost of chemical reagents and the large production of chemical sludge during phosphorus removal (Bertanza et al. 2013). Phosphorus recovery is generally achieved by magnesium ammonium phosphate (MAP) reaction (de-Bashan and Bashan 2004; Moed et al. 2015). The MAP reaction could remove both phosphorus and nitrogen simultaneously; meanwhile, the reaction product, namely struvite, could be recovered and employed as an ideal alternative fertilizer (Bi et al. 2014; Bouropoulos and Koutsoukos 2000). Thus, the MAP method was used to remove phosphorus from ozonized sludge supernatant in this study, as it can not only recover the limited phosphorus resource to alleviate the phosphorus resource crisis, but also reduce the phosphorus content. Nevertheless, the removal of phosphorus by MAP is complicated in relation to many factors, such as the initial Mg2+/PO4 3−-P, initial pH of reaction system, and reaction time (Wang et al. 2010; Bi et al. 2014). Therefore, it is necessary to study the removal performance of phosphorus from the ozonized supernatant by MAP.
Thus, the aim of this research was to study (1) the release of phosphorus from sewage sludge and the phosphorus fractions in sludge solid during ozonation, (2) the release contribution of each fraction of the phosphorus in sludge solid to the phosphorus in the supernatant during ozonation, (3) the optimal conditions for phosphorus removal from the ozonized sludge supernatant using MAP method, and (4) the characteristics of the ozonized sludge supernatant after MAP treatment. It is anticipated that this study would be meaningful not only for providing an insight into the release behavior of phosphorus from sludge during ozonation but also for offering a feasible method to remove phosphorus from ozonized sludge supernatant, which will be beneficial to the development of sludge reduction technology by ozonation.
Material and methods
Sewage sludge
Sewage sludge samples were obtained from a sewage disposal plant in Harbin, China. It was transported to the lab within 1 h after sampling. The sludge was subsequently screened through a sieve to remove big impurities then stored at 4 °C and used within 48 h. The basic characteristics of the sludge were shown in Table 1.
Experimental apparatus and sludge ozonation
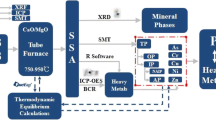
The apparatus used for sludge ozonation in this research was the same as that employed by Zhang et al. (2016), as shown in Fig. 1. The detailed experimental operations for ozonation of sludge and the corresponding calculation method of ozone consumption were also given by Zhang et al. (2016). The ozonized sludge mixtures were obtained from the reactor every 10 min then centrifuged at 5000 rpm for 5 min. The liquid phase was employed to measure TP(A) and total nitrogen (TN(A)) directly. The filtrate obtained by screening through a 0.45-μm membrane was employed to measure the PO4 3−-P, NH4 +-N, NO3 −-N, soluble chemical oxygen demand (SCOD), proteins, and polysaccharide contents. The sludge pellets were dried at ambient temperature, ground to stive, and sieved through a 100-mesh sieve. Then, it was stored in polyethylene tubes in a desiccator before use for phosphorus fraction analysis.
Phosphorus fractions in liquid phase and sludge solid
The supernatant of sludge mixtures under different doses of ozonation treatment was employed to analyze the phosphorus fractions in aqueous phase. There were three types of phosphorus in aqueous phase, including the TP(A), PO4 3−-P, and OP(A) (organic phosphorus in aqueous phase). TP(A) was acquired based on the method used by Xu et al. (2015). The content of OP(A) was calculated by the difference of TP(A) and PO4 3−-P.
The extraction protocol of the Standards in Measurements and Testing (SMT) program was used to study the phosphorus fractions in sludge solid. Details of the extraction process of the SMT protocol were described by Xie et al. (2011). According to the SMT protocol, there were five fractions of phosphorus in sludge solid: non-apatite inorganic phosphorus (NAIP), apatite phosphorus (AP), inorganic phosphorus (IP), organic phosphorus (OP), and total phosphorus (TP(S)), where TP(S) = OP + IP, IP = NAIP + AP (He et al. 2016). Generally, it was reasonable when the recovery rate of TP was in the range of 97.2 ~ 103.2% and the recovery rate of IP was within 95.5 ~ 104.7% (Pardo et al. 2004). The recovery rate (RR) of phosphorus during the SMT process was calculated by the following equations:
where RR(IP)(%) and RR(TPS)(%) are the recovery rates of IP and TP(S) during SMT process, respectively, and NAIP(X), AP(X), IP(X), OP(X), and TP(S) are the contents of certain phosphorus fraction in sludge solid after ozonation (mg/g).
RC analysis of different phosphorus fractions in sludge solid
The release of certain phosphorus fraction from sludge into the supernatant can be characterized by release contribution (RC). RC was calculated as follows:
where P (S)(raw) is a certain phosphorus fraction content in the raw sludge without ozonation (mg/g), P (X) is the content of certain phosphorus fraction in sludge solid after ozonation (mg/g), TP(A)(raw) is the TP content in the raw sludge supernatant (mg/L), TP(X) is the TP content in the ozonized sludge supernatant (mg/L), X is one kind of sludge, V is the volume of the sludge mixture (L), MLSS(X) is the mixed liquor suspended solid (MLSS) concentration in ozonized sludge (mg/L), and MLSS(raw) is the MLSS concentration in the raw sludge (mg/L).
Phosphorus removal by MAP method
The supernatant obtained from the ozonized sludge mixture was centrifuged at 5000 rpm for 5 min. The pH of the supernatant was adjusted with 1 mol/L sodium hydroxide (Huang et al. 2014). Magnesium chloride hexahydrate (MgCl2·6H2O) was employed as the source of Mg2+ in this study. Fifty milliliters of ozonized sludge supernatant was placed in a 150-mL conical flask. A certain amount of magnesium dichloride hexahydrate was added in the flask containing the ozonized sludge supernatant, and then, the mixture was agitated at 200 rpm. The mixture was sampled at a designed time interval and centrifuged at 5000 rpm for 5 min (Bi et al. 2014). After that, the liquid phase without filtration was used to measured TP(A) and TN(A) directly, and the supernatant screened through a 0.45-μm membrane was used to measured PO4 3−-P, NO3 −-N, SCOD, proteins, and polysaccharides contents.
Analysis methods
NH4 +-N, PO4 3−-P, SCOD, MLSS, and mixed liquor volatile suspended solid (MLVSS) were measured based on the Chinese NEPA standard methods (CEPB 2002). TP(A) was measured according to He et al. (2016). The pH of the sludge mixture was determined by a pH meter (FE20, INESA, China). The methods for TCOD, protein, and polysaccharide concentrations of extracellular polymeric substances (EPS) in the supernatant used in this study were described by Zhang et al. (2016). TN(A) was measured by a TN analyzer (OC-5000A, Shimadzu, Japan).
Results and discussion
Effect of ozone dose on TP(A), PO4 3−-P, and OP(A) in supernatant during ozonation
The TP(A) concentration rose from 9.0 to 70.9 mg/L with the dosage of ozone increased from 0 to 61.2 mg O3/gSS (Fig. 2). The reason for the TP(A) increase was that the cell wall and cell membrane of the microorganism in the sludge flocs were destroyed during ozonation, contributing to the release of phosphorus from sludge solid into the supernatant, such as phosphorus in EPS, lipid bilayer, polyphosphates, and nucleic acid. Meanwhile, the increasing tendency of TP(A) concentration became small as the dosage of ozone dose was over 61.2 mg O3/gSS. The reason for this phenomenon could be that as the dosage of ozone was higher than 61.2 mg O3/gSS, the materials in the supernatant released from sludge cells could react with ozone and expend a portion of ozone. As a consequence, the amount of ozone applied to destroy sludge flocs was reduced (Cesbron et al. 2003; Cui and Jahng 2004). Consequently, the TP(A) concentration stayed invariable when ozone dose was excessive.
The increasing tendency of PO4 3−-P in ozonized sludge supernatant was similar to that of TP(A), and the PO4 3−-P in ozonized sludge supernatant accounted for above 84.4% of TP(A) during ozonation. This meant that the vast majority of TP(A) in the supernatant was PO4 3−-P. During sludge ozonation, the intracellular and extracellular matters containing phosphorus were released into the supernatant, including organic phosphorus and inorganic phosphorus, while the inorganic phosphorus released accounted for a vast majority of the total phosphorus released (Latif et al. 2015). This would be verified in the “RC of different phosphorus fractions in sludge solid to TP(A) during ozonation S13” section. Meanwhile, ozone has a strong oxidation ability and can oxidize portions of organic phosphorus in the supernatant into phosphate (He et al. 2006). Therefore, the increased PO4 3−-P accounted for more than 84.4% of the increased TP(A) in the ozonized sludge supernatant during ozonation.
The OP(A) concentration in the supernatant increased first. As mentioned above, ozone could oxidize and destroy sludge cell, resulting to the release of organic phosphorus from the sludge solid into the supernatant. While the ozone dose increased from 40.8 to 61.2 mg O3/gSS (Fig. 2), the OP(A) concentration in the supernatant was nearly kept constant. This was because the quantity of organic phosphorus released from the sludge was approximately equal to that of the organic phosphorus oxidized into inorganic phosphorus. The OP(A) concentration in the supernatant decreased finally. This was mostly ascribed to the fact that part of OP(A) in supernatant was oxidized to PO4 3−-P.
Effect of ozone dose on TP(S) and phosphorus fractions in sludge during ozonation
The TP(S) and phosphorus fractions in the sludge during ozonation are presented in Table 2. It can be calculated that the recovery rates of TP and IP were 97.2 ~ 99.4% and 97.9 ~ 99.8%, respectively. This indicated that the SMT protocol is suitable for analyzing the phosphorus fractions in sludge solid in this study. It can be observed that TP(S) was mostly distributed in IP (more than 81.5% of TP(S)) and NAIP was the major component of IP in the sludge (more than 78.7% of IP) during ozonation. The contents of all kinds of phosphorus increasing with the ozone dose increase from 0 to 20.4 mg O3/gSS. This was attributed to the decrease of MLSS. As we know, the total amount of phosphorus in the sludge would not increase since there was no input of phosphorus, while the MLSS was decreased during ozonation (Zhang et al. 2016). Therefore, when the decreasing rate of MLSS exceeded the decreasing rate of phosphorus, there would be a pseudo-increase of phosphorus contents. All kinds of phosphorus contents decreased with the ozone dose increasing from 20.4 to 61.2 mg O3/gSS. During this stage, the MLSS was almost stable, while the phosphorus in sludge solid would be released into the supernatant due to ozonation. When the ozone dose increased from 61.2 to 81.6 mg O3/gSS, all kinds of phosphorus contents in sludge solid were nearly stable.
RC of different phosphorus fractions in sludge solid to TP(A) during ozonation
The RC of different phosphorus fractions in sludge solid to TP (A) were given in Fig. 3. It was clear that the RC of IP to TP(A) went up with increasing ozone dose. In terms of IP, the contribution of NAIP rose with increasing ozone dose and contributed more than 64.2% of RC to the IP. The contribution of AP increased when ozone dose increased from 20.4 to 40.8 mg O3/gSS and then was almost stable with the increase of ozone dose. This may be owing to the fact that AP was a relatively stable fraction (Xu et al. 2015). The RC of OP to TP(A) decreased when ozone dose increased from 20.4 to 40.8 mg O3/gSS and then stayed stable as ozone dose increased.
Removal of TP(A) and PO4 3−-P from ozonized sludge supernatant
It was known that PO4 3−-P in aqueous phase could react with Mg2+ and NH4 +-N to form struvite (MgNH4PO4·6H2O). The reaction formula was given as follows (Wang et al. 2010):
From the reaction formula, the phosphorus participating in the reaction was PO4 3−-P and the PO4 3−-P concentration was 65.4 ± 4.8 mg/L accounting for 92.8% of TP(A) in the ozonized sludge supernatant at the dose of 81.6 mg O3/gSS. Therefore, the ozonized sludge supernatant was in favor of MAP formation considering the phosphorus resource (Demirer and Othman 2009). The theoretical molar ratio of NH4 +, PO4 3−-P, and Mg2+ for struvite production is 1:1:1(Zhang et al. 2015), while the initial molar concentrations of Mg2+, NH4 +, and PO4 3−-P in the ozonized sludge supernatant were 0.76, 4.27, and 2.11 mmol/L, respectively. The results suggested that Mg2+ was the limiting factor and NH4 + was sufficient in terms of the ozonized sludge supernatant for struvite formation. The aim of this research was to remove TP(A) and PO4 3−-P from the ozonized sludge supernatant. Thus, Mg2+ should be added to the ozonized sludge supernatant to start the MAP reaction. The initial Mg2+/PO4 3−-P in reaction system, initial pH of reaction system, and reaction time were key factors influencing the removal efficiency of TP(A) and PO4 3−-P from reaction system (Wang et al. 2010; Bi et al. 2014). Thus, the effects of initial Mg2+/PO4 3−-P in the ozonized sludge supernatant, initial pH of the ozonized sludge supernatant, and reaction time on the removal efficiency of TP(A) and PO4 3−-P from the ozonized sludge supernatant were investigated in this study.
Effect of initial Mg2+/PO4 3−-P on removal of TP(A) and PO4 3−-P
The suitable pH for MAP reaction was about 9.5 (Bi et al. 2014). The pH of the ozonized sludge supernatant in this research was 5.63, which did not satisfy the pH condition for struvite formation reaction. So it was adjusted to 9.5 with 1 mol/L sodium hydroxide (Lee et al. 2003). The mixture was centrifuged at 5000 rpm for 5 min after the struvite formation reaction for 5 min with different initial molar ratios of Mg2+/ PO4 3−-P in the ozonized sludge supernatant (Bi et al. 2014). The removal efficiency of TP(A) and PO4 3−-P at different initial molar ratios of Mg2+/ PO4 3−-P is shown in Fig. 4. The removal efficiency of TP(A) and PO4 3−-P rose with increasing initial molar ratio of Mg2+/PO4 3−-P in the ozonized sludge supernatant. It was demonstrated that the increase of Mg2+/ PO4 3−-P initial molar ratio in the ozonized sludge supernatant was a feasible way to enhance the removal efficiency of TP(A) and PO4 3−-P especially as the initial molar ratio of Mg2+/PO4 3−-P was lower than 1.8. When the initial molar ratio of Mg2+/PO4 3−-P rose from 1.2 to 1.8, the removal efficiency of TP(A) and PO4 3−-P enhanced from 14.8 to 39.9 and 20.3 to 48.2%, respectively. When the initial molar ratio of Mg2+/PO4 3−-P was above 1.8, the removal efficiencies of PO4 3−-P stayed constant, while the removal efficiencies of TP(A) were increased slightly. Therefore, the optimal initial molar ratio of Mg2+/ PO4 3−-P for TP(A) and PO4 3−-P removal was 1.8:1 in this research. This was different from the theoretical initial molar ratio of Mg2+/ PO4 3−-P of 1:1 for struvite formation. The reasons for the discrepancy might be that a fraction of the Mg2+ added could be consumed by SCOD (for example proteins and lipids) in the ozonized sludge supernatant. Tong and Chen (2007) obtained similar results with this study. In addition, as for the slight increase of removal efficiency of TP(A) at a Mg2+/ PO4 3−-P ratio above 1.8, the reason could be that a few kinds of phosphorus except PO4 3−-P, for example, organic phosphorus, reacted with magnesium as well which was then removed from the supernatant fluid. Thus, the different removal efficiency of TP(A) and PO4 3−-P was observed when the initial molar ratio of Mg2+/PO4 3−-P was higher than 1.8.
Effect of initial pH on removal of TP(A) and PO4 3−-P
For the struvite formation, the ion forms and activities of Mg2+, NH4 +, and PO4 3−-P were influenced by the initial pH of liquid phase. For example, NH4 + may transform into NH3 when the initial pH of liquid phase was high. Then, the NH3 would be released from the liquid into the air, no longer participating in struvite formation reaction (Nelson et al. 2003; Song et al. 2007). Thus, the initial pH of the ozonized sludge supernatant is a significant parameter influencing the removal efficiency of TP(A) and PO4 3−-P. The struvite formation reaction happened in the pH range of 7.5–10 (Hao et al. 2008). Therefore, the initial pH of ozonized sludge supernatant was adjusted to 7.5, 8.0, 8.5, 9.0, 9.5, 10.0, 10.5, and 11.0 with 1 mol/L sodium hydroxide according to previous report (Jorge et al. 2017). Meanwhile, the initial molar ratio of Mg2+/ PO4 3−-P was kept at 1.8:1. The removal efficiency of TP(A) and PO4 3−-P at different initial pH was shown in Fig. 5. When the initial pH of the ozonized sludge supernatant increased from 7.5 to 9.5, the removal efficiencies of TP(A) and PO4 3−-P were enhanced from 4.0 to 39.1 and from 4.3 to 48.1%, respectively. Then, the removal efficiency of TP(A) and PO4 3−-P increased negligibly with the increasing initial pH of ozonized sludge supernatant. In addition, at a high pH, the free OH− could be reacted with Mg2+ and generated Mg(OH)2 precipitation, resulting in the decrease of Mg2+ concentration in the reaction system. From this perspective, the high initial pH of the reaction system would influence the removal efficiency of TP(A) and PO4 3−-P. For the cost reduction consideration, the optimal initial pH of ozonized sludge supernatant for TP(A) and PO4 3−-P removal was selected at 9.5 in this research.
Effect of reaction time on removal of TP(A) and PO4 3−-P
Under the initial pH of 9.5 and the initial Mg2+/ PO4 3−-P ratio of 1.8, the effect of reaction time on the removal efficiency of TP(A) and PO4 3−-P in the ozonized sludge supernatant at different reaction times was shown in Fig. 6. The maximal removal efficiency of TP(A) and PO4 3−-P was obtained at 5 min; after that, the removal efficiency of TP(A) and PO4 3−-P stayed almost constant until 50 min. It could be perceived that the reaction was finished in 5 min based on the above results. This result was consistent with the reports of Demirer and Othman (2009). Therefore, the optimal reaction time for TP(A) and PO4 3−-P removal was 5 min in this research.
The effects of TP(A) and PO4 3−-P removal on the ozonized sludge supernatant
A conclusion could be drawn that the optimum conditions for TP(A) and PO4 3−-P removal were at initial Mg2+/PO4 3−-P molar ratio of 1.8, pH of 9.5, and reaction time of 5 min from the single-factor experiment. The changes of sludge supernatant properties during TP(A) and PO4 3−-P removal are shown in Table 3. The removal efficiencies of NH4 +, PO4 3−-P, and TP(A) were 20.6, 52.2, and 43.1%, respectively. The molar ratio of NH4 +/PO4 3−-P removal was 0.76:1, while the theoretical molar ratio of NH4 +/PO4 3−-P for struvite formation was 1:1. The reason for excess PO4 3−-P consumption might be that PO4 3−-P could be adsorped and/or coprecipitated with struvite. It was found that the TN and NO3 −-N decreased after the MAP reaction, which was mostly due to the absorption of the precipitate. The SCOD, proteins, and polysaccharides in the ozonized sludge supernatant decreased from 2784.8 to 2059.3, from 696.3 to 556.7, and from 387.3 to 312.4 mg/L after reaction, respectively. The decrease of SCOD was mainly caused by the reduction of proteins and polysaccharides, as protein and polysaccharide could be coprecipitated with struvite. Meanwhile, several kinds of heavy metals (Al, Ca, and Fe) could also be precipitated with proteins and polysaccharides. This phenomenon was in accordance with that from Bi et al. (2014). The pH was decreased after the MAP reaction, which is attributed to the consumption of NH4 + during reaction. All in all, the vast majority of SCOD was still in the ozonized sludge supernatant after the MAP reaction, indicating it could be reused as a carbon resource in sewage treatment process.
Conclusions
Phosphorus was released from the sludge solid into the supernatant during ozonation, and the quantity of phosphorus released depended on ozone dose. In this study, TP(A) and PO4 3−-P reached a platform when ozone dose was 61.2 mg O3/gSS, at which the TP(A) and PO4 3−-P contents were 70.9 and 63.3 mg/L, respectively. The phosphorus in the supernatant was mainly derived from IP, in which NAIP was the major constituent in the sludge during ozonation. More than 81.5% of TP(S) was distributed in IP, and NAIP occupied over 78.7% of IP in the sludge during ozonation. The RC of IP to TP(A) accounted for over 73.9% and went up with increasing ozone dose. The phosphorus in the ozonized sludge supernatant could be removed effectively by the MAP reaction. The optimized conditions for removing phosphorus from ozonized sludge supernatant were at the initial Mg2+/ PO4 3−-P molar ratio of 1.8, pH 9.5, and reaction time of 5 min. Under the optimal condition, the removal efficiencies of TP(A) and PO4 3−-P were 43.1 and 52.2%, respectively.
References
Anjum M, Al-Makishah N, Barakat M (2016) Wastewater sludge stabilization using pre-treatment methods. Process Saf Environ Prot 102:615–632
de-Bashan L, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997-2003). Water Res 38:4222–4246
Bertanza G, Pedrazzani R, Manili L, Menoni L (2013) Bio-P release in the final clarifiers of a large WWTP with co-precipitation: key factors and troubleshooting. Chem Eng J 230:195–201
Bertanza G, Papa M, Canato M, Collivignarelli M, Pedrazzani R (2014) How can sludge dewatering devices be assessed? Development of a new DSS and its application to real case studies. J Environ Manag 137:86–92
Bi W, Li Y, Hu Y (2014) Recovery of phosphorus and nitrogen from alkaline hydrolysis supernatant of excess sludge by magnesium ammonium phosphate. Bioresour Technol 166:1–8
Bouropoulos N, Koutsoukos P (2000) Spontaneous precipitation of struvite from aqueous solutions. J Cryst Growth 213:381–388
CEPB (2002) Standard methods for examination of water and wastewater, Fourth edn. Chinese Environmental Science Press, Beijing
Cesbron D, Deleris S, Debellefontaine H, Roustan M, Paul E (2003) Study of competition for ozone between soluble and particulate matter during activated sludge ozonation. Chem Eng Res Des 81:1165–1170
Chu L, Yan S, Xing X, Sun X, Jurcik B (2009) Progress and perspectives of sludge ozonation as a powerful pretreatment method for minimization of excess sludge production. Water Res 43:1811–1822
Cui R, Jahng D (2004) Nitrogen control in AO process with recirculation of solubilized excess sludge. Water Res 38:1159–1172
Demirer S, Othman M (2009) Removal of ammonium and phosphate from the supernatant of an aerobically digested waste activated sludge by chemical precipitation. Bioresour Technol 100:3236–3244
Dytczak M, Londry K, Siegrist H, Oleszkiewicz J (2007) Ozonation reduces sludge production and improves denitrification. Water Res 41:543–550
Hao X, Wang C, Lan L, Loosdrecht M (2008) Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci Technol 58:1687–1692
He S, Xue G, Wang B (2006) Activated sludge ozonation to reduce sludge productionin membrane bioreactor (MBR). J Hazard Mater B135:406–411
He Z, Liu W, Wang L, Yang C, Guo Z, Zhou A, Liu J, Wang A (2016) Role of extracellular polymeric substances in enhancement of phosphorus release from waste activated sludge by rhamnolipid addition. Bioresour Technol 202:59–66
Huang H, Chen Y, Jiang Y, Ding L (2014) Treatment of swine wastewater combined with MgO-saponification wastewater by struvite precipitation technology. Chem Eng J 254:418–425
Jorge R, Carlos A, Tânia S, Dionysios D, Gianluca L, Marco S, José A (2017) Treatment of winery wastewater by sulphate radicals: HSO5 −/transition metal/UV-A LEDs. Chem Eng J 310:473–483
Kamiya T, Hirotsui J (1998) New combined system of biological process and intermittent ozonation for advanced wastewater treatment. Water Sci Technol 38:145–153
Latif M, Mehta C, Batstone D (2015) Low pH anaerobic digestion of waste activated sludge for enhanced phosphorous release. Water Res 81:288–293
Lee S, Weon S, Lee C, Koopman B (2003) Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 51:265–271
Manterola G, Uriarte I, Sancho L (2008) The effect of operational parameters of the process of sludge ozonation on the solubilisation of organic andnitrogenous compounds. Water Res 42:3191–3197
Moed N, Lee D, Chang J (2015) Struvite as alternative nutrient source for cultivation of microalgae Chlorella Vulgaris. J Taiwan Inst Chem Eng 56:73–76
Nelson N, Mikkelsen R, Hesterberg D (2003) Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg:P ratio and determination of rate constant. Bioresour Technol 89:229–236
Pardo P, Rauret G, López-sánchez J (2004) Shortened screening method for phosphorus fractionation in sediments: a complementary approach to the standards, measurements and testing harmonized protocol. Anal Chim Acta 508:201–206
Park K, Ahn K, Maeng S, Hwang J, Kwon J (2003) Feasibility of sludge ozonation for stabilization and conditioning. Ozone Sci Eng 25:73–80
Song Y, Yuan P, Zheng B, Peng J, Yuan F, Gao Y (2007) Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater. Chemosphere 69:319–324
Tong J, Chen Y (2007) Enhanced biological phosphorus removal driven by short-chain fatty acids produced from waste activated sludge alkaline fermentation. Environ Sci Technol 41:7126–7130
Wang C, Hao X, Guo G, Loosdrecht M (2010) Formation of pure struvite at neutral pH by electrochemical deposition. Chem Eng J 159:280–283
Wei Y, Van Houten R, Borger A, Eikelboom D, Fan Y (2003) Minimization of excess sludge production for biological wastewater treatment. Water Res 37:4453–4467
Xie C, Zhao J, Tang J, Xu J, Lin X, Xu X (2011) The phosphorus fractions and alkaline phosphatase activities in sludge. Bioresour Technol 102:2455–2461
Xu Y, Hu H, Liu J, Luo J, Qian G, Wang A (2015) pH dependent phosphorus release from waste activated sludge: contributions of phosphorus speciation. Chem Eng J 267:260–265
Yan S, Chu L, Xing X, Yu A, Sun X, Jurcik B (2009a) Analysis of the mechanism of sludge ozonation by a combination of biological and chemical approaches. Water Res 43:195–203
Yan S, Zheng H, Li A, Zhang X, Xing X, Chu L, Ding G, Sun X, Jurcik B (2009b) Systematic analysis of biochemical performance and the microbial community of an activated sludge process using ozone-treated sludge for sludge reduction. Bioresour Technol 100:5002–5009
Yang S, Guo W, Cao G, Zheng H, Ren N (2012) Simultaneous waste activated sludge disintegration and biological hydrogen production using an ozone/ultrasound pretreatment. Bioresour Technol 124:347–354
Zhang G, Yang J, Liu H, Zhang J (2009) Sludge ozonation: disintegration, supernatant changes and mechanisms. Bioresour Technol 100:1505–1509
Zhang Y, Zhang W, Pan B (2015) Struvite-based phosphorus recovery from the concentrated bioeffluent by using HFO nanocomposite adsorption: effect of solution chemistry. Chemosphere 141:227–234
Zhang J, Zhang J, Tian Y, Li N, Kong L, Sun L, Yu M, Zuo W (2016) Changes of physicochemical properties of sewage sludge during ozonation treatment: correlation to sludge dewaterability. Chem Eng J 301:238–248
Funding
This study was supported by the Major Science and Technology Program for Water Pollution Control and Management (No. 2013ZX07201007, 2014ZX07201012), the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No.2014DX03), and the Science Fund for Distinguished Young Scholars of Heilongjiang Province (JC201303). The authors also appreciate the Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology(QA201609-02) and the Heilongjiang Postdoctoral Science Foundation (LBH-Z14093).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Zhang, J., Tian, Y. & Zhang, J. Release of phosphorus from sewage sludge during ozonation and removal by magnesium ammonium phosphate. Environ Sci Pollut Res 24, 23794–23802 (2017). https://doi.org/10.1007/s11356-017-0037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0037-8