Abstract
To study the combined effects of multiple nitrogen (N) sources and salinity on the growth and physiology on macroalgae, we cultured Ulva prolifera under three N levels (N0, 0.1235 mg L−1; N1, 0.6 mg L−1; and N2, 4.4 mg L−1; the ratios were 18:74:8 for NH4–N, NO3–N, and NO2–N, respectively) and three salinity conditions (15, 25, and 35). Then, the growth, pigment content, photosynthetic performance, superoxide dismutase (SOD) activity, and contents of soluble protein and carbohydrates were measured. The results showed the following: (1) Compared to that grown at salinity 25, the growth of U. prolifera decreased under salinity 35, especially under the N0 and N2 levels, but there were no significant effects of salinity 15 under any of the N levels. (2) There were no significant effects of salinity on the chlorophyll a (Chla) content, but compared to the content at salinity 25, the chlorophyll b (Chlb) content was enhanced by salinity 15 and 35; lower ratio values between Chla and carotenoids (Car) occurred under the salinity 25 treatment. Under each salinity condition, the pigments were enhanced by a high N level. (3) A relatively higher salinity level decreased the photosynthetic oxygen evolution rate, while a higher N level increased this value. Compared to the rate at salinity 25, the dark respiration rate (Rd) significantly increased at salinity 15 under the N0 condition. (4) SOD activity was enhanced by a high N level, but no significant effects of salinity were observed. (5) The carbohydrate content was enhanced at salinity 35 under the N0 and N1 levels, and under salinity 15, this value increased with increasing N levels. In conclusion, although the growth of U. prolifera decreased at high N levels under high salinity conditions, a high N level induced an increase in photosynthesis, while no significant decrease in growth occurred. These findings indicate that low salinity and high N levels may be nonnegligible reasons why this species thrives, and low salinity was the better choice when this species was used for wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blooms of Ulva spp., also known as green tides, on the Yellow Sea coast are worrisome. Green tide outbreaks thrive under a variety of conditions, including suitable temperatures, light, and available nitrogen (N) concentrations (Kessing et al. 2011; Shi et al. 2015). Coastal eutrophication, which is caused by aquaculture activities, fertilizer, and manure use, occurs gradually over time. The N levels in most coastal areas in China are higher than those in other coastal zones of the world (Valiela et al. 2018). The supplement of nutrients not only enhanced the growth of macroalgae (Teichberg et al. 2010; Luo et al. 2012; Xu et al. 2014; Chen et al. 2016; Reidenbach et al. 2017) but also influenced their biochemical composition, for example, the protein and carbohydrate composition of macroalgae changed (Ross et al. 2018), as did levels of certain enzymes, including rubisco, carbonic anhydrase (CA), and nitrate reductase (NR), which are key enzymes in photosynthesis and N absorption (Jiménez del Rio et al. 1995; Zhu et al. 2016). Excessive nutrients are the main reason for the explosive proliferation of Ulva spp. (Valiela et al. 2018). Although there are many papers about the effects of nutrients on macroalgae, these studies have only investigated the effects of single N species, and relatively more attention has been paid to nitrate (NO3–N) (Teichberg et al. 2010; Zhu et al. 2016) and ammonium N (NH4–N) (Xu et al. 2014; Reidenbach et al. 2017); the effects of multiple N sources have been not been studied, including the interactive effects between N and other environmental factors. NO3–N and NH4–N are the main forms of inorganic N, but nitrite N (NO2–N) also exists in coastal waters, especially in coastal zones influenced by aquaculture (Ge et al. 2018). Macroalgae, including Ulva spp., have been used for wastewater treatment (Brito et al. 2014; Ge et al. 2018) due to their high nutrient uptake ability and storage capacity (Luo et al. 2012; Lubsch and Timmermans 2018).
Furthermore, macroalgae grow in nearshore ponds, estuaries, and intertidal zones, and the salinity varies in these areas. The pigment content, photosynthesis, growth, and antioxidant activity as well as reactive oxygen species in macroalgae could be affected by salinity (Choi et al. 2010; Luo and Liu 2011; Gao et al. 2016). Compared to optimal salinity levels, low and high salinities induce more oxidative damage, and relatively higher SOD activity has been observed under hyper- and hyposaline conditions (Luo and Liu 2011). Salinity-dependent morphological variations in physiology and biochemical composition were also observed in U. prolifera, i.e., there were more branches at low salinity levels (salinity 10), especially at 20 °C, and longer branches were observed under hypersaline conditions (salinity 30), especially at 25 °C; relatively higher NR activity occurred at salinity 20 at 25 °C (Gao et al. 2016). Additionally, the growth and nitrate uptake rates of U. pertusa decreased under low and high salinity levels (Choi et al. 2010).
As previously mentioned, Ulva prolifera is the dominant bloom-forming macroalga that thrives on the Yellow Sea coast in late April through early August every year. We wanted to investigate the extent to which coastal nutrient enrichment enhances the growth and photosynthesis of this species. Increases in the activity of reactive oxygen scavenging enzymes induced by low and high salinity levels are involved in oxidative stress (Luo and Liu 2011), and therefore, we also wondered whether and how this increase could mediate the effects of N enrichment in this species. Furthermore, this species has also been used as a biofilter and a monitor for metal pollution (Farias et al. 2017; Ge et al. 2018), and the advantages of the physiological characteristics of U. prolifera in addition to its higher nutrient uptake ability were also important. U. prolifera was chosen for this study and was cultured in the laboratory across a range of salinity levels and enriched with multiple N sources. After 6-day growth experiments, the algae growth, photosynthesis, pigment content, respiration, SOD activity, total soluble protein, and carbohydrate content in the thalli were determined. The aims of this study were to possibly understand which level of N enrichment enhanced the booming of this species, assess how this enhancement was affected by salinity fluctuations, and determine what we should pay attention to when this species is used for wastewater treatment.
Materials and methods
Species and culture conditions
Ulva prolifera in approximately 2–3 mm lengths was provided by Xiangshan Xuwen Seaweed Development Co. Ltd. Prior to the experiment, they were kept in non-N artificial seawater (ASW; salinity 25) enriched by f/2 medium (without Si, without N; Guillard and Ryther 1962) for 3–4 days at 25 °C and 80 μmol photons m−2 s−1 conditions (L:D = 12:12) by bubbling with ambient air at 600 mL min−1. The non-N ASW was changed every day, and the fresh weight was determined every day. Until the fresh weight was almost no longer increase, they were used for experiments.
Treatments
In previous studies, the ratios of NH4–N, NO3–N, and NO2–N have been ignored (Xu et al. 2014; Zhu et al. 2016). Therefore, in this study, we set three N levels (N0 (LN), 0.12 mg L−1; N1 (MN), 0.60 mg L−1; and N2 (HN), 4.4 mg L−1) with 18:74:8 ratios for NH4–N, NO3–N, and NO2–N, respectively, based on the China Offshore Sea Environmental Quality Bulletin 2016. N1 represented the average concentration of inorganic N in Zhejiang coastal waters, while N2 represented the total concentration of N in shrimp pond aquaculture wastewater. Three salinity levels were set up: salinity 15 (LS), salinity 25 (MS), and salinity 35 (HS). The salinity of ASW was 35. The salinity 25 and 15 treatments were prepared by diluting ASW with distilled water. Then, different volumes (28 and 136 μL for N0 and N1 treatments, and 1 mL for N2 treatments) of stock solutions (4.4 g L−1) were added. Approximately 0.2–0.3 g of U. prolifera thalli that were precultured in f/2 enriched non-N ASW were cultured (three replicates for each treatment) in treatment combinations as follows:
N concentration (mg L−1) | Salinity | ||
|---|---|---|---|
15 (LS) | 25 (MS) | 35 (HS) | |
0.12 (LN) | LNLS | LNMS | LNHS |
0.6 (MN) | MNLS | MNMS | MNHS |
4.4 (HN) | HNLS | HNMS | HNHS |
The fresh weight was measured every day, and the culture medium was renewed every day. To eliminate the effect of culture density (Jiang et al. 2017), new algal biomass was removed after the fresh weight was measured (Li et al. 2018). The growth rate was reported as the average of the last three days in each treatment during the culture. The pigments, photosynthesis, and biochemical constituents were determined after the algae were cultured under each treatment for 6 days.
Growth rate and pigment content
Changes in the fresh weight was evaluated every day, and the relative growth rate (RGR) was calculated using the following formula: \( \mathrm{RGR}\left(\%\cdotp {\mathrm{d}}^{-1}\right)=100\times \ln \frac{W_2}{W_1}\div 1 \), where W1 and W2 represent the fresh weight measured on the previous day and the current day.
The samples (ca. 0.01 g) used for determining the pigments were extracted in methanol (3 mL) overnight at 4 °C in darkness, and the absorption spectrum of the extraction solution was scanned from 250 to 750 nm using a scanning spectrophotometer (Yuanxi Instrument Co., Ltd, Shanghai). The chlorophyll a (Chla), chlorophyll b (Chlb), and carotenoid (Car) concentrations were calculated according to Lichtenthaler and Wellburn (1983) based on the absorbance of the methanol extracts at 470, 653, and 666 nm.
Photosynthesis
The net photosynthetic oxygen evolution rates (Pn) were measured using a Clark-type oxygen electrode (Hansatech Instruments Ltd, UK) at 25 °C under different irradiance levels (50, 100, 200, 400, and 600 μmol m−2 s−1; P-I curve). The thalli (ca. 0.01 g) were weighed and placed into 2 ml sample cups containing fresh medium, and the temperature was controlled by using a cooling circulator (Jinghong, Shanghai, China). The Pn was reported as the increase in the oxygen concentration of the medium at each light level from 5–8 min. The dark respiration rate (Rd) was measured by determining the oxygen consumption under dark conditions. The P-I curves were fitted by the following equation: Pn = Pmax × tanh (α I/Pmax) + Rd (Jasby and Platt 1976), where tanh and I represent the hyperbolic tangent and the irradiance (μmol m−2 s−1), respectively; α, Pmax, and Rd represent the apparent photosynthetic efficiency (α) (the slope of the initial linear region of the P-I curves), the maximal net photosynthetic rate, and the dark respiration rate, respectively. The compensation (Ic; μmol m−2 s−1) and saturating light intensity (Ik; μmol m−2 s−1) for photosynthesis were calculated as follows: Ic = − Rd/α; Ik = Pmax/α. The gross photosynthetic oxygen rate (Pg) was calculated according to Henley (1993) as follows: Pg = Pmax + Rd.
Superoxide dismutase (SOD) activity and biochemical constituent analysis
Aliquots of 0.15 g of thalli were extracted in phosphate buffer solution (PBS: 50 mmol L–1, pH 7.8) after being homogenized and were centrifuged for 15 min at 11,000g at 4 °C. The extraction solutions were used to determine their SOD activity and biochemical constituents.
SOD (EC 1.15.1.1) activity was determined by nitroblue tetrazolium (NBT) photoreduction (Giannopolitis and Ries 1977) with some modifications (Luo and Liu 2011; Li et al. 2017). In detail, a mixed solution that included methionine (14.5 mmol L−1, 94.5 mL), EDTA-Na2 (30 μmol L−1, 350 μL), PBS (50 mmol L−1, 3.15 mL, pH 7.8), NBT (2.25 mmol L−1, 3.5 mL), and riboflavin (60 mmol L−1, 3.5 mL) was prepared. Then, 3 mL of the mixed solution and 50 μL of crude SOD extract were transferred to test tubes and reacted at 25 °C and 80 μmol photons m−2 s−1 conditions. After 20 min, the absorbance at 560 nm was measured. The quantity of SOD required to produce a 50% reduction in NBT was defined as the SOD activity (U g−1 Fw). The total soluble protein content and carbohydrates were determined by coomassie brilliant blue G-250 dye and anthracene ketone sulfuric acid colorimetric methods according to Bradford (1976), and Loewus (1952), respectively.
Data analysis
Three replicates for each treatment were used in all the experiments, and the data were shown as the mean and the standard deviation. Origin 7.5 and SPSS 18.0 were used for plotting and the statistical analyses, respectively. The normal distribution of all data under each treatment and the homogeneity of variance were confirmed by a Shapiro-Wilk test (P > 0.05) and Levene’s test (P > 0.05), respectively. The effects of salinity, N, and their interactions were assessed by a two-way analysis of variance (ANOVA). A Tukey post hoc test (Tukey HSD) was performed to show differences between the salinity and N treatments. The significance level was set at P < 0.05.
Results
Effects of N and salinity on growth and pigments
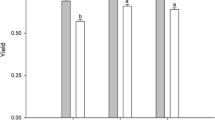
Compared to salinity 25, the growth of U. prolifera decreased by salinity 35, especially under the N0 and N2 levels, and there were no significant effects of salinity 15 under any N level. Under the salinity 25 and 35 conditions, a higher growth rate was observed at the N1 level, and under salinity 15, no effects of N were observed (Fig. 1). However, the interactive effects of salinity and N were significant (F4,18 = 3.779, P = 0.021; Table 1).
The relative growth rate (RGR) of U. prolifera grown under different treatments. Different uppercase letters indicate significant differences between different nitrogen levels for the same salinity, while different lowercase letters represent significant differences between salinity treatments at the same nitrogen level (P < 0.05)
Compared to changes in salinity, there were significant increases in the pigment contents in the thalli at higher N concentrations (Fig. 2, Table 1). In detail, higher Chla, Chlb, and Car contents were observed under the N2 level regardless of the salinity conditions. Significant effects of N on the Chla content (F2,18 = 90.992, P < 0.001) were observed, while no significant difference in salinity was observed (F2,18 = 2.869, P = 0.083). However, these two factors showed significant interactive effects (F4,18 = 3.723; P = 0.022). The thalli grown under the N2 level and salinity 25 conditions had the highest Car content (Fig. 2C). Significant effects of both N (ANOVA; Chlb: F2,18= 68.638, P < 0.001; Car: F2,18= 58.030, P < 0.001) and salinity (ANOVA; Chlb: F2,18= 48.759, P < 0.001; Car: F2,18= 51.487, P < 0.001) on the Chlb and Car contents were observed, but a significant interactive effect of these two pigments was found only in the Car content (F4,18 = 7.517, P = 0.001).
The Chla, Chlb, and Car contents of U. prolifera grown under the different treatments. Different uppercase letters indicate significant differences between different nitrogen levels for the same salinity, while different lowercase letters represent significant differences between salinity treatments at the same nitrogen level (P < 0.05)
Under each N level, compared to results at salinity 25, high and low salinity levels inhibited the ratio of Chla and Chlb (Chla/Chlb) and increased the ratio of Chla and Car (Chla/Car) as well as the ratio of Chlb and Car (Chlb/Car). There was no significant difference in the Chla/Chlb between salinity 15 and 35 (ANOVA; N0, F1,4= 0.519, P = 0.511; N1: F1,4= 1.218, P = 0.332; N2, F1,4= 3.566, P = 0.132), while the Chla/Car and Chlb/Car increased with increasing salinity (Table 2).
Effects of N and salinity on the photosynthesis
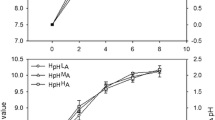
Net photosynthesis (Pn) increased gradually and then stabilized with increasing light intensity, and a higher maximal Pn (Pmax) occurred under the N2 level, especially at salinity 15 (Fig. 3, Table 3). Under the N0 level, compared to results in the salinity 25 treatment, the thalli grown at salinity 15 showed a higher α, Pmax, and Rd; the thalli grown at salinity 35 had a lower Rd and Pmax and no influence on α, which was lower than that for the salinity 15 treatment. The Ic and Ik decreased due to low and high salinity levels under the N0 level. Then, under the N1 and N2 conditions, the α and Pmax in the salinity 15 treatment increased, but the Ic and Ik decreased (Table 3). However, there were no significant interactive effects of salinity and N on these parameters, except for Ik (Table 4).
The ratio of Rd and Pg (Rd/Pg) decreased with increasing N concentrations under the salinity 15 and 25 conditions, but no differences between the N levels were observed for the salinity 35 condition. Additionally, under the N0 level, no effects of salinity on the Rd/Pg occurred, while this value increased with increasing salinity for the N1 and N2 levels. Significant effects of N (ANOVA; F2,18= 16.422, P < 0.001) and salinity (ANOVA; F2,18= 16.989, P < 0.001) on the Rd/Pg were observed, but there were no significant interactive effects between these two factors (F4,18 = 1.852, P = 0.163; Table 4).
Effects of N and salinity on SOD activity
No significant effects of salinity on the SOD activity were observed (ANOVA; F2,24= 2.098, P = 0.145; Fig. 4), although higher values occurred at salinity 35 under all the three N levels. Compared to the N0 level, the N2 level significantly increased the SOD activity, especially under the salinity 15 (ANOVA; F1,4= 33.300, P = 0.004) and salinity 35 conditions (ANOVA; F1,4= 6154.119, P < 0.001). The interactive effects of these two factors were statistically insignificant (F4,18 = 2.847, P = 0.054; Fig. 4).
The SOD activity of U. prolifera grown under different treatments. Different uppercase letters indicate significant differences between different nitrogen levels for the same salinity, while different lowercase letters represent significant differences between salinity treatments at the same nitrogen level (P < 0.05)
Effects of N and salinity on soluble protein and carbohydrates
The soluble protein content was maintained between 1.1 and 1.7 mg g−1 under the different treatments, and no significant effects of N and salinity (ANOVA; salinity: F2,18 = 2.379, P = 0.121; N: F2,18 = 0.896, P = 0.425) or the interactive effects of these two factors were observed (F4,18 = 0.648; P = 0.635) (Fig. 5A).
The contents of soluble protein (A) and carbohydrates (B) of U. prolifera under different treatments. Different uppercase letters indicate significant differences between different nitrogen levels for the same salinity, while different lowercase letters represent significant differences between salinity treatments at the same nitrogen level (P < 0.05)
Under the salinity 25 condition, no significant effects of N on carbohydrates were observed, but under salinity 15, this value increased with increasing N concentrations; the opposite trend occurred under the salinity 35 condition. Additionally, the thalli grown at salinity 35 under the N0 and N1 levels showed higher carbohydrate contents. The results showed that there were significant effects of salinity (ANOVA; F2,18 = 54.521, P < 0.001) but not of N (ANOVA; F2,18 = 3.201, P = 0.065) on carbohydrates; however, the interactive effects of salinity and N were significant (F4,18 = 17.251; P < 0.001) (Fig. 5B; Table 4).
Discussion
Ulva spp. in estuaries, intertidal zones and other areas often experience salinity fluctuations as well as eutrophication, including the enrichment of NH4–N, NO3–N, and NO2–N. This study was the first to attempt to evaluate the effects of salinity and multiple N sources on Ulva prolifera. The results showed that compared to that grown in a hypersaline environment, the growth of Ulva prolifera in hyposaline conditions was greater; high N concentrations lowered the growth of this species, but the photosynthetic rate was enhanced by a high N level, especially under low salinity conditions.
Previous studies have shown that Ulva spp. have a strong salinity tolerance, especially U. prolifera (Larsen and Sand-Jensen 2006; Rybak 2018). In this study, the growth of U. prolifera decreased due to high salinity, especially under low and high N concentrations. The reasons for this phenomenon include that higher salinity levels induced the formation of generative cells (Lin et al. 2011) and more oxidative stresses (Luo and Liu 2011); however, in this study, there were no significant effects of salinity on the Chla content and SOD activity, indicating the euryhaline nature of U. prolifera. The nonnegligible reason for this result may be the increased frequency of changing salinity conditions. However, in this study, the photosynthetic rate decreased significantly under the high salinity levels. To adapt to hypersaline conditions, algae rapidly accumulate the organic osmolytes proline, tyrosine, and histidine (Kakinuma et al. 2006; Angell et al. 2015) as well as carbohydrates (increased due to high salinity in this study), which is related to osmotic regulation ability (Bohnert et al. 1995), and the algae consumed more metabolites and energy necessary for growth. All the reasons mentioned above induced the lower growth rate under the high salinity condition. An inhibitory effect of extreme salinity stress (salinity 40) on growth was also observed in U. pertusa (Choi et al. 2010). In published papers, relatively higher growth was observed under lower salinity conditions (Choi et al. 2010; Lin et al. 2011; Li et al. 2017); this result was also found in this study, indicating strong adaptability to bay and coastal intertidal zones where the salinity varies due to rainfall and freshwater inputs. Low salinity levels increased the α as well as the Rd, especially under low N concentrations; in turn, more energy was provided that could be used for uptake and assimilation of N where NR plays an important role. Previous studies have shown that high N uptake occurred at salinity 20 (Choi et al. 2010), and compared to activity at salinities 5 and 30, high NR activity was observed at salinity 15 (Zhu et al. 2016).
Fluctuations in seawater salinity induced by rivers inputs, rainfall, and tidal periods often co-occur with eutrophication, and increasing N concentrations are the most important cause. A high NO3− concentration (2.24 mg L−1) increased the growth and number of fragments of U. lactuca (Van Alstyne 2018), and a high NH4–N concentration (2.8 mg L−1) also increased the growth of U. prolifera (Xu et al. 2014); however, in this study, a nonsignificant increase in growth was observed at a high N concentration (4.4 mg L−1), especially under salinity 35. Previous papers have shown that a relatively higher N concentration could stimulate maturity and induce the formation of reproductive cells in U. rigida, thus leading to a decline in growth (Gao et al. 2017, 2018). In addition, because of life stage-specific sensitivity differences, compared to adults, seedlings were more sensitive to changes in environmental factors (Cui et al. 2015). Moreover, the toxic effects of NH4− and NO2–N may be another reason. A high NH4–N concentration (> 0.5 mg L−1) reduced net primary production in Enteromorpha compressa adults (Kautsky 1982) and depressed the growth of Cladophora vagabunda and Gracilaria tikvahiae (Peckol and Rivers 1995). Although growth decreased at the highest N level, the pigment content increased significantly under all the three salinity conditions; in turn, the photosynthetic rate increased under high light levels in this study, which was also observed in previous publications (Gordillo et al. 2003; Zou and Gao 2014; Chen et al. 2016). However, this enhancement in photosynthesis was more significant at salinity 15 than at salinity 35, indicating that the synthesis or degradation of osmolytes under the high salinity condition consumed more energy (Angell et al. 2015).
In conclusion, the growth of U. prolifera decreased due to high N levels, especially under high salinity conditions; however, high N levels induced increases in photosynthesis, and no significant decreases in growth were observed. These results indicate that low salinity and high N levels may be nonnegligible reasons why this species thrives, and low salinity was the better choice when this species was used for wastewater treatment. Additionally, the nitrate concentrations in Chinese coastal waters were greater than those in other coastal regions of the world. Considering the complexity of life stage-specific responses to environmental factors as well as the annual periodicity and regularity of green tides, we speculate that the large-scale growth of U. prolifera is related to annual rainfall and industrial and domestic wastewater discharge. Therefore, information regarding nutrient sources as well as changes in salinity is needed to understand the basis of macroalgal blooms.
References
Angell AR, Mata L, de Nys R, Paul NA (2015) Indirect and direct effects of salinity on the quantity and quality of total amino acids in Ulva ohnol (Chlorophyta). J Phycol 51:536–545
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brito LO, Arantes R, Magnotti C, Derner R, Pchara F, Olivera A, Vinatea L (2014) Water quality and growth of Pacific white shrimp Litopenaeus vannamei (Boone) in co-culture with green seaweed Ulva lactuca (Linaeus) in intensive system. Aquacult Int 22:497–508
Chen BB, Zou DH, Ma JH (2016) Interactive effects of elevated CO2 and nitrogen-phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 28:1235–1243
Choi TS, Kang EJ, Kim JH, Kim KY (2010) Effect of salinity on growth and nutrient uptake of Ulva pertusa (Chlorophyta) from an eelgrass bed. Algae 25:17–26
Cui JJ, Zhang JH, Huo YZ, Zhou LJ, Wu Q, Chen LP, Yu KF, He PM (2015) Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar Pollut Bull 101:660–666
Farias DR, Hurd CL, Eriksen RS, Simioni C, Schmidt E, Bouzon ZL, Macleod CK (2017) In situ assessment of Ulva australis as a monitoring and management tool for metal pollution. J Appl Phycol 29:2489–2502
Gao G, Clare AS, Rose C, Caldwell GS (2017) Intrinsic and extrinsic control of reproduction in the green tide-forming alga, Ulva rigida. Environ Exp Bot 139:14–22
Gao G, Clare AS, Rose C, Caldwell GS (2018) Ulva rigida in the future ocean: potential for carbon capture, bioremediation, and biomethane production. GCB Bioenergy 10:39–51
Gao G, Zhong ZH, Zhou XH, Xu JT (2016) Changes in morphological plasticity of Ulva prolifera under different environmental conditions: a laboratory experiment. Harmful Algae 59:51–58
Ge HX, Ni Q, Li J, Li JT, Chen Z, Zhao FZ (2018) Integration of white shrimp (Litopenaeus vannamei) and green seaweed (Ulva prolifera) in minimum-water exchange aquaculture system. J Appl Phycol 31:1425–1432. https://doi.org/10.1007/s10811-018-1601-4
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gordillo FJ, Figueroa FL, Niell FX (2003) Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218:315–322
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) gran. Can j microbiol 8:229–239
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Jasby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jiang H, Zou DH, Lou WY, Ye CP (2017) Effects of stocking density and decreased carbon supply on the growth and photosynthesis in the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 29:3057–3065
Jiménez del Rio M, Ramazanov Z, García Reina G (1995) Effects of nitrogen supply on photosynthesis and carbonic anhydrase activity in the green seaweed Ulva rigida (Chlorophyta). Mar Biol 123:687–691
Kakinuma M, Coury DA, Kuno Y, Itoh S, Kozawa Y, Inagaki E, Yoshiura Y, Amano H (2006) Physiological and biochemical responses to thermal and salinity stresses in a sterile mutant of Ulva pertusa (Ulvales, Chlorophyta). Mar Biol 149:97–106
Kautsky L (1982) Primary production and uptake kinetics of ammonium and phosphate by Enteromorpha compressa in an ammonium sulphate industry outlet area. Aquat Bot 12:23–40
Kessing JK, Liu DY, Fearns P, Garcia R (2011) Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar Pollut Bull 62:1169–1182
Larsen A, Sand-Jensen K (2006) Salt tolerance and distribution of estuarine benthic macroalgae in the Kattegat-Baltic Sea area. Phycologia 45:13–23
Li YH, Wang D, Xu XT, Gao XX, Sun X, Xu NJ (2017) Physiological response of a green algae (Ulva prolifera) exposure to simulated acid rain and decreased salinity. Photosynthetica 55:623–629
Li YH, Zhong JL, Zheng MS, Zhuo PL, Xu NJ (2018) Photoperiod mediates the effects of elevated CO2 on the growth and physiological performance in the green tide alga Ulva prolifera. Mar Environ Res 141:24–29
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:59–592
Lin AP, Wang C, Pan GH, Song LY, Gao S, Xie XJ, Wang ZY, Niu JF, Wang GC (2011) Diluted seawater promoted the green tide of Ulva prolifera (Chlorophyta, Ulvales). Phycol Res 59:295–304
Loewus FA (1952) Improvement in anthrone method for determination of carbobydrates. Anal Chem 24:219
Lubsch A, Timmermans K (2018) Uptake kinetics and storage capacity of dissolved inorganic phosphorus and corresponding N: P dynamics in Ulva lactuca (Chlorophyta). J Phycol 54:215–223
Luo MB, Liu F (2011) Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. J Exp Mar Biol Ecol 409:223–228
Luo MB, Liu F, Xu ZL (2012) Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquatic Botany 100:18–24
Peckol P, Rivers JS (1995) Physiological responses of the opportunistic macroalgae Cladophora vagabunda (L.) van den Hoek and Gracilaria tikvahiae (McLachlan) to environmental disturbances associated with eutrophication. J Exp Mar Biol Ecol 190:1–16
Reidenbach LB, Fernandez PA, Leal PP, Noisette F, McGraw CM, Revill AT, Hurd CL, Kübler JE (2017) Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS ONE 12:e0188389
Ross ME, Davis K, McColl R, Stanley MS, Day JG, Semião AJC (2018) Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: influence on growth, nitrogen preference and biochemical composition. Algal Research 30:1–10
Rybak AS (2018) Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol Indic 85:253–261
Shi XY, Qi MY, Tang HJ, Han XR (2015) Spatial and temperature nutrient variations in the Yellow Sea and their effects on Ulva prolifera blooms. Estuarine Coastal Shelf Sci 63:36–43
Teichberg M, Fox SE, Olsen YS, Valiela I, Martinetto P, Iribarne O, Muto EY, Petti MAV, Corbisier T, Soto-Jiménez M, Páez-Osuna F, Castro P, Freitas H, Zitelli A, Cardinaletti M, Tagliapietra D (2010) Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Global Change Biol 16:2624–2637
Valiela I, Liu DY, Lloret J, Chenoweth K, Hanacek D (2018) Stable isotopic evidence of nitrogen sources and C4 metabolism driving the world’s largest macroalgal green tides in the Yellow Sea. Sci Rep 8:17437
Van Alstyne KL (2018) Seawater nitrogen concentration and light independently alter performance, growth, and resource allocation in the bloom-forming seaweeds Ulva lactuca and Ulvaria obscura (Chlorophyta). Harmful Algae 78:27–35
Xu ZG, Wu HY, Zhan DM, Sun FX, Sun JZ, Wang GC (2014) Combined effects of light intensity and NH4+-enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin J Oceanol Limnol 2:1016–1023
Zhu M, Liu ZP, Shao HB, Jin Y (2016) Effects of nitrogen and phosphate enrichment on the activity of nitrate reductase of Ulva prolifera in coastal zone. Acta Physiol Plant 38:169–161 6
Zou DH, Gao KS (2014) The photosynthetic and respiratory responses to temperature and nitrogen supply in the marine green macroalga Ulva conglobata (Chlorophyta). Phycologia 53:86–94
Funding
This study was supported by the National Natural Science Foundation (41606129 to YL; 41876181 to NX), the Natural Science Foundation of Zhejiang Province (LY19D060002 to YL; LZ17D060001 to NX), and the K.C. Wong Magna Fund at Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, M., Lin, J., Zhou, S. et al. Salinity mediates the effects of nitrogen enrichment on the growth, photosynthesis, and biochemical composition of Ulva prolifera. Environ Sci Pollut Res 26, 19982–19990 (2019). https://doi.org/10.1007/s11356-019-05364-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05364-y