Abstract
The ongoing increases in atmospheric CO2 concentrations and coastal eutrophication have affected the coastal environment and marine macroalgae. In this study, the interactive effects of CO2 (390 and 1000 μL L−1) and nutrient levels (nitrogen and phosphorus supplied simultaneously) on the physiological properties of the maricultured macroalga Pyropia haitanensis (Bangiales, Rhodophyta) were investigated. The results showed that elevated CO2 significantly enhanced P. haitanensis growth and NO3 − uptake, but lowered the pH compensation points, regardless of nutrient levels, and enhanced photosynthesis and apparent photosynthetic efficiency (α) when the nutrient levels were high. However, CO2 had little effect on photosynthetic rates at low nutrient levels. At each nutrient level, CO2 elevation lowered both the phycobiliproteins (PB) and soluble protein contents, but enhanced biomass accumulation. Chlorophyll a (Chl a) and carotenoid (Car) contents were markedly increased by high CO2 concentrations at low nutrient levels. Increasing nutrient supply significantly enhanced growth, pH compensation points, and photosynthesis in P. haitanensis at each CO2 level, but the differences of the effects between intermediate and high nutrient levels on this alga were not significant for photosynthesis, pigment content, and nutrient uptake, regardless of CO2 levels. Our results suggest that the growth and physiological responses of P. haitanensis to CO2 levels are largely dependent on the supplement of nutrients. However, the interactive effects of elevated CO2 and nitrogen–phosphorus supplies on the physiological properties of P. haitanensis were limited through respective regulation by the CO2 levels in the atmosphere and the nutrient concentrations in the seawater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is predicted that the atmospheric CO2 concentration will continue to rise throughout this century (IPCC 2007), and great attention has been paid to how global climate change may affect the global environment. The current CO2 concentration is reaching the highest it has been for the last 800,000 years (Lüthi et al. 2008). Increased CO2 levels in the atmosphere not only cause global warming (Florides and Christodoulides 2009) but also cause a decrease in the marine surface pH because more atmospheric CO2 is dissolved in the ocean (Caldeira and Wickett 2003).
Increasing atmospheric CO2, together with seawater acidification, has the potential to greatly affect marine organisms (for a review, see Fabry et al. 2008). Marine macroalgae have a considerable biomass production and CO2 bioremediation potential because the ocean is one of the largest carbon sinks of atmospheric CO2 on the earth (Gao and McKinley 1994; Zhang et al. 2005). Furthermore, rising CO2 levels have been shown to affect photosynthesis and other physiological processes in marine macroalgae (Zou and Gao 2002a; 2010; Wu et al. 2008).
Coastal eutrophication, caused by human activity, economic development, and overuse of the coastal environment, has become more and more serious (Fei 2004; Neori et al. 2004). Nutrients, such as nitrogen (N) and phosphorus (P), directly influence the biochemical composition of macroalgae, such as nitrogenous compounds such as Rubisco, a key enzyme in photosynthesis, which affects the storage of organic compounds (Lohman and Priscu 1992).
It is thought that the large scale cultivation of macroalgae in marine areas that are affected by eutrophication may act as a nutrient buffer and help lower N–P contamination risks, such as industrial and agricultural waste water, and/or the economic production of aquatic animals (Fei 2004; Troell et al. 2003). However, excessive nutrients from terrestrial sources cause anthropogenic eutrophication, which can result in the explosive proliferation of marine algae (Lin and Lin 2000; Yabe et al.2009). Moreover, under suitable light and temperature conditions, excessive nutrient levels may stimulate the coastal production of harmful algae and lead to expansion in toxic phytoplankton blooms (Paerl 1997).
Pyropia haitanensis (T.J. Chang and B.F. Zheng) N. Kikuchi and M. Myata (Bangiales, Rhodophyta) is a Chinese nori species that is artificially bred to be grown on a large scale in the South China Sea area (Tseng 1983). This species naturally grows in the mid intertidal zone and is greatly affected by fluctuating environmental condition, such as light, temperature, pH, and nutrient levels.
Previous research has shown that elevated CO2 levels can enhance photosynthesis of emersed P. haitanensis (Zou and Gao 2002b). When the CO2 concentration was between 60 and 1440 μL L−1, increased CO2 levels enhanced both intracellular and extracellular carbonic anhydrase (CA) (Zou and Gao 2004), by which the HCO3 − is dehydrated to CO2 which is then transported into algal cells (Zou et al. 2004). This suggested that CO2 transport had increased, consequently leading to a rise in CO2 assimilation by P. haitanensis (Zou and Gao 2004). Moreover, the nitrate reductase (NR) in P. haitanensis is positively regulated by seawater NO3 − concentration and negatively regulated by seawater NH4+ (Xu et al. 2007). However, previous laboratory studies have shown that NR levels were not directly positively correlated with NO3 − concentration in the culture medium (Xu et al. 2007). If the predicted rises in CO2 and coastal eutrophication occur, then the physiological properties of P. haitanensis growing at elevated CO2 and enriched nitrogen–phosphorus levels need to be investigated.
In this study, we cultured P. haitanensis in the laboratory at different levels of CO2 and at different nutrient concentrations. During the experiments, CO2 concentration was controlled at either 390 or 1000 μL L−1, with low, medium, and high nitrogen–phosphorus supplies. The aim was to investigate how the elevated CO2 and different nutrient concentration combinations influenced P. haitanensis and whether or not elevated CO2 levels and N–P supply would have any long-term interactive effects of on this alga.
Materials and methods
Samples of P. haitanensis were collected from the mid intertidal rocky shore along the coast of Nan’ao Island, Shantou, China (23° 20′ N, 116° 55′ E). Collected algae were cleaned on-site and brought to laboratory under low temperature conditions. Healthy individuals were selected and rinsed again in sterile seawater. Then, they were pre-cultured in incubators in 5 L aquaria in filtered seawater (salinity 32 ‰) at a density of 0.5 g fresh weight per liter. The cultured thalli were continuously aerated using ambient air at a rate of about 9 L h−1 (i.e., a renewal rate of more than 90 % per hour) under 100 μmol photons m−2 s−1 illumination supplied by fluorescent tubes with a 12:12 (light/dark) photoperiod. Temperature was controlled at 18 °C.
The environmental CO2 concentrations were respectively controlled at 390 μL L−1 (ambient air, AC) and 1000 μL L−1 (elevated CO2, EC) in two CO2 incubators, in which the CO2 concentrations were automatically adjusted by controlling the inflow of ambient air and pure CO2 gas, and each CO2 incubator had three levels of nutrient gradients (NaNO3 and NaH2PO4 supplied simultaneously): (i) low nutrient supplied (LN): three flasks contained filtered natural seawater (NO3 − concentration ≤ 40 μmol L−1, PO4 3− concentration <1 μmol L−1) with no additional N and P; (ii) intermediate N–P supply (IN): three flasks contained filtered natural seawater with additional nitrogen (300 μmol L−1 NO3 −) and phosphorus (15 μmol L−1 H2PO4 −); (iii) high nutrient supply (HN): three flasks contained filtered natural seawater with additional nitrogen (600 μmol L−1 NO3 −) and phosphorus (30 μmol L−1 H2PO4 −). Final concentrations of 20 mmol L−1 Tris and 20 mmol L−1 maleate solution buffers were used in seawater culture (Sober 1974), and the pH in every tank was adjusted with 1 mol L−1 HCl or 1 mol L−1 NaOH to maintain constant pH on the total scale (AC: pHnbs = 8.17; EC: pHnbs = 7.78). The measured concentrations of dissolved inorganic carbon (DIC), estimated using the CO2SYS program (Lewis and Wallace 1998), were 2032.1 (±30.6) and 2208.5 (±38.3) μmol L−1 in the seawater aerated with ambient air and elevated CO2, respectively. The culture medium was renewed every second day. The photosynthetic traits and biochemical criteria of each sample were determined after 10 days of culture, a period which could be enough for acclimation in marine macroalgae (Zou 2005; Zou et al. 2003; Mercado et al. 1999).
Growth rate estimation
Changes in algal biomass (Fw) were measured at the end of culture to estimate growth. Mean relative growth rate (RGR) was calculated using the formula: RGR (% day−1) = ln (W t /W 0 )/t × 100, where W 0 is the initial Fw and W t is the Fw after t days.
Photosynthetic oxygen evolution
Approximately 0.06 g (Fw) of algae was utilized to obtain the net photosynthetic O2 evolution rate (P n ) at different photon irradiances (P vs. E curve) using a Clark-type oxygen electrode (YSI Model5300, USA), with a water jacket connected to a cooling circulator for maintaining the temperature at 18 °C. Light (provided by a halogen lamp) intensity, which was measured with a spherical quantum sensor (SKP 200, ELE International, UK), was set at six levels from 0 to 600 μmol photos m−2 s−1 by altering the distance between the electrode chamber and the light source. The dark respiration rate (R d ) was measured by determining the dark O2 consumption. The electrode chamber contained 8 mL culture solution for each growth treatment and was magnetically stirred. The samples of algae were allowed to acclimate to the electrode cuvette environment for 10 min before measurement. Photosynthetic rates are all expressed in μmol O2 g−1Fw h−1. Photosynthetic parameters were calculated according to Henley (1993). The gross photosynthetic rate (P g ) was the sum of the maximum net photosynthetic O2 evolution rate (P m ) and the respiration rate. Each treatment had three replications.
pH drift experiment
The pH drift experiment of P. haitanensis was carried out according to Zou and Gao (2009) in order to estimate the pH compensation point of the alga grown under each treatment, indicating the ability to use HCO3 −. Exactly 0.15 g (Fw) of alga sample was transferred into sealed glass bottle containing 20 mL natural seawater that was air-filled and stored about 1 h at 18 °C before use. The bottles were incubated at 18 °C with 100 μmol photons m−2 s−1 illumination, and pH values were determined at regular intervals until the value remained constant.
Pigment estimation
Approximately 0.1 g (Fw) of alga was ground in 10 mL 100 % methanol and extracted at 4 °C in darkness for 24 h. This extract was centrifuged at 5000 rpm for 10 min and then used to determine the contents of chlorophyll a (Chl a) and carotenoid (Car) using an ultraviolet spectrophotometer (UV-1800, Shimadzu, Japan). Chl a and Car concentrations were estimated according to Porra (2002) and Parsons and Strickland (1963).
The phycobiliprotein (PB), phycoerythrin (PE), and phycocyanin (PC) contents were estimated by the equation proposed by Beer and Eshel (1985). The algal thallus was washed with deionized water and blotted dry. A sample of 0.2 g fresh weight (Fw) was homogenated in an ice bath with 5 mL phosphate buffer (0.1 mol L−1, pH = 6.8). The crude extract was centrifuged at 10,000 rpm for 20 min at 4 °C. The supernatant was transferred into centrifuge tube and the absorbances measured.
Soluble protein determination
Content of soluble protein was determined by the Coomassie Brilliant Blue G-250 dye method according to Kochert (1978). Fw of 0.1 g was homogenized in mortar with 5 mL distilled water. The extract was centrifuged at 5000 rpm for 10 min before analysis. Bovine serum albumin (BSA) was used as standard.
NO3 − uptake estimation
Rates of NO3 − uptake were estimated by determining the decrease in NO3 − in 24 h in culture medium. NO3 − concentrations were determined according to the method of Strickland and Parsons (1972). The NO3 − uptake rate was calculated by the following equation: ΔNO3 − = (N 0 − N t ) × V/(W 0 × t), where N 0 is the initial nitrate concentration, N t is the nitrate concentration after t hours, V is the volume of the culture medium, and W 0 is the initial Fw of the alga. The NO3 − uptake rate is expressed in μmol NO3 − g−1 h−1.
Statistical analyses
Origin 8.0 (Origin Lab Corp, USA) was used for data processing and statistical analysis. One-way (ANOVA) and two-way analysis of variance (ANOV2A) and the Tukey test were used to analyze differences among treatments. All the data are expressed as means ± SD (n ≥ 3). A p value of 0.05 was considered as statistically significant.
Results
Relative growth rate
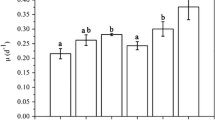
The RGR and biomass accumulation of P. haitanensis growing under different CO2 and nitrogen–phosphorus (N–P) supplied conditions are shown in Fig. 1. At each nutrient level, elevated CO2 significantly enhanced the algal growth and biomass accumulation (p < 0.01). Intermediate N–P supply significantly enhanced algal growth (AC: F 1,4 = 81.56, p < 0.01; EC: F 1,4 = 17.35, p = 0.14) and biomass accumulation (AC: F 1,4 = 76.48, p < 0.01; EC: F 1,4 = 18.85, p = 0.12) at both CO2 levels, but there was no significant influence on algal growth (AC: F 1,4 = 1.66, p = 0.27; EC: F 1,4 = 1.68, p = 0.26) and biomass accumulation (AC: F 1,4 = 1.68, p = 0.26; EC: F 1,4 = 1.69, p = 0.26) between the treatments of intermediate and high nutrient levels at the two CO2 levels.
Relative growth rate (RGR) (a) and biomass accumulation (b) of Pyropia haitanensis grown at different CO2 concentrations (CO2 level of ambient air, AC, ca. 390 μL L−1; elevated CO2, EC, 1, 500 μL L−1) and different nitrogen–phosphorus levels. Significant differences among the treatments are indicated by different lowercase letters (the Tukey test, p < 0.05). Values are means ± SD (n = 3)
Photosynthesis and respiration
The P n values in P. haitanensis at different irradiances (P–E curves) are shown in Fig. 2. Increased CO2 substantially increased P m under intermediate and high N–P supplies (p < 0.01), but hardly enhanced the P m under low nutrient treatment (F 1,4 = 3.92, p = 0.12) (Table 1). Increased N–P supply significantly increased the P m at each CO2 concentration (Table 2, p < 0.01), but there were no significant differences between the two increased nutrient levels at both CO2 levels (AC: F 1,4 = 0.64, p = 0.47; EC: F 1,4 = 1.87, p = 0.24). It is worth noting that the highest P m values were observed at elevated CO2 and intermediate supply treatments (F 5,12 = 46.18, p < 0.01).
Dark respiration (R d ) increased with increased nutrient levels at ambient air (IN: F 1,4 = 36.99, p < 0.01; HN: F 1,4 = 141.20, p < 0.01), and significant enhancements were also observed at elevated CO2 (IN: F 1,4 = 24.73, p < 0.01; HN: F 1,4 = 23.68, p < 0.01). It was noticed that R d under low N–P level at ambient CO2 had the lowest value among all the treatments (F 5,12 = 29.73, p < 0.01). The culture conditions of CO2 and nutrient levels significantly influenced the P m and R d in P. haitanensis (Table 2).
The apparent photosynthetic efficiency (α) values were significantly increased at intermediate and high N–P levels at both CO2 levels (p < 0.01), but no significant difference was observed in α values between intermediate and high nutrient levels, regardless of CO2 concentration (AC: F 1,4 = 0.33, p = 0.60; EC: F 1,4 = 1.09, p = 0.35). Elevated CO2 markedly increased α values regardless of nutrient level treatments (p < 0.01).
Increasing values of the compensation irradiance point (E c ) and the irradiance saturation point (E k ) were observed at ambient air with N–P supply (p < 0.01; Table 1). In the elevated CO2 treatment, E c (IN: F 1,4 = 35.10, p < 0.01; HN: F 1,4 = 18.09, p = 0.01) and E k (IN: F 1,4 = 191.42, p < 0.01; HN: F 1,4 = 48.53, p < 0.01) also increased with intermediate and high nutrient supply, whereas at elevated CO2 level, E c and E k values all decreased in algae cultured at the intermediate to high nutrient levels (E c : F 1,4 = 9.47, p = 0.04; E k : F 1,4 = 15.13, p = 0.02). The values of α, E c , and E k were all significantly influenced by CO2 and nutrient supply conditions (Table 2).
As shown in Fig. 3, under low nutrient level, the value of R d /P g at ambient air condition was remarkably higher than that at high CO2 level (F 1,4 = 24.53, p < 0.01); however, it was substantially lower than that at elevated CO2 under high nutrient supply (F 1,4 = 645.67, p < 0.01). The R d /P g values were markedly increased with increased nutrient supplies with ambient air (F 1,4 = 28.66, p < 0.01), but apparently declined at elevated CO2 condition (F 1,4 = 2.00, p = 0.22).
pH compensation point
The pH compensation points were obtained during 8 h pH drift period (Fig. 4). At each CO2 level, the pH compensation points under low N–P supply were all lower than at increased nutrient levels (AC: F 1,4 = 64.76, p < 0.01; EC: F 1,4 = 64.92, p < 0.01). Under each nutrient level, the pH compensation points at ambient air (Fig. 4a) were all higher than the values at elevated CO2 (Fig. 4b) (F 2,5 = 554.86, p < 0.01).
Pigment contents
At each CO2 level, increased nutrient supply significantly increased Chl a and Car contents in the algae (p < 0.01) (Table 3). The Chl a and Car contents were increased by elevated CO2 only under low nutrient level (Chl a: F 1,4 = 25.42, p < 0.01; Car: F 1,4 = 80.32, p < 0.01), while these contents were respectively increased by the additional nutrients (Chl a: p < 0.01; Car: p < 0.01).
Contents of PE in P. haitanensis significantly increased as the N–P supply increased at each CO2 level (p < 0.01), and the PC contents also increased with intermediate nutrient supply at both CO2 levels (p < 0.01) (Tables 3 and 4), while elevated CO2 apparently lowered the PE and PC contents under each nutrient level, although the declines were not significant at intermediate nutrient supply (PE: F 1,4 = 5.91, p = 0.07; PC: F 1,4 = 5.78, p = 0.07). The culture conditions of CO2 and nutrient levels exhibited significant influences on the pigment contents in P. haitanensis (Table 4).
NO3 − uptake and protein contents
As shown in Fig 5, the intermediate and high nutrient supplies significantly increased the ΔNO3 − and the SP contents, regardless of CO2 level (p < 0.01). At each nutrient level, elevated CO2 markedly increased the ΔNO3 − (p < 0.01), with a significant decline in the SP contents in the alga (p < 0.01). At each CO2 level, an increase in nutrient supply from intermediate to high levels hardly influenced the ΔNO3 − and the SP contents (p > 0.05). The values of ΔNO3 − in this alga exhibited a low correlation with SP contents (Fig. 5c).
Rates of NO3 − uptake (ΔNO3 −) (a) and soluble protein (SP) contents (b) and the relationships between log ΔNO3 − and log SP (c) in Pyropia haitanensis grown at different CO2 levels (AC ambient air, EC elevated CO2 level) and different nitrogen–phosphorus concentrations. Significant differences among the treatments are indicated by different lowercase letters (the Tukey test, p < 0.05). Linear regressions in (c) were used to test the correlation between the ΔNO3 − and SP (p < 0.05). Values are means ± SD for triplicate samples
Discussion
This study showed that P. haitanensis growth was significantly enhanced by elevated CO2, and this increase was not related to nutrient supply. Similar findings have been reported for Pyropia yezoensis (Gao et al. 1991) and for Hizikia fusiforme (Zou 2005). Moreover, at each CO2 concentration, increasing the nutrient supply also enhanced the growth and biomass accumulation by the alga. Elevated CO2 also lowered the P. haitanensis pH compensation points, regardless of nutrient levels, which indicated that there had been a reduction in activities of the CO2 concentrating mechanisms (CCMs). The growth response of the alga may partly depend on the presence of CCMs, which may have substantial energetic and metabolic costs (Israel and Hophy 2002), but may eventually contribute to algal biomass accumulation.
Under the low N–P supply treatment, the P. haitanensis photosynthetic rates were not markedly affected by CO2 levels. In contrast, under the low N–P supply treatment, the P. haitanensis dark respiration, photosynthetic efficiency, and the compensation irradiance significantly increased when CO2 levels were high, but the algal irradiance saturation point was decreased by CO2 elevation. The Chl a and Car contents in the alga also rose.
When nutrient supplies were increased, photosynthesis and the photosynthetic efficiency in P. haitanensis were markedly enhanced by elevated CO2. The Chl a and Car contents in the algae at the intermediate and high nutrient levels were also increased by elevated CO2. It has been reported that N enrichment could enhance photosynthesis rate and photosynthetic efficiency in algae (Dawes and Koch 1990; Crawford 1995; Chen et al. 2011). High nutrient supplies increased the contents of photosynthetic pigments and nitrogenous compounds, such as Rubisco, a key enzyme in photosynthesis, and finally enhanced photosynthesis. However, the PB contents, including PE and PC, declined under elevated CO2 and low nutrient conditions, but increased when additional nutrients were supplied. It has also been reported that PE and PC contents in Pyropia leucosticta (Mercado et al. 1999) and in Gracilaria lemaneiformis (Zou and Gao 2009) cultured at high levels of inorganic carbon (Ci) also decreased.
The DIC in seawater increased under elevated CO2 conditions, and the increased CO2 levels enhanced both the intracellular and extracellular CA in P. haitanensis (Zou and Gao 2004). Thus, more nutrients and increased light were required to meet the demands of the enhanced photosynthesis activity caused by CO2 elevation. Under the low nutrient treatment, P. haitanensis photosynthesis was depressed by the lack of N and P. Nitrogen plays an important role in photosynthesis and is an important component of many plant compounds, such as Rubisco, the key enzyme in photosynthesis (Dawes and Koch 1990; Crawford 1995), and phosphorus is required for various chloroplast functions, including ATP generation and photosynthetic protein and enzyme phosphorylation (Zer and Ohad 2003). Under the low nutrient level treatment, newly synthesized carbohydrates could not be used in protein synthesis and organic structure development due to the lack of N and P. This led to excessive carbohydrate accumulation, which probably depressed the relative expressions of photosynthetic enzyme genes that normally upregulated for the photosynthate production. Moreover, PB is used as an N reservoir in case N was needed for algal growth (Kursar and Alberte 1983; Zou and Gao 2009). This probably resulted in an increased allocation of energy to nutrient uptake and assimilation processes and ultimately affected dark respiration.
When nutrients levels were high, increased CO2 levels enhanced photosynthesis and consequently led to a rise in photosynthetic pigment contents, which indicated more carbohydrates were being synthesized. Nitrogen enrichment can stimulate pigment synthesis by promoting nitrogen metabolism (Fujii et al. 2012), which competes for energy and electrons sinks with photosynthesis (Falkowski and Raven 2007). When N and P levels were high, the photosynthate in P. haitanensis at elevated CO2 concentrations was functionally utilized for algal growth and protein synthesis, and when combined with sufficient Rubisco and phosphorylation intermediates, this led to photosynthesis enhancement in P. haitanensis.
It was noted that, under low nutrient level, the value of R d /P g at ambient air was remarkably higher than that at high CO2 level. This result was inconsistent with the results of Zou et al. (2011), who found that the R d /P g in Hizikia fusiformis was not significantly influenced by CO2 elevation. Under low nutrient level, CO2 enrichment slightly enhanced the photosynthesis of P. haitanensis, and this stimulated the nutrient demands for algal growth. Thus, more energy was probably needed for nutrient uptake and active transport or utilization of the N reservoir (such as PB) (Kursar and Alberte 1983). Thus, dark respiration was enhanced by CO2 elevation. However, the value of R d /P g at ambient air was significantly lower than that at elevated CO2 under high nutrient supply, which was consistent with Zou et al. (2011). Under high nutrient level, a high CO2 level decreases photorespiration in algae (Stitt and Krapp 1999; Zou et al. 2011), and CO2 enrichment enhanced the nutrient uptake and assimilation, which would permit decreased investment of nitrogen in the nitrogen-intensive process of photosynthesis and photorespiration (Zou 2005).
Nitrogen metabolism in P. haitanensis was highly correlated with the NO3 − concentration in the culture medium. At each CO2 concentration, increased nutrient levels markedly enhanced NO3 − uptake and soluble protein synthesis. Increased nutrient uptake at high nutrient level has also been reported in some other species of Pyropia (Pedersen et al. 2004) and in Gracilaria lemaneiformis (Xu et al. 2008). At each nutrient level, CO2 elevation increased NO3 − uptake, especially at the intermediate and high nutrient levels. It is possible that CO2 enrichment of the culture medium increased both NO3 − uptake and NR activity (Mercado et al. 1999; Zou 2005). However, the soluble protein contents in the algae fell when the CO2 concentration was high. This is consistent with the results for some Gracilaria species (Andria et al. 1999; Xu et al. 2008). In this study, NO3 − uptake by P. haitanensis had a low correlation with soluble protein contents. It could be that the enhanced algal growth caused by high CO2 levels accelerated the assimilation and fixation of inorganic nitrogen or the NR activity was not directly related to NO3 −–N concentration in the culture, although NO3 − enhanced NR activity in P. haitanensis (Xu et al. 2007).
This research showed that increased nutrient supply greatly influenced the physiological properties of P. haitanensis, especially in combination with elevated CO2. The interactive effect of elevated CO2 and nutrient supply has also been shown in G. lemaneiformis (Xu et al. 2008). It also has been shown that the photosynthesis and growth of Gracilaria gaditana (Andria et al. 1999) were enhanced only under sufficient N nutrient condition. However, there must be an upper limit to the positive growth improvements brought about by increasing nutrient concentrations. The positive effects included rises in photosynthesis, pigment content, and nitrogen metabolism, but the differences in these improvements between the intermediate and high nutrient levels were not great. Moreover, excessive nutrient levels may result in the expansion of harmful blooms (Paerl 1997; Lin and Lin 2000; Yabe et al. 2009). Thus, a controlled fertilization strategy should be used during P. haitanensis mariculture. Additionally, as considered by Schanz and Juon (1983), N is the limiting factor for algal growth at NO3 −–N/PO4 3−–P ratios less than 10, while P is limiting at ratios greater than 20. In this study, the N/P ratios in three nutrient treatments were all greater than 20:1. It is worth to further investigate if P was a limited factor for P. haitanensis growth and photosynthesis.
In summary, the growth and physiological properties of P. haitanensis to elevated CO2 levels were considerably affected by the nutrient concentrations in the seawater. When nutrient levels were insufficient, increasing the CO2 concentration hardly had any effect on biomass accumulation and photosynthetic rate, whereas when nutrients were enriched, enhanced CO2 levels significantly enhanced P. haitanensis photosynthesis and growth. However, the interactive effect of elevated CO2 and nitrogen–phosphorus supplies on the physiological properties of P. haitanensis was limited because the processes were regulated by the CO2 levels in the atmosphere and the nutrient concentrations in the seawater. Further studies need to explore whether P. haitanensis growth and photosynthesis would be further enhanced by the high nutrient level treatment combined with even higher CO2 levels compared to the increase seen under the low and intermediate nutrient level treatments and the two CO2 concentrations used in this study.
References
Andria JR, Vergara JJ, Perez-Llorens JL (1999) Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cádiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur J Phycol 34:497–504
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshwat Res 36:785–792
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Chen L, Zou D, Liu Z, Zhao G, Li F, Zheng Q (2011) Effects of different level of nitrogen and phosphorus on growth and physiological biochemical characteristics of Gracilaria lemaneiformis. Mar Environ Sci 30(2):211–215 (In Chinese with English abstract)
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7:859–868
Dawes CJ, Koch W (1990) Physiological responses of the red algae Gracilaria verrucosa and G. tikvahiae before and after nutrient enrichment. Bull Mar Sci 46:335–344
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Falkowski PG, Raven JA (2007) Aquatic photosynthesis. Princeton University Press, Princeton, p 484
Fei X (2004) Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 512:145–151
Fujii R, Kita M, Doe M, Iinuma Y, Oka N, Takaesu Y, Taira T, Iha M, Mizoguchi T, Cogdell RJ, Hashimoto H (2012) The pigment stoichiometry in a chlorophyll a/c photosynthetic antenna. Photosynth Res 111:165–172
Florides GA, Christodoulides P (2009) Global warming and carbon dioxide through sciences. Environ Int 35:390–401
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1991) Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J Appl Phycol 3:356–362
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
IPCC (Intergovernmental Panel on Climate Change (2007) Climate Change 2007 Synthesis Report. Cambridge University Press, New York
Israel A, Hophy M (2002) Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentration. Global Chang Biol 8:831–840
Kochert G (1978) Protein determination by dye binding. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 92–93
Kursar TA, Alberte RS (1983) Photosynthetic unit organization in a red alga: relationships between light-harvesting pigments and reaction centres. Plant Physiol 72:409–414
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tenessee
Lin Y, Lin RC (2000) Research on red tide occurrences using enclosed experimental ecosystem in West Xiamen Harbor, China—relationship between nutrients and red tide occurrence. Chin J Oceanol Limnol 18:253–259
Lohman K, Priscu JC (1992) Physiological indicators of nutrient deficiency in Cladophora (Chorophyta) in the Clark Fork of the Columbia River, Montana. J Phycol 28:443–448
Lüthi D, Floch ML, Bereiter B, Blunier T, Barnola JM, Siegenthaler U, Raynaud D, Jouzel J, Fischer H, Kawamura K, Stocher TF (2008) High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature 453:379–82
Mercado JM, Javier F, Gordillo L, Niell X, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticte. J Appl Phycol 11:455–461
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391
Paerl HW (1997) Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol Oceanogr 42:1154–1165
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. J Mar Res 21:155–163
Pedersen A, Kraemer G, Yarish C (2004) The effects of temperature and nutrient concentrations on nitrate and phosphate uptake in different species of Porphyra from Long Island Sound (USA). J Exp Mar Biol Ecol 312:235–252
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Schanz F, Juon H (1983) Two different methods of evaluating nutrient limitation of periphyton biomassays using water from the River Rhine and eight of its tributaries. Hydrobiologia 102:187–195
Sober HA (1974) Handbook of biochemistry, Selected Data for Molecular Biology, 2nd ed., p:J-234
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrient: the physiological and molecular background. Plant Cell Environ 22:583–621
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa, pp 71–79
Tseng CK (1983) Common seaweeds of China. Science Press, Beijing
Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yarish C (2003) Integrated mariculture: asking the right questions. Aquaculture 226:69–90
Wu H, Zou D, Gao K (2008) Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. Sci China C Life Sci 51:1144–1150
Xu Z, Zou D, Zhang X, Liu S, Gao K (2007) Effect of light and different forms of nitrogen on the activity of nitrate reductase of Porphyra haitanensis (Rhodophyta). J Fish China 31(1):90–96 (In Chinese with English abstract)
Xu Z, Zou D, Zhang X et al (2008) Effects of increased atmospheric CO2 and N supply on growth, biochemical compositions and uptake of nutrients in Gracilaria lemaneiformis (Rhodophyta). Acta Ecol Sin 28:3752–3759 (In Chinese with English abstract)
Yabe T, Ishii Y, Amano Y, Koga T, Hayashi S, Nohara S, Tatsumoto H (2009) Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 10:239–245
Zer H, Ohad I (2003) Light, redox state, thylakoid-protein phosphorylation and signaling gene expression. Trends Biochem Sci 28:467–470
Zhang J, Fang J, Tang Q (2005) The contribution of shellfish and seaweed mariculture in China to the carbon cycle of coastal ecosystem. Adv Earth Sci 20(3):359–365 (In Chinese with English abstract)
Zou D (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou D, Gao K (2002a) Effects of elevated CO2 concentration on the photosynthesis and related physiological processes in marine macroalgae. Acta Ecol Sin 22(10):1750–1757 (In Chinese with English abstract)
Zou D, Gao K (2002b) Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. Eur J Phycol 37:587–592
Zou D, Gao K, Xia J (2003) Photosynthetic utilization of inorganic carbon in the economic brown alga, Hizikia fusiforme (Sargassaceae) from the South China Sea. J Phycol 36:1095–1100
Zou D, Gao K (2004) Exogenous carbon acquisition of photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta) under emersed state. Prog Nat Sci 14:138–144
Zou D, Xia J, Yang Y (2004) Photosynthetic use of exogenous inorganic carbon in the agarophyte Gracilaria lemaneiformis (Rhodophyta). Aquaculture 237:421–431
Zou D, Gao K (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) growing at different irradiance levels. Phycologia 48:510–517
Zou D, Gao K (2010) Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. Cell Orig Life Extrem Habitats Astrobiology 15:115–126
Zou D, Gao K, Luo H (2011) Short- and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia fusiformis (Sargassaceae, Phaeophyta) grown at low and high N supplies. J Phycol 47:87–97
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41276148 and 41076094).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Zou, D. & Ma, J. Interactive effects of elevated CO2 and nitrogen–phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 28, 1235–1243 (2016). https://doi.org/10.1007/s10811-015-0628-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0628-z