Abstract
Deltamethrine (DLM) is a synthetic pyrethroid with broad spectrum activities against acaricides and insects. Widely used for agricultural and veterinary purposes, its human and animal exposure occurs by ingestion of contaminated water and food and leads to serious health problems. Kefir is fermented milk with numerous health favors counting restorative properties of bacterial flora, immune system stimulation, cholesterol reduction, as well as anti-mutagenic and anti-tumor properties. The present study was undertaken to examine the hepatoprotective and antioxidant potential of kefir against DLM toxicity in male Wistar albino rats. DLM-treated animals revealed a significant increase in serum biochemical parameters as well as hepatic protein and lipid oxidations but caused an inhibition in antioxidant enzymes. Additionally, we have observed an increase in hepatocyte DNA damages. This toxic effect was confirmed by histological study. Kefir administration normalized the elevated serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T bilirubin), and cholesterol. It also reduced DLM-induced protein carbonyl (PC) and malondialdehyde (MDA) formations. Furthermore, Kefir treatment restored catalase (CAT) and superoxide dismutase (SOD) activities. The co-treatment as well as the pre-treatment by kefir showed an improvement of oxidative status as well as suppressed inflammation and DNA damages. However, the pre-treatment seems to be the most efficient. Therefore, it could be concluded that kefir is a natural product able to protect against the hepatotoxic effects of DLM by its free radical-scavenging and potent antioxidant activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
What and how pesticides are used represent the most researched topics in the agro fields. Knowing that pesticides can significantly increase crop productivity, they are widely used to protect vegetables and fruits against pests. They are also used as an ectoparasiticide in farm animals and even used in residential homes against garden insects. Due to their low environmental persistence and toxicity, pyrethroids have replaced organophosphorus insecticides (Pérez et al. 2010). Pyrethroids are pyrethrin-synthetic derivatives, natural toxic substances obtained from Chrysanthemum cinerariaefolium flowers (Tewari and Gill 2014). Among pyrethroids, deltamethrin (DLM) is firstly thought to be the safest available insecticide (Shukla et al. 2002). Sadly, numerous recent reports considered DLM as an environmental and industrial pollutant that is toxic to non-target organisms living in the same ecosystem, leading to serious hazards (Yonar and Sakin 2011; Chandra et al. 2013). Contaminated water and food are considered as the main DLM population exposure sources because of its rapid oral absorption (Barlow et al. 2001).
The insecticidal effect of DLM resulted from its binding to a distinct receptor site on voltage gate sodium channels and blocking nerve impulse by modifying the open-state kinetics (Du et al. 2010; Mani et al. 2014). In addition to its neurotoxicity (Nieradko-Iwanicka and Borzecki 2015), DLM metabolites were found accumulated in the liver and the kidneys since DLM is mainly metabolized by liver microsomal enzymes and excreted by kidneys (El-Maghraby 2007; Abdelkhalek et al. 2015). Therefore, liver and kidneys are among the most chemically affected organs. Indeed, DLM through increasing oxidative stress status (Mazmancı et al. 2011; Abdel-Daim et al. 2014, 2015) can cause liver tissue alteration by inducing liver fibrosis and cirrhosis (Abdel-Daim et al. 2013, 2016). It also causes severe renal injury including inflammation, tubular cellular toxicity, and nephrotoxicity in rat models (Abdel-Daim and El-Ghoneimy 2015; Abdou and Abdel-Daim 2014).
Kefir is the result of fermenting milk with kefir grains. It is the beverage remaining after the removal of kefir grains (Bensmira et al. 2010). These grains resemble to small clumps of cauliflower forming a polysaccharide matrix of microbial symbiotic mixture. It contains yeasts and acetic acid and lactic acid bacteria (Jianzhong et al. 2009; Chen et al. 2015). During milk fermentation, many components are found in kefir such as lactic acid, acetic acid, ethanol, and aromatic compounds. Kefir holds equally essential amino acids, vitamins, minerals, and exopolysaccharides (Garrote et al. 2001; Ismaiel et al. 2011). However, kefir consumption has highly interesting healthy properties as the treatment of gastrointestinal disorders, hypertension, allergies, and ischemic heart disease (Farnworth 2005; Otles and Cagindi 2003; de Oliveira Leite et al. 2013). Additionally, immune-modulating (Vinderola et al. 2005), anti-microbial (Silva et al. 2009), anti-inflammatory (Prado et al. 2016), and anti-proliferative (Rizk et al. 2009) activities have been reported.
Therefore, the aim of the present study was to investigate the hepatoprotective effect of kefir against DLM-inducing hepatic alterations associated with oxidative stress in rats. This study is the first, to our knowledge, in which the protective effect of kefir was evaluated against DLM-induced hepatotoxicity.
Materials and methods
Chemicals
Deltamethrin (C22H19Br2NO3) (˃ 99% pure) was dissolved in corn oil. The CAS chemical name is a-cyano-3-phenoxybenzyl (1R, 3R)-3-(2,2-dibromovinyl)-2,2 dimethyl cyclopropa-necarboxylate. It is manufactured by a Tunisian company of fertilizers, El Afrane-1009, Elouardia, Tunisia. All serum biochemical parameters were performed on the Synckrom CX7 Clinical System using the Beckman reagents (Beckman, Fullerton, CA). All products used in this study were of analytical grade.
Production and preparation of kefir
Traditional cultured and original grains from Monastir, Tunisia, were used. Kefir culture was as follows: (10% w/v); 10 g kefir grains were inoculated in 100 ml of commercial liquid pasteurized full-fat cows’ milk. The mixture was subsequently cultivated at 23 °C for 12 h, then was filtered. Grains were removed and kefir was stored at 4 °C. The beverage was freshly given to animals directly after the fermentation period.
Kefir quality control
Knowing that, the most important parameter that can affect kefir quality is its geographical origin. However, variability in the microbial community composition has been reported in kefir grains from different geographical origins (Miguel et al. 2010; Garofalo et al. 2015). Thus, our Tunisian kefir, as reported by Ben Taheur et al. (2017), used equally a traditional culture containing lactic acid bacteria as the predominant microbial population (9.5 ± 0.15 × 1010 CFU/g) (Ben Taheur et al. 2017). Where, yeast were present at 9.2 ± 0.14 × 106 CFU/g. The milk nature (goat, cow, or ewe milk) can also significantly affect the microbial quality of kefir (Satir and Guzel-Seydimb 2016; Yilmaz-Ersan et al. 2018). Finally, the environmental production conditions as the proportion kefir grains (g)/milk (ml), the fermentation time, and the temperature can make differences in the kefir microflora. (Tamine 2006; Kim et al. 2018; Altuntas and Hapoglu 2019).
In all manipulations, we have kept our experimental conditions stable (kefir grains and milk origins, the proportion kefir grains (g)/milk (ml), the fermentation time and the temperature). Therefore, we estimated that we have maintained the invariability of microflora and a stable kefir quality.
Animals and experimental design
A total of 25 male Wister Albino rats, weighing 150 ± 20 g, purchased from the Central Pharmacy (SIPHAT, Tunis, Tunisia). Rats were housed in a ventilated room of normal 12:12 h light/dark cycle, relative humidity (55 ± 5%) and temperature (25 ± 2 °C). Food and water were provided ad libitum. All experimental design and animal manipulations were approved according to the National Institute of Health Guidelines for Animal Care and approved by the local Ethics Committee. All prerequisites were taken to avoid animal stress.
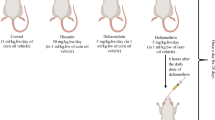
After 2 weeks of acclimation, rats were randomly subdivided into five different groups, five animals each:
The first group (G1) administered corn oil and acted as a control group.
The second group (G2) received kefir.
The third group (G3) received DLM at a dose of 25 mg/kg bw (1/6 LD50) (Manna et al. 2006).
The fourth group (G4) received simultaneously kefir + DLM at a dose of 25 mg/kg bw.
The fifth group (G5) was given kefir 1 h before DLM administration at the same dose of groups 3 and 4.
All treatments were given orally, at a dose of 1 ml of vehicle/DLM/kefir/day, in the morning (between 0900 hours and 1000 hours) and continued for 3 days.
Serum and liver collections
Animals were sacrificed at the end of experiments (24 h after the last DLM dose) by cervical dislocation. From the rats’ brachial artery, blood serum was withdrawn in heparinized tubes. Then, centrifuged at 2200g for 15 min (4 °C). Livers were immediately removed and washed with ice-cold physiological saline solution for histological analysis, biochemical analysis, and comet assay.
Liver extract preparations
Using Tris-HCl solution (10 mM, pH 7.4) and a potter (glass-Teflon), we have milled treated liver rats of each group. To obtain rich protein supernatants, a centrifugation (15,000g, 30 min, 4 °C) was performed. Protein extracts were stored at − 80 °C until used. Protein concentrations were calculated using commercial Bio-Rad protein assay, where bovine serum albumin was taken as a standard (Bradford 1979).
Serum biochemical analysis
Serum liver biomarkers (aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, total bilirubin (T bilirubin), and cholesterol) were measured using an automatic biochemistry analyzer at the Biochemistry Department, Sahloul University Hospital, Sousse, Tunisia.
Oxidative stress parameter assays
Protein carbonyl measurement
Oxidative stress generation can attack proteins via ROS, leading to the formation of carbonyl groups, and the loss of their structure and activity. Our method measured the carbonyl groups reactivity by 2,4dinitrophenylhydrasine (DNPH). Two hundred microliters of each liver extract was mixed with 800 μl of DNPH. After 1-h incubation and in order to precipitate proteins, we added 1 ml of trichloro-acetic acid. Then, to remove cell debris, we added 800 μl of ethanol-ethylacetate preparation. Finally, 500 μl of guanidine hydrochloride allowed solubilizing proteins complexed with the DNPH. The optical density was read at 340 nm (Mercier et al. 2004).
Lipid peroxidation status
Malondialdehyde (MDA) is the ultimate fragment of the degradation of cell membrane polyunsaturated fatty acids. This assay was founded on the formation of a fluorescent complex between thiobarbituric acid and MDA fragment. For that, 250 μl of each liver extract was mixed with 750 μl of both thiobarbituric acid and acetic acid. Then, the mixture was heated 2 h at 90 °C and chilled in ice for 10 min. Before optical density reading (540 nm), 2.5 ml of n-butanol pyridine (15:1 v/v) was added (Ohkawa et al. 1979).
Catalase activity evaluation
Catalases (CAT) are enzymes that neutralize oxygen peroxide (H2O2). In a quartz cuvette of spectrophotometer, we put, respectively, 20 μl of each liver extract, 780 μl of phosphate buffer solution, and 200 μl of H2O2 (the substrate of the enzyme). CAT activity was calculated after optical density measurement at 240 nm for 1 min of time interval (Clairbone 1985).
Superoxide dismutase activity evaluation
Superoxide dismutases (SOD) are ubiquitous metallo-enzymes that catalyzes superoxide ion disproportionation into molecular oxygen and hydrogen peroxides. Indeed, the SOD method referring to the nitroblue tetrazolium (NBT) test is a photo-reduction assay. Therefore, the basis for SOD activity detection is the reduction of NBT by the anion O2−. However, white light was triggered for 30 min on a reaction mixture containing 50 μl of liver homogenate, 950 μl of phosphate buffer, 1 ml of EDTA-methionine, 85.2 μl of NBT, and 22.6 μl of riboflavin. The riboflavin/methionine mixture undergoes photo-reduction-forming free radicals that will be oxidized by NBT resulting in a blue substrate. Thus, to calculate the enzyme activity, the optical density was measured at 540 nm (Beauchamp and Fridovich 1971).
Histopathological analysis
Pieces of livers were firstly incubated for 24 h in 10% formaldehyde at room temperature. Then, they were dehydrated using graded ethanol and were embedded in paraffin. Sections were stained with eosin and hematoxylin. For liver tissue histopathological analysis, we examined 30 slides per group (6 slides per rat). Light microscopy was used to distinguish organ lesions and was classified as follows:
-
Mild (+) = slight lymphocytic inflammatory infiltrate.
-
Moderate (++) = moderate lymphocytic inflammatory infiltrate.
-
Severe (+++) = marked lymphocytic inflammatory infiltrate.
DNA damage assessed by comet assay
DNA damage was determined using the alkaline comet assay according to Tice et al. (2000). Each liver piece was placed in 0.5 ml of cold phosphate-buffered saline and thoroughly chopped to obtain a cellular suspension. Hepatocyte suspensions (5 μl) were embedded in 60 μl of 1% low-melting-point agarose and spread on a microscope slide previously covered with a 1% normal-melting agarose. For cellular and nuclear membranes lysis of the embedded cells and for DNA unwinding in alkaline conditions, slides were immersed overnight in freshly prepared ice-cold lysis solution (Banath et al. 2002). Then, slides were placed in an electrophoresis alkaline buffer for 30 min to allow for DNA unwinding. Using the same alkaline buffer, electrophoresis was performed for 20 min at an electric field of 25 V. Slides were then neutralized with 0.4 M of Tris (pH 7.5) and DNA was marked with 50 μl of ethidium bromide (20 μg/ml). The comets were detected and scored using a fluorescence microscope. The previous steps have been done in darkness to eliminate supplementary DNA damage. Images of 100 randomly selected cells (50 cells from each of two replicate slides) were analyzed from each rat. Based on the tail size, cells were visually scored into five classes. From undamaged (0) to maximally damaged (4), a single DNA damage score was resulted to each animal and consequently, to each group. Thus, the total score was calculated according to the following equation: (percentage of cells in class 0 × 0) + (percentage of cells in class 1 × 1) + (percentage of cells in class 2 × 2) + (percentage of cells in class 3 × 3) + (percentage of cells in class 4 × 4).
Statistical analysis
Data were expressed as the mean ± standard deviation of the means. The data were statistically analyzed by one-way ANOVA, using Tukey’s multiple comparisons test. In all cases, p < 0.05 was considered statistically significant.
Results
Serum biochemical analysis
The effects of DLM-induced toxicity and kefir preventive effects on serum biochemical parameters are summarized in Table 1. Serum levels of liver function markers, AST, ALT, T bilirubin, and cholesterol, significantly increased in DLM-administrated rats (G3) as compared to control ones (G1/G2). Co-administration of kefir + DLM (G4) and 1-h pre-administration of kefir followed by DLM intoxication (G5) reduced the elevation in serum liver biomarkers. However, this amelioration in measured serum parameters depends on the treatment mode. Thus, the kefir pre-treatment seems to be the efficient one and kept the serum levels of analyzed markers within the normal levels.
Oxidative stress parameters
PC and MDA productions
Proteins and membrane lipid oxidations by DLM treatment as well as the preventive effects of kefir on liver tissue homogenate are represented in Table 2. We observed a significant increase (p ≤ 0.05) in protein carbonyl (PC) and MDA contents (229.7% and 181.55%, respectively) in DLM-treated group (G3) as compared to the control group (G1). Kefir itself does not induce stress oxidative markers as shown with the G2 group. Concerning co-administration of kefir + DLM (G4) and 1-h pre-administration of kefir followed by DLM intoxication (G5), liver PC amounts were decreased (168.94% and 119.72% respectively). It was the same for MDA amounts, which decreased to 134.5% and 107.69%, respectively, for the co-treatment and the pre-treatment.
CAT and SOD activities analysis
Table 2 represented equally the effects of DLM intoxication and the preventive effects of kefir on liver antioxidant enzymes. Animals treated with DLM alone (G3) showed a significant decrease (p ≤ 0.05) in CAT and SOD activities (40.43% and 38.08%, respectively) as compared to the control group (G1). In contrast, those co-treated by kefir + DLM (G4) showed an increase in CAT and SOD activities to 70.61% and 60.39%, respectively. One more time, the pre-treatment 1 h by kefir followed by DLM intoxication (G5) seems to be the most efficient; it restores antioxidant enzymes activities to 91.4% and 79.95%, respectively, for CAT and SOD.
Histopathology study
The histological changes in liver were evaluated as described in the “Materials and methods” section. Results are presented in Fig. 1. Histopathology of liver demonstrated that DLM administration caused an extensive histological damage (+++) (Fig. 1c) as compared to the control groups (normal hepatocytes shape) (Fig. 1a, b). Thus, we observed a lymphocytic inflammatory infiltrate in peri-portal and intra-lobular zones. Kefir administration decreased liver failure caused by DLM intoxication as shown in Fig. 1d (++) and Fig. 1e (+), respectively, for the co- and the pre-treatment.
DNA damage
Comet assay is estimated as a well-sensitive technique for the measuring of DNA damages and apoptosis in individual cells. Figure 2 showed that DLM-treated rats (G3) represented a significant increase (p ≤ 0.01) in hepatocytes DNA damage score, which reached 190.23% when compared to the control group (G1). The co-treatment (G4) as well as the pre-treatment (G5) by kefir reduced significantly DNA fragmentation to 132.96% and 108.25%, respectively.
Total DNA damage induced in rat hepatocytes treated with DLM alone (25 mg/kg bw) (G3). Preventive effect of kefir on DNA fragmentation; performed in co-administration with DLM (25 mg/kg bw) (G4) or in pre-administration of 1 h followed by DLM treatment (25 mg/kg bw) (G5). Data are expressed as means ± SD for five rats per group. Control animals (G1), animals received kefir alone (G2). Treated (G3–G5) vs control G1: *p ≤ 0.05; **p ≤ 0.01. Treated (G4 and G5) vs treated G3: #p ≤ 0.05
Discussion
Several pathological processes including cardiovascular and neurodegenerative diseases and even cancer have been closely related to the cellular imbalance between free radical production and antioxidant defense systems (Pizzino et al. 2017). It is commonly called the oxidative stress and can attack macromolecules via increasing phospholipids, proteins, and DNA oxidations (Lopez-Pedrera et al. 2016). Therefore, free radicals are among the major etiological factors implicated in several pesticides toxicity (Satpute et al. 2017). However, through pyrethroids toxic mechanisms, oxidative invasion may play critical roles. Among pyrethroids, DLM was shown to have some toxic effects. Otherwise, DLM is mainly metabolized by liver microsomal enzymes and DLM toxic metabolites were found accumulated in the liver (El-Maghraby 2007; Abdelkhalek et al. 2015). So, in rat liver, Tuzmen et al. (2008) detected that hepatic lipid peroxidation and the hepatic antioxidant defense system were significantly altered after 16 weeks of exposure to low and high DLM doses and ultimately caused the formation of oxygen free radicals and hepatic toxicity (Tuzmen et al. 2008). Likewise, Swiss mice treated with DLM 5 mg/kg bw and 25 mg/kg bw exhibited degenerative changes in the liver and kidneys (Tos-Luty et al. 2001). Rats’ exposure to DLM (0, 2, 5, 10, 20, or 40 mg/kg bw) for 7 days caused dose-dependent neurotoxicity and liver dysfunction that was accompanied by elevated ROS levels (Ding et al. 2017). A similar study reported that administration of DLM (5.6 and 18 mg/kg bw) significantly increased mean lipid peroxidation values in mouse liver and kidney (Rehman et al. 2006). Insofar as, the wide usage of DLM as an insecticide (Mehlhorn et al. 2011), investigations that would provide prevention by natural products with antioxidant effects in response to such pyrethroid toxicity are still needed. In this line, we have been interested in probiotics as living organisms, which upon ingestion exert health benefits. Especially kefir, a fermented beverage product produced by a symbiotic microbial with powerful antioxidant compounds. However, there is a strong connection between the composition of the gut microbiota and liver disease (Gkolfakis et al. 2015). Therefore, the modulation of the gut microbiota using probiotics has been suggested as a novel therapeutic strategy (Aron-Wisnewsky et al. 2013; Seo et al. 2015). Thereby, the aim of the present study was to investigate the protective potential of kefir against DLM deleterious oxidative effects leading to hepatotoxicity in rats.
Our selected dose of DLM, 25 mg/kg bw (1/6 LD50), for 3 days was based on anterior reports where highest dose, 30 mg/kg bw (1/5 LD50) for 5 days, induced biochemical alterations in rats without causing morbidity (Abdel-Daim et al. 2013). In these conditions, abnormally elevated serum ALT and AST levels is a sensitive marker of impaired liver function. Moreover, serum T bilirubin and cholesterol increased significantly. These perturbations in hepatic biochemical parameters were probably the consequences of DLM-induced liver pathological modifications. Our results are consistent with other studies which showed that oral administration of DLM to male rats for 5 or 30 days increased significantly liver biochemical parameters and particularly, ALT and AST enzymes (Abdel-Daim et al. 2013; Yousef et al. 2006; Maalej et al. 2017; Abdel-Daim et al. 2014).
PC and MDA, products of protein oxidation and lipid peroxidation, were significantly increased in response to DLM administration. Antioxidant enzymes such CAT and SOD that normally act to reduce and prevent tissue damages caused by free radicals were decreased in our conditions. All these alterations are implicated in DLM-mediated hepatic toxicity; such toxicity is the consequence of oxidative injuries induced by oxidative stress occurrence causing protein dysfunctions and lipid peroxidation. Moreover, the DLM-induced hepatic failure is supported by the impairment of antioxidant enzymes’ activities. Our results are in line with Abdel-Daim et al. (2013) that showed an increased in lipid peroxidation across high hepatic MDA level and inactivation of hepatic antioxidant enzymes (CAT, SOD) in response to orally DLM administration (30 mg/kg bw for 5 days). Saoudi et al. (2017) also reported that lipid peroxidation significantly increased while CAT and SOD decreased in the kidney and brain of rats due to DLM intraperitoneal administration at 7.2 mg/kg for 15 days. Our findings are equally concordant with those of Abdou and Abdel-Daim (2014) demonstrating increased serum MDA, but decreased CAT and SOD levels in rats treated intraperitoneally with DLM (30 mg/kg) for 5 days. Abdel-Daim et al. (2014) showed the same results in hepatic and renal tissues of rats administrated orally with DLM (15 mg/kg) for 30 days.
Excepting proteins and lipids, excessive ROS production can attack and disrupt the stability of DNA. Following our findings, DLM oral administration significantly advanced the DNA fragmentation score as assessed by comet test; a powerful technique in genotoxicity purview (Tice et al. 2002). DNA damage results are consistent with data reported by Ogaly et al. (2015). These authors showed that DLM significantly induced DNA damage expressed by the elevated tail moment and the extensive bright comet tail. Therefore, the ability of DLM to provoke DNA fragmentation may be due to its potential to outset a series of cell death signaling processes, which finally lead to apoptosis (Hossain and Richardson 2011).
All above results were supported by histopathological findings. However, the administration of DLM to rats showed disrupted hepatic architecture. Thus, histopathological changes covered inflammatory disorder, which might be due to the generation of ROS that provoked damages to different membrane constituents in hepatocytes (Rjeibi et al. 2016). Other reports also showed that DLM-induced hepatic lesions indicating acute and subacute hepatic damages in rats treated with 30 mg/kg bw of DLM for 5 days (Abdel-Daim et al. 2013) and with 15 mg/kg bw for 30 days (Maalej et al. 2017). However, all alterations attributed to DLM administration might be attenuated or pronounced from one study to another depending on the dose and the exposure time.
Because oxidative stress is considered a key mechanism of DLM toxicity, the use of antioxidants to counteract ROS production and to prevent liver damages could be investigated. To this end, we have used kefir as a highly antioxidant beverage against DLM-induced hepatotoxicity in rats. However, some kefir probiotic strains can alleviate oxidative status. So, Lactobacillus fermentum ME-3 has a manganese superoxide dismutase capable to decrease redox damage (Kullisaar et al. 2002; Songisepp et al. 2004). It was the same for lactobacilli and bifidobacteria strains that reduced especially lipid peroxidation and scavenged free radicals (Lin and Chang 2000; Wang et al. 2006; Savini et al. 2010). Moreover, the kefir polysaccharide, the kefiran, has many antioxidant activities (Mahapatra and Banerjee 2013). Additionally, this drink contained vitamins such as vitamins E and C and beta carotene with synergic antioxidant effects (Satir and Guzel-Seydimb 2016; Yilmaz-Ersan et al. 2018). Our results indicate that Kefir has a supreme antioxidant power and it prevents liver ROS invasion. Consequently, kefir protects against protein, lipid, and DNA oxidations by the prevention of PC, MDA, and DNA fragmentation. This prevention reached with the pre-treatment mode about 84%, 90%, and 91%, respectively. We have also demonstrated that the co-treatment as well as the pre-treatment by kefir reduced the serum hepatic injury biomarkers (AST, ALT, T bilirubin, and cholesterol). In addition, there was restoration of liver antioxidant enzyme (CAT and SOD) activities and prevention of histopathological changes in liver tissue to maintain the ultrastructure almost similar to the control one. Nevertheless, the pre-treatment remains the most efficient; it markedly improved or even totally reversed all toxicities observed with DLM administration. Recently, the hepatoprotective effects of natural antioxidant compounds of olive extracts and there critical roles in the improvement of liver functions have been reported (Mahmoudi et al. 2015; Maalej et al. 2017).
It is important to mention that kefir by itself reduces the intrinsic rats stress (G2); it ameliorates all biological parameters investigated within our study as compared to animals receiving the vehicle only (G1).
Conclusion
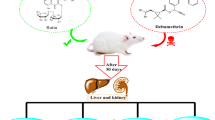
Accordingly, our data indicated that kefir effectively abolished DLM-induced hepatotoxicity mediated by oxidative stress (Fig. 3). Thus, this fermented milk could be given as a dietary supplement to individuals who are constantly exposed to such insecticides.
References
Abdel-Daim MM, El-Ghoneimy A (2015) Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren Fail 37(2):297–304
Abdel-Daim MM, Abuzead SM, Halawa SM (2013) Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One 8:72991
Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM (2014) Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol 92:679–685
Abdel-Daim MM, Abdelkhalek NK, Hassan AM (2015) Antagonistic activity of dietary allicin against deltamethrin-induced oxidative damage in freshwater Nile tilapia; Oreochromis niloticus. Ecotoxicol Environ Saf 111:146–152
Abdel-Daim M, El-Bialy BE, Rahman HG, Radi AM, Hefny HA, Hassan AM (2016) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother 77:79–85
Abdelkhalek NK, Ghazy EW, Abdel-Daim MM (2015) Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res 22:3023–3031
Abdou RH, Abdel-Daim MM (2014) Alpha-lipoic acid improves acute deltamethrin-induced toxicity in rats. Can J Physiol Pharmacol 92: 773-779.
Altuntas S, Hapoglu H (2019) 7-Kefir-type drinks from whey. Non-alcoholic beverages: The Science of Beverages 6:185–226
Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K (2013) Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect 19:338–348
Banath JP, Kim A, Olive P (2002) Overnight lysis improves the efficiency of DNA damage detection in the alkaline comet assay. Radiat Res 1155:564–571
Barlow SM, Sullivan FM, Lines J (2001) Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem Toxicol 39:407–422
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Ben Taheur F, Fedhila K, Chaieb K, Kouidhi B, Bakhrouf A, Abrunhosa L (2017) Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int J Food Microbiol 251:1–7
Bensmira M, Nsabimana C, Jiang B (2010) Effects of fermentation conditions and homogenization pressure on the rheological properties of kefir. Food Sci Technol 43:1180–1184
Bradford M (1979) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chandra N, Jain NK, Sondhia S, Srivastava AB (2013) Deltamethrin induced toxicity and ameliorative effect of alpha-tocopherol in broilers. Bull Environ Contam Toxicol 90:673–678
Chen Z, Shi J, Yang X, Nan B, Liu Y, Wang Z (2015) Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int Dairy J 43:15–21
Clairbone A (1985) Catalase activity. Handbook of Methods for Oxygen Radical Research. CRC, Press, Boca Raton FL, pp 283–284
de Oliveira Leite AM, Miguel MA, Peixoto RS, Rosado AS, Silva JT, Paschoalin VM (2013) Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz J Microbiol 44:341–349
Ding RQ, Cao ZF, Wang YH, Gao XB, Luo HY, Zhang CY, Ma SC, Ma X, Jin HY, Lu CL (2017) The implication of p66shc in oxidative stress induced by deltamethrin. Chem Biol Interact 278:162–169
Du Y, Song W, Groome JR, Nomura Y, Luo N, Dong K (2010) A negative charge intransmembrane segment 1 of domain II of the cockroach sodium channel is critical for channel gating and action of pyrethroid insecticides. Toxicol Appl Pharmacol 247:53–59
El-Maghraby S (2007) Metabolism of deltamethrin in rats. Biomed Environ Sci 20:212–216
Farnworth ER (2005) Kefir—a complex probiotic. Food Sci Technol Bull: Functional Foods 2:1–17
Garofalo C, Osimani A, Milanović V, Aquilanti L, De Filippis F, Stellato G, Di Mauro S, Turchetti B, Buzzini P, Ercolini D, Clementi F (2015) Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol 49:123–133
Garrote GL, Abraham AG, De Antoni GL (2001) Chemical and microbiological characterization of kefir grains. J Dairy Res 68:639–652
Gkolfakis P, Dimitriadis G, Triantafyllou K (2015) Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 14:572–581
Hossain MM, Richardson JR (2011) Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci 122:512–525
Ismaiel AA, Ghaly MF, El-Naggar AK (2011) Milk kefir: ultrastructure, antimicrobial activity and efficacy on aflatoxin b1production by Aspergillus flavus. Curr Microbiol 62:1602–1609
Jianzhong Z, Xiaoli L, Hanhu J, Mingsheng D (2009) Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol 26:770–775
Kim DH, Jeong D, Song KY, Seo KH (2018) Comparison of traditional and backslopping methods for kefir fermentation based on physicochemical and microbiological characteristics. LWT 97:503–507
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72:215–224
Lin MY, Chang FJ (2000) Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci 45:1617–1622
Lopez-Pedrera C, Barbarroja N, Jimenez-Gomez Y, Collantes-Estevez E, Aguirre MA, Cuadrado MJ (2016) Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology (Oxford) 55:2096–2108
Maalej A, Mahmoudi A, Bouallagui Z, Fki I, Marrekchi R, Sayadi S (2017) Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem Toxicol 106:455–465
Mahapatra S, Banerjee D (2013) Fungal exopolysaccharide: production, composition and applications. Microbiol Insights 6:1–16
Mahmoudi A, Ghorbel H, bouallegui Z, Marrekchi R, Isoda H, Sayadi S (2015) Oleuropein and hydroxytyrosol protect from bisphenol A effects in livers and kidneys of lactating mother rats and their pups'. Exp Toxicol Pathol 67:413–425
Mani VM, Asha S, Sadiq AMM (2014) Pyrethroid deltamethrin-induce developmental neurodegenerative cerebral injury and ameliorating effect of dietary glycoside naringin in male wistar rats. Biomed Aging Pathol 4:1–8
Manna S, Bhattacharyya D, Mandal TK, Dey S (2006) Neuropharmacological effects of deltamethrin in rats. J Vet Sci 7:133–136
Mazmancı B, Mazmanci MA, Unyayar A, Unyayar S, Cekic FO, Deger AG, Yalin S, Comelekoglu U (2011) Protective effect of Funalia trogii crude extract on deltamethrin- induced oxidative stress in rats. Food Chem 125:1037–1040
Mehlhorn H, Schumacher B, Jatzlau A, Abdel-Ghaffar F, Al-Rasheid KA, Klimpel S, Pohle H (2011) Efficacy of deltamethrin (Butox(R) 7.5 pour on) against nymphs and adults of ticks (Ixodes ricinus, Rhipicephalus sanguineus) in treated hair of cattle and sheep. Parasitol Res 108:963–971
Mercier Y, Gatellier P, Renerre M (2004) Lipid and protein oxidation in vivo, and antioxidant potential in meat from Charolais cows finished on pasture or mixed die. Meat Sci 66:467–473
Miguel MGDCP, Cardoso PG, Lago LDA, Schwan RF (2010) Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res Int 43:1523–1528
Nieradko-Iwanicka B, Borzecki A (2015) Subacute poisoning of mice with deltamethrin produces memory impairment reduced locomotor activity, liver damage and changes in blood morphology in the mechanism of oxidative stress. Pharmacol Rep 67:535–541
Ogaly HA, Khalaf AA, Ibrahim MA, Galal MK, Abd-Elsalam RM (2015) Influence of green tea extract on oxidative damage and apoptosis induced by deltamethrin in rat brain. Neurotoxicol Teratol 50:23–31
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Otles S, Cagindi O (2003) Kefir: A probiotic dairy-composition, nutritional and therapeutic aspects. Pak J Nutr 2:54–59
Pérez JJ, Williams MK, Weerasekera G, Smith K, Whyatt RM, Needham LL, Barr DB (2010) Measurement of pyrethroid, organophosphorus, and carbamate insecticides in human plasma using isotope dilution gas chromatography-high resolution mass spectrometry. J Chromatogr B 878:2554–2562
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxidative Med Cell Longev 2017:8416763
Prado MR, Boller C, Zibetti RG, de Souza D, Pedroso LL, Soccol CR (2016) Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc Res 108:29–33
Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S (2006) The modulatory effect of deltamethrin on antioxidants in mice. Clin Chim Acta 369:61–65
Rizk S, Maaloof K, Baydoun E (2009) The antiproliferative effect of kefir cell-free fraction on HuT-102 malignant T lymphocytes. Clin Lymphoma Myeloma 9:198–203
Rjeibi I, Ben Saad A, Hfaiedh N (2016) Oxidative damage and hepatotoxicity associated with deltamethrin in rats: the protective effects of Amaranthus spinosus seed extract. Biomed Pharmacother 84:853–860
Saoudi M, Badraoui R, Bouhajja H, Ncir M, Rahmouni F, Grati M, Jamoussi K, El Feki A (2017) Deltamethrin induced oxidative stress in kidney and brain of rats: protective effect of Artemisia campestris essential oil. Biomed Pharmacother 94:955–963
Satir G, Guzel-Seydimb ZB (2016) How kefir fermentation can affect product composition? Small Rumin Res 134:1–7
Satpute RM, Pawar PP, Puttewar S, Sawale SD, Ambhore PD (2017) Effect of resveratrol and tetracycline on the subacute paraquat toxicity in mice. Hum Exp Toxicol 36:1303–1314
Savini M, Verdenelli MC, Cecchini C, Silvi S, Orpianesi C, Cresci A (2010) Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients 2:330–339
Seo KH, Kim H, Chon JW, Kim DH, Nah SY, Arvik T, Yokoyama W (2015) Flavonoid-rich Chardonnay grape seed flour supplementation ameliorates diet-induced visceral adiposity, insulin resistance, and glucose intolerance via altered adipose tissue gene expression. J Funct Foods 17:881–891
Shukla Y, Yadav A, Arora A (2002) Carcinogenic and cocarcinogenic potential of 646 cypermethrin on mouse skin. Sci Irel 182:33–41
Silva KR, Rodrigues SA, Filho LX, Lima AS (2009) Antimicrobial activity of broth fermented with kefir grains. Appl Biochem Biotechnol 152:316–325
Songisepp E, Kullisaar T, Hutt P, Elias P, Brilene T, Zilmer M, Mikelsaar M (2004) A new probiotic cheese with antioxidative and antimicrobial activity. J Dairy Sci 87:2017–2023
Tamine AY (2006) Fermented milks. Blackwell, Oxford, UK
Tewari A, Gill JPS (2014) Assessment of hemato-biochemical parameters on exposure to low level of deltamethrin in mouse model. Vet World 7:152–157
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi Y, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Tice RR, Hook GG, Donner M, McRee DI, Guy AW (2002) Genotoxicity of radiofrequency signals I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics 23:113–126
Tos-Luty S, Haratym-Maj A, Latuszynska J, Obuchowska-Przebirowska D, TokarskaRodak M (2001) Oral toxicity of deltamethrin and fenvalerate in Swiss mice. Ann Agric Environ Med 8:245–254
Tuzmen N, Candan N, Kaya E, Demiryas N (2008) Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct 26:119–124
Vinderola CG, Duarte J, Thangavel D, Perdigon G, Farnworth E, Matar C (2005) Immunomodulating capacity of kefir. J Dairy Res 72:195–202
Wang YC, Yu RC, Chou CC (2006) Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol 23:128–135
Yilmaz-Ersan L, Ozcan T, Akpinar-Bayizit A, Sahin S (2018) Comparison of antioxidant capacity of cow and ewe milk kefirs. J Dairy Sci 101:3788–3798
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99:226–231
Yousef MI, Awad TI, Mohamed EH (2006) Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by vitamin E. Toxicology 227:240–247
Funding
This research was supported by “Le Ministère Tunisien de l’Enseignement Supérieur et de la Recherche Scientifique” through the Laboratoire de Recherche sur les Substances Biologiquement Compatible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Golli-Bennour, E., Timoumi, R., Annaibi, E. et al. Protective effects of kefir against deltamethrin-induced hepatotoxicity in rats. Environ Sci Pollut Res 26, 18856–18865 (2019). https://doi.org/10.1007/s11356-019-05253-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05253-4