Abstract
Hydrothermal treatment (HTT) of sewage sludge (SS) with pig manure biochar (PMB) addition at 160–200 °C was conducted in this study. The effects of PMB addition on the dewaterability of SS and the speciation evolution, leaching toxicity, and potential ecological risk of heavy metals were investigated. The results showed that the solid contents of the filter cakes after adding PMB increased from 20.24%, 24.03%, and 28.69% to 21.57%, 27.69%, and 32.91% at 160, 180, and 200 °C, respectively, compared with traditional HTT of SS. Furthermore, PMB could reduce the bioavailable fractions of Cr, Ni, As, and Cd in the filter cakes obtained at 160 and 180 °C compared with the theoretical value. The leaching toxicity of heavy metals in the filter cakes after adding PMB decreased significantly at 160 and 180 °C and the potential ecological risk index (RI) declined from 62.13 and 44.83 to 55.93 and 42.11, respectively. The obtained filter cake had low potential ecological risk when used in the environment. The mechanisms on the improvement of the dewaterability and heavy metals immobilization were related that PMB acted as the skeleton builder providing the outflow path for free water and implanting heavy metals into SS structure. And the optimal results were obtained at 180 °C during HTT of SS with PMB addition. This work provides a novel and effective method for the treatment of SS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of rapid economic development, large amounts of sewage sludge (SS) are produced from wastewater treatment plants. It has been reported that more than 30 million tons of SS (with 80 wt% of moisture) are annually generated in China (Zhang et al. 2017b). In general, the difficulty in low-cost dewatering is a limiting factor for the further utilization of SS owing to its special properties and structural stability (Li et al. 2011; Mikkelsen 2003). On the other hand, the pollutants in SS, especially heavy metals, are another tough problem affecting the residual utilization from treated SS (Antonkiewicz et al. 2018; Suanon et al. 2018). Therefore, developing a proper method to enhance both dewaterability and heavy metals immobilization is the most important step for the treatment of SS.
At present, hydrothermal treatment (HTT) is a simple and effective technique which is widely used to the treatment of solid waste with high moisture content (Li et al. 2018a; Yu et al. 2014; Zhang et al. 2014). During HTT, the extracellular polymeric substances are decomposed and the chemical bonds of the cell wall are also destroyed to release and solubilize the intracellular material into water phase (Appels et al. 2008). The organic pollutants and microorganisms are also degraded in the HTT process and the heavy metals may be immobilized in the solid residue (Shi et al. 2013a; Weiner et al. 2013). After HTT, the obtained residue has better dewaterability and stabilization, and it can also be further treated and utilized by combustion, pyrolysis, composting, land application (Chen et al. 2012; Hua et al. 2009; Wang et al. 2019; Yu et al. 2016).
To better improve the SS dewatering performance on the basis of HTT, the investigations about the effects of alkali additives have been performed by many researchers. Neyens et al. (2003) investigated the influence of monovalent/divalent cations (K+/Na+ and Ca2+/Mg2+) on the SS dewaterability and confirmed that alkaline hydrothermal treatment using Ca(OH)2 was the most efficient method in reducing the residual amount. Our previous study also confirmed that HTT with alkali addition was a practical method for SS dewatering due to the destruction of cell walls by high alkalinity and the bridge of bivalent cations with negative charges, and it was verified by a pilot-scale research in the Tong’an wastewater treatment plant, Xiamen (Li et al. 2017). Furthermore, alkaline additives also affect heavy metals immobilization during HTT on SS. Zhai et al. (2016) reported that alkaline condition promoted the more stable transformation of heavy metals during HTT. However, there are notable drawbacks of adding alkalis during HTT such as the corrosion of equipment, changes of product properties, and consumption of reagents. On the other hand, Shi et al. (2013b) investigated the synergistic effect of rice husk addition during HTT of SS and found that heavy metals were redistributed, fixed in the mixed matrix, and transferred from weakly bound state to stable state after treatment. Zhai et al. (2014) found that the addition of sewage sludge-based active carbon could decrease the risk of Cu and Zn during HTT of SS. While, there are still few researches on the effects of biomass or biochar on SS dewatering efficiency. Therefore, it is important to develop another effective method to achieve the dual effect of dewaterability enhancement and heavy metals immobilization during HTT of SS.
Pig manure biochar (PMB) with good porous structure and rich oxygen containing functional groups can be obtained from the pyrolysis of pig manure (Tsai et al. 2012; Xu et al. 2014), and its promotion of heavy metals immobilization was also verified in soil remediation (Wang et al. 2017). Hence, adding PMB during HTT of SS may positively affect the dewatering and heavy metals immobilization performance. In this study, bench-scale experiments were conducted to investigate the effects of PMB addition on the dewaterability during HTT of SS. The migration, speciation evolution, and leaching toxicity of heavy metals were studied after HTT. Furthermore, the potential ecological risk was also assessed for further application.
Materials and methods
Materials
The raw SS with 83.09% of moisture in this study was obtained from the double effluent oxidation ditch process in a wastewater treatment plant in Tong’an, Xiamen, Fujian Province, China. The SS was stored in 4 °C before HTT. The used PMB was obtained from pyrolysis of pig manure at 600 °C for 30 min in a rotary furnace under anaerobic atmosphere. The PMB was sieved (less than 0.15 mm) after cooling down and stored in a glass desiccator for further use. The physiochemical properties of the SS and PMB are shown in Table 1.
HTT experiments
All HTT experiments were conducted in an electrically heated autoclave with an inner volume of 1.0 L (Fig. 1). Every HTT test was started by loading 500 g of raw SS or the mixture of SS and PMB (mass ratio of 90%:10% on dry basis) and 100 mL of ultrapure water referred from the previous study (Wang et al. 2016). After the autoclave was sealed, it was heated to a pre-set temperature at a heating rate of 5 °C/min and then maintained for 60 min. The sample inside was agitated by a stirrer at 200 revolutions per minute. The conditions were designated as SS160, SS180, and SS200 for the HTT of SS in 160, 180, and 200 °C, respectively. And, the corresponding comparable conditions were designated as SB160, SB180, and SB200 for the HTT of SS with PMB addition under the above temperature, separately. After the HTT test, the treated SS was centrifuged at 8000 r/min for 15 min. The filter cake was obtained after solid-liquid separation and stored at 4 °C for further analysis.
Analysis of dewaterability
The improvement of the dewaterability can be verified from the solid content of filter cake, which was obtained by drying at 105 °C for 24 h (Wang et al. 2016). The drying property is further used to characterize the dewatering effect of HTT. The drying property was analyzed by drying at 75 °C; the mass was recorded once hourly, and the result was presented as change of the moisture content over the drying time (Cai et al. 2017). To investigate PMB role in SS dewatering, the particle size distributions of samples were measured by a laser diffraction particle analyzer (Mastersizer 2000, UK) at a stirring rate of 200 r/min and the microstructures of samples were analyzed by scanning electron microscopy (SEM, S-4800, Hitachi, Japan).
Analysis of heavy metals
The European Community Bureau of Reference (BCR) three-step extraction procedure was widely used to analyze the speciation of heavy metals (Zhang et al. 2017a). The detailed extraction steps are as follows: exchangeable fraction (F1): 0.5 g of dry sample was mixed with 20 mL of 0.11 mol/L acetic acid by shaking for 16 h (25 °C). Reducible fraction (F2): the dry residue of F1 was mixed with 20 mL of 0.5 mol/L hydroxylamine hydrochloride by shaking for 16 h (25 °C). Oxidizable fraction (F3): the dry residue of F2 was mixed with 5 mL 30% hydrogen peroxide for 1 h at room temperature and then another 5 mL of hydrogen peroxide was added after digestion for 1 h at 85 °C. When the solution was evaporated to near dried, the residue was mixed with 25 mL of 1 mol/L ammonium acetate by shaking for 16 h (25 °C). The residual fraction (F4) and total concentration of heavy metals in dry sample were extracted after digestion in an acid mixture (HNO3–HClO4–HF, 5:5:2, v/v/v) (Li et al. 2018b). All extracted liquid samples were centrifuged, filtered, and measured by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500cx, USA) to obtain the concentrations of Cr, Ni, As, Cd, and Pb. All measurements were triplicated to guarantee veracity and average values were applied.
The residual rate (R, %) of each heavy metal in the filter cake was calculated with the following equation (Li et al. 2018b):
where C1 is the concentration of individual heavy metal in the dry SS (mg/kg), M1 is the mass of the dry SS (kg), C2 is the concentration of individual heavy metal in the dry filter cake (mg/kg), and M2 is the mass of the dry filter cake (kg).
The leaching characteristics of heavy metals of SS and filter cake were determined with the toxicity characteristic leaching procedure (TCLP) recommended by the USEPA (Wang et al. 2019). Leachable heavy metals were extracted using a glacial acetic acid solution (pH = 2.88 and dry solid/liquid ratio of 1:20) in a shaking incubator at 200 r/min for 18 h. All the extracted samples were centrifuged, filtered, and then stored at 4 °C for further analysis by ICP-MS.
The assessment of the potential ecological risk has been widely used to evaluate the bioavailability and environmental risk of heavy metals, which can be calculated by the following equations (Jin et al. 2016):
where Cf is the contamination factor of the individual heavy metal; Ci and Cn are the potential mobile fractions (F1 + F2 + F3) and stable fraction (F4) of the individual heavy metal, respectively; Tr represents the toxicity response factor of the individual heavy metal and the order is Cd (30) > As (10) > Ni (6) > Pb (5) > Cr (2); Er is the potential ecological risk index for the individual heavy metal, and RI is the potential ecological risk index of the SS or filter cake based on the addition of the potential ecological risk index of each heavy metal.
Results and discussions
Effects of HTT with PMB addition on the SS dewatering
Effects of HTT with PMB addition on the solid content are shown in Fig. 2a. The solid content of the raw SS after centrifugation was low with 18.48%, which indicates that water is difficult to flow out from SS flocs. After HTT, the solid content of filter cake increased remarkably with the rise of temperature because the extracellular polymeric substances are thoroughly decomposed and the internal bound water is released at high temperature (Neyens et al. 2004; Yu et al. 2014). And also, the solid contents of the filter cakes after adding PMB increased from 20.24%, 24.03%, and 28.69% to 21.57%, 27.69%, and 32.91% at 160, 180, and 200 °C, respectively, compared with the HTT without PMB addition. This shows that PMB has a positive effect on the improvement of SS dewaterability during HTT and the higher solid content of filter cake also facilitates its subsequent disposal. In particular, the solid content of the filter cake obtained under SB180 was very close to that obtained under SS200. Considering the energy conservation, 180 °C is a suitable HTT temperature for the SS dewatering.
The effects of HTT with PMB addition on the drying property are shown in Fig. 2b. If the physical and chemical properties of flocs in SS are thoroughly disrupted, the filter cake will present a short drying time. The drying time of the raw SS after centrifugation was as long as 27 h, which presents the difficulty in removal of water. The drying time of the filter cake shortened continuously with elevating HTT temperature because the HTT at high temperature and high pressure can break cells and cause a shift from bound water to free water (Cai et al. 2017). Compared with the HTT without PMB addition, the drying times after adding PMB further decreased from 26, 19, and 16 h to 23, 16, and 11 h at 160, 180, and 200 °C, separately. These results are in accordance with the analysis of solid content, which proves that adding PMB during HTT of SS can reduce the drying time of the filter cake and present better dewatering effect.
PMB role in the SS dewatering
The particle size distributions of SS and treated SS are presented in Fig. 3. The diameter of the soluble colloidal matter in SS was large and distributed mainly in 20–100 μm. After HTT, the particles larger than 800 μm disappeared and the particles smaller than 1 μm appeared. The distribution trend of particle size moved to the left and d(0.5) decreased from 53.756 μm to 17.283–24.277 μm. It can be concluded that the large particles are degraded into smaller particles after HTT (Li et al. 2017). However, the particles between 200 and 300 μm in the treated SS under SB200 showed the high proportion due to Maillard reaction to generate Amadori compounds and melanoidins (Chevalier et al. 2001). Additionally, the particle size in the treated SS became larger after adding PMB at 160 °C, and the d(0.5) increased from 19.931 to 21.144 μm. The particle size became smaller after adding PMB at 180 and 200 °C, and the d(0.5) decreased from 24.277 and 17.739 μm to 20.170 and 17.283 μm, respectively. It may be explained that PMB acts as a skeleton builder to embed into SS structure at low temperature and this structure is collapsed at high temperature so that the dewaterability of SS is improved (Wu et al. 2016).
The microstructures of SS, PMB, and the filter cakes obtained under different HTT conditions are shown in Fig. 4. The surface of raw SS was compact and less porous. There were rod-like structure and several pores in the surface of PMB. The compact structure in the surface of filter cake obtained under the HTT of SS was gradually broken and collapsed with the rise of temperature. However, the surface of the filter cake obtained at 200 °C formed large sheet structure due to pyrogenation at high temperature. After adding PMB, the surfaces of filter cakes (Fig. 4d, f, and h) presented more small cracks and pores compared with those of the HTT without PMB addition (Fig. 4c, e, and g). These results imply that PMB are implanted into the SS residue during HTT. Considering on the changes of particle size distribution and microstructure, we can conclude that the cracks and pores in filter cake are formed because PMB acted as a skeleton builder which can transmit the acting force to the SS internal parts, provide the outflow path for free water, and enhance the dewaterability of SS (Liu et al. 2013).
The distribution and speciation of heavy metals
Effect of PMB addition on the distribution of heavy metals
Table 2 presents the concentrations of heavy metals in the filter cakes obtained under different HTT conditions. The concentrations of heavy metals in the raw SS were described as follows: Cr > Ni > Pb > As > Cd, and Cr occurs at high concentration, which was closely related to sewage. The concentrations of heavy metals in the filter cake showed an increasing trend after HTT. This enrichment can be attributed to that the organic matters in SS are degraded, and the heavy metals bound to the organic matter are released and co-precipitated with the residue matrix during HTT (Wang et al. 2019). Compared with the HTT without PMB addition, the concentrations of Cr, Ni, As, and Pb in the filter cake slightly went down after adding PMB because the concentrations of heavy metals in PMB are low. Additionally, the concentration of Cd in the filter cake had no significant change.
The residual rates of heavy metals in Fig. 5 show that the heavy metals were mostly accumulated in the filter cake after HTT and the change of the residual rate of individual heavy metal differed depending on the HTT temperature and heavy metal speciation (Huang and Yuan 2016). The similar result was made by other researchers (Leng et al. 2014; Shi et al. 2013a; Wang et al. 2016). The residual rates of Cr, Ni, As, and Pb in the filter cake after adding PMB remarkably increased compared with the HTT without PMB addition, which can be explained that PMB adsorbs part unbonded heavy metals from liquid phase by the complexation with ionized hydroxyl-O groups and the precipitation with CO32− and/or PO43− (Xu et al. 2013a; Xu et al. 2013b). Additionally, the residual rates of high toxic Cd increased to over 95% after adding PMB at 160 and 180 °C. To further find the effect of PMB addition, the speciation distributions of heavy metals will be comprehensively investigated based on the relation to the bioavailability and ecotoxicity (Huang and Yuan 2016).
Effects of PMB addition on the speciation of heavy metals
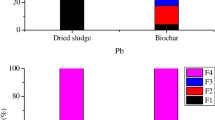
According to the BCR method, the speciation of heavy metals is apportioned into four fractions. F1 is adsorbed on the particle surface or in the form of carbonates, and F2 is the combination of iron and manganese oxides. F1 + F2 are recognized as bioavailable fraction because it is easily adsorbed by plants or the water system (Ščančar et al. 2000). F3 is a form of metal sulfides or metal combined with organic matters, and F4 is recognized as stable fraction due to the combination with silicate minerals (Fuentes et al. 2008).
The speciation distributions of heavy metals in the raw SS and filter cakes obtained under different HTT conditions are shown in Fig. 6a. After HTT of SS with PMB, the F4 of Cr, Ni, As, and Cd in the filter cake remarkably increased compared with the raw SS and the F4 of Pb was 100%. In the case of Cr, the main speciation was F3 in SS and F3 was transferred to F4 after HTT of SS with PMB. When the temperature rose up from 160 to 200 °C, the F4 of Cr increased from 52.70 to 84.40%. For Ni, the F1 + F2 in SS was high and reached up to 48.45%. The F1 + F2 of Ni in the filter cake decreased and F4 remarkably increased after HTT of SS with PMB. The F4 of Ni rose up from 15.13 to 23.12% with the increase of temperature. The As was transferred from F1 + F2 to F3 + F4 after HTT of SS with PMB and the F4 of As increased from 83.47 to 86.77–88.21% in the filter cake compared with the raw SS. In case of Cd, the F1 + F2 in SS was high and reached up to 45.71% and the F1 + F2 of Cd in the filter cake remarkably decreased and F4 increased from 36.57% in SS to 60.75–61.06% in the filter cake after HTT of SS with PMB. These observations imply a positive effect of the HTT of SS with PMB on the immobilization of heavy metals.
Figure 6a also shows that PMB could promote notably the immobilization of Cr in the filter cake. The F4 of Cr in the filter cakes after adding PMB increased from 46.94%, 61.84%, and 80.23% to 52.70%, 69.00%, and 84.40% at 160, 180, and 200 °C, respectively, compared with the HTT without PMB addition. Additionally, the addition of PMB during HTT of SS promoted the immobilization of Ni, Cd, and As at the low HTT temperature. Under the HTT at 160 °C, the F1 + F2 of Ni, As, and Cd in the filter cake after adding PMB dropped from 37.03%, 11.20%, and 31.09% to 34.00%, 10.51%, and 28.37%, respectively, and the F4 rose from 14.89%, 87.27%, and 55.44% to 15.13%, 88.21%, and 61.06%, respectively. Under the HTT at 180 °C, the F1 + F2 of Ni, As, and Cd in the filter cake after adding PMB decreased from 36.74%, 12.14%, and 30.73% to 31.23%, 10.79%, and 28.18%, respectively, and the F4 increased from 21.62%, 86.75%, and 59.63% to 22.57%, 87.85%, and 60.91%, respectively. In short, PMB has a positive effect on the immobilization of heavy metals during HTT and the heavy metals are transferred from the F1 + F2 into the more stable F4.
This study used the synergistic strength (η) to express the influence of PMB on the speciation of heavy metal during the HTT of SS. When η < 0%, adding PMB can promote the immobilization of heavy metal. When η > 0%, PMB can activate the speciation of heavy metal. The η value and the theoretically calculated value of the F1 + F2 were described as follows:
where FSB is the experimental value of the F1 + F2 for individual heavy metal in the filter cake after HTT of SS with PMB; FSB-cal. is the theoretical calculating value of the F1 + F2 for individual heavy metal in the filter cake after HTT of SS with PMB; FSS or FPMB are the experimental value of the F1 + F2 for individual heavy metal in the filter cake after HTT of SS or PMB; 90% and 10% are the mixed ratio of SS and PMB on a dry basis. The results are presented in Fig. 6b. The η values of Cr, Ni, As, and Cd were lower than 0% at 160 and 180 °C and the η values of Ni and As were higher than 0% at 200 °C. The η value of Pb was always 0% because it exists in the filter cake as F4. Therefore, 180 °C is the most suitable HTT temperature for promoting the immobilization of heavy metals during HTT of SS by adding PMB according to η value. The possible mechanisms for the immobilization of heavy metals by PMB addition can be summed up as shown in Fig. 7. Firstly, the unstable heavy metals in SS are dissolved under HTT condition; secondly, the dissolved heavy metals combine with PMB via precipitation, ion exchange, and electrostatic attraction (Ahmad et al. 2014; Zhu et al. 2017); finally, these heavy metals are implanted into the SS structure together with PMB as a skeleton builder (Dou et al. 2017; Shi et al. 2013b). However, the water that contained higher H+ and OH− ion concentrations with elevating HTT temperature can be an effective medium for acid- and base-catalyzed reactions based on PMB to activate heavy metal, which results in the increase of the F1 + F2 of Ni and As at 200 °C compared with the theoretical value (Zhai et al. 2014).
Leaching toxicity and potential ecological risk assessment of heavy metals
Leaching toxicity
Table 3 shows the leaching concentrations of heavy metals in the filter cakes for TCLP tests. The leaching concentrations of Cr, Ni, As, Cd, and Pb in the SS were 1.768, 14.067, 0.218, 0.008, and 0.035 mg/L, respectively, and the leaching concentration of Ni was approximately 2.81 times of the threshold value. After HTT of SS with PMB, the leaching concentrations of Cr, Ni, As, and Pb in the filter cake decreased significantly and reached to 0.460–0.940, 8.372–9.358, 0.147–0.178 mg/L, and undetectable amounts, respectively. The leaching concentration of Cd had unobvious change. Moreover, the leaching concentrations of Cr, Ni, and As in the filter cakes after adding PMB reduced at 160 and 180 °C compared with the HTT without PMB addition. In particular, the leaching concentrations of heavy metals in the filter cake were lowest at 180 °C and the leaching concentrations of Cr, Ni, and As decreased by 27.90%, 19.81%, and 10.37%, which indicates that the addition of PMB leads to the reduction of the leaching toxicity and environment risk of heavy metals in the filter cake and 180 °C can be used as an optimization condition for further application.
Potential ecological risk assessment
Based on the potential ecological risk assessment method, Cf, Er, and RI of heavy metals and indices for ecological risk assessment are shown in Table 4. The RI of SS was 122.10, mainly due to the contributions of Ni (63.87) and Cd (52.03) and their potential ecological risks reached to moderate levels. After HTT of SS with PMB, the Er of Ni and Cd in the filter cake declined to 19.95–33.66 and 19.13–19.38, respectively, and the potential ecological risks of these heavy metals reached to low levels. Accordingly, the RI of the filter cake was 41.23–55.93 and decreased with elevating HTT temperature. This presents that the filter cake has a very low potential ecological risk when used in the environment. Furthermore, the RI of heavy metals in the filter cakes after adding PMB decreased from 62.13 and 44.83 to 55.93 and 42.22 at 160 and 180 °C, respectively, but the result was opposite at 200 °C compared with the HTT without PMB addition. These results are consistent with the conclusions about the speciation distributions and leaching toxicity of heavy metals.
Conclusions
PMB addition notably promoted the SS dewaterability during HTT. The solid content of filter cake after adding PMB increased and the drying time decreased compared with the HTT without PMB addition. Additionally, PMB could reduce the bioavailable fractions of Cr, Ni, As, and Cd in the filter cakes obtained at 160 and 180 °C compared with the theoretical value. The leaching toxicity and RI of heavy metals after adding PMB decreased significantly at 160 and 180 °C. The obtained filter cake had very low potential ecological risk when used in the environment. To sum up, 180 °C is the optimal temperature for the enhancement of dewatering and the immobilization of heavy metals during HTT of SS with PMB addition because PMB acted as the skeleton builder can provide the outflow path for free water and implant heavy metals into SS structure. This work provides a useful process for the treatment of SS with high moisture and heavy metals concentrations.
Abbreviations
- HTT:
-
Hydrothermal treatment
- SS:
-
Sewage sludge
- PMB:
-
Pig manure biochar
- BCR:
-
European Community Bureau of Reference
- F1:
-
Exchangeable fraction
- F2:
-
Reducible fraction
- F3:
-
Oxidisable fraction
- F4:
-
Residual fraction
- TCLP:
-
Toxicity characteristic leaching procedure
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Antonkiewicz J, Pełka R, Bik-Małodzińska M, Żukowska G, Gleń-Karolczyk K (2018) The effect of cellulose production waste and municipal sewage sludge on biomass and heavy metal uptake by a plant mixture. Environ Sci Pollut R 25:31101–31112. https://doi.org/10.1007/s11356-018-3109-5
Appels L, Baeyens J, Degrève J, Dewil R (2008) Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energy Combust 34:755–781. https://doi.org/10.1016/j.pecs.2008.06.002
Cai C, Liu HL, Wang MM (2017) Characterization of antibiotic mycelial residue (AMR) dewatering performance with microwave treatment. Chemosphere 174:20–27. https://doi.org/10.1016/j.chemosphere.2017.01.121
Chen H, Yan SH, Ye ZL, Meng HJ, Zhu YG (2012) Utilization of urban sewage sludge: Chinese perspectives. Environ Sci Pollut R 19:1454–1463. https://doi.org/10.1007/s11356-012-0760-0
Chevalier F, Chobert JM, Popineau Y, Nicolas MG, Haertlé T (2001) Improvement of functional properties of β-lactoglobulin glycated through the Maillard reaction is related to the nature of the sugar. Int Dairy J 11:145–152. https://doi.org/10.1016/S0958-6946(01)00040-1
Dou XM, Chen DZ, Hu YY, Feng YH, Dai XH (2017) Carbonization of heavy metal impregnated sewage sludge oriented towards potential co-disposal. J Hazard Mater 321:132–145. https://doi.org/10.1016/j.jhazmat.2016.09.010
Fuentes A, Lloréns M, Sáez J, Isabel Aguilar MA, Ortuño JF, Meseguer VF (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99:517–525. https://doi.org/10.1016/j.biortech.2007.01.025
Hua L, Wu WX, Liu YX, Mcbride M, Chen YX (2009) Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ Sci Pollut R 16:1–9. https://doi.org/10.1007/s11356-008-0041-0
Huang HJ, Yuan XZ (2016) The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour Technol 200:991–998. https://doi.org/10.1016/j.biortech.2015.10.099
Jin JW, Li YN, Zhang JY, Wu SC, Cao YC, Liang P, Zhang J, Wong MH, Wang MY, Shan SD, Christie P (2016) Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J Hazard Mater 320:417–426. https://doi.org/10.1016/j.jhazmat.2016.08.050
Leng LJ, Yuan XZ, Huang HJ, Jiang HW, Chen XH, Zeng GM (2014) The migration and transformation behavior of heavy metals during the liquefaction process of sewage sludge. Bioresour Technol 167:144–150. https://doi.org/10.1016/j.biortech.2014.05.119
Li J, Liu X, Liu Y, Ramsay J, Yao CC, Dai RH (2011) The effect of continuous exposure of copper on the properties and extracellular polymeric substances (EPS) of bulking activated sludge. Environ Sci Pollut R 18:1567–1573. https://doi.org/10.1007/s11356-011-0492-6
Li CX, Wang XD, Zhang GY, Yu GW, Lin JJ, Wang Y (2017) Hydrothermal and alkaline hydrothermal pretreatments plus anaerobic digestion of sewage sludge for dewatering and biogas production: bench-scale research and pilot-scale verification. Water Res 117:49–57. https://doi.org/10.1016/j.watres.2017.03.047
Li CX, Wang XD, Zhang GY, Li J, Li ZW, Yu GW, Wang Y (2018a) A process combining hydrothermal pretreatment, anaerobic digestion and pyrolysis for sewage sludge dewatering and co-production of biogas and biochar: Pilot-scale verification. Bioresour Technol 254:187–193. https://doi.org/10.1016/j.biortech.2018.01.045
Li J, Yu GW, Xie SY, Pan LJ, Li CX, You FT, Wang Y (2018b) Immobilization of heavy metals in ceramsite produced from sewage sludge biochar. Sci Total Environ 628–629:131–140. https://doi.org/10.1016/j.scitotenv.2018.02.036
Liu H, Yang JK, Zhu NR, Zhang H, Li Y, He S, Yang CZ, Yao H (2013) A comprehensive insight into the combined effects of Fenton’s reagent and skeleton builders on sludge deep dewatering performance. J Hazard Mater 258–259:144–150. https://doi.org/10.1016/j.jhazmat.2013.04.036
Mikkelsen LH (2003) Applications and limitations of the colloid titration method for measuring activated sludge surface charges. Water Res 37:2458–2466. https://doi.org/10.1016/S0043-1354(03)00021-6
Neyens E, Baeyens J, Creemers C (2003) Alkaline thermal sludge hydrolysis. J Hazard Mater 97:295–314. https://doi.org/10.1016/S0304-3894(02)00286-8
Neyens E, Baeyens J, Dewil R, De heyder B (2004) Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J Hazard Mater 106:83–92. https://doi.org/10.1016/j.jhazmat.2003.11.014
Ščančar J, Milačič R, Stražar M, Burica O (2000) Total metal concentrations and partitioning of Cd, Cr, Cu, Fe, Ni and Zn in sewage sludge. Sci Total Environ 250:9–19. https://doi.org/10.1016/S0048-9697(99)00478-7
Shi WS, Liu CG, Ding DH, Lei ZF, Yang YN, Feng CP, Zhang ZY (2013a) Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour Technol 137:18–24. https://doi.org/10.1016/j.biortech.2013.03.106
Shi WS, Liu CG, Shu YJ, Feng CP, Lei ZF, Zhang ZY (2013b) Synergistic effect of rice husk addition on hydrothermal treatment of sewage sludge: fate and environmental risk of heavy metals. Bioresour Technol 149:496–502. https://doi.org/10.1016/j.biortech.2013.09.114
Suanon F, Chi QQ, Yang XY, Wang HJ, Rashid A, Asefi B, Mama D, Yu CP, Sun Q (2018) Diagnosis and ecotoxicological risk assessment of 49 elements in sludge from wastewater treatment plants of Chongqing and Xiamen cities, China. Environ Sci Pollut R 25:29006–29,016. https://doi.org/10.1007/s11356-018-2888-z
Tsai WT, Liu SC, Chen HR, Chang YM, Tsai YL (2012) Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 89:198–203. https://doi.org/10.1016/j.chemosphere.2012.05.085
Wang XD, Li CX, Zhang B, Lin JJ, Chi QQ, Wang Y (2016) Migration and risk assessment of heavy metals in sewage sludge during hydrothermal treatment combined with pyrolysis. Bioresour Technol 221:560–567. https://doi.org/10.1016/j.biortech.2016.09.069
Wang T, Sun HW, Ren XH, Li B, Mao HJ (2017) Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci Rep 7(12114). https://doi.org/10.1038/s41598-017-12503-3
Wang XD, Chi QQ, Liu XJ, Wang Y (2019) Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 216:698–706. https://doi.org/10.1016/j.chemosphere.2018.10.189
Weiner B, Baskyr I, Poerschmann J, Kopinke FD (2013) Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 92:674–680. https://doi.org/10.1016/j.chemosphere.2013.03.047
Wu Y, Zhang PY, Zeng GM, Ye J, Zhang HB, Fang W, Liu JB (2016) Enhancing sewage sludge dewaterability by a skeleton builder: biochar produced from sludge cake conditioned with rice husk flour and FeCl3. ACS Sustain Chem Eng 4:5711–5717. https://doi.org/10.1021/acssuschemeng.6b01654
Xu XY, Cao XD, Zhao L, Wang HL, Yu HR, Gao B (2013a) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy; manure-derived biochar. Environ Sci Pollut R 20:358–368. https://doi.org/10.1007/s11356-012-0873-5
Xu XY, Cao XD, Zhao L (2013b) Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961. https://doi.org/10.1016/j.chemosphere.2013.03.009
Xu XY, Cao XD, Zhao L, Sun TH (2014) Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 111:296–303. https://doi.org/10.1016/j.chemosphere.2014.04.014
Yu J, Guo MH, Xu XH, Guan BH (2014) The role of temperature and CaCl2 in activated sludge dewatering under hydrothermal treatment. Water Res 50:10–17. https://doi.org/10.1016/j.watres.2013.11.034
Yu GW, Wang Y, Zhang X, Tang XD, Li J, Yu Z, Wang XD, You FT (2016) Influence of sludge and sludge biochar on the transfer of available heavy metals in soil. J Solid Waste Technol Manage 42:814–823
Zhai YB, Chen HM, Xu BB, Xiang BB, Chen Z, Li CT, Zeng GM (2014) Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresour Technol 159:72–79. https://doi.org/10.1016/j.biortech.2014.02.049
Zhai YB, Liu XM, Zhu Y, Peng C, Wang TF, Zhu L, Li CT, Zeng GM (2016) Hydrothermal carbonization of sewage sludge: the effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour Technol 218:183–188. https://doi.org/10.1016/j.biortech.2016.06.085
Zhang GY, Ma DC, Peng CN, Liu XX, Xu GW (2014) Process characteristics of hydrothermal treatment of antibiotic residue for solid biofuel. Chem Eng J 252:230–238. https://doi.org/10.1016/j.cej.2014.04.092
Zhang J, Tian Y, Zhang J, Li N, Kong L, Yu M, Zuo W (2017a) Distribution and risk assessment of heavy metals in sewage sludge after ozonation. Environ Sci Pollut R 24:5118–5125. https://doi.org/10.1007/s11356-016-6313-1
Zhang QG, Hu JJ, Lee DJ, Chang YJ, Lee YJ (2017b) Sludge treatment: current research trends. Bioresour Technol 243:1159–1172. https://doi.org/10.1016/j.biortech.2017.07.070
Zhu XM, Chen BL, Zhu LZ, Xing BS (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115. https://doi.org/10.1016/j.envPol.2017.04.032
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA23020500), the Natural Science Foundation of Fujian Province (2019J01135), the China-Japanese Research Cooperative Program (2016YFE0118000), the Industry Leading Key Projects of Fujian Province (2015H0044), the Scientific and Technological Major Special Project of Tianjin City (16YFXTSF00420) and the Key Project of Young Talent of the Institute of Urban Environment, Chinese Academy of Sciences (IUEZD201402).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, S., Yu, G., Li, C. et al. Dewaterability enhancement and heavy metals immobilization by pig manure biochar addition during hydrothermal treatment of sewage sludge. Environ Sci Pollut Res 26, 16537–16547 (2019). https://doi.org/10.1007/s11356-019-04961-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04961-1