Abstract
The synergistic effects of pyrolysis byproduct, biochar (BC) on heavy metal consolidation, and H2S removal during and after from microwave pyrolysis of municipal sludge were studied in this paper. The results showed that above 80% of heavy metals (Zn and Pb) were enriched in the biochar and the leaching toxicity of both heavy metals was lower than the national emission standards. The chemical specification analysis found the sum of acid-soluble/exchangeable fraction (F1) and reducible fraction (F2) for Pb and Zn metals decreased by 26 and 40%; however, the residual fraction (F4) increased 33 and 46%, which contributed to the good stabilization of heavy metals in biochar. Besides, biochar achieved high H2S removal efficiency of 78.4% compared with the commercial activated carbon (AC). Furthermore, the biochar prepared by microwave pyrolysis had excellent adsorption performance, which was attributed to its larger specific surface area of 476.87m2/g under nitrogen atmosphere at 650oC compared with traditional pyrolysis. The mechanism analysis showed that microwave pyrolysis resulted in the high alkaline condition and formation of a large number of microparticles containing large metal elements on the biochar surface, which mainly contributed to the stabilization of heavy metals. The metal oxides adsorbed on the surface of biochar can catalyze the oxidation of H2S absorption, which will change the pH atmosphere of biochar reducing the leaching behavior of heavy metals. This study provided the good application potential of solid waste (biochar) for simultaneous heavy metal stabilization and H2S capture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the formulation of the restrictive legislation and the improvement of living standards, sewage treatment has been entailed more stringent requirements of environmental protection while providing the rapid and efficient performance. Global solid waste generation is on the rise, with 2 billion tons of solid waste being produced annually and expected to increase by 70% to 3.4 billion tons a year by 2050 (Chen et al. 2020). Although municipal sludge is a kind of low-grade biomass resources with high utilization value, the huge amount of heavy metals and other harmful substances have restricted the further unitization as resources. The traditional sludge treatment methods, such as direct combustion and landfill, present obvious some problems and defects, which could hardly be accepted in practice application of environmental protection. From the viewpoint of following harmless treatment and resource utilization, among which gasification and pyrolysis as alternative candidates have received widespread attention. (Bulushev and Ross 2011; Chen et al. 2015; Zhang et al. 2013). It is reported that the main pyrolysis product above 650 °C is biogas, in which H2 and CO accounts contributed to more than 50% of the total gas production (Zuo et al. 2011). Therefore, the development of municipal sludge treatment technology has important practical significance to achieve sustainable development.
The decomposition products of organic matter in sludge are seriously complex, and the content of H2S remained in a high level, which limits the application of pyrolysis biogas as fuel (Zhang et al. 2017b; Zhang et al. 2017c). Moreover, biogas with high H2S content have reduced the calorific value of the gas, which drastically affect the reliability of transportation due to the strong corrosion effect on the metal pipeline. Therefore, gas desulfurization technology has been attracted more attention in research field of harmful gas removal. Wen et al. investigated that activated carbon based on municipal sludge with relatively higher surface area could exhibit excellent adsorption performance during adsorption of gaseous formaldehyde compared with commercial activated carbon (Wen et al. 2011). Additionally, an efficient H2S adsorbent was synthesized by pyrolysis of municipal sludge and mineral oil, which showed more effective than 230% coconut shell-based activated carbon (Bagreev and Bandosz 2004). The relevant research indicates that the synthesis of adsorbents was mainly concentrated on the conventional heating equipment such as electric furnace, while the studies in the production of activated carbon by microwave pyrolysis were relatively few. Compared with traditional electric heating processes, microwave pyrolysis technology has many advantages, including uniform heating at the molecular level, process flexibility and equipment portability, lower thermal inertia, faster response, and energy saving (Zhang et al. 2017; Motasemi and Afzal 2013). Studies have shown that the adsorbent SBET and pore volume prepared by microwave are superior to conventional pyrolysis/carbonization and physical activation methods (Zhang et al. 2018). Studies have shown that the leaching rates of heavy metals Zn, Cd, Cr, and Pb in the solid products of sludge pyrolysis in the traditional electric furnace are 67.35%, 52.17%, 0.27% and 0.39%, respectively, while the leaching rates of heavy metals in the solid products obtained by microwave pyrolysis are, respectively, 9.38, 2.11, 0.04, and 0.11%. It shows that the leaching rate of various heavy metals in the microwave pyrolysis sludge is lower, which is better than the traditional pyrolysis method for fixing heavy metals (Wu et al. 2015; Ma et al. 2018).
The secondary pollution problem such as heavy metals in the sludge has been given increasing attention recently (Cai et al. 2007; Li et al. 2014; Abdelhafez et al. 2014). The activity, bioavailability, and ecotoxicity of heavy metals mainly depend on their chemical forms and binding modes (Fuentes et al. 2004). During the pyrolysis of municipal sludge, Yuan et al. (2015) studies the effect of pyrolysis temperature on the biochar characteristics and found that the contents of Cr, Ni, and Zn in the biochar reached the maximum values at temperatures between 500 and 700 °C, while Cu showed different trends during pyrolysis, but the change of metal speciation and risk assessment of heavy metals have been few reported. Bridle and Pritchard (2004) also reported that a relatively high amount of heavy metals including Zn, Cu, Ni, and Cr were observed in pyrolysis solid residuals from municipal sludge; however, the article did not mention the changes of heavy metal speciation during sludge pyrolysis. There were also a number of published articles investigating changes in the chemical forms of heavy metals in municipal sludge in various processes (Dong et al. 2013; Yuan et al. 2011; Xiao et al. 2015). The above researches showed that different sludge treatment processes had an impact on the distribution and ecological toxicity of heavy metals. Thus, the migration and transformation characteristic of heavy metals in sludge pyrolysis deserved special concern.
Previous studies on the heavy metal concentration and H2S release during sludge pyrolysis were separated, and little was known about the performances under microwave pyrolysis condition. Correspondingly, the heavy metal consolidation behavior of biochar in sludge microwave pyrolysis was first investigated in this study. Then, obtained biochar from sludge pyrolysis was tested for the H2S adsorption performance. Finally, the mechanism of heavy metal stabilization and H2S removal was conducted in this study.
Material and methods
Materials
The dewatered sludge was collected from the Municipal Wastewater Treatment Plant of Harbin city, P.R. China. The reagents of this experimental, including the commercial activated carbon (AC), were all analytically pure (Tianjin Benchmark Chemical Reagent Co., Ltd, China), and the characteristic of municipal sludge was shown in Table 1. The pyrolysis byproduct, biochar, was collected from the solid residue after pyrolysis experiments, in which dewater municipal sludge (moisture content about 80%) was used as raw material for microwave pyrolysis.
Microwave pyrolysis experiments
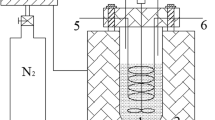
The sludge pyrolysis experiment was carried out in a microwave device, HAMILAB-C1500 microwave oven produced by Changsha Longtai Microwave Heating Co., LTD, and its operation method was given in previous studies (Zhang et al. 2017b, c). In brief, a fixed-bed microwave heating apparatus was applied to conduct the pyrolysis experiments. First, put the dried sludge samples into the quartz reactor in the microwave cavity. Then, the pyrolysis experiments would start after heating up at a rate of 50 °C/min to the desired temperature of 650 °C. Keep constant after reaching this temperature; the samples would still in the cavity for 10 min, and then the microwave generator was shut off to ensure the complete finish of pyrolysis reactions. Finally, collect the remaining coke and store it in a closed container for next use.
To ensure the inert environment of the pyrolysis experiment, the sample in the quartz reactor was purged with nitrogen at 150 ml/min for 10 min before the start of the pyrolysis. The nitrogen was turned off at the beginning of the experiment. After the end of pyrolysis, continue to inject nitrogen at 150 ml/min for 2 min to exhaust the gas from the pyrolysis system.
Adsorption performance of H2S
In the experiment, H2S containing pyrolysis gas is used as the vapor source, and the original biogas first passes through the tar collection system to avoid the influence of biochar adsorption on H2S. After the cooling system, the biogas was discharged into 0.1 mol/L sulfuric acid solution to absorb alkaline gas. The desulfurization experiments were conducted in a column fixed bed device with a known adsorbent content and the adsorption process is kept in a sealed state. The bed diameter is 100 mm, filling height is 1000 mm, and packing density is 470–520 g/L. 50 L with H2S concentration of 0.091 mg/m3 biogas flow rate of 50 mm/s entered the desulfurization device, passed through the adsorption layer from the bottom to the top and then discharge at the outlet valve. The operation temperature was maintained at 35 °C, and the contact time between biochar and biogas was around 20 s. The outlet gas was collected in the gas sampling bag and H2S concentration analyzed by gas chromatograph method.
Heavy metal extraction and leaching toxicity analysis
Heavy metal speciation analysis was based on the BCR sequential extraction procedure (Yuan et al. 2011). Using 0.5 g dried sludge and 0.5 g biochar samples to extract heavy metals in different forms and concentrations, take the extraction of residual fraction; for example, 5 ml of HNO3, 5 ml of HClO4, and 3 mL of hydrogen peroxide (30%) were added to the biochar. The mixture evaporated to near dryness on a hot plate. After cooling to room temperature, 2% HNO3 was used to dissolve the obtained residues to 10 ml, and then use it to measure the residual fraction.
The overall concentrations of Pb and Zn in solutions were measured by ICP-OES (PerkinElmer Optima 5300DV). Furthermore, the leaching toxicity of heavy metals using TCLP method was also tested according to the US EPA standard, SW-846.
Characterization analysis
Surface morphology was observed by a scanning electron microscopy (SEM-EDS) technique (JSM-6700F, Japan). Porous structure characteristics of adsorbents were determined from N2 adsorption and desorption isotherms using standard volumetric techniques (ASAP 2010, Micromeritics, USA). In addition, H2S concentration was analyzed in a gas chromatograph equipped with a TCD detector (HP 5890, Agilent China). In the experiment, HP 3 FT Molecular Sieve 13×45/60 column was used.
Results and discussion
Heavy metal consolidation during microwave pyrolysis of municipal sludge
To understand the biochar safety on the stabilization of heavy metals (Pb and Zn), the heavy metal concentrations and leaching toxicity of biochar produced during microwave pyrolysis of municipal sludge was measured. The leaching toxicity of solid waste is an important basis for determining the methods of treatment, disposal or resource utilization of solid wastes. The results can be seen in Table 2.
The overall concentrations of Pb and Zn in the biochar were higher than those in the raw municipal sludge. Especially, above 80% of two metals were still maintained in the biochar product after pyrolysis. This meant that heavy metals were enhanced in the biochar owing to the decomposition of organic compounds in sludge during sludge pyrolysis at high temperature. This was similar to the studies of Zorpas et al. (2001), who reported that above 90% of Cd, Co, Cr, Cu, Fe, Ni, Pb and Zn metals were remained in the residue after sludge pyrolysis. Table 2 shows that the leaching toxicity of both metals was lower than china emission standards (GB 5085.3—2007). What is more, the leaching concentrations of Pb and Zn in biochar product accounted for about 5% of those in dried municipal sludge, indicating the heavy metal ions present in the biochar were effectively stabilized during sludge pyrolysis process.
The mobility, bioavailability, and environmental damage of heavy metals are mainly affected by the chemical speciation in sludge (Jin et al. 2005). Thus, the changes on the chemical speciation of Pb and Zn during municipal sludge pyrolysis were studied in order to further elucidate the mechanism of low leaching toxicity of heavy metals in the biochar, and the results can be seen in Fig. 1 (acid-soluble/exchangeable fraction F1, reducible fraction F2, oxidizable fraction F3 residual fraction F4).
From Fig. 1, the content of Pb and Zn in the acid-soluble/exchangeable fraction (F1) of biochar was decreased significantly after sludge pyrolysis. It was found that F1 fraction of Zn was negligible and that of Pb was also below 4% in biochar. F1 mainly refers to the weak adsorption on the surface of solid particles and the carbonate formation of metals (Zhang et al. 2017a). The results evidence that the proportion of carbonate-bound heavy metals in the biochar was small after sludge pyrolysis, indicating the heavy metals in biochar were not easily affected and not sensitive to environmental conditions after pyrolysis, especially for pH influence. The proportion of Zn bound to the reducible fraction (F2) increased by 6%, while that of Pb was decreased by 19% compared with the corresponding F2 fractions in the dried sludge. The F2 fraction was primarily the iron-manganese oxide and hydrated oxide compounds, which were also vulnerable to reducing environmental conditions. Generally, the sum of F1 and F2 fractions were used to describe the ecotoxicity of solid waste. From Fig. 1, the sum of acid-soluble/exchangeable fraction (F1) and reducible fraction (F2) for Pb and Zn metals were decreased by 26 and 40% respectively in comparison with those in the dried sludge. Therefore, the Pb and Zn in biochar had a lower direct toxicity after sludge pyrolysis.
Besides, Pb and Zn distributed in the oxidizable fraction (F3) were reduced while both metals in the residual fraction (F4) in biochar were raised significantly 33 and 46%, respectively. The increase of F2 fraction for Zn may be due to the decomposition of organic matter (F3) during sludge pyrolysis leading to a more extractible speciation present in F2 form. In the study of heavy metal distribution of municipal sludge after ozonation, Zhang et al. (2017a) also found the decrease of F3 fraction accompanied by the increase of F2 fraction in sludge. It was well known that heavy metals associated with the F4 fraction were incorporated into silicate minerals and it is very difficult to release them through dissociation (Nemati et al. 2011). Thus, the significant increase of F4 fractions for Zn and Pb after sludge pyrolysis demonstrated that both heavy metals were stabilized in biochar effectively. This conclusion was consistent with the results of total concentration and leaching concentrations of heavy metals. Heavy metals can be retained in biochar for two main reasons. The immobilized precipitation of alkaline substances (especially Ca-compounds) and phosphate generated by pyrolysis will reduce the leaching toxicity of heavy metals to below the safe level (Hu et al. 2013). Besides, the vitrification formed during high-temperature pyrolysis will embed heavy metals into the solid solution (Chen et al. 2014)
Adsorption performances of H2S by biochar from sludge pyrolysis
According to our previous studies, the primary sulfur-containing pollutant in the process of pyrolysis biogas was regarded as H2S (Zhang et al. 2017b, c). In order to further explore the post-removal efficiency of H2S, the 50-L biogas with H2S concentration of 0.091 mg/m3 was used as the absorbates, which is prepared by microwave pyrolysis using municipal sludge as raw materials. And the adsorption performance was tested by passing the gas flow through a fixed-bed reactor containing the same mass of adsorbent. After the adsorption, the biogas concentrations of activated carbon and biochar are 0.0083 mg/m3 and 0.0196 mg/m3, respectively (in Fig. 2). The results indicated that the desulfurization efficiency of activated carbon (90.3%) is higher than that of BC (78.4%), showing high selective adsorption performance.
Previous researches had shown that porous microstructure was one of the important factors affecting the adsorption performance of carbon adsorbents (Stüber et al. 2011). As shown in Fig. 3, the specific surface area of the prepared biochar and commercial activated carbon was determined by BET method. The adsorption isotherm of both the BC and AC was corresponded to type I according to the taxonomy of BDDT, which increased sharply at low relative pressure and formed a hysteresis loops in the N2 adsorption curve. The results show that the carbonaceous structure of the two adsorbents is mainly characterized by micropores, while the hysteresis loop is related to capillary condensation of mesoporous solids (Wen et al. 2011). And the adsorption capacities of BC and AC separately reached 72 cm3/g and 100 cm3/g, which increased gradually by the growth of P/P0 in the range of 0.1–0.75.
The detailed parameters for adsorption and desorption isotherms were listed in Table 3. The results showed that SBET and SLangmuir of BC were 476.87 and 565.22 m2/g, respectively, which were similar to that of the activated carbon. Compared with biochar obtained in other studies (55 and 49 m2/g), the surface area prepared in this study was significantly higher (Ros et al. 2007). With regard to the conventional pyrolysis, the increase of surface area was attributed to the rapid release of volatiles during microwave pyrolysis, resulting in the abundant and high porosity after pyrolysis. According to research reports, it takes less time to reach the required high temperature in the microwave pyrolysis by simultaneous heating of sludge inside and outside compared with the traditional heating (Zhang et al. 2017b, c).
Through the above analysis, it can be clearly concluded that the desulfurization efficiency was closely related to a large specific surface area of adsorbents. The research of Wen et al indicated that the average pore size of adsorbent was another important parameter affecting the removal efficiency in adsorption of gaseous formaldehyde (Wen et al. 2011). The parameters of specific surface area were described in Table 3. The average pore size of BC was 2.34 nm, which was larger than that of AC (1.67 nm). Generally, the larger pore size distribution could provide support and guarantee for the absorbates to reach the adsorption sites easily, improving the removal efficiency. In conclusion, the above results suggest that the H2S adsorption properties of biochar were mainly determined by porous structure of adsorbents, among which the surface area was the most important influence factor.
Mechanism of heavy metals stabilization and H2S removal by biochar
To explore the mechanism of heavy metal consolidation in the biochar, the SEM-EDS analysis of dried sludge and biochar particles was carried out. The results can be seen in Table 4 and Fig. 4. From Table 4, it was shows that compared with the dried sludge, a large amount of Si, Al, Cu, and Fe elements were enriched on the surface of the biochar particle during the microwave pyrolysis process. Smoother and denser microparticles were formed on the surface of biochar. Meanwhile, after pyrolysis, the biochar pH was found to increase from 7.36 of raw dried sludge to 11.05 presenting significantly alkaline ability. Thus, it was referred that the high alkaline environment and large number of metal elements on the biochar surface mainly contributed to the stabilization of Pb and Zn in the biochar. During study on the combustion of heavy oil, Rink et al. (1995) also discovered the generation of a large number of microparticles in which a large number of metal elements were enriched. The authors suggested that the chemical binding of different metals might explain the concentrating mechanism of heavy metals. In an alkaline environment, the repulsive force of positively charged metal ions decreases as H+ decreases. At the same time, part of Pb2+ and Zn2+ are converted into PbOH+ and ZnOH+, respectively. Therefore, the electrostatic interaction between heavy metal ions and biomass carbon is enhanced, which in turn increases the amount of heavy metal adsorption (Lu et al. 2012). In addition, mineral components such as phosphate and carbonate in biochar can precipitate with heavy metals as the pH value increases, reducing the leaching toxicity of heavy metals (Hu et al. 2013).
In order to investigate the changes of structure morphology of biochar before and after adsorption of H2S, the SEM of adsorbents was illustrated in Fig. 5. The surface porosity of biochar prepared by microwave pyrolysis of municipal sludge exhibited a superb pore-scale uniformity in Fig. 5a. Besides, the great development of the porous structure was primarily explained in the following aspects: The gas substances produced by high temperature pyrolysis of sludge certainly contribute to the formation of porous structure. On the other hand, the shrinkage and cracking of sludge surface caused by microwave heating conduction, which greatly increased the surface area and pore volume of biochar (Zhang et al. 2017b). The micromorphology of biochar after adsorption of H2S was shown in Fig. 5b, the results showed that part of micro-porous channel of BC was destroyed and microporous and mesoporous structures were further eroded with the increase of H2S adsorption amount, resulting in the decrease of pore volumes. The research (Wallace et al. 2014; Wu et al. 2017) pointed out that the metal elements on the surface of biochar were beneficial to the catalytic oxidation of H2S during the adsorption process to form metal sulfates or metal sulfides. The XPS analysis on the sulfur element also confirmed that the contents of sulfide and sulfate in the biochar before and after adsorption were increased correspondingly (Fig. 6). After H2S adsorption, the leachability of Zn and Pb metals decreased below the toxic limit. It is suggested that the main reason for reducing the leaching capacity of heavy metals in biochar through H2S adsorption was the formation of metal sulfides as shown Fig. 7. Consequently, BC provided an exercisable strategy for preparing efficient adsorbents to decrease the content of H2S from pyrolysis biogas.
Conclusions
In the present study, the interconnective effects of biochar on heavy metal consolidation and H2S removal from microwave pyrolysis of municipal sludge were investigated. Biochar achieved effective stabilization of heavy metals during sludge pyrolysis. Most (above 80%) of Pb and Zn metals were concentrated in the solid biochar, and the chemical speciation of heavy metals changed significantly. The contents of acid-soluble/exchangeable fraction (F1) plus reducible fraction (F2) for both metals were decreased by 26 and 40%, respectively, compared with the raw sludge. The consolidation performance of biochar was Pb> Zn under the same reaction conditions. The desulfurization efficiency of biochar during the post-treatment of H2S-containing pyrolysis biogas could reach 78.4%, which is relatively lower than 90.3% of activated carbon. The excellent adsorption performance is attributed to the higher specific surface area with SBET and SLangmuir of 476.87 m2/g and 565.22 m2/g, respectively. Biochar has double superposition effects on heavy metal fixation and H2S adsorption due to the formation of metal sulfide on the surface of biochar reducing the leachability of heavy metals. The research results found that reuse of biochar from sludge pyrolysis displayed great application potential due to its comparable pollutants-control capabilities and low-cost advantage.
References
Abdelhafez AA, Li J, Abbas MHH (2014) Feasibility of biochar manufactured from organic wastes on the stabilization of heavy metals in a metal smelter contaminated soil. Chemosphere 117:66–71
Bagreev A, Bandosz TJ (2004) Efficient hydrogen sulfide adsorbents obtained by pyrolysis of sewage sludge derived fertilizer modified with spent mineral oil. Environ Sci Technol 38:345–351
Bridle TR, Pritchard D (2004) Energy and nutrient recovery from sewage sludge via pyrolysis. Water Sci Technol 50:169–175
Bulushev DA, Ross JRH (2011) Catalysis for conventional of biomass to fuels via pyrolysis and gasification: A review. Catal Today 171:1–13
Cai QY, Mo CH, Wu QT et al (2007) Concentration and speciation of heavy metals in six different sewage sludge-composts. J Hazard Mater 147:1063–1072
Chen DMC, Bodirsky BL, Krueger T, Mishra A, Popp A (2020) The world’s growing municipal solid waste: Trends and impacts. Environ Res Lett 15(7):074021
Chen H, Chen D, Hong L (2015) Influences of activation agent impregnated sewage sludge pyrolysis on emission characteristics of volatile combustion and De-NOx performance of activated char. Appl Energy 156:767–775
Chen T, Zhang Y, Wang H, Lu W, Zhou Z, Zhang Y, Ren L (2014) Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol 164:47–54
Dong B, Liu X, Dai L, Dai X (2013) Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour Technol 131:152–158
Fuentes A, Llorens M, Saez J et al (2004) Phyto-toxicity and heavy metal speciation of stabilized sewage sludges. J Hazard Mater 108:161–169
Hu HY, Liu H, Shen WQ, Luo GQ, Li AJ, Lu ZL, Yao H (2013) Comparison of CaO’s effect on the fate of heavy metals during thermal treatment of two typical types of MSWI fly ashes in China. Chemosphere 93(4):590–596
Jin C, Zheng S, He Y, Zhou G, Zhou Z (2005) Lead contamination in tea garden soils and factors affecting its bioavailability. Chemosphere 59:1151–1159
Li R, Zhao W, Li Y et al (2014) Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J Hazard Mater 283:423–431
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46(3):854–862
Ma R, Sun S, Geng H, Fang L, Zhang P, Zhang X (2018) Study on the characteristics of microwave pyrolysis of high-ash sludge, including the products, yields, and energy recovery efficiencies. Energy 144:515–525
Motasemi F, Afzal MT (2013) A review on the microwave-assisted pyrolysis technique. Renew Sust Energ Rev 28:317–330
Nemati K, Bakar N, Abas M, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Rink KK, Kozinski JA, Lighty JS (1995) Biosludge incineration in FBCs: behavior of ash particles. Combus Flame 100:121–126
Ros A, Lillo-Roadenas MA, Canals-Batlle C, Fuente E, Montes-Moran MA, Martin MJ, Linares-Solano A (2007) A new generation of sludge-based adsorbents for H2s abatement at room temperature. Environ Sci Technol 41:4375–4381
Stüber F, Smith KM, Mendoza MB, Marques RRN, Fabregat A, Bengoa C, Font J, Fortuny A, Pullket S, Fowler GD, Graham NJD (2011) Sewage sludge based carbons for catalytic wet air oxidation of phenolic compounds in batch and trickle bed reactors. Appl Catal B Environ 110:81–89
Wallace R, Seredych M, Zhang P, etc (2014) Municipal waste conversion to hydrogen sulfide adsorbents: Investigation of the synergistic effects of sewage sludge/fish waste mixture. Chem Eng J 237:88–94
Wen Q, Li C, Cai Z, Zhang W, Gao H, Chen L, Zeng G, Shu X, Zhao Y (2011) Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde. Bioresour Technol 102:942–947
Wu D, Tian Y, Wen X, Zuo W, Liu H, Lee DJ (2015) Studies on the use of microwave for enhanced properties of glass-ceramics produced from sewage sludge pyrolysis residues (SSPR). J Taiwan Inst Chem Eng 48:81–86
Wu H, Zhu Y, Bian S, Ko JH, Li SFY, Xu Q (2017) H2S adsorption by municipal solid waste incineration (MSWI) fly ash with heavy metals immobilization. Chemosphere 195:40–47
Xiao Z, Yuan X, Li H, Jiang L, Leng L, Chen X, Zeng G, Li F, Cao L (2015) Chemical speciation, mobility and phyto-accessibility of heavy metals in fly ash and slag from combustion of pelletized municipal sewage sludge. Sci Total Environ 536:774–783
Yuan X, Huang H, Zeng G, Li H, Wang J, Zhou C, Zhu H, Pei X, Liu Z, Liu Z (2011) Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge. Bioresour Technol 102:4104–4110
Yuan X, Leng L, Huang H, Chen X, Wang H, Xiao Z, Zhai Y, Chen H, Zeng G (2015) Speciation and environmental risk assessment of heavy metal in bio-oil from liquefaction/pyrolysis of sewage sludge. Chemosphere 120:645–652
Zhang J, Tian Y, Cui Y, Zuo W, Tan T (2013) Key intermediates in nitrogen transformation during microwave pyrolysis of sewage sludge: a protein model compound study. Bioresour Technol 132:57–63
Zhang J, Tian Y, Zhang J, Li N, Kong L, Yu M, Zuo W (2017a) Distribution and risk assessment of heavy metals in sewage sludge after ozonation. Environ Sci Pollut Res 24:5118–5125
Zhang J, Zuo W, Tian Y, Chen L, Yin L, Zhang J (2017b) Sulfur transformation during microwave and conventional pyrolysis of sewage sludge. Environ Sci Technol 51:709–717
Zhang J, Zuo W, Tian Y, Yin L, Gong Z, Zhang J (2017c) Release of hydrogen sulfide during microwave pyrolysis of sewage sludge: effect of operating parameters and mechanism. J Hazard Mater 331:117–122
Zhang Y, Chen P, Liu S, Peng P, Min M, Cheng Y et al (2017) Effects of feedstock characteristics on microwave-assisted pyrolysis–a review. Bioresour Technol 230:143–151
Zhang J, Tian Y, Yin LL, Zhang J, Drewes JE (2018) Insight into the effects of biochar as adsorbent and microwave receptor from one-step microwave pyrolysis of sewage sludge. Environ Sci Pollut Res 25(19):18424–18433
Zorpas AA, Vlyssides AG, Zorpas GA, Karlis PK, Arapoglou D (2001) Impact of thermal treatment on metal in sewage sludge from the Psittalias wastewater treatment plant, Athens, Greece. J Hazard Mater 82:291–298
Zuo W, Tian Y, Ren N (2011) The important role of microwave receptors in bio-fuel production by microwave-induced pyrolysis of sewage sludge. Waste Manag 31:1321–1326
Acknowledgments
This research was supported by the National Key R&D Program of China (2019YFD1100300), National Natural Science Foundation of China (41877396, 51708157). The authors also appreciate State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (ES201905, 2020TS02) and Shenzhen Key Technology R&D Program of China (JSGG20180507183210868).
Availability of data and materials
The data and materials used or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Qingyuan Lin: Writing draft preparation
Methodology and Assembly of data:
Jun Zhang: Research concept and design
Linlin Yin: Contributed significantly to analysis and manuscript preparation
Hao Liu: Investigation and data analysis
Wei Zuo: Revised the manuscript
Yu Tian: Critical revision of the article
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
This work does not involve potential conflicts of interest research involving Human Participants and/or Animals. Our institution’s committee on research gave approval for this study, and all participants gave informed consent.
Consent to publish
The authors declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. The authors confirm that the manuscript has been read and approved by all named authors. The author agrees to publication in the Journal of Environmental Science and Pollution Research and also to publication of the article in English by Springer in Springer's corresponding English-language journal. The copyright transfer covers the exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online), or any other reproductions of similar nature. After submission of the agreement signed by the corresponding author, changes of authorship or in the order of the authors listed will not be accepted by Springer.
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Q., Zhang, J., Yin, L. et al. Relationship between heavy metal consolidation and H2S removal by biochar from microwave pyrolysis of municipal sludge: effect and mechanism. Environ Sci Pollut Res 28, 27694–27702 (2021). https://doi.org/10.1007/s11356-021-12631-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12631-4