Abstract
Papillary thyroid cancer (PTC) has inflicted huge threats to the health of mankind. Metal pollution could be a potential risk factor of PTC occurrence, but existing relevant epidemiological researches are limited. The current case-control study was designed to evaluate the relationships between exposure to multiple metals and the risk of PTC. A total of 262 histologically confirmed PTC cases were recruited. Age- and gender-matched controls were enrolled at the same time. Urine samples were used as biomarkers to reflect the levels of environmental exposure to 13 metals. Conditional logistic regression models were adopted to assess the potential association. Single-metal and multi-metal models were separately conducted to evaluate the impacts of single and co-exposure to 13 metals. The increased concentration of urinary Cd, Cu, Fe, and Pb quartiles was found significant correlated with PTC risk. We also found the decreased trends of urinary Se, Zn, and Mn quartiles with the ORs for PTC. These dose-response associations between Pb and PTC were observed in the single-metal model and remained significant in the multi-metal model (OR25-50th=1.39, OR50-75th=3.32, OR>75th=7.62, p for trend <0.001). Our study suggested that PTC was positively associated with urinary levels of Cd, Cu, Fe, Pb, and inversely associated with Se, Zn, and Mn. Targeted public health policies should be made to improve the environment and the recognition of potential risk factors. These findings need additional studies to confirm in other population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, the past several decades have observed a surge in papillary thyroid cancer (PTC) incidence (Kilfoy et al. 2009; Siegel et al. 2018). PTC is the most common subtype of thyroid cancer (TC) and comprises approximately 80% of incident cases (Lim et al. 2017). Some authors suggest that the increasing trend seems predominantly ascribe to an increased detection of small tumors using neck ultrasonography and ultrasound-guided fine-needle aspiration biopsies (Sanabria et al. 2018). However, accumulating evidence reveals that a substantial increase of PTC exists in all sizes and stages and a real rise is indeed occurring (Wang and Wang 2015). While family history, radiation exposure during childhood, and the metabolic syndrome are well-established risk factors, little research has focused on the role of other environment exposure, such as heavy metal, which may be important contributors to the increasing incidence of PTC (Almquist et al. 2011; Leux et al. 2012; Taylor et al. 2009).
Normal physiological function and development of the thyroid gland rest upon a balanced endocrine system. As ubiquitous ingredients of the natural environment, multiple metals reach the public through dietary intake, drinking water, medications, inhalation of ambient air, and frequent contact of consumer products. Heavy metals, characterized by carcinogenicity and bioaccumulation, irreversibility and perform as endocrine disruptors. Excessive exposure to heavy metals could produce negative health consequences, for example, liver and kidney dysfunction, cardiovascular disease, nervous system impairment, and malignant tumor (Kim et al. 2015; Rokadia and Agarwal 2013; Zimeri et al. 2015). Given the relationship between heavy metal exposure and endocrine disrupting effects, we hypothesize that chronic exposure to toxic heavy metal increase TC risk, in particular PTC risk. Indeed, the International Agency for Research on Cancer (IARC) has classified Cd as human carcinogen, Hg as probable carcinogen, and Pb as suspected human carcinogen.
In the recent years, Japanese epidemiological studies have indicated that a high level of Cd is connected with breast cancer, a major member of endocrine carcinoma (Itoh et al. 2014). Meanwhile, the ratio of Cu to Zn is associated with head and neck cancer (Kucharzewski et al. 2003). Animal evidence indicates that intake of B and Mo enhance rat thyroid cell transformation (Luca et al. 2017). All of these studies suggest that toxic metal that disrupt hormone homeostasis in an important way could lead to cancer risk.
In recent years, the pace of China’s economic development has shocked the world. But the degradation of ecological environment, especially metals pollution, has been the most painful price in the course of Chinese modernization. Given China’s 1.3-billion-person population base and drastic incidence rate of TC, thus TC has placed a heavy burden on the country. Although some researchers have made attempts to investigate whether multiple heavy metals were potential risk factors for thyroid-related diseases worldwide, most of the studies were conducted in a special population, such as residents living nearby the volcano and people with occupational exposure history. Thus, there is insufficient epidemiological study on the environmental exposure to heavy metals on the onset of PTC as well as on clinical characteristics of PTC in Chinese population. To address this limitation, we conducted a case-control study to fills this knowledge gap between various heavy metal level and PTC and to evaluate a possible link to clinical characteristics of PTC in Chinese.
Methods
Study population
Study protocols were reviewed and approved by the Anhui Medical University Biomedical Ethics Committee (Approval No. 20170305). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Prior to the research beginning, patients completed an informed consent form, containing a detailed explanation of the study.
The study population was selected continuously from September 2016 to March 2018 in the First Affiliated Hospital of Anhui Medical University, Second Affiliated Hospital of Anhui Medical University, and Anhui Provincial Cancer Hospital. Enrollment activities mainly included a urine sample collection and an interviewed-administrated questionnaire gathering. Subjects newly diagnosed with PTC were approached and invited to participate in this study, based on a definitive pathological diagnosis record. Cases with critical illness, such as severe hepatic and renal dysfunction, malignant anemia, and mental disorder, were excluded. We also exclude subjects who had a past history of malignant tumor. Pregnant and breastfeeding females were also excluded due to the various level of thyroid hormone during pregnancy. Ultimately, a total of 262 PTC patients were selected and comprised the case group.
The moment the PTC cases were confirmed, patients in other departments undergoing routine health care were randomly selected as control participants. Inclusion of controls was restricted to individuals free of thyroid diseases, severe hepatic and renal dysfunction, malignant anemia, mental disorder, and malignant tumor. Individuals who have thyroid dysfunction were also excluded, based on the thyroid function indicators, including free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH). Former studies indicated the correlation of gender and sex with PTC (Rago et al. 2010) and control individuals were matched to eliminate confounding factors based on sex and age (within 2 years of the cases’ age at recruitment). Finally, 262 healthy controls were enrolled in our study.
Covariate definition
Data on demographic characteristics, lifestyle, behavior habits, radioactive exposure history, and family history of thyroid cancer were obtained by trained investigators with interviewer-administered questionnaires. Height and weight were approached by admission registration information. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of a person’s height in meters.
People who are current smokers and have a history of cigarette smoking for greater than or equal to six consecutive months were defined as smokers. World Health Organization (WHO) indicates that passive smoking, sometimes, referred to as secondhand smoke, was defined as cigarette, cigar, or pipe smoke that is inhaled unintentionally for longer than 15 min once a week by nonsmokers other than intended “active” smoker. Alcoholic drinkers were considered as having ever drinking at least once a week for 12 months and consuming over 40 g of alcohol on any single week. We defined physical activities as at least 30 min of exercises for 1 day. Frequency of activities were divided into three groups (never, 1–4 times, ≥5 times). Information concerning the history of radioactive exposure was self-reported during the phase of study enrollment.
Urine metals
Upon enrollment, first-morning urine samples were collected by a 50-ml centrifuge tube and preserved at −80 °C until assayed. Urine value of 12 metals, including Fe, Zn, Cr, Cu, As, B, Mn, Hg, Pb, Se, Mo, and Mg, in both patients and controls were measured by inductively coupled plasma optical emission spectrometry (ICP-OES; PerkinElmer Optima 7000DV, USA). Prior to analysis, frozen urine samples were removed from −80 °C refrigerator and thawed at room temperature gradually. Twelve sets of calibration standards in nitric acid (HNO3) with internal standards were adopted to test the concentration corresponding metals in urine. An aliquot of 5-ml urine sample was transferred to 15-ml centrifuge tube, and then diluted with 5 ml 1% (v/v) HNO3 (guarantee reagent, GR) and then centrifuged with 4500 r/min for 8 min to obtain liquid supernatant for subsequent analysis. The element Cd was assayed by graphite furnace atomic absorption spectrometry (GFAAS, Analytik Jena AG ZEEnit®700P, Germany). Recovery test were conducted to examine the accuracy of experiment in every 10 urinary samples and the recovery rate ranged from 92 to 105%. Values of urinary concentrations below the limit of detection (LOD) were replaced with LOD. For urinary creatinine assay, colorimetric method was used with a Beckman DXC 800 automatic biochemistry analyzer.
Statistical analysis
Statistical analysis was performed by SPSS software (version 23.0. IBM Corp., Armonk, NY, USA). Descriptive analysis were performed to describe the demographic characteristics of study population. Comparisons of means between groups were made using paired t test. Chi-square tests were adopted for categorical variables. Spearman’s rank correlation test was conducted to calculate the correlations between urinary metals.
Wilcoxon signed-rank tests were used to evaluate differences of urine concentration in multiple metals between PTC and control groups. In addition, all urinary metal levels were adjusted by creatinine. The method of correction is calculated as the concentration of metals divided by corresponding concentration of creatinine. Geometric mean and percentile (5th, 25th, 50th, 75th, and 95th) was used to better understand the distribution of elements in each groups.
We used separate conditional logistic regression models to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for PTC risk. Individual urinary metals were categorized into quartiles according to their distributions among the controls and the lowest quartiles were deemed to be reference. Mode 1 was adjusted for matching factors of age and sex. Mode 2 was additionally adjusted for other potential confounders: BMI (continuous), annual household income (RMB, 10000–30,000; 30,000–60,000; and 60,000–120,000), smoking status (ever or never), frequency of physical activity weekly (0, 1–4, and ≥5), and family history of thyroid diseases (yes or no). Linear trend p values were derived by entering the quartiles of creatinine standardized urinary metal concentrations into the models as an ordinal categorical variable.
Given the interference effects of the co-exposure to multiple metals on the risk of PTC, we constructed a logistic regression model and all the urinary metals and potential confounders were considered. Backward elimination procedure was used and alpha at 0.10 was set for variables to be retained in the final model. Similarly, in the multi-metals model, potential covariates were adjusted. Moreover, subgroup analysis was also conducted to examine the associations according to gender (male or female), tumor size (≤1 cm and >1 cm) and smoking status (never, ever). All p values were test in two-sided and p<0.05 was considered statistically significant.
Results
Characteristics of study participants
The present study included 262 PTC cases and 262 corresponding non-PTC healthy control subjects. Basic anthropometric and clinical characteristics of the 524 participants are presented in Table 1. The mean age was 47.1 years in the cases and 46.8 years in the controls, and 67.2% of them were women. In comparison with the controls, PTC cases were more likely to have an elevated BMI and exercise frequency. In addition, they were apt to have a family history of thyroid diseases. On average, cases were similar to controls in the distributions of passive smoking, alcohol consumption, sleeping time, hypertension, and family history of malignant tumor. In addition, no statistical difference was observed between PTC patients and controls concerning regional distribution.
Concentrations of various metals
The geometric means were 0.22, 4.45, 190.59, 10.46, 3.14, 30.05, 445.86, 0.30, 12.61, 5.91, 38.28, and 18.83 μg/g creatinine for Cd, Fe, Zn, Cr, Cu, As, B, Mn, Hg, Pb, Se, and Mo and 26.02 mg/g creatinine for Mg, respectively. The distribution of 13 elements in urine of 524 individuals is summarized in Table 2. Concentrations of Cd, Cu, Pb, Hg, and Mo were significant higher in cases than in controls whereas Zn, Se, and Mn concentrations were significantly lower in PTC patients (p<0.05). No significant association was found for Fe, Cr, As, B, and Mg between the groups of case and control. Among the 13 metals, most of them were significantly but modestly correlated with each other. The spearman’s rank correlation coefficients ranged from −0.013 to 0.675 (see Table 3).
Metals exposure and PTC
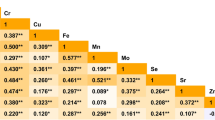
Conditional logistic regression analyses were performed to evaluate the associations between metals exposure level and PTC risk in single-metal model, and the results are listed in Table 4. After adjusting for potential confounders, the PTC cases were significantly associated with elevated urinary Cd, Fe, Cu, Hg, and Pb concentrations and these associations rely on creatinine standardization. Urinary concentration of Se was negatively associated with risk of PTC. An obvious association was observed between Cd exposure and PTC in highest quartile (OR=2.90, 95% CI 1.50–5.59). As for Cu, notably significant correlation was found in the second, third, and highest quartiles (OR25th–50th =2.54, 95% CI 1.43–4.51; OR50th–75th =1.81, 95% CI 1.01–3.24; 0R>75th =2.97, 95% CI 1.61–5.47). For Fe, increasing level of Fe was observed significant related to PTC risk and the OR in the highest quartile was 2.08 (95% CI 1.09–3.95). In addition, we observed prominently dose-response relationships between Pb and Hg and PTC risk (p for trend <0.05). Although concentrations of Se were inversely correlated with PTC occurrence in each quartile, these significant correlations failed to be a dose-response relationship (OR25th–50th =0.50, 95% CI 0.29–0.84; OR50th–75th =0.25, 95% C: 0.13–0.49; 0R75th- =0.58, 95% CI 0.35–0.98). Results of the associations of urinary metals with the risk of PTC are also depicted in Fig. 1.
In view of the simultaneous effect of shared exposure of various metal elements, we further evaluate the associations between urinary metals and the risk of PTC in the multi-metal model, in which all 13 metals and potential covariates were all taken into consideration synchronously (Table 5, Fig. 2). Cd, Fe, Se, Mn, Pb, Zn, and Cu were retained in the final model. The most results of the multi-metal model were in line with those of single-metal model. Increasing Cd, Cu, and Pb quartiles remained to be positively related with the increased risk of PTC, with the ORs in highest quartile of 2.60 (95% CI 1.20–5.64), 2.83 (1.27–6.30), and 7.62 (95% CI 3.46–16.83). In addition, elevated concentration of Fe was observed statistically correlated with PTC in quartiles 3 and 4 and the ORs were 3.22 (95% CI 1.49–6.95) and 2.86 (95% CI 1.22–6.70). Of note, Se was constantly retained in the model, indicating a protective role in PTC occurrence. Meanwhile, Mn and Zn were also presented an inversely correlation with the risk of PTC, with the ORs of 0.43 (95% CI 0.21–0.88) and 0.35 (95% CI 0.15–0.78), respectively. Moreover, mercury was not retained in the final multi-meta model, while it is significant in single-metal model. Moreover, considering the small number of ever smokers in this study, we also conduct a multivariate analysis restricted to nonsmokers and overall results remain stable (Table S2).
Subgroup analyses
Subgroup analysis was conducted by gender and tumor diameter (see Table 6). When stratified by gender, slight difference was found in the present association. Increasing trends of Fe and Cu with the risk of TC were only found in female individuals, while increasing trend of Cd quartiles with decreased TC risk was only found in male (p for trend <0.05). Within the subgroup classified by tumor diameter, significant association was exclusively observed between papillary thyroid micro-carcinoma (PTMC) and Mn exposure in highest quartile, with the value of OR to be 0.26 (95% CI 0.10–0.73). In addition, the result was presented in Table S1, when stratified by smoking status.
Discussion
Over the past 50 years, the economy in China has expanded more than 80 times, but it was at great ecological cost, especially metal pollution. Heavy metals could be enriched in the process of biological migration, for example, moving from soil to plants, bottom mud to aquatic organism, then transfer to human beings, resulting in elevated level of exposure (Cui et al. 2017). Metallic elements are essential for human health due to their unique role in metabolism. Normally, various metal elements lie in a homeostatic balance, nevertheless, which may be disturbed on account of suboptimal intake of one or more elements, contributing to consequences that multiple organs are not in the best condition of optimal health, including thyroid gland (Vye and Vtyurin 1995). In this study, we explored the associations of 13 urine metals with PTC risk with a number of noticeable findings fund in multiple statistical models. Taken together, urinary concentrations of Cd, Cu, Fe, and Pb were positively associated with PTC occurrence, whereas urinary Se, Mn, and Zn concentrations were inversely associated with PTC risk.
The majority of the subjects included in this study were from areas along the Yangtze River in China, which has always been polluted by heavy metals. Research conducted in residents of rural areas along the Yangtze River suggested that environmental level of multiple metals remained high (Cui et al. 2017). In this area, the geometric mean concentration of As was 80.3 μg/g in women, the 95th percentile of Cr among women was 111.02 μg/g and the concentrations of Cd were in high level, men and women both. Compared with our present study, one cohort study carried out in Wuhan City presented higher levels of urinary Fe, Cr, Mn, Zn, and Cu, but lower levels of As, Hg, Pb, and Se (Wu et al. 2018). Previous report indicated highest As level was in Japan (114.9 μg/g) (Hata et al. 2007; Wu et al. 2018). According to the data from 170 Spanish adults (Castaño et al. 2012), except Cd, the median concentration of Pb and Hg were both higher than that in our study. In fact, the levels of heavy metals in China were apparently higher than developed countries. The level of urinary Pb in Korea and Mexican seasonal farmworkers were clearly in a low level (Choi et al. 2017; Quandt et al. 2010). Additionally, if PTC patients came from more polluted areas than the controls, this kind of regional difference may contribute to an unreliable result. However, in our statistical analysis, no regional difference was observed between the two groups.
In our current study, low-to-moderate Cd exposure, as measured in urine, has no association with PTC. However, the obvious association was observed in high Cd exposure group. Epidemiological studies about the relationship between Cd and PTC were rare. Research conducted in a basaltic volcanic area indicated that TC incidence has increased in the volcanic area of high concentration of Cd compared with the control area, which was consistent with our study conclusion (Malandrino et al. 2016). In experimental studies (Jancic and Stosic 2014), Cd was proved to be an endocrine disruptor, supporting the hypothesis that this metal element could potentially induce hormone-dependent malignant tumors (Benbrahim-Tallaa et al. 2009). In occupational-exposed population (Pollán and Gustavsson 1999), Cd has been suggested to be associated with increased breast cancer incidence and mortality. Endocrine toxicity can result in dysfunction of the thyroid gland. The effect of Cd exposure on the thyroid can be proposed in most studies that presented unchanged T3 levels but decreased T4 levels, as the thyroid is the only organ involved in T4 synthesis (Buha et al. 2018). Possible mechanisms under this Cd effect on the thyroid gland may due to oxidative stress and its changes in serum levels suggest that Cd influences the hormone production and secretion by follicular cells (Hammouda et al. 2008).
Thyroid hormone levels affect the metabolism of Cu. Likewise, abnormal levels of trace elements Cu can interfere with the synthesis of thyroid hormone and its physiological functions. A meta-analysis of five case-control studies summarized the association between Cu exposure and TC risk, indicating that patients with TC had elevated levels of Cu than controls (SMD=2.372, p =0.001) (Shen et al. 2015). Data from the National Health and Nutrition Examination Survey showed levels of Cu were associated with increased levels of total triiodothyronine (TT3), FT4 and total thyroxine (TT4) (Jain 2014). Besides, in a population epidemiological study, the researchers found that the urinary copper and blood copper concentrations in the goiter group were both higher than in healthy people. This study confirmed a possible correlation between increased urinary copper levels and the risk of PTC. Studies on mechanisms elucidated that normal thyroid function depends on certain nutritional status, especially iodine, Se, and tyrosine. Cu is an essential trace element in the metabolism of tyrosine. The apoptosis of normal thyroid cells is an imbalance outcome between the exceed production of oxygen free radicals and H2O2 and the body’s ability to neutralize them, which eventually leads to be carcinogenesis (Mittag et al. 2012).
Underlying mechanisms for the associations of PTC with Fe are not clear. Most of the studies recently suggested that Fe deficiency may affect thyroid hormone status (Yavuz et al. 2004). An Iran study presented that subjects with low serum ferritin had a higher ratio of T3/T4 (Eftekhari et al. 2006). A cross-sectional study conducted in Nepalese children found that anemic and Fe-deficient children had poor thyroid function (Khatiwada et al. 2016). In our study, nonetheless, the result was at odds with the above conclusions. The outcome from multi-metal model indicated elevated concentration of urinary Fe could enhance the risk of PTC. The discrepancy of the study results may ascribe to the difference of study population. Previous studies preferred to explore the relationship between thyroid diseases and special groups (e.g., pregnant women, adolescents).
Although humans may be widely exposed to Pb in daily life, there is very little information on the potential thyroid health effects of chronic exposure to Pb. Pb is a chronic toxic substance, long-term low-level Pb exposure can cause serious damage to human organs and tissues. The field epidemiological study in Argentina Pb working population found that after long-term exposure to Pb, thyroid function was impaired severely, and the level of TSH was in notable high condition (López et al. 2000). In the present study, we found a significant association between Pb exposure and PTC risk, with a clear dose-response curve, indicating that Pb plays an important role in the pathogenesis of thyroid disease. The exact biological mechanisms that link Pb exposure and PTC risk remained uncertain. Rana et al. pointed out that Pb had a certain accumulation in the thyroid gland and disturbed the normal physiological function of the hypothalamic-pituitary-thyroid (HPT) axis, which may be the primary explanation (Rana 2014).
Hg threatens human health and can impair many physiological processes. As previously stated, mercury is insidious and toxic even at a very low dose of exposure. With respect to the association between urinary Hg level and PTC risk, we identified a positive relationship in single-metal mode. Compared with lowest quartile, the relative risks of second, third and highest quartile were 1.76, 2.37, and 2.98, respectively, revealing a strong and significant dose-response relationship between Hg level and PTC risk. An epidemiological study conducted in Ghana supported our finding, which found elevated TSH level was associated with boosted blood Hg concentration (Afrifa et al. 2018). An existing study on zebra fish demonstrated that environmentally relevant concentrations of Hg2+ exposure could change the levels of thyroid hormone and the transcription of related HPT axis genes, interfering with the normal metabolism of thyroid hormone (Sun et al. 2018).
Although accumulating evidence has shown a lower TC risk associated with high Se level, it is still of considerable debate about whether this represent a causal relationship or arises from potential confounding (Kohrle and Gartner 2009; Schomburg and Kohrle 2008). Based on the results of multivariate analysis, our research confirmed a prominent inverse association between PTC occurrence and Se. A pooled-analysis including six case-control studies suggested that patients with TC had lower levels of Se than the healthy controls (Vye and Vtyurin 1995). The thyroid gland is the organ with the most abundant Se in the human body. Se can be involved in the expression of various enzymes in the thyroid tissue. The reason why Se could reduce the relative risk of TC is indistinct. The mechanism underlying the effects of Se is that everlasting Se deficiency severely impairs the biosynthesis of thyroid hormone and enhances the destruction of follicular structures and accelerates their replacement by fibrotic tissue (Duntas 2010). In addition, lack of Se could result in increased TSH level. Thus, chronic TSH stimulation was correlated with exacerbated tumor proliferation and aggressiveness.
Zn is a common trace element but also a key component of the enzyme superoxide dismutase. Zn is important for normal thyroid balance, including effects on hormone synthesis and the way it works, but its role is extremely complex and little research kept watchful eye on the correlation in between. Malfunction of its transporters have been related to chronic diseases such as malignant tumors. According to the NIH-AARP diet and health study, a non-significant protective effect was observed in correlation between dietary Zn intake and TC risk (O'Grady et al. 2014). A nutrition survey conducted in MTC patients found higher concentration of Zinc in MTC groups compared with healthy controls (Emami et al. 2017). Additionally, data from National Health and Nutrition Examination Survey revealed that, for males, levels of zinc were associated with decreased levels of FT4 and TT4. In our study, compared with the lowest quartile group, the highest quartile group presented an inversely relationship between zinc exposure and PTC. More profound mechanism study were warranted to clarify this phenomenon.
Thus far, Mn has been well known for its significant role in immunity improvement and CVD prevention, little has been known about the association between the urinary level of Mn and PTC (Xu et al. 2006). In an in vivo experiment, Eder et al. found Mn deficiency could alter thyroid-hormone metabolism in rats (Eder et al. 1996). Additionally, researchers indicated that Mn concentration was presented with downtrend in breast cancer patients (Kilic et al. 2004). Our study was consistent with this experiment and indicated that deficiency of Mn could eventually develop into PTC. Why could Mn be a protective role in cancer occurrence? A potential mechanism hypothesis is that Mn can effectively change carcinogens into easily excreted metabolites with polar gene; meanwhile, it can also significantly enhance the ability of detoxification in liver, thus effectively inhibits the occurrence of multiple tumors (Mitrunen et al. 2001).
Tobacco smoking was a vital source of contamination of heavy metals and has more than 40 kinds of ingredients proven to be carcinogenic. Large amounts of studies have confirmed the association between smoking and malignant tumors, especially lung cancer. However, there are complicated but interesting relations taken on between tobacco smoking and TC. A meta-analysis indicated that smoking, particularly current smoking, may be a protective factor of TC and smoking may influence susceptibility to TC and result in a decreased TSH level (Cho and Kim 2014). Another cohort study reached a non-significant association between former smoking and development of TC (Cho et al. 2018). In our study, smoking was considered as a risk factor of PTC. Many kinds of heavy metals were largely extracted from the soil by tobacco plants. In addition, due to soil having high ambient heavy metal concentrations, level of heavy metals in tobacco were naturally higher. Of note, Cd and Pb were important matters presented in tobacco smoke and contribute substantially to cancer risk (Khlifi and Hamza-Chaffai 2010). However, because more than two thirds of the PTC cases were female and Chinese women has the tradition of non-smoking, only few smokers were included in our study, limiting the power of statistical analysis.
To our best knowledge, this study is the first study to evaluate the association between internal exposures of multiple metals and PTC among Chinese population. As we all know, China is probably the fastest developing country the world has ever seen. A downside of the economic boom has been environmental degradation. China is home to many kinds of pollution, among which, discharge of metals is the most important concern. For the present research, we focused on the effects of non-occupational exposure to multiple metals in individual urinary sample on PTC risk in Chinese population. This could provide little but valuable suggestion for the public health department in China. Moreover, multi-metal models were applied in the analysis, examining the independent associations between metal elements and PTC risk. We performed stringent quality control standards in data collection and laboratory detection to reduce the potential of systemic bias and measurement error. In addition, an age, sex matched, case-control design improve the comparability of groups.
However, several limitations need to be noticed. First, we did not distinguish metals species among metabolites in urine, for example, organic and inorganic Hg and our study hence represented the total level of each element. Second, due to the mechanisms of homeostasis and regulation, urine sample may not be the most reliable biomarker for measuring the status of all metal elements. For example, blood serum is most suitable for Fe measurement while urine is a better choice for As. However, it was reported that there existed a significant correlation between plasma and urine concentrations for multiple metals in Chinese adults. Therefore, urine sample could accurately reflect the load of various metals in human body. Third, as a result of the nature of a retrospective study, the ability of causal inference was limited. Finally, due to the characteristic of a hospital-based case-control design, selection bias, especially admission rate bias, may exist in the process of investigation.
Conclusion
In summary, we observed significant associations between urinary concentrations of several metals and PTC in Chinese. Our findings could require further clarification but have crucial implications for public health, given the high burden of PTC, both in China and the rest of the world.
References
Afrifa J, Ogbordjor WD, Duku-Takyi R (2018) Variation in thyroid hormone levels is associated with elevated blood mercury levels among artisanal small-scale miners in Ghana. PLoS One 13:e0203335. https://doi.org/10.1371/journal.pone.0203335
Almquist M, Johansen D, Björge T, Ulmer H, Lindkvist B, Stocks T, Hallmans G, Engeland A, Rapp K, Jonsson H, Selmer R, Diem G, Häggström C, Tretli S, Stattin P, Manjer J (2011) Metabolic factors and risk of thyroid cancer in the metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control : CCC 22:743–751. https://doi.org/10.1007/s10552-011-9747-2
Benbrahim-Tallaa L, Tokar EJ, Diwan BA, Dill AL, Coppin JF, Waalkes MP (2009) Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ Health Perspect 117:1847–1852. https://doi.org/10.1289/ehp.0900999
Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri E, Tsatsakis A, Schweitzer A, Wallace D (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 19. https://doi.org/10.3390/ijms19051501
Castaño A, Sánchez-Rodríguez JE, Cañas A, Esteban M, Navarro C, Rodríguez-García AC, Arribas M, Díaz G, Jiménez-Guerrero JA (2012) Mercury, lead and cadmium levels in the urine of 170 Spanish adults: a pilot human biomonitoring study. Int J Hyg Environ Health 215:191–195. https://doi.org/10.1016/j.ijheh.2011.09.001
Cho YA, Kim J (2014) Thyroid cancer risk and smoking status: a meta-analysis. Cancer causes & control : CCC 25:1187–1195. https://doi.org/10.1007/s10552-014-0422-2
Cho A, Chang Y, Ahn J, Shin H, Ryu S (2018) Cigarette smoking and thyroid cancer risk: a cohort study. Br J Cancer 119:638–645. https://doi.org/10.1038/s41416-018-0224-5
Choi W, Kim S, Baek YW, Choi K, Lee K, Kim S, Yu SD, Choi K (2017) Exposure to environmental chemicals among Korean adults—updates from the second Korean National Environmental Health Survey (2012–2014). Int J Hyg Environ Health 220:29–35. https://doi.org/10.1016/j.ijheh.2016.10.002
Cui Y, Zhong Q, Hu M, Sheng J, Yang Y, Liang L, Wang X, Yang Y, Zhou M, Huang F (2017) Human biomonitoring of eight trace elements in urine of residents living in rural areas along the Yangtze River. China Environ Sci Pollut Res Int 24:27963–27973. https://doi.org/10.1007/s11356-017-0414-3
Duntas LH (2010) Selenium and the thyroid: a close-knit connection. J Clin Endocr Metab 95:5180–5188. https://doi.org/10.1210/jc.2010-0191
Eder K, Kralik A, Kirchgessner M (1996) The effect of manganese supply on thyroid hormone metabolism in the offspring of manganese-depleted dams. Biol Trace Elem Res 55:137–145. https://doi.org/10.1007/bf02784175
Eftekhari MH, Keshavarz SA, Jalali M, Elguero E, Eshraghian MR, Simondon KB (2006) The relationship between iron status and thyroid hormone concentration in iron-deficient adolescent Iranian girls. Asia Pac J Clin Nutr 15:50–55
Emami A, Nazem MR, Shekarriz R, Hedayati M (2017) Micronutrient status (calcium, zinc, vitamins D and E) in patients with medullary thyroid carcinoma: a cross-sectional study. Nutrition 41:86–89. https://doi.org/10.1016/j.nut.2017.04.004
Hammouda F, Messaoudi I, El Hani J, Baati T, Saïd K, Kerkeni A (2008) Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res 126:194–203. https://doi.org/10.1007/s12011-008-8194-8
Hata A, Endo Y, Nakajima Y, Ikebe M, Ogawa M, Fujitani N, Endo G (2007) HPLC-ICP-MS speciation analysis of arsenic in urine of Japanese subjects without occupational exposure. J Occup Health 49:217–223. https://doi.org/10.1539/joh.49.217
Itoh H, Iwasaki M, Sawada N, Takachi R, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Yokoyama K, Tsugane S (2014) Dietary cadmium intake and breast cancer risk in Japanese women: a case-control study. Int J Hyg Environ Health 217:70–77. https://doi.org/10.1016/j.ijheh.2013.03.010
Jain RB (2014) Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol Trace Elem Res 159:87–98. https://doi.org/10.1007/s12011-014-9992-9
Jancic SA, Stosic BZ (2014) Cadmium effects on the thyroid gland. Vitam Horm 94:391–425. https://doi.org/10.1016/B978-0-12-800095-3.00014-6
Khatiwada S, Gelal B, Baral N, Lamsal M (2016) Association between iron status and thyroid function in Nepalese children. Thyroid Res 9:2–2. https://doi.org/10.1186/s13044-016-0031-0
Khlifi R, Hamza-Chaffai A (2010) Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: a review. Toxicol Appl Pharmacol 248:71–88. https://doi.org/10.1016/j.taap.2010.08.003
Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y (2009) International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control : CCC 20:525–531. https://doi.org/10.1007/s10552-008-9260-4
Kilic E, Saraymen R, Demiroglu A, Ok E (2004) Chromium and manganese levels in the scalp hair of normals and patients with breast cancer. Biol Trace Elem Res 102:19–25. https://doi.org/10.1385/Bter:102:1-3:019
Kim NH, Hyun YY, Lee KB (2015) Environmental heavy metal exposure and chronic kidney disease in the general population. J Korean Med Sci 30:272–277. https://doi.org/10.3346/jkms.2015.30.3.272
Kohrle J, Gartner R (2009) Selenium and thyroid. Best Pract Res Cl En 23:815–827. https://doi.org/10.1016/j.beem.2009.08.002
Kucharzewski M, Braziewicz J, Majewska U, Gozdz S (2003) Copper, zinc, and selenium in whole blood and thyroid tissue of people with various thyroid diseases. Biol Trace Elem Res 93:9–18. https://doi.org/10.1385/bter:93:1-3:9
Leux C, Truong T, Petit C, Baron-Dubourdieu D, Guenel P (2012) Family history of malignant and benign thyroid diseases and risk of thyroid cancer: a population-based case-control study in New Caledonia. Cancer Causes Control : CCC 23:745–755. https://doi.org/10.1007/s10552-012-9944-7
Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM (2017) Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. Jama 317:1338–1348. https://doi.org/10.1001/jama.2017.2719
López CM, Piñeiro AE, Núñez N, Avagnina AM, Villaamil EC, Roses OE (2000) Thyroid hormone changes in males exposed to lead in the Buenos Aires area (Argentina). Pharmacol Res 42:599–602. https://doi.org/10.1006/phrs.2000.0734
Luca E, Fici L, Ronchi A, Marandino F, Rossi ED, Caristo ME, Malandrino P, Russo M, Pontecorvi A, Vigneri R, Moretti F (2017) Intake of boron, cadmium, and molybdenum enhances rat thyroid cell transformation. J Exp Clin Cancer Res: CR 36:73. https://doi.org/10.1186/s13046-017-0543-z
Malandrino P, Russo M, Ronchi A, Minoia C, Cataldo D, Regalbuto C, Giordano C, Attard M, Squatrito S, Trimarchi F, Vigneri R (2016) Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 53:471–479. https://doi.org/10.1007/s12020-015-0761-0
Mitrunen K, Sillanpää P, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Uusitupa M, Hirvonen A (2001) Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 22:827–829. https://doi.org/10.1093/carcin/22.5.827
Mittag J, Behrends T, Nordstrom K, Anselmo J, Vennstrom B, Schomburg L (2012) Serum copper as a novel biomarker for resistance to thyroid hormone. Biochem J 443:103–109. https://doi.org/10.1042/bj20111817
O'Grady TJ, Kitahara CM, DiRienzo AG, Gates MA (2014) The association between selenium and other micronutrients and thyroid cancer incidence in the NIH-AARP diet and health study. PLoS One 9:e110886. https://doi.org/10.1371/journal.pone.0110886
Pollán M, Gustavsson P (1999) High-risk occupations for breast cancer in the Swedish female working population. Am J Public Health 89:875–881
Quandt SA, Jones BT, Talton JW, Whalley LE, Galván L, Vallejos QM, Grzywacz JG, Chen H, Pharr KE, Isom S, Arcury TA (2010) Heavy metals exposures among Mexican farmworkers in eastern North Carolina. Environ Res 110:83–88. https://doi.org/10.1016/j.envres.2009.09.007
Rago T, Fiore E, Scutari M, Santini F, di Coscio G, Romani R, Piaggi P, Ugolini C, Basolo F, Miccoli P, Pinchera A, Vitti P (2010) Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol 162:763–770. https://doi.org/10.1530/eje-09-0895
Rana SVS (2014) Perspectives in endocrine toxicity of heavy metals—a review. Biol Trace Elem Res 160:1–14. https://doi.org/10.1007/s12011-014-0023-7
Rokadia HK, Agarwal S (2013) Serum heavy metals and obstructive lung disease: results from the National Health and nutrition. Examination Survey. Chest 143:388–397. https://doi.org/10.1378/chest.12-0595
Sanabria A, Kowalski LP, Shah JP, Nixon IJ (2018) Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck 40:855–866. https://doi.org/10.1002/hed.25029
Schomburg L, Kohrle J (2008) On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res 52:1235–1246. https://doi.org/10.1002/mnfr.200700465
Shen F, Cai WS, Li JL, Feng Z, Cao J, Xu B (2015) The association between serum levels of selenium, copper, and magnesium with thyroid cancer: a meta-analysis. Biol Trace Elem Res 167:225–235. https://doi.org/10.1007/s12011-015-0304-9
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Sun YL, Li YW, Liu ZH, Chen QL (2018) Environmentally relevant concentrations of mercury exposure alter thyroid hormone levels and gene expression in the hypothalamic-pituitary-thyroid axis of zebrafish larvae fish. Physiol Biochem 44:1175–1183. https://doi.org/10.1007/s10695-018-0504-2
Taylor AJ, Croft AP, Palace AM, Winter DL, Reulen RC, Stiller CA, Stevens MCG, Hawkins MM (2009) Risk of thyroid cancer in survivors of childhood cancer: results from the British childhood Cancer survivor study. Int J Cancer 125:2400–2405. https://doi.org/10.1002/ijc.24581
Vye ZTAF, Vtyurin BM (1995) Trace Elements and Thyroid Cancer. Analyst 120:817
Wang Y, Wang W (2015) Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia-Pac J Public Health 27:Np223–Np229. https://doi.org/10.1177/1010539512436874
Wu W, Jiang S, Zhao Q, Zhang K, Wei X, Zhou T, Liu D, Zhou H, Zhong R, Zeng Q, Cheng L, Miao X, Lu Q (2018) Associations of environmental exposure to metals with the risk of hypertension in China. Sci Total Environ 622-623:184–191. https://doi.org/10.1016/j.scitotenv.2017.11.343
Xu SQ, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schöneich C, Cohen RA (2006) Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol-Heart C 290:H2220–H2227. https://doi.org/10.1152/ajpheart.01293.2005
Yavuz O, Yavuz T, Kahraman C, Yesildal N, Bundak R (2004) The relationship between iron status and thyroid hormones in adolescents living in an iodine deficient area. J Pediatr Endocrinol Metab 17. https://doi.org/10.1515/JPEM.2004.17.10.1443
Zimeri AM, Robb SW, Hassan SM, Hire RR, Davis MB (2015) Assessing heavy metal and PCB exposure from tap water by measuring levels in plasma from sporadic breast cancer patients, a pilot study. Int J Environ Res Public Health 12:15683–15691. https://doi.org/10.3390/ijerph121215013
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81373071) and the Project for Anhui Province Academic Technology Leader Reserve Candidates’ Academic Research Activities (2017H108).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Anhui Medical University Biomedical Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, C., Wu, HB., Cheng, MX. et al. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ Sci Pollut Res 26, 20560–20572 (2019). https://doi.org/10.1007/s11356-019-04733-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04733-x