Abstract
Exposure to heavy metals in the environment exerts serious effects on kidney health. However, the effects of joint exposure on the kidneys have been rarely studied, particularly in non-occupational exposure high-risk populations. This study provided a reference threshold range of heavy metals in urine and explored the effect of joint exposure on nephrolithiasis in men. The data were obtained from the China Multi-Ethnic Cohort database, and 1502 men were included in the study. A two-piece-wise regression model was used to assess the dose–response relationship between heavy metal exposure and nephrolithiasis. The least absolute shrinkage and selection operator regression model was used to calculate the score of joint exposure to heavy metals. The threshold effect analysis revealed a linear relationship between the concentration of arsenic (As) in the urine and the prevalence of nephrolithiasis, whereas a nonlinear relationship was observed with cadmium (Cd), chromium (Cr), mercury (Hg), and lead (Pb). In addition, As, Cd, Cr, Hg, and Pb may significantly affect the joint exposure effect. Moreover, the final risk of nephrolithiasis increased by 123% (P for trend < 0.001). This study found a threshold relationship between heavy metals (Cd, Cr, Hg, Pb) in male urine and the occurrence of nephrolithiasis. Joint exposure to heavy metals in urine caused a high-risk effect on nephrolithiasis. The study provided a reference threshold value of related studies and indicated that environmental pollution caused by heavy metals should be reduced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are natural metals with an atomic number greater than 20 and element density greater than 5 g/cm3 [1]. With the rapid development of the economy and society, heavy metal pollution has become one of the widespread environmental problems worldwide, with adverse effects on the aquatic, terrestrial, and atmospheric environments [2]. Among them, toxic heavy metals, including arsenic (As), cadmium (Cd), chromium (Cr), mercury (Hg), and lead (Pb), are of utmost concern [3]. According to the International Agency for Research on Cancer, these metals were classified as human carcinogens (known or probable) [4]. Their toxicity depends on the dose, route of exposure, and chemical species, as well as the age, sex, genetics, and nutrition of the individual exposed. They affect multiple organs of the human body and were associated with several diseases [5]. Kidneys are particularly sensitive to contamination because filtrates can be concentrated (> threefold) in the proximal tubule during their transportation along with the renal tubules [6]. Heavy metals affect the kidneys through increased excretion of low-molecular-weight proteins in the urine due to damage to proximal tubular cells [7]. Oxidative stress generating reactive oxygen species (ROS) is the major mechanism underlying heavy metal-induced nephrotoxicity [8]. For example, hydronephrosis occurs when arsenic oxides deposited in the kidney are converted to methylated metabolites by As3+ methyltransferase [9]. The high levels of exposure to Cd cause increased lipid peroxidation and decreased antioxidant supply, resulting in its accumulation in the proximal tubules of kidneys and impairing tubular function and protein reabsorption [10, 11]. The analysis of the Cr metabolism pathway revealed that Cr and Cr compounds induced the oxidation and functional degradation of the kidneys, thereby damaging blood cells [12]. Hg2+ has long been known to cause necrosis of the renal unit [13]. Several functional dysregulations have been linked to Pb exposure, such as enzymuria, proteinuria, impaired anion, and glucose transportation, eventually leading to apoptosis and damage to kidney structure and function [14, 15]. In addition, the exposure to even low levels of these metals can cause them to slowly accumulate and result in chronic kidney disease and proteinuria [16].
Most of the effects of heavy metals on the kidneys are associated with nephrolithiasis, a common kidney disease with a prevalence rate of 15 to 20% worldwide [17]. It is caused by dehydration, decreased urine output or fluid flow rate, or increased mineral excretion [18]. It occurs because of the joint effect of environment and genes. The occurrence of the disease is higher in men than in women [19], because estrogen induces proteomic alterations in renal tubular cells, which decrease the expression of calcium oxalate crystal receptors and thus prevent nephrolithiasis [20]. Therefore, the analysis of heavy metal exposure in men’s urine may contribute to explore the reasons for the consistently higher prevalence of nephrolithiasis in men than in women.
Although several studies have focused on heavy metals in the blood, studies on urine heavy metals are relatively rare. However, to evaluate renal diseases, the most useful information was acquired by measuring glomerular filtration rate and examining urine sediment [21]. Metal content in the urine is frequently used as a biomarker in human studies, especially in large group studies [22]. The 24-h urine test is widely considered as the gold standard to assess the excretion of various analytes over time [23]. Urinary heavy metal concentrations can reflect the excretion of heavy metals in the body and indirectly reflect the amount accumulated in the organism [24]. Therefore, urine element concentration forms an important basis for clinical monitoring and evaluation of heavy metal poisoning. A few studies exist on the normal dosage range of heavy metals in urine after being metabolized by the human body. Moreover, studies on nephrolithiasis are often restricted to single or interactive effects, with few reports on joint urinary metal exposure.
Most studies in the cross-sectional direction of heavy metals have been conducted on occupationally exposed populations, with insufficient studies on populations at high risk of non-occupational exposure [16, 25,26,27]. In addition, studies on nephrolithiasis are usually limited to single or interactive effects, with a few reports on joint urinary metal exposure [28,29,30]. Although the levels of heavy metals in the urine vary from region to region [31], significant exposure can adversely affect kidney function [32, 33]. Thus, studying the relationship between exposure and nephrolithiasis in non-occupational, high-risk populations is of high relevance. Although an increasing number of study groups are working on nephrolithiasis and heavy metals, a few have indicated their threshold ranges. This study investigated the threshold effect of heavy metals in the urine of Chinese Dong men under a stable genetic background. In addition, the effect of joint exposure to heavy metal elements in the environment on the prevalence of nephrolithiasis in men was investigated to understand the reasons for the differences in the occurrence rate between men and women with nephrolithiasis.
Materials and Methods
Research Subjects
From July to December 2018 in the Qiandongnan Prefecture, China (Kaili City, Liping County, Defeng Town, Yandong Town, Zhaoxing Town, and Zhongchao Town), the Dong people in the Guizhou Province of China aged 30 to 79 years who had been living for three generations were included in the study. A cross-sectional study was conducted using the multistage stratified cluster sampling method. The data of Dong population were obtained from the China Multi-Ethnic Cohort (CMEC) Study database. A total of 5792 people from the Dong ethnic group were selected from the database, and non-nephrolithiasis who lacked urine samples and whose abdominal B-ultrasound examination showed renal calcification were excluded. The selected nephrolithiasis-free population had no history of nephrolithiasis, no kidney disease conditions such as kidney calcification, with an intact urine sample. The final study population consisted of 4479 individuals, including 1502 men (Fig. 1). All participants signed an informed consent form before the investigation. This study was approved by the Sichuan University Medical Ethical Review Board (K2016038) and the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University (2018[094]).
Basic Data Collection
We used a tablet computer with a self-developed application (CMEC application) to collect questionnaire information. It was collected through face-to-face interviews conducted by well-trained interviewers, who typically had medical backgrounds and were local college students [34]. Baseline information included gender, age, smoking status, alcohol intake, and physical examination. The physical examination was performed by a professional doctor; it included height, weight, blood pressure, chest X-ray, and abdominal ultrasound. The participant’s body mass index (BMI) was calculated using weight (kg) and height (m2). The electronic sphygmomanometer measured blood pressure thrice in total, with an interval of 1 min between each measurement. The average value was considered the final systolic (SBP) and diastolic blood pressure (DBP). Glomerular filtration rate (GFR) was calculated based on gender, age, and serum creatinine (CR) according to the Chronic Kidney Disease Epidemiology Collaborative Group method [35, 36].
All participants fasted for more than 8 h, and blood samples were collected on the spot in the morning. Venous blood was collected, and one tube of serum and two tubes of ethylenediaminetetraacetic acid anticoagulant were prepared. The blood samples were transported under cold chain logistics (− 80 °C) to the Golden Field Medical Inspection Center on the same day for testing. Roche Modular P800 automatic biochemical analyzer (Roche, Basel, Kanton Basel, Switzerland) was used to measure CR, urea (UREA), and uric acid (UA). Britest 200B (URIT, Guilin, Guangxi, China) was used to measure urine protein (UPRO) and urine-specific gravity.
Assessment of Heavy Metal Elements in Urine
A 5 mL sample of urine was collected in the middle of the morning and stored at − 20 °C. The sample was placed at room temperature before use. Next, 1 mL of the original urine supernatant sample was diluted with 9 mL of nitric acid solution (5%) (GR, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) in a 10 mL polyethylene centrifuge tube, which was well-mixed and filtered through a 0.45 μm membrane. The diluted sample was measured within 24 h. Using an inductively coupled plasma mass spectrometer (NexION 2000, PerkinElmer, Waltham, MA, USA), the concentrations of heavy metal elements, including As, Cd, Cr, Hg, and Pb, were determined. Internal standard solutions of Bi, Ge, In, 6Li, Sc, Tb, and Y at a concentration of 10 µg/L provided by PerkinElmer were used for internal online standardization, and the regression coefficient of the standard curve was maintained above 0.999. Seronorm™ Trace Elements Urine L-2 RUO (Sero, Billingstad, Norway) was used for checking the accuracy. Percent recovery was between 80 and 120% (Table 2). The detection limit falls within 0.0001 and 0.0168 µg/L for the metals tested, and the concentration of the sample below the detection limit was expressed as half of the limit of quantification. The final concentration was corrected by urine-specific gravity and multiplied by 10 [37].
Assessment of Nephrolithiasis
Nephrolithiasis was diagnosed by a specialized physician at the Second Affiliated Hospital of Guizhou Medical University using ultrasonography (Apogee 1200, Shantou Ultrasound, Shanghai, China) that revealed strong urinary light spots or clusters with an echogenic diameter ≥ 4 mm or results of a chest X-ray (1250 Shimadzu 500 mA X-ray machine, Shimane Prefecture, Japan) were used [34, 38].
Statistical Analysis
We performed a statistical analysis of the final population data. Continuous variables are represented as mean ± standard deviation. Categorical variables are expressed in proportion. Nonparametric methods were used to compare the difference between continuous variables in the nephrolithiasis and non-nephrolithiasis groups. After dividing the measured quartiles of five heavy metals into the groups, using the first group as a reference, multiple regression equations were used to adjust multiple variables (age, BMI, SBP, DBP, CR, GFR, UREA, UA, smoking status, and alcohol intake). The results showed the independent effect of each group on the prevalence of nephrolithiasis by odds ratio (OR), with a confidence interval of 95% (95% CI). A linear trend test was performed by entering the median of the concentration of each group of heavy metal elements in the model as a continuous variable. After logarithmic transformation of the concentration data of five heavy metals, smooth curve fitting was performed according to the generalized additive model.
The threshold effect analysis was used to describe the linear/nonlinear relationship between the concentration of heavy metal elements and the prevalence of nephrolithiasis. First, we determined whether the P-value of the log-likelihood ratio test (LRT) was significant (P < 0.05). If model 1 was not significantly selected, model 2 was selected significantly. Model 1 was a univariate linear regression, and model 2 was a two-piece-wise regression model. Next, the bootstrap resampling method was used to determine the credible interval of the threshold [39, 40].
To solve the collinearity problem among heavy metal elements, we adopted the least absolute shrinkage and selection operator (Lasso) regression method. A formula was obtained for calculating the score value of five heavy metal elements and their regression coefficients. The score value was then subjected to smooth curve fitting and threshold effect analysis to obtain the final relationship between the five heavy metal elements in urine and nephrolithiasis.
All statistical analyses were performed by statistical packages R (The R Foundation; http://www.r-project.org; version 3.4.3.) and Empower (R) (www.empowerstats.com, X&Y solutions, Inc. Boston, MA, USA). A P value of < 0.05 was considered statistically significant.
Results
Participants’ Characteristics
The characteristics of the total participants are given in Supplementary Table 1. A total of 4479 people were included (1502 men and 2977 women), and the total prevalence of nephrolithiasis was 13.89% (622/4479). The prevalence of nephrolithiasis was 23.77% (357/1502) in men, which was 2.67-fold higher than that of 8.90% in women (265/2977) and the overall prevalence of the high-risk group of nephrolithiasis. The age, BMI, SBP, DBP, CR, UREA, and UA values of men in the nephrolithiasis group were overall 1 to 7% higher to various degrees than those in the non-nephrolithiasis group. The GFR of men in the nephrolithiasis group was generally 6% lower than that of the non-nephrolithiasis group. Compared with the nonpatient group, the prevalence of nephrolithiasis was 10 to 20% higher in men with UPRO and smoking and drinking habits (Table 1).
Correlation Between Heavy Metal Elements and Nephrolithiasis
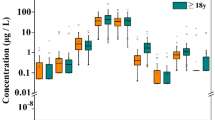
Compared with the non-nephrolithiasis group, the concentrations of As, Cd, and Hg in men in the nephrolithiasis group were about 8 to 16% lower than those in the non-nephrolithiasis group, whereas those of Cr and Pb were higher (Table 2). As and Cd may be significantly associated with the occurrence of nephrolithiasis (P < 0.001). Figure 2 showed a small difference between the unadjusted and adjusted OR values, indicating stable results. The relationship between Cr, Hg, and Pb and nephrolithiasis was not clear.
Forest chart of the prevalence of five heavy metal elements and male nephrolithiasis. The multiple regression model shows the independent relationship between metal elements and the prevalence of male nephrolithiasis, and the trend test of each metal element was performed. The quartile of heavy metal elements was based on the first group. Adjusted factors included age, BMI, SBP, DBP, CR, GFR, UREA, UA, UPRO, smoking status, and alcohol intake. OR, odds ratio; CI, confidence interval
Threshold Effect Analysis of Heavy Metals in Urine and Nephrolithiasis
According to the smooth fitting curve between the urinary heavy metal concentration and the prevalence of nephrolithiasis as shown in Fig. 3, a linear relationship was observed between As and the prevalence of nephrolithiasis, whereas Cd, Cr, Hg, and Pb showed a nonlinear relationship. The dose–response relationship was consistent with the results of threshold effect analysis, and P was < 0.05 for the maximum likelihood ratio test (Table 3). When the concentration of Cd in urine was in the range of 0.20 to 0.34 µg/L, the risk of nephrolithiasis was significantly increased by 101% (P < 0.05). After reaching the key point (0.34 µg/L), for every 1 µg/L increase in urine Cd concentration, the risk of nephrolithiasis was significantly reduced by 61% (P < 0.05). When the Cr concentration in urine was in the range of 17.78 to 25.12 µg/L, the risk of nephrolithiasis was significantly increased by 24% (P < 0.05). Similarly, when the Hg concentration in urine was in the range of 0.04 to 0.08 µg/L, the risk of nephrolithiasis disease increased by 3.00-fold higher (P < 0.05). When the Pb concentration in urine was in the range of 4.90 to 6.17 µg/L, the risk of nephrolithiasis was significantly reduced by 42% (P < 0.05). However, after reaching the key point (6.17 µg/L), for every 1 µg/L increase in urine Pb, the risk of nephrolithiasis disease increased by 3.53-fold higher (P < 0.05).
The smooth fitting curve of five heavy metals in urine and the prevalence of nephrolithiasis. Curve-adjusted age, BMI, SBP, DBP, CR, GFR, UREA, UA, UPRO, smoking status, and alcohol intake. The red curve in the middle represents the estimated value, and the blue curves on both sides represent the 95% confidence intervals
Joint Effects of Heavy Metal Elements on the Prevalence of Male Nephrolithiasis
We used the Lasso regression model to eliminate the possible collinearity problem between heavy metal elements. Furthermore, the effect of the joint exposure of five heavy metal elements on nephrolithiasis was evaluated. We calculated the score according to the model formula for subsequent analysis (Formula 1). When a threshold effect analysis was performed on the score, a dose–response relationship was consistent with that shown in Fig. 4a. When the score reached the key point, the risk of nephrolithiasis significantly increased by 5.85-fold higher (Table 4). After performing multiple regression analysis using the score and after adjusting for confounding factors, the risk of nephrolithiasis increased by 123% in the high-risk group compared with the low-risk group (OR: 2.23, 95% CI: 1.57–3.17) (Fig. 4b).
The relationship between the score of metal combination and the risk of nephrolithiasis. a The smooth fitting curve of five environmental heavy metal elements score and the prevalence of nephrolithiasis. The red curve in the middle represents the estimated value, and the blue curves on both sides represent 95% confidence intervals. b The multiple regression model shows the independent relationship between metal elements and the prevalence of male nephrolithiasis, and the trend test of each metal element was performed. The quartile of heavy metal elements was based on the first group. Adjusted factors included age, BMI, SBP, DBP, CR, GFR, UREA, UA, UPRO, smoking status, and alcohol intake
Score = − 0.0038*As − 0.133*Cd + 0.00538*Cr − 0.00109*Hg + 0.01818*Pb (1).
Discussion
This study revealed the relationship between heavy metal pollutants in the urine and nephrolithiasis in males. According to Fig. 3, it can be initially determined that the As curve is linear, whereas the Cd, Cr, Hg, and Pb curves are nonlinear. The LRT shown in Table 3 indicated that when the P-value was < 0.05, there was an inflection point; therefore, model 2, representing a nonlinear relationship, was selected. When the P-value was nonsignificant (> 0.05), model 1 representing a linear relationship was selected. In this study, the P-value in the LRT of As was 0.111, indicating a linear relationship. The P values of Cd, Cr, Hg, and Pb were all < 0.05, implying a nonlinear relationship, with the presence of inflection points and segments. The dose–response relationship between the levels of heavy metal elements, such as Cd, Cr, Hg, Pb, and nephrolithiasis in urine was two-piece-wise linear, with a threshold effect. The effect of individual elements on nephrolithiasis was not evident. However, when the five elements were evaluated together, the higher concentration of Cr and Pb, the lower concentration of As, Cd, and Hg, and all five heavy metal elements significantly increased the risk of nephrolithiasis. Therefore, the joint exposure of multiple heavy metal elements may play a crucial role in the occurrence of nephrolithiasis.
Exposure to heavy metals was associated with the occurrence of nephrolithiasis [41]. Studies have shown that it could be because heavy metals can induce renal cell apoptosis, leading to kidney damage [42, 43]. A high or small amount of them in the human body can affect health. Among them, As is an essential element for the human body. It exists in different forms in the environment. A higher or smaller dose of it can affect the health of the human body. A study on Taiwan people reported a J-shaped relationship between As intake and kidney damage. As the daily intake of inorganic substances increased, the damage intensified [44]. However, the dose–response of arsenous acid in non-Hispanic white individuals was negatively associated with nephrolithiasis [28]. In our study, the concentration of As in the nephrolithiasis group was significantly lower than that in the non-nephrolithiasis group in this study, and the risk of nephrolithiasis was reduced as the urine concentration increased. A study of China’s Tibetans, Hans, and Huis showed that different individuals showed different urinary arsenic metabolism patterns, and the bioaccumulation of arsenic in the kidneys of different individuals might be different [5, 45]. In addition, the level of UPRO (renal biomarker) in the nephrolithiasis group was higher, indicating that the kidneys of the Dong male nephrolithiasis population might have been damaged, thereby reducing the excretion capacity of As [30, 46]. Urine As is an interactive term that regulates several associations between renal biomarkers and urine Pb, Cd, and Hg [47]. The As in the nephrolithiasis group might interact with other heavy metals in the body, resulting in a decrease in urine content.
Cd is an extremely toxic heavy metal even at low concentrations [48]. Cd accumulation in the human body can lead to kidney damage by potentially harming the proximal tubular epithelial cells [49]. Cd exposure was related to a significant decrease in the glomerular filtration rate [50]. Table 1 showed that the glomerular filtration rate of the nephrolithiasis group was lower than that of the non-nephrolithiasis group. It could be inferred that when the Cd concentration was greater than 0.34 µg/L, renal function was significantly impaired. The concentration of cadmium in urine reflected the burden on the kidneys after long-term exposure to cadmium. Therefore, the threshold for nephrotoxicity observed in diseased kidneys might be lower than that observed in healthy kidneys [51]. In addition, the interaction of elements in the body would affect the excretion of urinary Cd. For example, due to the competition with metallothionein binding, an appropriate concentration of zinc exerts an antagonistic effect on the toxicity of cadmium [52].
We found that the dose–response relationship of Cr exposure was nonlinear, and the toxicity of < 25.12 µg/L Cr exposure increased the risk of nephrolithiasis by 24%. The literature pointed out that it was because Cr (+ 6) was reduced to Cr (+ 3) in the stomach and gastrointestinal tract and was excreted by the human body [53]. The concentration of Cr in urine was used as an internal Cr exposure biomarker [54], and it was affected by many factors. Studies had shown that metabolic disorders of obesity might affect changes in Cr in the body [55]. We found that the BMI of people with nephrolithiasis was 2% higher than that of people without nephrolithiasis, suggesting that increased Cr excretion in people with nephrolithiasis might partly lead to a decrease in body storage. Moreover, the co-exposure of Cr, Pb, and Cd might cause a further decrease in glomerular filtration rate [56], leading to an increased risk of nephrolithiasis.
Hg and Pb are heavy metal elements that are extremely harmful to the human body and can accumulate in human tissues, including the brain [57]. Most of the Hg in the environment that affects the human body is inorganic Hg [58]. The toxic effects of inorganic Hg were mainly seen in the kidneys of humans and animals, and experiments in rats had shown effects including increased kidney weight, tubular necrosis, UPRO, and hypoalbuminemia [53]. Our results also showed that UPRO was significantly higher in the nephrolithiasis group, suggesting that Hg might have started to impair kidney function, leading to excretory dysfunction with more Hg accumulation in the body and less in the urine. Pb causes toxicity in living cells by generating ions and oxidative stress [59]. This oxidative stress was manifested as an increase in the level of malondialdehyde in the kidneys, a decrease in the level of intracellular glutathione, and an increase in the infiltration of inflammatory cells into the organs [60]. Our results confirmed the damage of Pb to the kidneys. When the urine Pb concentration was ≥ 6.17 µg/L, the risk of nephrolithiasis disease increased by 3.53-fold higher. Therefore, the urine Pb concentration < 6.17 µg/L might be the result of normal kidney detoxification in Dong men.
The occurrence of stones may occur mainly because of environmental metal exposure [61]. However, a few studies have indicated the joint exposure effect between heavy metals. In a 2015–2016 cross-sectional study on the blood levels of Co, Cr, Hg, and Pb and renal function, exposure to a mixture of heavy metals was associated with decreased renal function [30]. Low-dose metal mixtures (Pb, Hg, As, and Cd) interacted with toxic and essential metals, and the interactions between metal mixtures had a synergistic effect on kidney toxicity to a large extent [62]. Studies had found that long-term exposure to Cd would produce greater renal toxicity than As, and the combination of Cd and As could cause kidney damage even greater than the damage caused by using any chemical alone [63]. Rat studies had shown that the combination of Pb and Cd had an enhanced effect and produced renal subcellular changes [64]. The results of a large-scale prospective study of middle-aged and elderly people in China showed that exposure to multiple metals could cause a decline in kidney function in middle-aged and elderly people, and simultaneous exposure to multiple metals might have a synergistic effect on the decline in kidney function [65]. Our study found that the joint effect of heavy metals significantly increased the risk of nephrolithiasis by calculating the score using the Lasso regression model. In other words, the effect of individual heavy metals on nephrolithiasis may not be clear. Using the prediction formula derived from the collinearity study, we found that the joint exposure of five heavy metals affected the risk of nephrolithiasis.
There are several limitations to this study. First, these results were based on a cross-sectional study and could not explain the cause and effect. Second, the influence of genetics, eating habits, and other urinary metal elements were not discussed. Finally, the difference in male and female participants also needs further investigation. Most of the baseline characteristics were significantly different (P < 0.05) in the analyzed group (Supplementary Table 1). In contrast to males, we did not observe a significant difference (P > 0.05) in metal exposure levels between non-nephrolithiasis (N = 2,712) and nephrolithiasis (N = 265) female participants (Supplementary Table 2). The relationship between the baseline markers and metal exposure level remains unclear. Therefore, dietary intake and genetics should be considered to improve the monitoring system in future studies.
This study also had several advantages. The effect of joint exposure to heavy metals in the research environment on people with a high incidence of nephrolithiasis is rare. We included three generations of Dong people, with fixed diet and living habits, and high genetic stability. Their data were more valuable than those of other floating population studies. Moreover, the male population was selected, which reduced the influence of gender on the study. Although several studies have examined nephrolithiasis and heavy metals, only a few have indicated their threshold ranges. This study will serve as a reference for evaluating and monitoring potential heavy metals in different environmental areas and residential bioregions.
Conclusions
The potential association between urinary heavy metal concentrations and the prevalence of nephrolithiasis in men among the ethnic minority (Dong) population of China was demonstrated. Urinary As showed a linear relationship with the risk of nephrolithiasis. However, Cd, Cr, Hg, and Pb had a nonlinear relationship with the risk of nephrolithiasis. This result provided a reference for the studies of other high-risk groups. Moreover, a single heavy metal in the urine may not have a large effect on the risk of nephrolithiasis. However, the joint exposure of As, Cd, Cr, Hg, and Pb in urine may play a crucial role in the high incidence of nephrolithiasis in men. The results showed that exposure to heavy metals in the environment is of great significance to the human body. Therefore, we should reduce heavy metal pollution in the environment to prevent the occurrence of diseases.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ali H, Khan E (2018) What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’—proposal of a comprehensive definition. Toxicol Environ Chem 100:6–19. https://doi.org/10.1080/02772248.2017.1413652
Mishra S, Bharagava RN, More N, Yadav, Zainith S, Mani S, Chowdhary P (2019) Heavy metal contamination: an alarming threat to environment and human health. Environmental biotechnology: for sustainable future 103:125. https://doi.org/10.1007/978-981-10-7284-0_5
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb) on the total environment: an overview. Environ Monit Assess 191:419. https://doi.org/10.1007/s10661-019-7528-7
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology 133:164. https://doi.org/10.1007/978-3-7643-8340-4_6
Gunawardena SA, Gunawardana JW, Chandrajith R, Thoradeniya T, Jayasinghe S (2020) Renal bioaccumulation of trace elements in urban and rural Sri Lankan populations: a preliminary study based on post mortem tissue analysis. J Trace Elem Med Biol 61:126565. https://doi.org/10.1016/j.jtemb.2020.126565
Cabral M, Garçon G, Touré A, Bah F, Dewaele D, Bouhsina S, Cazier F, Faye A, Fall M, Courcot D, Verdin A (2021) Renal impairment assessment on adults living nearby a landfill: early kidney dysfunction biomarkers linked to the environmental exposure to heavy metals. Toxicol Rep 8:386–394. https://doi.org/10.1016/j.toxrep.2021.02.009
Joint W, World Health Organization (2007) Health risks of heavy metals from long-range transboundary air pollution. WHO Regional Office for Europe, Copenhagen
Rana MN, Tangpong J, Rahman MM (2018) Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review. Toxicol Rep 5:704–713. https://doi.org/10.1016/j.toxrep.2018.05.012
Yokohira M, Arnold LL, Pennington KL, Suzuki S, Kakiuchi-kiyota S, Herbin-Davis K, Thomas DJ, Cohen SM (2011) Effect of sodium arsenite dose administered in the drinking water on the urinary bladder epithelium of female arsenic (+ 3 oxidation state) methyltransferase knockout mice. Toxicol Sci 121(2):257–266. https://doi.org/10.1093/toxsci/kfr051
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23(5):783–792. https://doi.org/10.1007/s10534-010-9328-y
Al-Rikabi ZGK, Al-Saffar MA, Abbas AH (2021) The accumulative effect of heavy metals on liver and kidney functions. Medico Legal Update 21(1):1114–1119
Kim HS, Kim YJ, Seo YR (2015) An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev 20:4–232. https://doi.org/10.15430/JCP.2015.20.4.232
Zalups RK, Joshee L, Bridges CC (2014) Novel hg2+-induced nephropathy in rats and mice lacking mrp2: evidence of axial heterogeneity in the handling of hg2+ along the proximal tubule. Toxicol Sci 142(1):250–260. https://doi.org/10.1093/toxsci/kfu171
Missoun F, Slimani M, Aoues A (2010) Toxic effect of lead on kidney function in rat Wistar. Afr J Biochem Res 4(2):021–027
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90(5):1193–1209. https://doi.org/10.1007/s00204-015-1547-0
Scammell MK, Sennett CM, Petropoulos ZE, Kamal JK, Kaufman JS (2019) Environmental and occupational exposures in kidney disease. Seminars in nephrology WB Saunders 39(3):230–243. https://doi.org/10.1016/j.semnephrol.2019.02.001
Assadi F, Moghtaderi M (2017) Preventive kidney stones: continue medical education. Int J Prev Med 8:67. https://doi.org/10.4103/ijpvm.IJPVM_17_17
National Center for Health Statistics (US), Council on Clinical Classifications (1980) The International classification of diseases, 9th revision, clinical modification: ICD-9-CM. Public Health Service, Health, US Department of Health and Human Services
Lin H, Zhu X, Long J, Chen Y, Xie Y, Liao M, Chen J, Tian J, Huang S, Tang R, Xian X, Wei S, Wang Q, Mo Z (2018) HIPK2 polymorphisms rs2058265, rs6464214, and rs7456421 were associated with kidney stone disease in Chinese males not females. Gene 653:51–56. https://doi.org/10.1016/j.gene.2018.02.020
Peerapen P, Thongboonkerd V (2019) Protective cellular mechanism of estrogen against kidney stone formation: a proteomics approach and functional validation. Proteomics 19(19):1900095. https://doi.org/10.1002/pmic.201900095
Post TW, Rose BD (2006) Urinalysis in the diagnosis of renal disease. Up To Date 13(3).
Wang YX, Feng W, Zeng Q, Sun Y, Wang P, You L, Yang P, Huang Z, Yu SL, Lu WQ (2016) Variability of metal levels in spot, first morning, and 24-hour urine samples over a 3-month period in healthy adult Chinese men. Environ Health Perspec 124(4):468–476. https://doi.org/10.1289/ehp.1409551
Côté AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA (2008) The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol 199(6):625. e1-625. e6. https://doi.org/10.1016/j.ajog.2008.06.009
Xie KP, Tian Z, Wu TH, LI Z, Zhao YG, Shi XY (2010) Co-precipitation-flame atomic absorption spectrophotometric determination of lead, cadmium and manganese in urine. Chin J Health Lab Tech 20(9):2154–2155
Michalek IM, Martinsen JI, Weiderpass E, Hansen J, Sparen P, Tryggvadottir L, Pukkala E (2019) Heavy metals, welding fumes, and other occupational exposures, and the risk of kidney cancer: a population-based nested case-control study in three Nordic countries. Environl Res 173:117–123. https://doi.org/10.1016/j.envres.2019.03.023
Jordanova M, Rebok K, Dragun Z, Ramani S, Ivanova L, Kostov V, Valić D, Krasnići N, Marijić VF, Kapetanović D (2017) Effects of heavy metal pollution on pigmented macrophages in kidney of V ardar chub (S qualius vardarensis K araman). Microsc Res Tech 80(8):930–935. https://doi.org/10.1002/jemt.22884
Bot YS, Nwanjo HU, Nwosu DC, Olumide OB, Ifenkwe JC (2020) Derangement of kidney biomarkers associated with blood cadmium, lead and chromium in artisans and petrol hawkers in Jos. Nigeria Inter J Nephrol Kidney Fail 6(1):1–6
Sun Y, Zhou Q, Zheng J (2019) Nephrotoxic metals of cadmium, lead, mercury and arsenic and the odds of kidney stones in adults: An exposure-response analysis of NHANES 2007–2016. Environ Int 132:105115. https://doi.org/10.1016/j.envint.2019.105115
Ferraro PM, Gambaro G, Curhan GC, Taylor EN (2018) Intake of trace metals and the risk of incident kidney stones. J Urol 199(6):1534–1539. https://doi.org/10.1016/j.juro.2018.01.077
Luo J, Hendryx M (2020) Metal mixtures and kidney function: an application of machine learning to NHANES data. Environ Res 191:110126. https://doi.org/10.1016/j.envres.2020.110126
World Health Organization (2015) Human biomonitoring: facts and figures. WHO Regional Office for Europe, Copenhagen
Jain RB (2019) Co-exposures to toxic metals cadmium, lead, and mercury and their impact on unhealthy kidney function. Environ Sci Pollut Res 26(29):30112–30118. https://doi.org/10.1007/s11356-019-06182-y
Lee MY, Kim YJ, Hwang DG, Kang YY, Shin SK, Jeon TW (2021) Potential risk of exposure to heavy metals from co-processing of secondary wastes in the Republic of Korea. J Environ Manag 286:112164. https://doi.org/10.1016/j.jenvman.2021.112164
Zhao X, Hong F, Yin J, Tang W, Zhang G, Liang X, Li J, Cui C, Li X (2020) Cohort profile: the China Multi-Ethnic cohort (CMEC) study. J. Epidemiol, Int. https://doi.org/10.1093/ije/dyaa185
Stockman JA (2011) A new equation to estimate glomerular filtration rate. Yearb Pediatr 2011:193–194. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Lente FV, Zhang YL, Coresh J, Levey AS (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20. https://doi.org/10.1056/NEJMoa1114248
Moore RE, Rehkämper M, Strekopytov KK, S, Larner F, (2018) Determination of major and trace element variability in healthy human urine by ICP-QMS and specific gravity normalisation. RSC Adv 8(66):38022–38035. https://doi.org/10.1039/C8RA06794E
Wu J, Yang Z, Wei J, Zeng C, Wang Y, Yang T (2020) Association between serum magnesium and the prevalence of kidney stones: a cross-sectional study. Biol Trace Elem Res 195:20–26. https://doi.org/10.1007/s12011-019-01830-3
Zhang X, Yao ZQ, Karuna T, He XY, Wang XM, Li XF, Liu WC, Li R, Guo SQ, Chen YC, Li GC, Duan CZ (2018) The role of wall shear stress in the parent artery as an independent variable in the formation status of anterior communicating artery aneurysms. Eur Radiol 29(2):689–698. https://doi.org/10.1007/s00330-018-5624-7
Lin L, Chen CZ, Yu XD (2013) The analysis of threshold effect using Empower Stats software. Chin J Epidemiol 34(11):1139–1141. https://doi.org/10.3760/cma.j.issn.0254-6450.2013.011.021
Hara A, Yang WY, PetitT ZZY, Gu YM, Wei FF, Jacobs L, Odiliab AN, Thijs L, Nawrot TS, Staessen JA (2016) Incidence of nephrolithiasis in relation to environmental exposure to lead and cadmium in a population study. Environ Res 145:1–8. https://doi.org/10.1016/j.envres.2015.11.013
Kosiba AA, Wang Y, Chen D, Wong CKC, Gu J, Shi H (2020) The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life Sci 242:117183. https://doi.org/10.1016/j.lfs.2019.117183
Barnett LMA, Cummings BS (2018) Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci 164:379–390. https://doi.org/10.1093/toxsci/kfy159
Lin YJ, Hsiao JL, Hsu HT (2020) Integration of biomonitoring data and reverse dosimetry modeling to assess population risks of arsenic-induced chronic kidney disease and urinary cancer. Ecotoxicol Environ Saf 206:111212. https://doi.org/10.1016/j.ecoenv.2020.111212
Fu S, Wu J, Li Y, Liu Y, Gao Y, Yao F, Qiu C, Song L, Wu Y, Liao Y, Sun D (2014) Urinary arsenic metabolism in a Western Chinese population exposed to high-dose inorganic arsenic in drinking water: influence of ethnicity and genetic polymorphisms. Toxicol Appl Pharmacol 274(1):117–123. https://doi.org/10.1016/j.taap.2013.11.004
Kolbach-Mandel AM, Mandel NS, Hoffmann BR, Kleinman JG, Wesson JA (2017) Stone former urine proteome demonstrates a cationic shift in protein distribution compared to normal. Urolithiasis 45:337–346. https://doi.org/10.1007/s00240-017-0969-y
de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovský Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A (2006) Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect 114(4):584–590. https://doi.org/10.1289/ehp.820
Idrees N, Tabassum B, Abd Allah EF, Hashem A, Sarah R, Hashim M (2018) Groundwater contamination with cadmium concentrations in some West U.P. Regions. India Saudi J Biol Sci 25:1365–1368. https://doi.org/10.1016/j.sjbs.2018.07.005
Jin Y, Lu Y, Li Y, Zhao H, Wang X, Shen Y, Kuang X (2020) Correlation between environmental low-dose cadmium exposure and early kidney damage: a comparative study in an industrial zone vs. a living quarter in Shanghai, China. Environ Toxicol Pharmacol 79:103381. https://doi.org/10.1016/j.etap.2020.103381
Satarug S, Boonprasert K, Gobe GC (2019) Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin Kidney J 12(4):468–475. https://doi.org/10.1093/ckj/sfy113
Orr SE, Bridges CC (2017) Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 18(5):1039. https://doi.org/10.3390/ijms18051039
Wang Y, Tang Y, Li Z, Hua Q, Wang L, Song X, Zou B, Ding M, Zhao J, Tang C (2020) Joint toxicity of a multi-heavy metal mixture and chemoprevention in Sprague Dawley rats. Int J Environ Res Public Health 17(4):1451. https://doi.org/10.3390/ijerph17041451
Edition F (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Zhao M, Xu J, Li A, Mei Y, Ge X, Liu X, Wei L, Xu Q (2020) Multiple exposure pathways and urinary chromium in residents exposed to chromium. Environ Int 141:105753. https://doi.org/10.1016/j.envint.2020.105753
Tinkov AA, Skalnaya MG, Ajsuvakova OP, Serebryansky EP, Chao JC, Aschner M, Skalny AV (2021) Selenium, zinc, chromium, and vanadium levels in serum, hair, and urine samples of obese adults assessed by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 199:490–499. https://doi.org/10.1007/s12011-020-02177-w
Tsai TL, Kuo CC, Pan WH, Chung YT, Chen CY, Wu TN, Wang SL (2017) The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int 92:710–720. https://doi.org/10.1016/j.kint.2017.03.013
Wang Y, Tang Y, Li Z, Hua Q, Wang L, Song X, Tang C (2020) Joint toxicity of a multi-heavy metal mixture and chemoprevention in sprague dawley rats. Int J Environ Res Public Health 17(4):1451. https://doi.org/10.3390/ijerph17041451
Adu-Poku B, Asiedu N, Akoto O, Ataki J (2019) Modelling the distribution of arsenic and mercury in urine using chemometric tools. Cogent Chemistry 5:1. https://doi.org/10.1080/23312009.2019.1586064
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Abdel-Zaher AO, Abd-Ellatief RB, Aboulhagag BA, Farghaly HSM, Al-Wasei FMM (2019) The interrelationship between gasotransmitters and lead-induced renal toxicity in rats. Toxicol Lett 310:39–50. https://doi.org/10.1016/j.toxlet.2019.04.012
Wang L, Chen M, He P, Yu H, Block KA, Xie Z (2019) Composition and spatial distribution of elements and isotopes of a giant human bladder stone and environmental implications. Sci Total Environ 650:835–846. https://doi.org/10.1016/j.scitotenv.2018.09.028
Cobbina SJ, Chen Y, Zhou Z, Wu X, Feng W, Wang W, Mao G, Xu H, Zhang Z, Wu X, Yang L (2015) Low concentration toxic metal mixture interactions: Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere 132:79–86. https://doi.org/10.1016/j.chemosphere.2015.03.013
Liu J, Liu Y, Habeebu SM, Waalkes MP, Klaassen CD (2000) Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology 147(3):157–166. https://doi.org/10.1016/S0300-483X(00)00194-3
Ramesh G, Madhuri D, Lakshman M, Reddy AG (2019) Histopathological and ultrastructural changes of liver and kidney induced by lead and cadmium alone and combined exposure in male wistar rat. The Pharma Innov J 8(2):407
Liu Y, Yuan Y, Xiao Y, Li Y, Yu Y, Mo T, Jiang H, Li X, Yang H, Xu C, He M, Guo H, Pan A, Wu T (2020) Associations of plasma metal concentrations with the decline in kidney function: a longitudinal study of Chinese adults. Ecotoxicol Environ Saf 189:110006. https://doi.org/10.1016/j.ecoenv.2019.110006
Acknowledgements
The author would like to thank the Second Affiliated Hospital of Guizhou Medical University, University Town Hospital of Gui’an New District, Guizhou Province, and the Guiyang Center for Disease Control and Prevention.
Funding
This study was supported by the National Key R&D Program of China (NO.2017YFC0907301), the Science and Technology Support Plan of Science and Technology Department of Guizhou Province ([2018] No.5403), Guizhou Province Graduate Research Fund Project, Qianjiaohe YJSCXJH [2019] 076, and First-Class Discipline Construction Project in Guizhou Province-Public Health and Preventive Medicine (No. 2017[85]).
Author information
Authors and Affiliations
Contributions
YL prepared the data, conducted the analysis, and drafted and revised the manuscript. CZ and QY conducted the experiments. ZQ, JL, XT, and QW provided technical support and contributed to the project. FH monitored all aspects of research implementation and contributed to the writing of the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Sichuan University Medical Ethical Review Board (K2016038) and the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University (2018[094]).
Consent to Participate
All participants provided the written informed consent for the current study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(Docx 68.0 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, C., Qin, Z. et al. Analysis of Threshold Effect of Urinary Heavy Metal Elements on the High Prevalence of Nephrolithiasis in Men. Biol Trace Elem Res 200, 1078–1088 (2022). https://doi.org/10.1007/s12011-021-02740-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02740-z