Abstract
This study evaluated the reutilization of waste materials (scrap tires, sewage sludge, and wood chips) to remove volatile organic compounds (VOCs) benzene/toluene/ethylbenzene/xylenes/trichloroethylene/cis-1,2-dichloroethylene (BTEX/TCE/cis-DCE), plasticizer di(2-ethylhexyl) phthalate (DEHP), and pharmaceutically active compound carbamazepine from artificially contaminated water. Different hybrid removal processes were developed: (1) 300 mg/L BTEX + 20 mg/L TCE + 10 mg/L cis-DCE + tires + Pseudomonas sp.; (2) 250 mg/L toluene + sewage sludge biochar + Pseudomonas sp.; (3) 100 mg/L DEHP + tires + Acinetobacter sp.; and (4) 20 mg/L carbamazepine + wood chips + Phanerochaete chrysosporium. For the hybrid process (1), the removal of xylenes, TCE, and cis-DCE was enhanced, resulted from the contribution of both physical adsorption and biological immobilization removal. The hybrid process (2) was also superior for the removal of DEHP and required a shorter time (2 days) for the bioremoval. For the process (3), the biochar promoted the microbial immobilization on its surface and substantially enhanced/speed up the bioremoval of toluene. The fungal immobilization on wood chips in the hybrid process (4) also improved the carbamazepine removal considerably (removal efficiencies of 61.3 ± 0.6%) compared to the suspended system without wood chips (removal efficiencies of 34.4 ± 1.8%). These hybrid processes would not only be promising for the bioremediation of environmentally concerned contaminants but also reutilize waste materials as sorbents without any further treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Even though both scientific advances and technological innovations dramatically improved the quality of human life, they also brought huge negative impacts on the environment. Approximately 80% of wastewater generated globally would flow back into the ecosystem without being treated or reused (UNESCO 2017). As a consequence, by 2025, it is expected that 1.8 billion people would face water scarcity and around two-third of the world population would be living under water-stressed conditions due to the poor water quality (UNESCO 2012). The water bodies worldwide have been contaminated with a mixture of persistent and eco-toxic organic and inorganic substances, mainly resulted from the anthropogenic activities (Wong et al. 2018). One typical example is the detection of volatile organic compounds (VOCs) including petroleum hydrocarbons such as benzene, toluene, ethylbenzene, and xylenes (BTEX) and methyl tert-butyl ether (MTBE), polycyclic aromatic hydrocarbons (PAHs), and chlorinated aliphatic hydrocarbons (CAHs) such as trichloroethylene (TCE) and cis-1,2-dichloroethylene (cis-DCE) in ground and surface waters (Moran et al. 2007; Sun et al. 2018; Williams 2014; Yu et al. 2015b). They are listed as priority pollutants due to their hazardous and carcinogenic natures (Arshadi et al. 2016). More recently, attention has also been paid to the detection of emerging organic contaminants (e.g., pharmaceuticals and personal care products, pesticides, natural and synthetic hormones, plasticizers) even in drinking water supply sources (Gabarrón et al. 2016; Magi et al. 2018), resulted from the inefficient conventional activated sludge process in removing those contaminants (Buttiglieri and Knepper 2008) and the non-existing respective regulations (Carmalin and Lima 2018). The presence of emerging organic contaminants in water bodies could be considered a threat to human and environmental health considering their bioaccumulation (Wilkinson et al. 2018) and microbial drug resistance issues (Ye et al. 2017).
Many remediation methods have been developed to remove organic pollutants from surface and ground waters and wastewater using physical, chemical, and biological technologies. The application of advanced oxidation processes is one alternative but the generation of secondary pollution and the high investment and operation costs limit their applications at wastewater treatment facilities (Salimi et al. 2017). Adsorption as a physical treatment, on the other hand, shows a great potential on the remediation of organic/inorganic pollutants in contaminated waters especially when the waste materials could be applied as non-conventional adsorbents providing simple and low-cost operations (Vukelic et al. 2018). However, adsorption is still considered only transferring the contaminant from one medium to another and consequently a secondary treatment would still be needed to mineralize the contaminant even though the advantage of desorption process is to get rid of the pollutant in a relatively easy manner. The biological strategies are environmentally friendly, mostly utilizing the indigenous microbial strains to completely mineralize the contaminants even though they still show some limitations toward the efficiency and the time needed, relatively longer compared to the chemical methods (Azubuike et al. 2016; Kang 2014). The possibility to sorb/immobilize microbes on the sorbent surface can be an alternative to facilitate the contaminant mineralization and has been receiving an increasing attention. Other technologies with great potential to remove organic contaminants from water include photocatalytic processes (Balcha et al. 2016), microencapsulation (Krause et al. 2012), and nanofiltration/reverse osmosis (Schäfer et al. 2003).
There have been many studies dealing with the synthesis of adsorbents using different materials to remove organic/inorganic contaminants (San Miguel et al. 2006), but the current work focuses on reutilizing waste materials to improve the bioremoval of contaminant (biosorption) while also providing an alternative for the waste disposal (Bhatnagar and Sillapãã 2010). Different waste materials such as natural zeolites (Wang and Peng 2010), Moringa oleifera seed cake (Almeida et al. 2012), plant-derived cellulosic nanofibers (Dey 2012), sewage sludge and municipal solid waste compost (Simantiraki et al. 2013), and rice husk ash (Deokar et al. 2016) have been directly applied as adsorbents to remove organic contaminants. On the other hand, some waste materials have been considered as precursors for the inexpensive absorbents, including soybean stover and peanut shell waste (Ahmad et al. 2013), waste tires (Gupta et al. 2014), ostrich bone waste (Arshadi et al. 2016), and waste cherry kernels (Vukelic et al. 2018).
Over 1 billion waste tires are generated per year globally and the key environmental issues associated with this waste are their disposal, pollution emissions, and recalcitrant nature (Forrest 2014). Waste tires have been mainly used to remove inorganic species from aqueous solutions after the thermal and/or chemical treatment to generate the alternative activated carbon and biochar (Belgacem et al. 2014; Karmacharya et al. 2016; Ramola et al. 2014). They were also successfully used, without any further pretreatment, as microbial immobilization matrices to biologically remove VOCs (Lu et al. 2015, 2017). Biochar is another adsorbent commonly used in the environmental applications. As a carbon-rich solid material produced by the thermal decomposition of different biomass types in the absence of oxygen (Yu et al. 2015a), it has gained substantial attention as an environmentally sustainable electron mediator, acceptor, and donor (Yuan et al. 2017), accelerating the bioremoval of contaminants to some extent. The current study focuses on the potential reutilization of waste scrap tires/sewage sludge/wood chips, as biosorbents, to biologically remove VOCs (BTEX/TCE/cis-DCE) mixture and newly emerging contaminants such as plasticizer di(2-ethylhexyl) phthalate (DEHP) and pharmaceutically active compound carbamazepine (CBZ), the anticonvulsant 5H-dibenzo[b,f]azepine-5-carboxamide, from the artificially contaminated water.
Materials and methods
Reagents
All the reagents used were of analytical grades and were used as received without any further purification. Carbamazepine (CBZ; 99%) and DEHP (99%) were purchased from Sigma-Aldrich (USA). Benzene (99.7%), ethylbenzene (99%), toluene (99%), and o,m,p-xylenes (99%) were obtained from International Laboratory (USA) while TCE (99%) and cis-DCE (99%) were purchased from Damao Chemical Manufacture (China). All other solvents (LC-MS grades) used to prepare the stock solutions (methanol, dimethylformamide) and to use as mobile phase (acetonitrile) were also purchased from Sigma-Aldrich (USA). CBZ (1 g/L), DEHP (1 g/L), BTEX (10 g/L), TCE (1 g/L), and cis-DCE (1 g/L) stock solutions were kept at 4 °C until used.
Preparation of waste scrap tires, sewage biochar, and wood chips
The waste motorcycle scrap tire (Bridgestone; Battlax BT-39 tubeless) was cut into small pieces (0.2 cm × 0.2 cm × 0.2 cm), washed (with the deionized (DI) water for 1 h), and dried (at 60 °C). Without any other thermal or chemical treatment, the tire pieces were used directly in the adsorption experiments. According to our previous thermogravimetric analysis (Lu et al. 2017), the waste scrap tire used is mainly made of oil (11%), filler (38%), and rubber (52%), and silicon is the main additive element, with the surface of hydrophobic nature (contact angle value, 93.1° ± 4.1). The detailed information about the tire characterization can be found elsewhere (Lu et al. 2017). The sewage sludge was obtained from a local wastewater treatment plant (Macau Special Administrative Region, China) and was dried overnight (80 °C). After being adjusted to the room temperature, the sludge samples were crushed and sieved. The biochars (BCs) were prepared at different pyrolysis temperatures (300, 500, and 700 °C) for 4 h (5 °C min−1) under the nitrogen atmosphere (using a tube furnace OTF-1200X-II-100, Kejing). The wood chips (Cunninghamia; 10–20 mm × 5–10 mm × 0.2 mm) were washed in the DI water and dried overnight (80 °C) before use.
Microbial cultures
Pseudomonas plecoglossicida and Acinetobacter sp. were previously isolated from a petroleum contaminated site (Xiamen, China) and from a local wastewater treatment plant, respectively. The details about microbial isolation, identification, and subculture can be found elsewhere (Lu et al. 2017; Xu et al. 2017). Phanerochaete chrysosporium was purchased from the Guangdong Institute of Microbiology (China) and the details about the fungal subculture and the preparation of fungal spore suspension are given by Li et al. (2015).
Experimental setup
VOCs (BTEX/TCE/cis-DCE) mixture removal
The microcosms for VOCs (BTEX/TCE/cis-DCE mixture; Henry’s law constants from 1.03 × 10−2 to 7.88 × 10−3 atm m3/mol at 25 °C) were prepared in serum bottles (160 mL) containing 45 mL of the mineral salts medium (MSM which contained, in g/L, KH2PO4 1.0; K2HPO4 1.0; NH4NO3 1.0; MgSO4·7H2O 0.2; Fe2(SO4)3 0.05; and CaCl2 0.02) (pH 7) and 10% (v/v) inoculum size (5 mL, Pseudomonas sp.). The microcosms were individually spiked with different concentrations of the contaminants (300 mg/L BTEX, 20 mg/L TCE, and 10 mg/L cis-DCE). The BTEX solution was prepared based on the mass fraction (%) of each component in crude oil (benzene/toluene/ethylbenzene/o-xylene/m-xylene/p-xylene at 22.7:48.3:4.6:6.3:6.9:11.1) (Li et al. 2017). The concentrations of each BTEX compound in 300-mg/L mixture were 68.1 mg/L benzene, 144.9 mg/L toluene, 13.8 mg/L ethylbenzene, 18.9 mg/L o-xylene, 20.7 mg/L m-xylene, and 33.3 mg/L p-xylene. The adsorbent used was waste scrap tires (1.5 g). The serum bottles were covered with stopper (90% Teflon/10% silicone; Ohio Valley Specialty, CO), sealed with aluminum crimp, and incubated (30 °C and 150 rpm) for 5 days. The liquid samples (1 mL) were collected from the microcosms on a daily basis, filtered (Millipore; 0.45 μm), and analyzed by GC-FID (Lu et al. 2017). The microcosms containing contaminants and MSM solution but without tires and Pseudomonas sp. were set as controls to check the abiotic losses (mainly due to volatilization) of the BTEX/TCE/cis-DCE mixture.
Toluene removal
The microcosms for the toluene (Henrys law constant, 6.64 × 10−3 atm m3/mol at 25 °C) removal were prepared following the same procedure as for the VOC mixture, but the contaminant was spiked at 250 mg/L and the adsorbent used was sewage sludge biochar (50 mg) produced at different pyrolysis temperatures (300, 500, and 700 °C). The serum bottles were covered with stopper (90% Teflon/10% silicone; Ohio Valley Specialty, CO), sealed with aluminum crimp, and incubated (30 °C and 150 rpm) for 3 days. The liquid samples (1 mL) were collected from the microcosms on a daily basis, filtered (Millipore; 0.45 μm), and analyzed by GC-FID (Lu et al. 2017). The microcosms containing toluene and MSM solution but without sewage sludge biochar and Pseudomonas sp. were set as controls to check the abiotic losses (mainly due to volatilization) of toluene.
DEHP removal
The microcosms for DEHP (Henry’s law constant, 1.71 × 10−5 atm m3/mol at 25 °C) were prepared in serum bottles (160 mL) containing 45 mL of the basal salts medium (BSM which contained, in g/L, NaCl 1.0; K2HPO4 1.0; NH4Cl 0.5; and MgSO4·7H2O 0.4, pH 7) and 10% (v/v) inoculum size (5 mL, Acinetobacter sp.). The microcosms were individually spiked with 100 mg/L DEHP and the adsorbent used was waste scrap tires (2.5 g). The serum bottles were covered with stopper (90% Teflon/10% silicone; Ohio Valley Specialty, CO), sealed with aluminum crimp, and were incubated (30 °C and 150 rpm) for 5 days. The liquid samples (1 mL) were collected from the microcosms on a daily basis, filtered (Millipore; 0.45 μm), and analyzed by LC-MS (Xu et al. 2017). The microcosms containing DEHP and BSM solution but without tires and Acinetobacter sp. were set as controls to check for any abiotic losses of the plasticizer.
Carbamazepine removal
The microcosms for carbamazepine (Henry’s law constant, 1.08 × 10−10 atm m3/mol at 25 °C) were prepared in shake flasks containing 150 mL synthetic wastewater (per liter of tap water: 2 g glucose, 0.66 g ammonium tartrate, 1 mL Tween 80, 1 mg thiamine, 1 g KH2PO4, 0.5 g MgSO4·7H2O, and 0.1 g CaCl2·2H2O), 1 g/L of the adsorbent (wood chips), and the fungal spore suspension (at 1:40, v/v). After the flasks were incubated (120 rpm, 30 °C) for a week for the fungal cultivation, carbamazepine (10 mg/L) was spiked into the microcosms. The shake flasks were sealed with aluminum foil and incubated (30 °C and 150 rpm) for 7 days. The liquid samples (1 mL) were collected from the microcosms on a daily basis, filtered (Millipore; 0.45 μm), and analyzed by LC-MS (Li et al. 2015). The microcosms containing carbamazepine and synthetic wastewater but without wood chips and Phaenerochaete chrysosporium were set as controls to check for any abiotic losses of carbamazepine.
Desorption experiments
The desorption experiments were carried out in microcosms containing scrap tires (1.5 g) only and scrap tires (1.5 g) + inoculum to further evaluate whether the sorbed VOCs (especially the BTEX/TCE/cis-DCE mixture) were biologically removed. The VOCs were added into the microcosms at the same concentration level (100 mg/L). The tire pieces were added to the serum bottles with the water/ethanol solution (at 1:1, v/v) and shaken for 24 h (150 rpm) (Garoma and Skidmore 2011). The desorption recovery for each contaminant was calculated by Eq. (1):
where Cd, Ce, and C0 are the equilibrium concentration of each contaminant (in mg/L) after the desorption process, after the adsorption and immobilization process, and the initial concentration, respectively.
Adsorption capacity
The adsorption capacities (qe) of the contaminants on different adsorbents were assessed for different durations (5, 24, 48, and 72 h for toluene; 15, 30, 60, 120, 360, 720, 1440, 2880, 4320, and 5760 min for DEHP, and 15, 30, 45, 60, 75, 90, 1440, 2880, and 4320 min for BTEX/TCE/cis-DCE mixture) and were calculated by Eq. (2):
where Ce and C0 refer to the contaminant concentrations at equilibrium and at time zero, respectively. V and m are the volume of medium solution (L) and the amount of adsorbent added (g), respectively.
Freundlich and Langmuir isotherm models
The sorption of the contaminants on different adsorbents was assessed by the Freundlich and Langmuir isotherm models. The Langmuir isotherm model (Eq. (3)) accounts for an adsorbate single layer onto a homogeneous adsorbent surface (Chung et al. 2015).
where Ce and qe are the contaminant concentration in solution at equilibrium (mg/L) and the contaminant amount adsorbed at equilibrium (mg/g), respectively. The Langmuir constants KL (L/mg) and Cm (mg/g) refer to energy and adsorption capacity, respectively. In comparison, the Freundlich isotherm model considers the heterogeneous surface nature of the sorbent (Eq. (4)) (Chung et al. 2015).
where qe is the contaminant amount sorbed at equilibrium (mg/g) and Ce is the contaminant concentration in solution at equilibrium (mg/L). The Freundlich constants 1/n and Kfrefer to the adsorption intensity and the contaminant adsorption capacity, respectively.
Analytical methods
BTEX, TCE, cis-DCE, and toluene were analyzed by gas chromatography (GC; Thermo ISQ™, Thermo Scientific, USA) equipped with a flame ionization detector (FID) and a TG-5MS analytical column (0.25 mm × 15 mm × 0.25 μ m). The injector and detector temperatures were 120 and 260 °C, respectively. The oven temperature was initially set at 40 °C and incrementally increased to 110 °C (6 °C/min). CBZ and DEHP were analyzed by liquid chromatography (LC; Dionex UltiMate 3000 HPLC, Thermo Scientific, USA) equipped with a Syncronis C18 reversed-phase analytical column (4.6 mm × 100 mm × 5 μm). The ratio of mobile phase (acetonitrile/deionized water; pH 3) was 95:0.5 and the oven temperature was 45 °C. The flow rate of eluent and the injection volume were 0.5 mL and 20 μL, respectively. The analytical curves for each contaminant were obtained in triplicate by the external standard method. The scanning electron microscopy (SEM; Hitachi S-3400 N, Type I) was used for the micrographs for the tire and biochar surfaces. The accelerating voltages used were 15 kV for tires + inoculum and 10 kV for biochar + inoculum. The samples were sputter coated with gold (10 nm thickness) before the SEM observation (Ion Sputter, Hitachi E1010). The amount of Acinetobacter sp. immobilized onto the tyre surface was counted using the colony forming units (CFUs). The samples were first ultrasonicated for 60 min to detach microorganisms from the tire surface and then were further diluted and spread on the nutrient agar plates (3.0 g/L beef extract, 5.0 g/L peptone, and 15 g/L agar) for counting.
The samples were analyzed in triplicate and the statistical significances of different factors on the contaminant removal were evaluated using the one-way analysis of variance (ANOVA) at 95% confidence.
Results and discussion
One important factor for a successful microbial immobilization is the affinity of the microbial cells with the support material. Many studies have already provided a clear evidence of the hydrophobic nature of Pseudomonas sp. and Acinetobacter sp. (Gohl et al. 2006; Pijanowska et al. 2007; Tribedi and Sil 2013). Therefore, those isolates were preferentially immobilized on the hydrophobic surfaces (waste scrap tire and biochar). The scrap tires were used to immobilize Phanerochaete chrysosporium but without success to remove carbamazepine, while the wood chips were not considered an ideal immobilization matrix (due to the poor microbial colonization) for Pseudomonas sp. and Acinetobacter sp. for the removal of VOCs and DEHP, respectively.
Waste scrap tires
BTEX/TCE/cis-DCE mixture removal
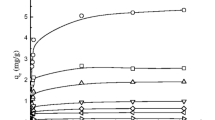
The effectiveness of waste scrap tires on the contaminant sorption was first evaluated with a mixture of VOCs (300 mg/L BTEX, 20 mg/L TCE, and 10 mg/L cis-DCE). Figure 1a shows that the contaminants had different affinities for the tire sorption sites and the differences observed were mainly associated with the contaminant concentration in the BTEX mixture but not with a specific physicochemical property. The multicomponent adsorption equilibrium was reached after 1 day and the adsorption capacities of the individual VOC in mixture followed the order of 5.3 ± 0.6 mg/g (toluene) > 2.9 ± 0.7 mg/g (benzene) > 2.2 ± 0.3 mg/g (m,p-xylene) > 1.1 ± 0.2 mg/g (o-xylene) > 0.6 ± 0.3 mg/g (ethylbenzene) > 0.4 ± 0.02 mg/g (TCE) > 0.2 ± 0.05 mg/g (cis-DCE). TCE showed a higher sorption affinity to the tire surface than cis-DCE, as expected due to the lower water solubility and smaller dipole moment (0.8 D) of TCE than cis-DCE (Avila and Breiter 2008). Figure 1b, on the other hand, shows the removal efficiency for each VOC in different microcosms, with tires only, tires with inoculum, and inoculum only. Pseudomonas sp. showed high removal capabilities for benzene, toluene, and ethylbenzene (as carbon and energy sources) but the removal of xylenes (o,m,p-), TCE, and cis-DCE was not that impressive and mainly occurred by cometabolism, which is in line with previous literature (Li et al. 2017). The tires were still effective on adsorbing xylenes and also improved the physical removal of TCE and cis-DCE to some extent. When tires and inoculum were simultaneously added, the removal of xylenes, TCE, and cis-DCE was enhanced mainly due to the hybrid (physical and biological) biosorption process.
BTEX/TCE/cis-DCE mixture sorption on waste scrap tire surface (1.5 g). a Adsorption capacity of BTEX/TCE/cis-DCE mixture after 1 day. Inset: adsorption capacity vs. contact time curve for each contaminant. b Removal efficiency for BTEX/TCE/cis-DCE mixture in different systems (inoculum only; tires + inoculum; tires only) after 5 days of incubation. Initial concentrations: 300 mg/L BTEX, 20 mg/L TCE, and 10 mg/L cis-DCE
Table 1 summarizes some recent applications of different types of adsorbents to remove BTEX and TCE/cis-DCE from the artificially contaminated water. The sorbents chosen for BTEX have been mostly synthetized (surfactant-modified zeolites and ordered mesoporous carbon) (Carvalho et al. 2012; Vidal et al. 2012; Konggidinata et al. 2017) or bio-originated material with some modification (ostrich bone waste immobilized with CTABr as cationic surfactant) (Shakeri et al. 2016). The adsorption capacity of BTEX varies greatly and some materials have even showed higher adsorption capacities compared to the waste scrap tires used in this study. Even though the physicochemical treatments of adsorbents could improve the BTEX adsorption capacity substantially, those pretreatments are not considered cost effective in large scale. In addition, the contaminants are just transferred from one phase to another and additional treatments would still be necessary to mineralize BTEX. The hybrid technology developed in this study would have some advantages compared to those other adsorbents shown in Table 1, especially considering the possibility to mineralize the contaminants by the biosorption process. Some waste materials such as pine mulch and silica gel (Avila and Breiter 2008; Wei and Seo 2010) were also used to physically remove the TCE/cis-DCE mixture but the sorption capacities were not that significant compared to the waste scrap tires used in this study, further suggesting the superior performance of tires on removing the chlorinated aliphatic hydrocarbons. Although the tire performance on sorbing BTEX was lower and slower compared to some previous studies, it would not be considered a bottleneck for further reutilization of the waste scrap tires since the main purpose was the improved removal of the BTEX/TCE/cis-DCE mixture by a hybrid (biosorption) process.
Table 2 shows the Langmuir and Freundlich isotherm parameters for BTEX/TCE/cis-DCE mixture. The Freundlich isotherm adequately described the adsorption mechanism for all the VOCs in mixture, as shown by the coefficient of determination (r2) values (0.74–0.99). Accordingly, the adsorption sites of tire would have different affinities to the BTEX/TCE/cis-DCE mixture and those sites with strong affinities would be occupied first, as also suggested by Vidal et al. (2012). Benzene and toluene showed the highest Kf values of 28.91 mg/g (L/mg)1/n and 24.45 mg/g (L/mg)1/n, respectively, suggesting a high adsorption affinity to the tire surface and a high binding energy of adsorption. The Freundlish constant 1/n, associated with the adsorption intensity, varied between 0.41 and 0.71, suggesting that the sorption of all the VOCs on the tire surface was a favorable process.
After the sorption of VOCs on the tire surface was characterized, Pseudomonas sp. was inoculated into the microcosms to biologically remove the sorbed contaminants. Figure 2a shows the SEM images of tire surface with significant roughness, further characterizing its heterogeneous surface morphology. Pseudomonas sp. could adapt to the tire effectively within 1–3 days of cultivation. The tire surface showed the isolate effectively adsorbed and colonized (Fig. 2b; after 5 days of cultivation), implying that the biosorption removal of VOCs was successful with a biofilm formation on the tire surface.
The desorption experiments were carried out in microcosms containing inoculum and tire pieces (1.5 g) after 5 days of incubation to further confirm Pseudomonas sp. almost mineralized the contaminants sorbed on the surface. Figure 3a, b shows the removal and desorption efficiencies for tires and tires + inoculum systems, respectively, where each contaminant was added at the same concentration (100 mg/L) with 1.5 g tires. In the system with tires only (Fig. 3a), the adsorbent almost completely removed the contaminants in the order of o-xylene > m,p-xylene > ethylbenzene > toluene > TCE > benzene > cis-DCE. Around 80% of VOCs were desorbed from the adsorption process while only 18% were recovered from the tires + inoculum system, further confirming the biological process (biosorption) could remove the contaminants effectively when the hybrid process was applied. Benzene, toluene, and ethylbenzene were almost completely removed (mineralized) from the tire surface by the inoculum (Fig. 3b) while xylenes, TCE, and cis-DCE were mainly removed biologically with a little contribution from the physical adsorption. Even though the exact mechanism for the VOC removal by this hybrid process seems complicated, it can still be explained by both sorption of VOCs and Pseudomonas sp. on the tire surface. The isolate utilized those sorbed contaminants as carbon and energy sources while colonizing the surface accordingly and those sorption sites would be free for the additional sorption of contaminants. This hybrid process is considered promising for the bioremediation of VOCs and other contaminants of environmental concern because of reutilizing waste as adsorbent without any further treatment and the sorbed microorganisms mineralizing contaminants into harmless products.
DEHP removal
The main objective to reutilize waste scrap tires as a potential microbial immobilization matrix was to further enhance the microbial ability to remove DEHP in a shorter time. Since both DEHP and tire surface are mostly hydrophobic, the adsorption of the contaminant on the tire surface would be a favorable process. There has been no report on reutilizing waste materials (or other adsorbents) as microbial immobilization matrices to remove DEHP from the environmental samples, further justifying the development of a hybrid and environmentally friendly technology. The amount of tire pieces (adsorbent dosage, surface area, at pH 7) to be added into the microcosms containing 100 mg/L DEHP but without the isolate was further optimized and the adsorption capacities were also assessed at different pH values with 2.5 g of tires. Figure 4 shows that the DEHP adsorption increased with the increasing tire amount up to 2.5 g, as expected due to the increase of adsorption sites. There was no statistical significance (p = 0.936) when higher amounts of tires (5 g) were provided, suggesting that the DEHP adsorption capacity equilibrium was reached at higher sorbent dosages, which was also observed by Shakeri et al. (2016) using ostrich bone waste to remove BTEX. The overlapping or partial aggregation of sorption sites on the tire surface might be the reason for the decreased DEHP adsorption capacity at higher tire amount and this trend was also reported when other adsorbents and adsorbates were used (Garg et al. 2004; Chowdhury et al. 2011). The highest DEHP adsorption capacity was achieved at pH 7 (p < 0.05), and this pH was used in the subsequent experiments. Figure 5a shows the DEHP adsorption capacity at different contact times when 2.5 g of tires added. The amount of DEHP sorbed on the scrap tire surface was substantially affected by the contact time and the maximum adsorption capacity (2 mg/g) was reached in about 3 days with DEHP almost completely removed (94.9 ± 0.2%). The sorption of DEHP on the tire surface was not that impressive and fast. The obtained results are in accordance with Salim et al. (2010) showing the slower adsorption of DEHP and the lower adsorption capacity compared to other phthalate esters, even though DEHP showed the highest organic carbon-normalized partition coefficient. They attributed this behavior to the adsorbent structure, especially to the molecule size. The large molecular size of DEHP could exert a steric hindrance effect on the binding sites of tire surface, slowing down the sorption process to some extent.
Both Langmuir and Freundlich adsorption isotherm models were applied to the data obtained when different concentrations of DEHP (10–500 mg/L) were added to the fixed amounts of tires (2.5 g). Table 2 shows the DEHP sorption on tires fitted the Langmuir isotherm model (r2 = 0.998) better than the Freundlich (r2 = 0.908), suggesting DEHP adsorbed on the tire surface as a monolayer. The values of Langmuir constants KL and Cm, associated with the energy sorption and the maximum adsorption capacity, respectively, were 176.50 L/mg and 10.33 mg/g. The similar results were also reported for the DEHP removal on vermiculite (Wen et al. 2013).
The DEHP concentration sharply decreased when both tires and inoculum were simultaneously provided (immobilized system) compared to the microcosms with inoculum only (suspended system) (Fig. 5b). The colony forming units (CFUs) increased while the concentration of DEHP decreased, further confirming the hybrid process (DEHP sorption and microbial immobilization) contributing to the almost complete DEHP removal in 2 days. The period for the Acinetobacter sp. adaptation was relatively fast (within 2 days) with biofilm formed on the tire surface. The obtained results further suggest both DEHP and Acinetobacter sp. sorbed/attached onto the tire surface and the isolate used the sorbed DEHP as carbon and energy source for growth and colonize the tire surface accordingly. The hybrid process showed clear advantages over the suspended system. It required a shorter time for the DEHP bioremoval (in 2 days) and would be a promising alternative for the remediation of different organic contaminants in the environmental sites.
Sewage sludge biochar
Toluene removal
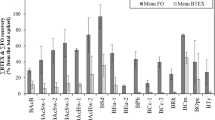
Some physicochemical parameters of sewage sludge biochar produced at different pyrolysis temperatures are shown in Table 3. The biochar pH increased significantly when higher temperatures were employed during their production (p < 0.05), in accordance with Gai et al. (2014) using biochars generated from wheat-straw, corn-straw, and peanut-shell at four different pyrolysis temperatures (400, 500, 600, and 700 °C) to remove ammonium and nitrate. The extractable COD, TN, and TP concentrations which could be used as additional nutrients for the microorganisms were higher and statistically significant (p < 0.05) for the biochar produced at the lower pyrolysis temperature (300 °C). Regardless of the pyrolysis temperature used, the generated biochars were classified as fine biochars (53–120 μm) but the fine portion decreased with the increasing pyrolysis temperatures (p < 0.05). According to Gu et al. (2017), the fine particles were effective on adsorbing both tomato root exudates and pathogen cells, resulting in lower pathogen densities in the rhizosphere. The fine particles of biochar were also responsible for removing Escherichia coli from the storm water under a wide range of field conditions (Moranty and Boehm 2014). The bacterial isolate was adsorbed on biochar and colonized its surface efficiently within 3 days of cultivation even though no visible biofilm was observed. The sewage sludge biochar generated in the current study could be used to sorb/immobilize the microbial isolate efficiently and would be a promising strategy for future application of hybrid technologies in the contaminated environmental sites as well.
The toluene adsorption (250 mg/L) on sewage sludge biochar (50 mg) increased with the contact time and reached equilibrium within 24 h with adsorption capacities of 34.7 ± 9.9, 22.7 ± 2.4, and 26.74 ± 2.7 mg/g for BC300, BC500, and BC700, respectively (Fig. 6a). Since the main purpose for the development of the hybrid removal process was the removal of toluene through biosorption instead of just physical adsorption, the biochar generated at 500 °C which showed that the lowest adsorption capacity for toluene, compared to 300 and 700 °C, was chosen to further study the adsorption mechanism.
Toluene removal efficiencies (%): a by sorption and b by hybrid process (adsorption + bioremoval), using sewage sludge biochars generated at different pyrolysis temperatures (300, 500, and 700°C). Toluene concentration, 250 mg/L; biochar amount, 50 mg. Inset: adsorption capacity of toluene in sewage sludge biochars at different pyrolysis temperatures at different contact times
The equilibrium adsorption isotherms for toluene (20–300 mg/L) on sewage sludge biochar (BC500) at 25 °C were fitted by using Langmuir and Freundlich isotherm models (Table 2) for the toluene removal mechanism. The Freundlich isotherm showed a better fit (r2 = 0.99) to the adsorption data than Langmuir (r2 = 0.60), implying that the biochar surface is heterogeneous in nature with the sorption sites showing different energies/affinities. The Freundlich constant (Kf) associated with the toluene adsorption capacity was low (0.002 mg/g), suggesting the poor adsorption capacity of toluene on the biochar surface, while relatively weak interactions between toluene and biochar were expressed by the 1/n value higher than unit 1. The capability of sewage sludge biochar of sorbing both contaminants and microorganisms was further evaluated using toluene (250 mg/L) as a representative VOC and Pseudomonas sp. as the microbial isolate. Figure 2c, d shows the surface morphology of the biochar (BC500) without and with Pseudomonas sp. attached to its surface, respectively. The biochar generated by the pyrolysis of sewage sludge showed a coarse and heterogeneous surface with the micrometric structures of irregular size and orientation, in agreement with Ahsaine et al. (2017) who used sewage sludge biochar as a low-cost adsorbent for cadmium. Regardless of the biochar used (BC300, BC500, or BC700), approximately 20% toluene were removed by sorption after 3 days of contact with the adsorbent (Fig. 6a), suggesting adsorption with a minor role in the toluene removal. In comparison, about 40% toluene were biologically removed during the same period. The relatively poor performance of Pseudomonas sp. on removing toluene might be associated with the lower temperature used for the microbial cultivation (24 °C), compared to the optimal value known for this strain (30 °C). The lower cultivation temperature was used to mimic the under sub-optimal condition for the growth of Pseudomonas sp., which would be the case in the contaminated site.
The toluene removal sharply increased (Fig. 6b) and was almost completely removed within 3 days when both biochar and microorganisms were provided simultaneously. The removal efficiencies were not statistically significant (p = 0.118) for the microcosms with biochar and microorganisms regardless of different temperatures used to produce biochar (99.80 ± 0.23, 98.66 ± 0.45, and 99.83 ± 0.17% for BC300, BC500, and BC700, respectively). Different performances of sewage sludge biochars on removing toluene were expected due to the associated adsorbent electrochemical properties. Klüpfel et al. (2014) reported that the biochars produced at intermediate to high heat treatment temperatures showed the highest capacities to accept and donate electrons, further confirming the pyrolysis temperature would play a major role in the adsorbent electrochemical properties. However, regardless of the sewage sludge biochar used (BC300, BC 500, and BC700) in the current study, no obvious differences in the toluene removal efficiency were obtained, further suggesting the electrochemical properties of the sewage sludge biochars generated at different pyrolysis temperatures were not that different.
As shown in Fig. 6b, it is clear that the toluene bioremoval was improved to a great extent by the biochar application. Previous studies have been emphasizing biochar as an efficient electron mediator in the bioremediation applications. In the current study, its redox fractions could substantially influence the electron transfer between Pseudomonas sp. and toluene, with both sorbed/attached onto the biochar surface. The improved toluene removal after the biochar incorporation in the microcosm is in agreement with the “electron shuttle” property of biochar. Yu et al. (2015a) reported that the reductive dechlorination of pentachlorophenol (PCP) by Geobacter sulfurreducens was greatly improved after the biochar addition, and the surface redox-active moieties and the conductive graphite regions played major roles during the microbial dechlorination process. They concluded that the phenolic and quinone moieties and the trace levels of surface redox-active metals (Fe3+ and Mn2+) were the main biochar surface redox-active moieties responsible for the dechlorination process. Therefore, the coexistence of microbial cells and PCP sorbed on the biochar surface could facilitate the electron transfer between them. Chen et al. (2018) also proved the biochar redox-active fractions speeded up the BDE-47 microbial reductive debromination in anaerobic mangrove sediments in three aspects: (1) biochar enriched the microbial communities involved in organohalide respiration, (2) biochar can act as an “electron shuttle” accelerating the electron transfer from the microbes to the contaminant, and (3) biochar may act as an active reductant to accelerate the abiotic reductive debromination. Zhang et al. (2017) also concluded that the total petroleum hydrocarbon (TPH) removal enhancement (up to 78.9%) was attributable to the immobilization of the isolate Corynebacterium variabile HRJ4 on biochar surface compared to the free-living bacteria after 7 days of incubation. The biochar addition created a sustainable micro-environment by an efficient mass transport of nutrients and oxygen to the microbial isolate which colonized the biochar surface and used TPH as carbon and energy source more efficiently.
Wood chips
Carbamazepine removal
The white-rot fungus (WRF) has been successfully applied to the remediation of trace organic contaminants (TrOCs), mostly under the sterile conditions, due to the presence of its non-specific intra- and extracellular enzymes. In this study, Phanerochaete chrysosporium was applied to remove a representative TrOC, carbamazepine, commonly present in water bodies worldwide by immobilizing/sorbing the WRF on wood chips to mimic its natural habitat.
After carbamazepine was spiked at 20 mg/L in synthetic wastewater containing P. chrysosporium spore suspension (1:40, v/v) sorbed on wood chips (1 g/L), two microcosms were tested, without (free growing/suspended system) and with (immobilized/attached system) wood chips. As shown in Fig. 7, the carbamazepine residual concentration after 7 days of incubation reached 13 mg/L (removal efficiency, 34.4 ± 1.8%) while 7.7 mg/L remained in the solution (removal efficiency, 61.3 ± 0.6%) after the fungal immobilization (p < 0.05). The WRF sorption on wood chips improved the carbamazepine removal to a great extent, and such improvement was also observed during the removal of phenolic compounds in cooking wastewater by P. chrysosporium immobilized on wood chips (Lu et al. 2011). Rubilar et al. (2011) also reported the increase of pentachlorophenol removal by the WRF immobilization on wheat grains. The outstanding performance on the humic acid removal in real and synthetic wastewater by the immobilized Trametes versicolor on sorghum was also reported by Zahmatkesh et al. (2018). In general, the extensive surface area of wood chips contributed to the fungal optimal growth, followed by the substantial enzyme production, and the presence of the adsorbent was critical to the carbamazepine removal. The WRF P. crysosporium showed a great adaptation to the wood chip surface and a thin biofilm was observed on the surface after 7 days of fungal cultivation. Two extracellular enzymes produced by P. chrysosporium, lignin peroxidase (LiP) and manganese peroxidase (MnP), were monitored on a daily basis during the fungal incubation (7 days). Laccase (Lac) enzyme was not monitored due to its low production in this fungal strain. Twenty-four hours after the carbamazepine spike, a sharp decline of the MnP activity was observed (from 4150 to 730 U/L), while the LiP activity peaked on day 3 (1430 U/L) and remained low (370–490 U/L) throughout the incubation period (Fig. 7).
Table 2 summarizes the Langmuir and Freundlich isotherm constants for carbamazepine (10 mg/L) at different amounts of wood chips (1, 5, 10, 15, and 20 g/L) in 50 mL synthetic wastewater at 30 °C. The carbamazepine adsorption data fitted the Langmuir model better (r2 = 0.86) and its maximum adsorption capacity (qmax) was 0.06 mg/g, further suggesting a poor affinity of the contaminant to the adsorbent surface and the bioremoval playing the main role. The intracellular enzymes would also play an important role in the carbamazepine degradation, together with biosorption, which was also confirmed by previous studies. Marco-Urrea et al. (2009) evaluated the carbamazepine bioremoval by different WRF strains and concluded the extracellular enzymes laccase and MnP failed to oxidize the contaminant while the intracellular cytochrome P450 system seemed to effectively remove carbamazepine to a great extent. When Vasiliadou et al. (2016) evaluated the biological removal of carbamazepine and other pharmaceuticals using Trametes versicolor and Ganoderma lucidum, the carbamazepine removal was around 32% using the combined fungal system and was negatively affected by the inhibition of the P450 system. The sharp decrease of carbamazepine in both suspended and immobilized systems during first 24 h in the current study could also be associated with the mechanism suggested by Haroune et al. (2017), where the fast pharmaceutical uptake by sorption occurred, followed by its active transformation by intracellular enzymes inside the fungal cell. The generated products could then be excreted back to the medium and further degraded by the extracellular enzymes (MnP and LiP).
Conclusions
The reutilization of waste materials (scrap tires, sewage sludge biochar, and wood chips) as adsorbents together with the immobilized microorganisms was effective on the removal of VOCs (BTEX/TCE/cis-DCE mixture), plasticizer (DEHP), and pharmaceutically active compound (carbamazepine) from the contaminated water. The development of this hybrid (physical/sorption and biological/immobilization) technology would be promising for the on-site application, and some important features of this technology can be highlighted as follows: it mainly deals with the indigenous microorganisms previously isolated from the contaminated sites, with high capabilities of the contaminant mineralization; the adsorbents used are waste materials so they could be obtained at no cost and easy acquisition and used as the microbial immobilization matrices without pretreatment; the contaminants are being mineralized by the respective microorganisms and the sorption sites of adsorbents are being constantly replaced by more contaminants (so working as ‘biosorbents’); and the removal period could also be shortened to some extent (e.g., DEHP). Further studies are still warranted to further improve the bioremoval of TCE/cis-DCE and carbamazepine as well as to evaluate the effectiveness of the hybrid system when the contaminants are spiked at different time intervals.
References
Ahmad M, Lee SS, Oh SE, Mohan D, Moon DH, Lee YH, Ok YS (2013) Modeling adsorption kinetics of trichloroethylene onto biochars derived from soybean Stover and peanut shell wastes. Environ Sci Pollut Res 20:8364–8373
Ahsaine HA, Zbair M, El Haouti R (2017) Mesoporous treated sewage sludge as outstanding low-cost adsorbent for cadmium removal. Desalin Water Treat 85:330–338
Almeida ILS, Antoniosi Filho NR, Alves MIR, Carvalho BG, Coelho NMM (2012) Removal of BTEX from aqueous solution using Moringa oleifera seed cake. Environ Technol 33:1299–1305
Arshadi M, Shakeri H, Savacion JWL (2016) A green adsorbent for the removal of BTEX from aqueous media. RSC Adv 6:14290–14305
Avila MAS, Breiter R (2008) Competitive sorption of cis-DCE and TCE on silica gel as a model porous mineral solid. Chemosphere 72:1807–1815
Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques - classification based on site application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol 32:180. https://doi.org/10.1007/s11274-016-2137-x
Balcha A, Yadav OP, Dey T (2016) Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ Sci Pollut Res 23:25485–25493
Belgacem A, Rebiai R, Hadoun H, Khemaissia S, Belmedani M (2014) The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ Sci Pollut Res 21:684–694
Bhatnagar A, Sillapãã B (2010) Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment – a review. Chem Eng J 157:277–296
Buttiglieri G, Knepper TP (2008) Removal of emerging contaminants in wastewater treatments: conventional activated sludge treatment. In: Barceló D, Petrovic M (eds) Emerging contaminants from industrial and municipal waste. Removal technologies. Springer-Verlag, Berlin, pp 1–36
Carmalin SA, Lima EC (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf 150:1–17
Carvalho MN, da Mota M, Benachour M, Sales DCS, Abreu CAM (2012) Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay. J Hazard Mater 239-240:95–101
Chen J, Wang C, Oan Y, Farzana SS, Tam NFY (2018) Biochar accelerates microbial reductive debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in anaerobic mangrove sediments. J Hazard Mater 341:177–186
Chowdhury S, Chakraborty S, Saha P (2011) Biosorption of basic green 4 from aqueous solution by Ananas comosus (pineapple) leaf powder. Colloids Surf B: Biointerfaces 84:520–527
Chung HK, Kim WH, Park J, Cho J, Jeong TY, Park PK (2015) Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J Ind Eng Chem 28:241–246
Deokar SK, Mandavgane SA, Kulkarni BD (2016) Agro-industrial waste: a low-cost adsorbent for effective removal of 4-chloro-2-methylphenoxyacetic acid herbicide in batch and packed bed modes. Environ Sci Pollut Res 23:16164–16175
Dey T (2012) Magnetic nanoparticles and cellulosic nanofibers to remove arsenic and other heavy metals from water. In: Dey T (ed) Nanotechnology for water purification. Universal Publishers, Boca Raton, pp 1–28
Forrest M (2014) Recycling and re-use of waste rubber. Smithers Rapra, Shawbury
Gabarrón S, Gernjak W, Valero F, Barceló A, Petrovic M, Rodríguez-Roda I (2016) Evaluation of emerging contaminants in a drinking water treatment plant using electrodialysis reversal technology. J Hazard Mater 309:192–201
Gai X, Wang H, Liu J, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 9:e113888. https://doi.org/10.1371/journal.pone.0113888
Garg VK, Kumar R, Gupta R (2004) Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigments 62:1–10
Garoma T, Skidmore L (2011) Modeling the influence of ethanol on the adsorption and desorption of selected BTEX compounds on bentonite and kaolin. J Environ Sci 23:1865–1872
Gohl O, Friedrich A, Hoppert M, Averhoff B (2006) The thin pili of Acinetobacter sp. strain BD413 mediate adhesion to biotic and abiotic surfaces. Appl Environ Microbiol 72:1394–1401
Gu Y, Hou Y, Huang D, Hao Z, Wang X, Wei Z, Jousset A, Tan S, Xu D, Shen Q, Xu Y, Friman VP (2017) Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 415:269–281
Gupta VK, Nayak A, Agarwal S, Tyagi I (2014) Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J Colloid Interface Sci 417:420–430
Haroune L, Saibi S, Cabana H, Bellenger JP (2017) Intracellular enzymes contribution to the biocatalytic removal of pharmaceuticals by Trametes hirsuta. Environ Sci Technol 51:897–904
Kang JW (2014) Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol Lett 36:1129–1139
Karmacharya MS, Gupta VK, Tyagi I, Agarwal S, Jha VK (2016) Removal of as(III) and as(V) using rubber tire derived activated carbon modified with alumina composite. J Mol Liq 216:836–844
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611
Konggidinata MI, Chao B, Lian Q, Subramanian R, Zappi M, Gang DD (2017) Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto ordered mesoporous carbon (OMC). J Hazard Mater 336:249–259
Krause RWM, Thwala LN, Dey T (2012) Silver-impregnated cyclodextrin nanocomposite for safe drinking water. In: Dey T (ed) Nanotechnology for water purification. Universal Publishers, Boca Raton, pp 51–70
Li X, Xu J, de Toledo RA, Shim H (2015) Enhanced removal of naproxen and carbamazepine from wastewater using a novel countercurrent seepage bioreactor immobilized with Phanerochaete chrysosporium under non-sterile conditions. Bioresour Technol 197:465–474
Li J, de Toledo RA, Shim H (2017) Multivariate optimization for the simultaneous bioremoval of BTEX and chlorinated aliphatic hydrocarbons by Pseudomonas plecoglossicida. J Hazard Mater 321:238–245
Lu Y, Yan L, Wang Y, Zhou S, Fu J, Zhang J (2011) Biodegradation of phenolic compounds from cooking wastewater by immobilized white rot fungus Phanerochaete chrysosporium. J Hazard Mater 165:1091–1097
Lu Q, de Toledo RA, Xie F, Li J, Shim H (2015) Combined removal of a BTEX, TCE, and cis-DCE mixture using Pseudomonas sp. immobilized on scrap tyres. Environ Sci Pollut Res 22:14043–14049
Lu Q, de Toledo RA, Xie F, Li J, Shim H (2017) Reutilization of waste scrap tyres as the immobilization matrix for the enhanced bioremoval of a monoaromatic hydrocarbons, methyl tert-butyl ether, and chlorinated ethenes mixture from water. Sci Total Environ 583:88–96
Magi E, Di Carro M, Mirasole C, Benedetti B (2018) Combining passive sampling and tandem mass spectrometry for the determination of pharmaceuticals and other emerging pollutants in drinking water. Microchem J 136:56–60
Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74:765–772
Moran M, Zogorski JS, Squillace PJ (2007) Chlorinated solvents in groundwater of the United States. Environ Sci Technol 41:74–81
Moranty SK, Boehm AB (2014) Escherichia coli removal in biochar-augmented biofilter: effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ Sci Technol 48:11535–11542
Pijanowska A, Kaczorek E, Chrzanowski T, Olszanowski A (2007) Cell hydrophobicity of Pseudomonas spp. and Bacillus spp. bacteria and hydrocarbon biodegradation in the presence of Quillaya saponin. World J Microbiol Biotechnol 23:677–682
Ramola S, Mishra T, Rana G, Srivastava RK (2014) Characterization and pollutant removal efficiency of biochar derived from bagasse, bamboo, and Tyre. Environ Monit Assess 186:9023–9039
Rubilar O, Tortella G, Cea M, Acevedo F, Bustamante M, Gianfreda L, Diez MC (2011) Bioremediation of a Chilean Andisol contaminated with pentachlorophenol (PCP) by solid substrate cultures of white-rot fungi. Biodegradation 22:31–41
Salim CJ, Liu H, Kennedy JF (2010) Comparative study of the adsorption on chitosan beads of phthalate esters and their degradation products. Carbohydr Polym 81:640–644
Salimi M, Esrafili A, Gholami M, Jafari AJ, Kalantary RR, Farzadkia M, Kermani M, Sobhi HR (2017) Contaminants of emerging concern: a review of new approach in AOP technologies. Environ Monitor Assess 189:414. https://doi.org/10.1007/s10661-017-6097-x
San Miguel G, Lambert SD, Graham NJD (2006) A practical review of the performance of organic and inorganic adsorbents for the treatment of contaminated waters. J Chem Technol Biotechnol 81:1685–1696
Schäfer AI, Nghlem LD, Waite TD (2003) Removal of the natural hormone estrone from aqueous solutions using nanofiltration and reverse osmosis. Environ Sci Technol 37:182–188
Shakeri H, Arshadi M, Salvacion JWL (2016) Removal of BTEX by using a surfactant – bio-originated composite. J Colloid Inter Sci 466:186–197
Simantiraki F, Kollias CG, Maratos D, Hahladakis J, Gidarakos E (2013) Qualitative determination and application of sewage sludge and municipal waste compost for BTEX removal from groundwater. J Environ Chem Eng 1:9–17
Sun S, Jia L, Li B, Yuan A, Kong L, Qi H, Ma W, Zhang A, Wu Y (2018) The occurrence and fate of PAHs over multiple years in wastewater treatment plant of Harbin, Northeast China. Sci Total Environ 624:491–498
Tribedi P, Sil AK (2013) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol 116:295–303
UNESCO (2012) The 4th edition of the UN world water development report. http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/wwdr4-2012/. Accessed 17 Jan 2018
UNESCO (2017) The United Nations world water development report. http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/. Accessed 15 Jan 2018
Vasiliadou IA, Sánchez-Vázquez R, Molina R, Martinez F, Melero JA, Bautista LF, Iglesias J, Morales G (2016) Biological removal of pharmaceutical compounds using white-rot fungi concomitant FAME production of the residual biomass. J Environ Manag 180:228–237
Vidal CB, Raulino GSC, Barros AL, Lima ACA, Ribeiro JP, Pires MJR, Nascimento RF (2012) BTEX removal from aqueous solutions by HDTMA-modified Y zeolite. J Environ Manag 112:178–185
Vukelic D, Boskovic N, Agarski B, Radonic J, Budak I, Pap S, Sekulic MT (2018) Eco-design of a low-cost adsorbent produced from waste cherry kernels. J Clean Prod 174:1620–1628
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Wei Z, Seo Y (2010) Trichloroethylene (TCE) adsorption using sustainable organic mulch. J Hazard Mater 181:147–153
Wen ZD, Gao DW, Li Z, Ren NQ (2013) Effects of humic acid on phthalate adsorption to vermiculite. Chem Eng J 223:298–303
Wilkinson JL, Hooda PS, Swinden J, Barker J, Barton S (2018) Spatial (bio)accumulation of pharmaceuticals, illicit drugs, plasticisers, perfluorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms. Environ Pollut 234:864–875
Williams PRD (2014) Methyl tertiary butyl ether (MTBE) and other volatile organic compounds (VOCs) in public water systems, private wells, and ambient groundwater wells in New Jersey compared to regulatory and human-health benchmarks. Environ Forensic 15:97–119
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375
Xu J, Lu Q, de Toledo RA, Shim H (2017) Degradation of di-2-ethylhexyl phthalate (DEHP) by an indigenous isolate Acinetobacter sp. SN13. Int Biodeterior Biodegrad 117:205–214
Ye J, Rensing C, Su J, Zhu YG (2017) From chemical mixtures to antibiotic resistance. J Environ Sci 62:138–144
Yu L, Yuan Y, Tang J, Wang Y, Zhou S (2015a) Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens. Sci Rep 5. https://doi.org/10.1038/srep16221
Yu S, Lee PK, Hwang S (2015b) Groundwater contamination with volatile organic compounds in urban and industrial area: analysis of co-occurrence and land use effects. Environ Earth Sci 74:3661–3677
Yuan Y, Bola N, Prévoteau A, Vithanage M, Biswas JK, Ok YS, Wang H (2017) Applications of biochar in redox-mediated reactions. Bioresour Technol 246:271–281
Zahmatkesh M, Spanjers H, van Lier JB (2018) A novel approach for application of white rot fungi in wastewater treatment under non-sterile conditions: immobilization of fungi on sorghum. Environ Technol 39:2030–2040
Zhang H, Tang J, Wang L, Liu J, Gurav RG, Sun K (2017) A novel bioremediation strategy for petroleum hydrocarbon pollutants using salt tolerant Corynebacterium variabile HRJ4 and biochar. J Environ Sci 47:7–13
Funding
This work was supported by the grants from the Macau Science and Technology Development Fund (FDCT/115/2016/A3; FDCT044/2017/AFJ) and the University of Macau Multi-Year Research Grant (MYRG2017-00181-FST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Rights and permissions
About this article
Cite this article
de Toledo, R.A., Hin Chao, U., Shen, T. et al. Development of hybrid processes for the removal of volatile organic compounds, plasticizer, and pharmaceutically active compound using sewage sludge, waste scrap tires, and wood chips as sorbents and microbial immobilization matrices. Environ Sci Pollut Res 26, 11591–11604 (2019). https://doi.org/10.1007/s11356-018-2877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2877-2