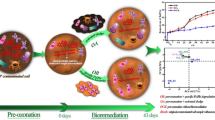
Abstract
The coexistence of various organic pollutants in soil always draws extensive attention because of their difficulties and complexity for remediation. Especially, the impacts of bioremediation on soil co-contaminated with benzene, toluene, and trichloroethylene (TCE) have seldom been comprehensively evaluated yet. In this study, the contributions of biostimulation, bioaugmentation, and their combination for the bioremediation of the co-contaminated soil containing benzene, toluene, and TCE were systematically investigated. The addition of nutrients ((NH4)2SO4 (as N source), K2HPO4 (as P source), vegetable oil and CH3COONa (as C sources)) enhanced the degradation efficiency of the co-contaminated soil by 10.19% to 49.62%. The optimal biostimulation condition involved using vegetable oil as the carbon source with a C: N: P ratio of 100: 10: 1. Meanwhile, the addition of the microbial cultures screened and domesticated from the co-contaminated soil, named B-T, effectively enhanced the removal rate of contaminants by 33.02% to 37.55%. The genera comprising Pseudomonas, Stenotrophomonas, and Chryseobacterium in B-T exhibited the highest relative abundance, suggesting their potential for the removal of benzene, toluene, and TCE. Besides, the coupling of biostimulation and bioaugmentation enhanced the degradation efficiency by 62.38% to 68.84%, showing the most effective biodegradation effects. The coupled strategy showed synergistic effects of both, increasing the quantity and activity of microorganisms and accelerating the biodegradation of target contaminants. The findings indicated that the coupling of bioaugmentation and biostimulation treatment strategy holds promise for the bioremediation of benzene, toluene, and TCE from co-contaminated soil.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rapid development of industrialization has increased the production of various chemicals. Benzene and toluene are the main constituents of gasoline and are commonly utilized as industrial solvents (Khodaei et al., 2017). Trichloroethylene (TCE) is extensively used in the industrial sector, primarily as a cleaning solvent and degreasing agent (Li et al., 2021). As the toxic, cumulative, carcinogenic, and mutagenic chemicals, they would pose great threats to human health and natural ecosystems. However, these chemicals would unavoidably enter soil and groundwater through leakages and discharges during production, transportation, and utilization, leading to serious environmental problems and adverse social impacts (Zhu et al., 2021). Since benzene, toluene, and TCE are commonly detected concurrently in many contaminated sites, their efficient treatment or remediation always attract great attentions (Meng & Wang, 2005; Youya et al., 2009).
Many physical and chemical remediation technologies have been developed for cleaning up organic contaminants in soil (Ding et al., 2019). Generally, physical and chemical methods are suitable for soil with high pollutant concentrations and low moisture content, while entailing higher economic costs. Among them, physical techniques like thermal desorption and vapor extraction are commonly used for benzene, toluene, or TCE removal. However, they tend to consume substantial energy and result in secondary pollution (Han et al., 2023). Chemical remediation techniques such as photocatalysis and chemical oxidation or reduction effectively facilitate benzene, toluene, or TCE degradation, yet often result in the generation of intermediate products that display greater toxicity (Qian et al., 2016). Additionally, benzene and toluene are aromatic hydrocarbons characterized by carbon–carbon single bonds, while TCE is a chlorinated hydrocarbon featuring carbon–carbon double bonds. The coexistence and the distinct redox reactions of these three types of contaminants increase the challenge of employing physical and chemical methods. However, bioremediation technology has garnered wide attention in the field of complex contaminants removal due to its simplicity, environmental friendliness, superior efficiency, and cost-effectiveness (Bai et al., 2015).
Biostimulation and bioaugmentation are two effective strategies for contaminated field bioremediation (Ji et al., 2008). The effectiveness of bioremediation employing indigenous microorganisms in remediating co-contaminated soil is restricted by pollutant concentration, nutrients, pH, temperature, oxygen, etc. (Smith et al., 2015). Deficiency of essential nutrients like carbon (C), nitrogen (N), and phosphorus (P), has been recognized as crucial factors that inhibit microbial bioremediation performance (Alvarez et al., 2015). Therefore, it is beneficial to establish the in-situ ideal ratios of C: N: P for bioremediation. The addition of organic carbon-based bioremediation stimulants (biostimulants), such as vegetable oil and CH3COONa, has been found as an efficient biostimulation approach (Borden et al., 2007). Ding et al. found that using vegetable oil as the exclusive additional organic carbon source facilitated the efficient degradation of benzene in groundwater (Ding et al., 2021). Additionally, the slow biodegradation efficiency of contaminants was also attributed to the deficiency or insufficient activities of functional enzymes within indigenous microorganisms. Therefore, the introduction of exogenous microorganisms for bioaugmentation could overcome the functional constraints of indigenous microorganisms (Simpanen et al., 2016). The effectiveness of bioremediation in co-contaminated soil is limited when only applying a single microbial species. Employing microbial communities for bioaugmentation would enhance biodiversity and promote adaptability and synergistic interactions among them (Wang et al., 2020). However, both methods exhibit specific limitations when individually applied for the removal of complex contaminants. Biostimulation may be limited by soil environmental factors and the activity of indigenous microorganisms, while bioaugmentation might face challenges associated with adaptability and competition upon introducing exogenous microorganisms (Roy et al., 2018).
The coupling of biostimulation and bioaugmentation is a promising method for remediating co-contaminated soil. The strategy maximizes their respective advantages, fostering microbial synergies and enhancing the degradation efficiency of co-contaminated soil (Raimondo et al., 2020; Varjani & Upasani, 2019). Currently, many reports have shown that the coupling of biostimulation and bioaugmentation enhances the co-bioremediation of complex contaminants. Li et al. indicated that bioaugmentation combined with biostimulation effectively removed BTEX (benzene, toluene, ethylbenzene, and xylenes) and chloroethylenes from contaminated soil (Li et al., 2021). Agarry et al. demonstrated that the coupling of biostimulation and bioaugmentation strategies showed a great remediation effect in kerosene-contaminated soil (Agarry et al., 2010). Therefore, the coupling of biostimulation and bioaugmentation holds significant promise in the practical application for removing benzene, toluene, and TCE in co-contaminated soil. There is currently insufficient research on biostimulant adjustment and the adaptability of exogenous microbial communities in soil co-contaminated with benzene, toluene, and TCE and further study is necessary to understand how to enhance the synergistic effect between biostimulation and bioaugmentation.
The main purpose of this study was to optimize the synergy effects of biostimulation and bioaugmentation for the bioremediation of the co-contaminated soil containing benzene, toluene, and TCE. The optimal C: N: P ratio and key limiting factors of biostimulants were performed. Simultaneously, the exogenous microbial communities were screened and cultivated. Furthermore, the effects of biostimulation, bioaugmentation, and their coupled application on the co-contaminated soil were compared by evaluating the degradation rate of contaminants and monitoring changes in soil microbial activity. The achievements obtained are expected to be beneficial for bioremediation strategy design of organic co-contaminated soil.
2 Materials and Methods
2.1 Soil Sample
The soil sample was collected from a site mainly contaminated with TCE, benzene, and toluene in Jiangsu province, China. It was then sieved through a 2 mm mesh to remove impurities. The soil was analyzed as a clay texture (9.23 ± 0.53% sand, 54.12 ± 6.2% silt, and 36.8 ± 3.5% clay), with pH 7.33 ± 0.37, 18 ± 2.5% moisture content, and the contents of 0.3 ± 0.02% total organic carbon, 0.07 ± 0.02 mg/kg total nitrogen, 2.35 ± 0.23 mg/kg total phosphorus.
2.2 Microcosms Experimental Design
2.2.1 Experiment for Biostimulation
Considering the probable nutrient deficiency in the contaminated soil, biostimulation experiments were conducted using vegetable oil and CH3COONa as carbon sources, (NH4)2SO4 as nitrogen source, and K2HPO4 as phosphorus source. The experiment was performed in 40 mL brown bottles, each containing 40 g soil and the designed proportions of biostimulants (Table 1) to determine the optimal nutrient addition ratio of C: N: P. Incubation was carried out at 25℃ for 40 days before analysis, with daily mixing 3 times.
2.2.2 Preparation of the Inoculum for Bioaugmentation Preparation of the Inoculum for Bioaugmentation Preparation of the Inoculum for Bioaugmentation and the Experiment Design
The microbial community was screened and domesticated from the soil, namely B-T. 50 g pre-treated soil contaminated by benzene, toluene and TCE was weighed and added to 200 mL medium (with the following components per liter: K2SO4 0.4 g, CaSO4 0.015 g, (NH4)2HPO4 0.50 g, NaH2PO4 0.20 g, MgSO4 0.30 g). The supernatant was collected after being incubated at 25℃ with agitation at 150 rpm for 50 days, followed by centrifugation. The pellet was suspended with sterile phosphate-buffered saline (PBS) and then added to 100 mL medium at a 5% dosage, for incubation at 25℃ for 40 days. The above procedures were repeated 2 to 3 times before the cultures were collected, namely B-T.
The bioaugmentation experiment was conducted in brown bottles and divided into a control group (namely CK) and an experimental group (namely BA). The 40 g soil and 5% sterile culture solution were added to the CK group, and 40 g soil and 5% B-T with a bacterial density of about 5 × 108 CFU/mL were added to the BA group. Both were incubated at 25℃ for 30 days before analysis.
2.2.3 Experiment for Combined Bioaugmentation and Biostimulation
The control group (S0), biostimulation group (S1), bioaugmentation group (S2), and the combined biostimulation and bioaugmentation treatment group (S3) were established to examine the potential advantages of combining biostimulation and bioaugmentation treatment for co-contaminated soil bioremediation with the design shown in Table 2. The experiments were conducted using 40 mL brown bottles with incubation at 25℃ for 30 days before analysis.
2.3 Microbial Community Analysis
The microbial community diversity was assayed by high-through sequencing. Total DNA of the samples was extracted using FastDNA® Spin Kit. The V3-V4 region of the bacterial 16S rDNA gene was amplified with a primer set of 338F (5’-ACTCCTA CGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The amplified products were detected by 2% agarose gel electrophoresis and recovered from the gel, using the AxyPrep DNA Gel Extraction Kit. The purified amplicons were quantified using the Quantus™ Fluorometer and sequenced on an Illumina Miseq PE300 platform by Sangon Biotech (Shanghai) Co., Ltd.
2.4 Chemical Analysis
VOC analysis was performed using a purge and trap concentrator (Eclipse 4552&4660, Aurora, USA) coupled with a gas chromatography-mass spectrometrometry system (7890A/5975C, Agilent, USA). The program of the concentrator was set as follows: purging with N2 at 45℃ for 11 min, desorbing at 190℃ for 5 min, and baking at 220℃ for 10 min. The temperature of the gas chromatograph was initially held at 45℃ for 2 min, increased at the rate of 6 ℃/min and held at 150℃ for 5 min, then increased at the rate of 10 ℃/min and held at 220℃ for 3 min.
Soil dehydrogenase activity (DHA) and microbial biomass carbon (MBC) respectively reflect the activity and quantity of microorganisms in the soil, determined by the triphenyltetrazolium chloride spectrophotometry for DHA and chloroform fumigation method for MBC, according to Chinese National Standards.
2.5 Data Analysis
The experimental data were analyzed using Origin 2018. All the assays were implemented with triplicates and the results were represented as means ± standard deviation. Analysis of variance (ANOVA) was employed to assess significant differences among various treatments, with significance expressed by values of p < 0.05.
3 Results
3.1 Degradation of Contaminants through Biostimulation
The addition of nutrients at various concentrations proved beneficial for enhancing the biodegradation efficiency of benzene, toluene, and TCE in co-contaminated soil (Fig. 1). After incubation for 40 days, group C1 showed the highest residue of contaminants, while group C5 exhibited the most effective biostimulation effect compared to C1, with a significant difference between them (p < 0.05). The degradation efficiency of benzene, toluene, and TCE in group C5 was 46.51% ± 4.42%, 49.62% ± 6.17%, and 48.78% ± 5.49% higher than that in group C1, respectively. Meanwhile, there was no significant difference between groups C5 and C8 (p > 0.05). Group C5 exhibited higher degradation rates for benzene, toluene, and TCE by 7.10% ± 0.62%, 13.80% ± 1.24%, and 3.01% ± 0.22%, respectively, compared to group C8. This may be attributed to the crucial role of vegetable oil as a carbon source in the effectiveness of biostimulation. CH3COONa was used as a carbon source and significantly increased the biodegradation efficiency of TCE (p < 0.05), but had no significant effect on the degradation of benzene and toluene compared to vegetable oil (p > 0.05) (Table 3). Therefore, vegetable oil demonstrated unique advantages over CH3COONa in biostimulation. Additionally, the degradation efficiency of TCE in groups C2 and C3 (lack of N source) and groups C4 and C7 (lack of P source) were significantly lower than in groups C5 and C8 (p < 0.05), which indicated the necessity of adding nitrogen and phosphorus sources. However, the addition of N and P sources in group C9 was higher than in group C5, yet the degradation performance exhibited the opposite trend. This indicated that the appropriate C: N: P ratio was a crucial factor in determining the effectiveness of biostimulation. When the ratio of C: N: P was 100: 10: 1, specifically with the addition of 2000 mg/kg vegetable oil, 200 mg/kg (NH4)2SO4, and 20 mg/kg K2HPO4, the biostimulation effect was optimal in co-contaminated soil.
Note: The same letter (a, b, c, d) indicates no significant difference between treatment groups (C1-C9) over 40 days (p > 0.05), while different letters indicate significant differences between treatment groups (C1-C9) over 40 days (p < 0.05).
Adding nutrients at different concentrations demonstrated a positive impact on enhancing both soil microbial biomass carbon (MBC) and soil dehydrogenase intensity (DHA) compared to group C1 (Fig. 2). Group C5 showed significant differences compared to all other treatment groups (p < 0.05). After 40 d incubation, DHA and MBC in group C5 were 9.60 and 18.23 times higher than those in group C1, respectively. Moreover, the growth rates of DHA and MBC in group C5 were 26.98% ± 3.14% and 46.89% ± 6.17% higher than those in group C8, respectively. This suggested that microorganisms probably effectively utilize vegetable oil as an additional carbon source to promote their own growth and activity. Additionally, the absence of nitrogen (groups C2 and C3) and phosphorus (groups C4 and C7) sources impeded the supply of essential growth nutrients for microorganisms, resulting in significantly lower levels of microbial activity and quantity compared to group C5 (p < 0.05). Nevertheless, group C5 showed significant differences compared to C9 (p < 0.05), with the growth rates of DHA and MBC in group C9 being 37.74% ± 3.90% and 53.65% ± 6.56% lower than those in group C5, indicating that excessively high concentrations of N and P may have inhibitory effects on microbial growth and reproduction. The microorganisms in co-contaminated soil exhibited high activity and quantity when the C: N: P ratio was 100: 10: 1, effectively promoting the degradation of contaminants.
Note: The same letter (a, b, c, d) indicates no significant difference between treatment groups (C1-C9) over 40 days (p > 0.05), while different letters indicate significant differences between treatment groups (C1-C9) over 40 days (p < 0.05).
Degradation intermediates, specifically dichloroethylene (DCE) and vinyl chloride (VC), were produced during the cultivation process (Fig. 3). The concentration of degradation intermediates showed an initial increase followed by a decreasing trend. The levels of DCE and VC reached their peaks at 20 days, with group C5 surpassing group C1 by 120.71% ± 12.99% and 11.73% ± 1.72%, respectively, and group C8 exceeding group C1 by 61.58% ± 6.89% and 24.41% ± 3.05%, respectively. However, the concentrations of DCE and VC tended towards zero at the end of the experiment. Moreover, throughout the entire experimental process, their concentrations consistently remained at relatively low levels. Therefore, the addition of biostimulants potentially facilitated the transformation of target pollutants, accompanied by the generation of more degradation intermediates, ultimately leading to mineralization.
3.2 Degradation of Contaminants Through Bioaugmentation
B-T addition induced notable increased biodegradation effects on benzene, toluene, and TCE in the co-contaminated soil (Fig. 4). Compared with group CK, group BA demonstrated 33.04% ± 2.56%, 37.55% ± 2.78%, and 33.02% ± 1.99% higher degradation rates of benzene, toluene, and TCE, respectively. Degradation intermediates DCE and VC were not detected in the process of the bioaugmentation experiment in group BA. Additionally, dehydrogenase activity and microbial carbon content (Fig. 4B) in group BA were significantly enhanced compared to group CK, with growth rates of 4.75 and 3.19 times, respectively. Thus, the screened B-T culture exhibited the capability to grow and propagate under nutrient-deficient conditions and promoted the biodegradation of benzene, toluene, and TCE.
Pseudomonas, Stenotrophomonas, Chryseobacterium, Rhodococcus, Bordetella, and norank_f_JG30-KF-CM45 were the predominant species in the B-T culture (Fig. 5). The relative abundance of Pseudomonas, Stenotrophomonas, and Chryseobacterium was relatively high, accounting for 24.64% ± 2.45%, 35.95% ± 1.98% and 11.32% ± 0.87%, respectively. In general, B-T exhibited rich biodiversity and probably played a significant role in the effective removal of benzene, toluene, and TCE from co-contaminated soil.
3.3 Coupling of Bioaugmentation and Biostimulation on Soil Bioremediation
The coupling of biostimulation and bioaugmentation (group S3) demonstrated a superior bioremediation effect compared to other treatment groups (Fig. 6A). There were significant differences between group S3 and groups S0, S1 and S2 (p < 0.05). The degradation efficiency of benzene, toluene, and TCE in group S3 was 3.52, 4.16, and 9.11 times higher than in group S0, respectively. Additionally, the degradation rates of benzene, toluene, and TCE in group S3 surpassed those in group S1 by 13.46% ± 2.47%, 15.80% ± 1.64%, and 20.84% ± 2.03%, respectively. Furthermore, compared to group S2, the degradation rates of three contaminants in group S3 were notably higher by 43.28% ± 3.73%, 43.99% ± 2.42%, and 47.23% ± 2.38%, respectively. Meanwhile, there were significant differences between groups S1 and S2 (p < 0.05). Biostimulation (S1) demonstrated significantly higher degradation efficiency compared to bioaugmentation (S2), indicating that the potentially key factor for the bioremediation efficacy of co-contaminated soil was the deficiency of essential nutrients elements containing carbon, nitrogen, and phosphorus. Therefore, while both biostimulation and bioaugmentation could enhance the degradation of three contaminants, their coupling strategy achieved higher degradation efficiency.
The coupling of biostimulation and bioaugmentation (group S3) exhibited the highest soil dehydrogenase activity and microbial biomass carbon compared to other treatment groups (Fig. 6B). There were significant differences between group S3 and group S0 (p < 0.05). After 30 d incubation, the dehydrogenase activity and microbial biomass in group S3 were 8.52 and 37.50 times higher than those in group S0, respectively. Additionally, there were significant differences between group S3 and group S1 (p < 0.05). The levels of DHA and MBC in group S3 increased by 22.59% ± 1.86% and 24.17% ± 1.43% compared to group S1, respectively. This suggested that the addition of exogenous microorganisms probably enhanced soil microbial activity and synergistic interactions among microorganisms. Furthermore, there remained significant differences between group S2 and group S3 (p < 0.05), with growth rates of DHA and MBC in group S3 being 6.24 and 8.3 times higher than group S2, indicating that the growth and reproduction of exogenous microorganisms may be limited in nutrient-deficient soil. In summary, the coupling of biostimulation and bioaugmentation had a synergistic effect in enhancing soil microbial activity, promoting microbial growth and metabolism, and facilitating pollutant degradation.
Note: There are significant differences between each pair of groups (S1-S3) over 30 days, marked with “*” (p < 0.05).
4 Discussion
The preservation of a balanced nutrient ratio (C: N: P = 100: 10: 1) in biostimulation strategies enhanced microbial community activity and contaminant degradation, with carbon sources playing a crucial role. The degradation efficiency of benzene, toluene, and TCE was positively correlated with microbial activity, and the decline in contaminant concentrations coincided with the increase in both the quantity and activity of soil microorganisms (Figs. 1 and 2). Vegetable oil significantly enhanced contaminant degradation rates and microbial activity compared with CH3COONa (p < 0.05), which emphasized the significance of selecting appropriate carbon sources in biostimulation strategies. Besides, the deficiency of nitrogen and phosphorus sources inhibited the effectiveness of biostimulation. Microorganisms utilize carbon sources as the primary source of energy to sustain their activities, while nitrogen and phosphorus sources primarily contribute to the synthesis of cell structures and functional proteins (Atlas & Hazen, 2011). Moreover, vegetable oil exhibited unique advantages over CH3COONa in biostimulation, probably because of its even soil distribution, slow release for microbial utilization, and effective aquifer retention as reported previously (Liu et al., 2012). In addition, vegetable oil exhibited a high degree of mutual solubility with benzene and toluene, contributing to the increased bioavailability of both (Hariz et al., 2017). Therefore, vegetable oil was recommended as the primary carbon source in bioremediation strategies for co-contaminated soil.
Bioaugmentation effectively enhanced the biodegradation of benzene, toluene, and TCE, exhibiting higher removal efficiency for toluene and benzene in comparison to TCE. Wu et al. reported that a consortium consisting of many different bacterial species was required to efficiently degrade complex contaminants (Wu et al., 2013). The addition of B-T as a consortium proved beneficial for enhancing soil biodiversity, boosting microbial activity, and demonstrating synergistic effects in the removal of benzene, toluene, and TCE in co-contaminated soil (Fig. 4). The dominant microbial genera in B-T have been proven to exhibit significant degradation capabilities towards aromatic hydrocarbons and chlorinated hydrocarbons, with the relative abundance of dominant genera capable of degrading benzene and toluene being higher than that of TCE. Among them, Pseudomonas, a bacteria genus commonly employed in the remediation of volatile organic compounds in wastewater and contaminated sites (Qian et al., 2012). Rhodococcus has been widely reported and employed in the bioremediation of soil contaminated with aromatic hydrocarbon, facilitating the simultaneous degradation of both benzene and toluene (Feng et al., 2021). Stenotrophomonas has been documented as a bacterium capable of degrading benzene and toluene, with tce300 and tce350 genes within its DNA playing a significant role in aerobic co-metabolism (Bashandy et al., 2020). Chryseobacterium, known as a dechlorination bacterium, has been applied to the co-degradation of TCE and nitrate in groundwater (Chen et al., 2022). Bordetella and norank_f_JG30-KF-CM45 have both been reported as bacteria capable of degrading benzene and toluene (Sun et al., 2019; Zhang et al., 2021). Furthermore, the main degradation pathways of benzene and toluene were divided into two categories: Toluene Ortho-Monooxygenase (TOL) and toluene Ortho-Dioxygenase (TOD), based on the plasmids encoding relevant enzymes (Nitz et al., 2020; Zhang et al., 2019). The apparent degradation trends of benzene and toluene probably suggested that both TOD and TOL pathways are concurrently active in the co-degradation of benzene and toluene in the soil. Therefore, the bioaugmentation strategy with B-T achieved superior biodegradation of benzene and toluene than TCE.
The coupling of biostimulation and bioaugmentation provided the most beneficial effects than either single treatment strategy for the co-metabolisms of benzene, toluene, and TCE. The biostimulation strategy resulted in the formation of degradation intermediates (DCE and VC) during the remediation process (Fig. 3). This is mainly because TCE bioremediation is primarily driven by aerobic degradation or anaerobic degradation (Wu et al., 2022). Notably, the production of DCE and VC occurs during the anaerobic degradation process, both of which are volatile organic pollutants with persistent toxic effects (Enzien et al., 1994). The toxicity of both is typically higher than TCE, potentially causing adverse effects on human health and the environment. Biostimulation significantly enhanced the removal efficiency of contaminants, while the toxicity of degradation intermediates posed a hindrance to further application. Besides, while the bioaugmentation strategy achieved effective biodegradation of benzene, toluene, and TCE, its biodegradation effectiveness and microbial activity were significantly lower than that of the biostimulation and coupling strategies (Fig. 6). However, the coupling strategy achieved the most effective co-metabolism of benzene, toluene, and TCE through the synergistic interaction among microbial communities. Microbial activity was significantly positively correlated with the degradation potential of contaminants in soil. The increased enzyme activity could activate TCE co-metabolism enzymes, facilitating the co-degradation of benzene, toluene, and TCE (Arcangeli & Arvin, 1997). The key catalyst within the toluene pathway was toluene dioxygenase, a pivotal enzyme that played a significant role in enhancing the co-metabolic degradation of TCE (Saviozzi et al., 2009). This co-contamination facilitates aerobic co-metabolism of TCE, utilizing benzene and toluene as primary substrates, enabling the simultaneous mineralization of both types of contaminants. In summary, the coupling of biostimulation and bioaugmentation strategy stimulated synergistic interactions within the microbial community and ultimately achieved the co-metabolism of benzene, toluene, and TCE.
5 Conclusion
In this study, the biostimulation strategy showed the most significant bioremediation effect with a C: N: P ratio of 100: 10: 1, with vegetable oil playing a crucial role as a carbon source. The bioaugmentation strategy involving the introduction of exogenous microorganisms (B-T) significantly enhanced the biodegradation of co-contaminated soil, with higher removal efficiency for benzene and toluene compared to TCE. Additionally, the coupling of biostimulation and bioaugmentation promoted synergistic interactions within the microbial community, thereby achieving the most effective degradation of contaminants. This study has clearly defined the requirements for coupling biostimulation and bioaugmentation treatment strategy requirements for the degradation of benzene, toluene, and TCE. In conclusion, it has probably provided valuable data to support its practical application in the field of co-contaminated soil.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agarry, S. E., Owabor, C. N., & Yusuf, R. O. (2010). Studies on Biodegradation of Kerosene in Soil under Different Bioremediation Strategies. Bioremediation Journal, 14(3), 135–149. https://doi.org/10.1080/10889868.2010.495364
Alvarez, L. M. M., Lo Balbo, A., Cormack, W. P., & Ruberto, L. A. M. (2015). Bioremediation of a petroleum hydrocarbon-contaminated Antarctic soil: Optimization of a biostimulation strategy using response-surface methodology (RSM). Cold Regions Science and Technology, 119, 61–67. https://doi.org/10.1016/j.coldregions.2015.07.005
Arcangeli, J. P., & Arvin, E. (1997). Modeling of the cometabolic biodegradation of trichloroethylene by toluene oxidizing bacteria in a biofilm system. Environmental Science & Technology, 31(11), 3044–3052. https://doi.org/10.1021/es9609112
Atlas, R. M., & Hazen, T. C. (2011). Oil Biodegradation and Bioremediation: A Tale of the Two Worst Spills in US History. Environmental Science & Technology, 45(16), 6709–6715. https://doi.org/10.1021/es2013227
Bai, L.-p, Luo, Y., Liu, L., Zhou, Y.-y, Yan, Z.-g, & Li, F.-s. (2015). Research on the Screening Method of Soil Remediation Technology at Contaminated Sites and Its Application. Environmental Science, 36(11), 4218–4224.
Bashandy, S. R., Abd-Alla, M. H., & Dawood, M. F. A. (2020). Alleviation of the toxicity of oily wastewater to canola plants by the N 2-fixing, aromatic hydrocarbon biodegrading bacterium Stenotrophomonas maltophilia-SR1. Applied Soil Ecology, 154, 103654. https://doi.org/10.1016/j.apsoil.2020.103654
Borden, R. C., Beckwith, W. J., Lieberman, M. T., Akladiss, N., & Hill, S. R. (2007). Enhanced anaerobic bioremediation of a TCE source at the Tarheel Army Missile Plant using EOS. Remediation, 17(3), 5–19. https://doi.org/10.1002/rem.20130
Chen, F., Ye, Y., Fan, B., Lv, M., Liang, B., Liu, W., Cheng, H.-Y., Chen, Y., Liu, Y., Wang, Y., Wang, A., & Li, Z. (2022). Simultaneous removal of tetrachloroethylene and nitrate with a novel sulfur-packed biocathode system: The synergy between bioelectrocatalytic dechlorination and sulfur autotrophic denitrification. Chemical Engineering Journal, 439, 135793. https://doi.org/10.1016/j.cej.2022.135793
Ding, D., Song, X., Wei, C. L., & LaChance, J. (2019). A review on the sustainability of thermal treatment for contaminated soils. Environmental Pollution, 253, 449–463. https://doi.org/10.1016/J.ENG.2016.04.005
Ding, L. J., Dong, J., Bai, J., & Chi, Z. F. (2021). Migration and evolution of an in situ bioreactive zone formed with emulsified vegetable oil for the long-term remediation of nitrobenzene-contaminated groundwater. Journal of Hydrology, 593, 125914. https://doi.org/10.1016/j.jhydrol.2020.125914
Enzien, M. V., Picardal, F., Hazen, T. C., Arnold, R. G., & Fliermans, C. B. (1994). Reductive Dechlorination of Trichloroethylene and Tetrachloroethylene under Aerobic Conditions in a Sediment Column. Applied and Environmental Microbiology, 60(6), 2200–2204. https://doi.org/10.1128/aem.60.12.4646-4646.1994
Feng, S., Gong, L., Zhang, Y., Tong, Y., Zhang, H., Zhu, D., Huang, X., & Yang, H. (2021). Bioaugmentation potential evaluation of a bacterial consortium composed of isolated Pseudomonas and Rhodococcus for degrading benzene, toluene and styrene in sludge and sewage. Bioresource Technology, 320, 124329. https://doi.org/10.1016/j.biortech.2020.124329
Han, Y. X., Xu, J., & Zhu, L. Z. (2023). Predicting soil concentrations and remediation target values of BTEX by an off-gas based mass transfer model. Science of the Total Environment, 900, 165731. https://doi.org/10.1016/j.scitotenv.2023.165731
Hariz R, Sanz J.I.d.R, Mercier C, Valentin R, Dietrich N, Mouloungui Z, Hebrard G. (2017). Absorption of toluene by vegetable oil-water emulsion in scrubbing tower: Experiments and modeling. Chemical Engineering Science. 157 264-271https://doi.org/10.1016/j.ces.2016.06.008
Ji, Y., Zhang, J., & Zhu, Z. (2008). Bioremediation Technology for Soil Contaminated by Polycyclic Aromatic Hydrocarbons. Environmental Protection of Chemical Industry, 28(3), 243–246.
Khodaei, K., Nassery, H. R., Asadi, M. M., Mohammadzadeh, H., & Mahmoodlu, M. G. (2017). BTEX biodegradation in contaminated groundwater using a novel strain (Pseudomonas sp BTEX-30). International Biodeterioration & Biodegradation, 116, 234–242. https://doi.org/10.1016/j.ibiod.2016.11.001
Li, J. H., Lu, Q. H., Odey, E. A., Lok, K. S., Pan, B. C., Zhang, Y. Y., & Shim, H. (2021). Coupling of biostimulation and bioaugmentation for enhanced bioremoval of chloroethylenes and BTEX from clayey soil. Ecotoxicology, 30(7), 1446–1453. https://doi.org/10.1007/s10646-020-02323-z
Liu, J. B., Amemiya, T., Chang, Q., Qian, Y., & Itoh, K. (2012). Toluene dioxygenase expression correlates with trichloroethylene degradation capacity in Pseudomonas putida F1 cultures. Biodegradation, 23(5), 683–691. https://doi.org/10.1007/s10532-012-9544-y
Meng, F., & Wang, Y. (2005). Remediation of Groundwater and Soil Polluted by Trichloroethylene. China Water & Wastewater, 21(4), 34–36.
Nitz, H., Duarte, M., Jauregui, R., Pieper, D. H., Mueller, J. A., & Kaestner, M. (2020). Identification of benzene-degrading Proteobacteria in a constructed wetland by employing in situ microcosms and RNA-stable isotope probing. Applied Microbiology and Biotechnology, 104(4), 1809–1820. https://doi.org/10.1007/s00253-019-10323-1
Qian, Y., Yue, F., & Chu, Y. (2012). Advances in environmental remediation technologies for trichloroethylene pollution. Environmental Chemistry, 31(9), 1335–1343.
Qian, Y., Wang, Q. Q., & Yue, F. F. (2016). Remediation of TCE-contaminated water by enhanced chemical oxidation using Na2S2O8/H2O2/red mud. Desalination and Water Treatment, 57(9), 4154–4161. https://doi.org/10.1080/19443994.2014.988648
Raimondo, E. E., Saez, J. M., Aparicio, J. D., Fuentes, M. S., & Benimeli, C. S. (2020). Coupling of bioaugmentation and biostimulation to improve lindane removal from different soil types. Chemosphere, 238, 124512. https://doi.org/10.1016/j.chemosphere.2019.124512
Roy, A., Dutta, A., Pal, S., Gupta, A., Sarkar, J., Chatterjee, A., Saha, A., Sarkar, P., Sar, P., & Kazy, S. K. (2018). Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresource Technology, 253, 22–32. https://doi.org/10.1016/j.biortech.2018.01.004
Saviozzi, A., Cardelli, R., & Cozzolino, M. (2009). Bioremediation with Compost of a Diesel Contaminated Soil: Monitoring by Dehydrogenase Activity and Basal Respiration. Compost Science & Utilization, 17(1), 55–60. https://doi.org/10.1080/1065657X.2009.10702400
Simpanen, S., Dahl, M., Gerlach, M., Mikkonen, A., Malk, V., Mikola, J., & Romantschuk, M. (2016). Biostimulation proved to be the most efficient method in the comparison of in situ soil remediation treatments after a simulated oil spill accident. Environmental Science and Pollution Research, 23(24), 25024–25038. https://doi.org/10.1007/s11356-016-7606-0
Smith, E., Thavamani, P., Ramadass, K., Naidu, R., Srivastava, P., & Megharaj, M. (2015). Remediation trials for hydrocarbon-contaminated soils in arid environments: Evaluation of bioslurry and biopiling techniques. International Biodeterioration & Biodegradation, 101, 56–65. https://doi.org/10.1016/j.ibiod.2015.03.029
Sun, H., Narihiro, T., Ma, X., Zhang, X.-X., Ren, H., & Ye, L. (2019). Diverse aromatic-degrading bacteria present in a highly enriched autotrophic nitrifying sludge. Science of Total Environment, 666, 245–251. https://doi.org/10.1016/j.scitotenv.2019.02.172
Varjani, S., & Upasani, V. N. (2019). Influence of abiotic factors, natural attenuation, bioaugmentation and nutrient supplementation on bioremediation of petroleum crude contaminated agricultural soil. Journal of Environmental Management, 245, 358–366. https://doi.org/10.1016/j.jenvman.2019.05.070
Wang, Y. X., Wang, G., Dai, Y. J., Wang, Y., Lee, Y. W., Shi, J. R., & Xu, J. H. (2020). Biodegradation of Deoxynivalenol by a Novel Microbial Consortium. Frontiers in Microbiology, 10, 02964. https://doi.org/10.3389/fmicb.2019.02964
Wu, M. L., Chen, L. M., Tian, Y. Q., Ding, Y., & Dick, W. A. (2013). Degradation of polycyclic aromatic hydrocarbons by microbial consortia enriched from three soils using two different culture media. Environmental Pollution, 178, 152–158. https://doi.org/10.1016/j.envpol.2013.03.004
Wu, Z., Man, Q., Niu, H., Lyu, H., Song, H., Li, R., Ren, G., Zhu, F., Peng, C., Li, B., & Ma, X. (2022). Recent advances and trends of trichloroethylene biodegradation: A critical review. Frontiers in Microbiology, 13, 1053169. https://doi.org/10.3389/fmicb.2022.1053169
Youya, Z., Xiaozhen, H. E., Hong, H. O. U., Li, W., Qingbao, G. U., & Fasheng, L. I. (2009). Removal of benzene and ethylbenzene from soil by soil vapor extraction. Journal of Chemical Industry and Engineering (China), 60(10), 2590–2595.
Zhang, Y., Liu, J., Qin, Y., Yang, Z., Cao, J., Xing, Y., & Li, J. (2019). Performance and microbial community evolution of toluene degradation using a fungi-based bio-trickling filter. Journal of Hazardous Materials, 365, 642–649. https://doi.org/10.1016/j.jhazmat.2018.11.062
Zhang, X., Li, R., Song, J., Ren, Y., Luo, X., Li, Y., Li, X., Li, T., Wang, X., & Zhou, Q. (2021). Combined phyto-microbial-electrochemical system enhanced the removal of petroleum hydrocarbons from soil: A profundity remediation strategy. Journal of Hazardous Materials, 420, 126592. https://doi.org/10.1016/j.jhazmat.2021.126592
Zhu, G., Ying, R., Ye, M., Zhang, S., Xia, B., Qian, J., & Jiang, X. (2021). Research Progress on Remediation Technology of Contaminated Soil in Pesticide Production Sites in China. Chinese Journal of Soil Science, 52(2), 462–473.
Funding
This work was supported by the National Key R & D Program of China (Grant NO. 2023YFC3709600) and the Open Foundation of Jiangsu Geological Engineering Environment Intelligent Monitoring Engineering Research Center (NO. 2023-ZNJKJJ-01).
Author information
Authors and Affiliations
Contributions
Huinan Liu: Writing – original draft. Runmin Wang: Investigation, Methodology. Moye Luo: Writing – review & editing. Chenghua Xu: Investigation, Methodology. Dandan Yu: Investigation, Methodology. Manjun Zhan: Data curation, Formal analysis, Resources. Tao Long: Formal analysis, Resources. Ran Yu: Supervision, Funding acquisition, Writing – review & editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Wang, R., Luo, M. et al. Coupling of Biostimulation and Bioaugmentation for Benzene, Toluene, and Trichloroethylene Removal from Co-Contaminated Soil. Water Air Soil Pollut 235, 665 (2024). https://doi.org/10.1007/s11270-024-07481-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07481-y