Abstract
Anaerobic co-digestion of sewage sludge and glycerol from the biodiesel industry was evaluated in three experimental stages. In the first step, the addition of higher proportions of crude glycerol (5–20% v/v) to the sludge was evaluated, and the results showed a marked decrease in pH and inhibition of methane production. In the second step, co-digestion of sludge with either a lower proportion (1% v/v) of crude glycerol or glycerol pretreated to remove salinity resulted in volatile acid accumulation and low methane production. The accumulation of volatile acids due to the rapid degradation of glycerol in the mixture was more detrimental to methanogenesis than the salinity of the crude glycerol. In the third step, much lower amounts of crude glycerol were added to the sludge (0.3, 0.5, 0.7% v/v), resulting in buffering of the reaction medium and higher methane production than in the control (pure sludge). The best condition for co-digestion was with the addition of 0.5% (v/v) crude glycerol to the sewage sludge, which equals 0.6 g glycerol/g volatile solids applied. Under this condition, the specific methane production (mL CH4/g volatile solids applied) was 1.7 times higher than in the control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioethanol and biodiesel have gained increased interest as renewable energy sources due to their environmental advantages and increased oil prices (Demirbas 2017). The use of biodiesel has grown enormously throughout the world. Between 2005 and 2011, biodiesel production increased from 736 to 2,530,000 m3 (Padula et al. 2012).
Transesterification is one of the processes used to produce biodiesel. It is based on a chemical reaction of a vegetable oil with a simple alcohol, usually methanol or ethanol, catalyzed by an acid or a base (sodium hydroxide or potassium hydroxide may be used). In this reaction, the main molecules of oils and fats, called triacylglycerols, are converted to fatty acids and glycerin (Chuah et al. 2016). Each mole of oil reacts with three moles of alcohol (methanol or ethanol), producing biodiesel and 1 mol of glycerol. About 1 kg of crude glycerol is generated for every 10 kg of biodiesel produced (Hu et al. 2012). The glycerol co-product is nearly 100% biodegradable and presents a high theoretical methane production equal to 0.43 Nm3 CH4/kg glycerol (López et al. 2009).
The significant production of glycerol generated by the biodiesel industry only finds a limited demand from other industrial processes, and it is considered as an important waste. The acceleration of biodiesel production in recent years resulted in an accumulation of glycerol, causing a significant drop in the profitability of the biodiesel industries (Yazdani and Gonzalez 2007). Due to its high availability, the market value of this co-product has drastically decreased.
In the other hand, the purity of the crude glycerol varies on average from 65 to 70% (w/w). In some cases, glycerol presents concentration below 50%. Hu et al. (2012), Athanasoulia et al. (2014), and Nghiem et al. (2014) report that crude glycerol contains between 23 and 80% of pure glycerol. The impurities mostly consist of soap, formed by the reaction of free fatty acids under an excess catalyst (saponification), which in turn gives the glycerol a viscous and dark appearance (Rivaldi et al. 2009; Luo et al. 2016). The presence of a catalyst in the glycerol can influence the pH, making it more basic or acidic, with values from 5 to above 10. Common components in the crude glycerol include glycerol; alcohol; water; salts; heavy metals; fatty acids; unreacted mono-, di-, and triglycerides; and methyl esters. The use of crude glycerol is also quite limited due to the presence of methanol, salts, and fatty acids (Hu et al. 2012; Jensen et al. 2014).
There are several industrial methods to purify crude glycerin, including conventional and ion exchange. Both involve unit operations (evaporation and distillation) and unit processes (acidification and neutralization), with high-energy consumption, time demand, and low yield. The high cost of purification is not feasible for small and medium-sized plants (Luo et al. 2016). An alternative way to use crude glycerol is as a substrate in industrial fermentation processes to obtain high value-added metabolites, such as hydrogen, methane, 1,3-propanediol, and ethanol (da Silva et al. 2009; Nitayavardhana and Khanal 2011). Studies have already been conducted with crude glycerol as the sole carbon source in microbial cultures (da Silva et al. 2009; Yang et al. 2012). Glycerol is considered a highly assimilable carbon source by bacteria and yeasts under aerobic and anaerobic conditions to obtain metabolic energy (Dills et al. 1980).
Crude glycerol has alkaline pH, high COD values from 1.0 to 2.4 g/L, high concentration of chlorides, and low methanol concentration (Ma et al. 2008; López et al. 2009; Maragkaki et al. 2017; Athanasoulia et al. 2014). The presence of residual concentrations of the alkaline catalyst and alcohol (usually methanol) used in the transesterification reaction as well as the presence of free fatty acids and phosphorus are important restrictions for anaerobic treatment of glycerol. The presence of inhibitory compounds, such as salts and methanol, may limit glycerol dosage levels (Robra et al. 2010; Castrillón et al. 2013). Inhibition by overload is usually recorded as the greatest risk for the stability of the glycerol co-digestion process (Jensen et al. 2014; Fountoulakis et al. 2010).
However, under co-digestion, the alkaline pH of the crude glycerol is not harmful to anaerobic process because it is reduced in mixtures with sewage sludge, and initial pH values are maintained within an optimum interval (7.0–8.0) for methane formation (Weiland 2010). When present in low concentration, due to its efficient recovery during the biodiesel production process, low values of methanol may not be inhibitory for anaerobic digestion. Bhatti et al. (1993) obtained good methanol removal and relatively stable biogas production in an upflow anaerobic sludge blanket (UASB) reactor operated with up to 20 g methanol/L in the feed. However, most of the crude glycerin also contains 34 up to 46 g Cl−/L, and these values are well above the supported limit of 9 g Cl−/L for a stable and efficient anaerobic digestion (Viana et al. 2012).
In a general view, anaerobic co-digestion technology increases the biogas productivity due to the addition of organic by-products in high, but limited, concentrations. Co-digestion of sewage sludge with one or more substrates has become an alternative to improve the yield of anaerobic digestion. The co-substrate can provide nutrients that are deficient in the sludge and, at the same time, have a positive synergistic effect on the medium, leading to stable digestion and improved biogas yield (Jensen et al. 2014; Mata-Alvarez et al. 2014). Fats, oils, and greases (FOG); food waste and scraps; organic fraction of municipal solid waste (OFMSW); food and beverage processing waste; energy crops; agricultural residues; livestock manure; biofuel by-products, including corn-ethanol stillage, crude glycerol, and spent microalgae are examples of high-strength wastes used as organic sources for anaerobic co-digestion processes (Shen et al. 2015; Chiu and Lo 2016; Maragkaki et al. 2017; Thorin et al. 2017).
In municipal wastewater treatment plants (WWTPs), anaerobic digestion is the most used technology for sludge stabilization, but energy recovery from biogas is not commonly practice in Brazil. In 2015, 127 biogas plants were in operation, but only seven of them used sewage sludge as the main source of organic substrate, contributing to only 5% of the total energy produced by biogas plants (Svensson and Baxter 2016). Only 10% of small to medium WWTPs in the USA inject purified biogas directly into natural gas pipelines (Shen et al. 2015). Efforts must be made to improve the management and treatment of sludge in WWTPs, including the enhancement of biogas production in anaerobic digesters and the implementation of technically feasible bioenergy plants (Wang et al. 2008; Brisolara and Qi 2015; Mininni et al. 2015). Growing concerns about energy security, environmental impacts, and rising energy costs of WWTPs have reestablished the anaerobic digestion process as a viable technology for the production of renewable energy (Barber 2012; Jenicek et al. 2013; Karthikeyan and Visvanathan 2013; Kacprzak et al. 2017).
The addition of between 1 and 4% (v/v) crude glycerol to the sludge was studied previously. Binary mixtures at ratios of 5 up to 20% (v/v) caused system instability due to volatile acid accumulation and pH drop (Fountoulakis et al. 2010; Razaviarani et al. 2013; Athanasoulia et al. 2014; Rivero et al. 2014; Razaviarani and Buchanan 2015). As stated before, salinity is considered an important inhibitory compound for anaerobic digestion and may play an important role for co-digestion of glycerol and sewage sludge. Owing to different compositions of glycerol in terms of organics and impurities, as well as distinct characteristics of the sludge and digestion conditions, there is no consensus on which concentration would be best to ensure that the anaerobic digestion process is stable in the medium and long term.
Thus, interest in research has increased about finding a way to add value to the crude glycerol due to its great importance to the long-term sustainability of the biodiesel industries. Therefore, this study concentrates on identifying inhibitory effects caused by salinity, based on the evaluation of methane production from mixtures of sewage sludge with different proportions of crude glycerol and treated glycerol in the absence of salinity.
Materials and methods
Waste collection and characterization
The sludge used was collected at the end of the sludge recirculation line, on the return to the aeration tank of an activated sludge system of a WWTP and stored at 4 °C until the moment of use. Three collections of this waste activated sludge (WAS) were carried out from January to March 2015. A more complete characterization, to determine pH, humidity, total solids (volatile and fixed), carbon, nitrogen, and phosphorus, was performed only for the first collection (January). For the other collections, only pH, humidity, and total solids concentration (volatile and fixed) were quantified. For standardization, the concentration of total volatile solids in the sludge hereafter will be termed only volatile solids (VS).
Crude glycerol (CG) was collected from a plant that produced biodiesel from a mixture of raw materials (40% bovine tallow and 60% soybean oil). CG was characterized in terms of density, moisture, and contents of glycerol, total organic carbon (TOC), chemical oxygen demand (COD), chlorides, and methanol.
Having verified a high concentration of chlorides (salinity) in the CG samples, a classical method of precipitation of chlorides (Mohr method) was employed to reduce this concentration. As soybean-based CG has a high viscosity (up to 162 mPa s at 25 °C) (Hu et al. 2012), it was necessary to dilute the CG samples in distilled water (1:10 v/v) prior to the precipitation of chlorides. After the precipitation step, the diluted glycerol with low salt concentration, pretreated glycerol (PTG), was used in the anaerobic co-digestion assays with secondary sludge. This glycerol was characterized only in terms of chloride concentration, and its glycerol content was considered identical to that of the crude glycerol. To compare the results of the co-digestion with CG and PTG, analytical grade glycerol (99.5% purity), designated pure glycerol (PG), was also used.
Anaerobic co-digestion assays
All assays were conducted in 100-mL penicillin flasks with 50 mL usable volume. Different proportions of WAS and CG, PTG, or PG were evaluated, with the residue mixtures corrected with NaHCO3 to pH values of 7.0 or 7.5. After introduction of the WAS and glycerol mixture, the flasks were sealed with rubber plugs and aluminum seals, coupled to 60-mL plastic syringes for measurement of the biogas volume produced via plunger displacement, and incubated at 30 °C without agitation until stabilization of the biogas production (20 to 30 days). The stabilization of the biogas production was established when the incubated flasks did not present any biogas production in a 24-h interval.
Initial aliquots were taken for pH verification and initial concentrations of volatile suspended solids, glycerol, and chlorides. For standardization, the concentration of volatile suspended solids in the residue mixtures hereafter will be termed only VS. After stabilization of the biogas production, the flasks were opened and final measurements of the same parameters, except chlorides, were carried out. In addition, soluble COD, total alkalinity, and total volatile acids were measured in the second and third steps of the experiments, when glycerol addition was between 0.3 and 1% (v/v). Samples of the accumulated biogas were subjected to gas chromatography to quantify the percentage of methane. The VS removal efficiency, biogas volume, % CH4, and specific methane production (SMP) were evaluated. The SMP was calculated by the equations below:
where V is the volume of biogas (mL), % CH4 is the content of methane in biogas (% v/v), VS is the concentration of volatile solids (i = initial, f = final, in g/L), and v is the useful volume of the penicillin flasks (mL).
In the first step of the study, to evaluate the effect of the addition of glycerol in the co-digestion with WAS, tests were carried out with two mixtures: WAS+GC and WAS+PG. In this step, the following proportions of WAS to glycerol (in % v/v) were evaluated: 100:0; 95:5; 90:10; 85:15; and 80:20. Tests with WAS and pure glycerol were conducted to evaluate the effect of the addition of glycerol to the sludge in the absence of crude glycerol contaminants, such as chlorides, methanol, soaps, and detergents (Castrillón et al. 2013). Both assays were conducted in quadruplicate, with incubation times of 19–30 days.
Because high inhibition in the anaerobic digestion process occurred in the first step, a second series of experiments was conducted to evaluate the effect of the addition of 1% (v/v) of different types of glycerol to the sludge. In this second step, salinity interference in the co-digestion with sludge was also verified by using crude glycerol with salinity (CG), crude glycerol pretreated to remove salinity (PTG), and pure glycerol (PG). For this step, CG and PG were diluted with distilled water (1:10 v/v) prior to addition to the sludge, whereas the PTG already underwent this same dilution during pretreatment. Two assays were performed, for eight replicates per mixture, with 15 days of incubation at 30 °C.
In the third step, lower percentages of CG in the mixture with WAS were evaluated (0.3, 0.5, and 0.7% v/v). As in the second step experiments, CG was diluted (1:10 v/v) prior to addition to the sludge, to facilitate the volume measurement to be added. Two assays were performed, totaling eight replicates for each condition, with 30 days of incubation at 30 °C.
For statistical analysis of data from the second and third steps, the analysis of variance with multiple factors was used (ANOVA multi-factor) with a confidence interval of 95% (α = 5%) for all variables. The Fisher least significant difference (LSD) was used for comparisons between means. Both analyses were done using the Statistica software 8.0.
Analytical methods
Humidity was measured using an infrared analyzer (IV 2000, Gehaka) at 160 °C for 10 min. Total organic carbon was analyzed by combustion (680 °C) catalytic oxidation/ non-dispersive infrared gas analyzer (NDIR) method using a Shimadzu TOC-VCSN analyzer (Greenberg et al. 2005). The determination of the C and N contents in the sludge was performed with a LECO Truspec Micro elemental analyzer equipped with an infrared detector. The samples were burned at 1075 °C in a quartz tube to quantify the C and N contents. Volatile fatty acids (VFA) and total alkalinity were determined according to Dilallo and Albertson (1961) and Ripley et al. (1986), respectively. Glycerol content was measured according to Bondioli and Della Bella (2005). Soluble COD (CODs), pH, chlorides, total phosphorus, and total suspended solids or total solids (volatile and fixed) were measured according to standard procedures (Greenberg et al. 2005). The biogas composition was analyzed in a Micro-GC Varian gas chromatograph (CP-4900). The conditions used in chromatography were as follows: 10 m × 0.32 mm PoraPLOT Q column, column temperature of 50 °C, thermal conductivity detector (TCD) at 250 °C, injector temperature of 80 °C, and helium as carrier gas.
Results and discussion
Table 1 summarizes the characterization of the waste activated sludge and crude glycerol. The VS/TS ratio of the sludge (62%) is similar to those obtained for WWTP sludge (primary and secondary), which range from 59 to 88% (Kacprzak et al. 2017). In relation to carbon and nitrogen concentrations of the sludge, values of 34.7 and 5.4% were obtained, respectively, resulting in a C/N ratio of 6.6, which is below the range considered adequate for anaerobic digestion (15–30) (Weiland 2010). The WAS and CG presented moisture concentrations compatible with the wet digestion process evaluated in the study, i.e., solids content below 10% (Weiland 2010).
Crude glycerol used during the experiments presented pure glycerol concentration equal to 74%, and this value agrees with those reported by other authors in the range of 23 up to 80% (Hu et al. 2012; Athanasoulia et al. 2014; Nghiem et al. 2014). The carbon content of the crude glycerol used during the experiments reached 32.8%, which is in accordance with the value of 26% reported by Thompson and He (2006) and related to experiments with crude glycerol of soybean biodiesel. The high contents of COD and chlorides in the crude glycerol may hinder its use as a substrate. However, in mixtures with sewage sludge, toxic and organic overloading can be avoided.
Table 2 shows the average values and respective standard deviations obtained in the first step experiments, in which the effect of the addition of glycerol on the co-digestion with waste activated sludge was evaluated for mixtures of WAS+CG or WAS+PG in the following sludge:glycerol proportions: 100:0, 95:5, 90:10, 85:15, and 80:20 (% v/v). For all the mixtures of WAS with the two types of glycerol (does not include the pure sludge digestion), the marked drop in pH leads to the inhibition of biogas production and virtually no methane in their compositions. The severe drop in pH observed in the WAS mixtures with higher proportions of crude glycerol or pure glycerol (first step; Table 2) is probably related to the rapid production and accumulation of volatile acids in the process, caused by the high initial concentrations of glycerol (47 to 186 g/L based on glycerol content of the CG). Glycerol alone is not toxic or inhibitory (López et al. 2009); however, high concentrations of glycerol combined with its high biodegradability favor the formation of intermediates via lactate, such as 1,3-propanediol and propionate (Liu and Fang 2007; Cho et al. 2015). When glycerol is rapidly degraded to propionic acid/propionate, it leads to the accumulation of this acid and the destabilization of the anaerobic co-digestion process (Jensen et al. 2014).
Fountoulakis et al. (2010), in the co-digestion of sludge with 1 and 3% (v/v) glycerol, verified a high imbalance in the process and a decrease in the biogas production with addition of 3% CG after the tenth day. On the other hand, the addition of 1% produced increased biogas production compared to that of the pure sludge. Holm-Nielsen et al. (2008) verified that concentrations of glycerol above 0.5% (v/v) generated instability in the anaerobic digestion process. Razaviarani et al. (2013), while evaluating the co-digestion of sewage sludge and glycerol in a semi-continuous reactor with an HRT of 20 days, verified that above 2% (v/v) glycerol inhibited biogas production and led to instability in the process. Baba et al. (2013), while evaluating the co-digestion of sewage sludge with 1 to 15% (v/v) of crude glycerol in a semi-continuous reactor (working volume: 30 m3; HRT: 60 days), verified the accumulation of volatile acids and decrease in methane production for crude glycerol loads above 1 mL/L days (6% v/v equivalent).
In addition to the high concentration of glycerol and chlorides, the crude glycerol may also contain long-chain fatty acids, which are known inhibitors of the anaerobic digestion process (Alves et al. 2009; Viana et al. 2012). Nghiem et al. (2014) obtained higher specific methane production and absence of inhibition in methanogenesis when low concentrations of crude and pure glycerol (0.25 and 0.5% v/v) were applied in the co-digestion with sewage sludge. According to the authors, even when employed in low concentrations, the high COD and biodegradability of glycerol favor methane production. Although the biogas production in the co-digestion of sludge with PG was higher than that with CG, the production of methane in the sludge and GC or PG mixtures was practically nil (as evidenced by statistical analysis, Table 2), when both substrates were added at high concentrations, proving a marked inhibition of methanogenesis. The organic content of glycerol based on long-chain fatty acids and the presence of other inhibitory compounds may imposed an alternative and easier biochemical route for the formation of methane, which may explain this specific behavior as previously reported by Liu and Fang (2007), and Cho et al. (2015).
Nghiem et al. (2014) also achieved a higher biogas production with addition of PG compared to that of CG during the first 10 days of sludge co-digestion. This result is probably associated with the high initial concentrations of salts in the CG used in this first stage (1.8 to 7.4 g Cl−/L for CG concentrations of 5 to 20% v/v), as the PG did not contain any salinity. Salinity consists mostly of sodium, chloride, potassium, and magnesium ions; the high concentration of these salts increases ionic strength. The more concentrated outer hypertonic medium influences cellular osmoregulation and the ability of the cells to import and excrete materials and water. Salinity increases the solubility of proteins and reduces the activity of enzymes and bacteria that use intra- and extracellular enzymes in their metabolism. Inhibition of NaCl on non-halophilic bacteria may be due to dehydration as a consequence of increased osmotic pressure, inhibition of the activity of intra- and extracellular enzymes that affect bacterial metabolism, and the physical effect leading to inhibition of bacterial cell wall functions (Larsen 1962). Therefore, salinity is another important inhibition factor of methanogenesis, with concentrations between 5 and 9 g Cl−/L sufficient to inhibit the process (Vijayaraghavan and Ramanujam 1999; Vallero et al. 2003; Riffat and Krongthamchat 2006).
The VS removal was consistent with the biogas production, obtaining lower values in relation to the control (pure sludge) for sludge mixtures with PG (Table 2). Higher VS removals in the sludge and GC mixtures (compared to the sludge and PG mixtures) can be attributed to an interference in the quantification of volatile solids. Contaminants of crude glycerol, such as methanol and fatty acids, remain adsorbed to the sludge and are volatilized and counted as volatile solids in the analysis. In addition, during the co-digestion experiments, glycerol interfered in the analysis of volatile suspended solids, as it adsorbed into the sludge and, consequently, increased the removal values. Glycerol contributed up to 11.7% of the VS removals. Thus, a prewash sequence with distilled water was added to the analysis of solids to reduce this interference.
To evaluate the contribution of CG salinity to the inhibition of methane production in the co-digestion with WAS, a second step was conducted with only 1% (v/v) pure glycerol, crude glycerol, or glycerol pretreated to remove salinity. Table 3 shows that the addition of a lower proportion of glycerol led to higher methane production after 15 days of digestion (1.9 to 3.1 mL) than that obtained in step 1 (0 to 0.6 mL). However, these values are still lower than that obtained in the control (pure sludge), which was 5.5 mL that was verified after the same period, confirming the inhibitory effect of glycerol even at lower concentration. Consistent with the low methane production, the removal of VS in the experiments with addition of glycerol also presented low values (3–14%), as did the pH, which remained below 7 (around 5). Statistical analysis (Table 3) conducted with the variables biogas volume, % CH4, and VS removal confirms that the values obtained in control experiments were always significantly higher than in experiments with addition of CG, PTG, or PG.
The specific methane production (SMP) varied from 49 to 317 mL CH4/g VSremoved in the second step of the experiments with glycerol addition, values much higher than those obtained in the first step (0 to 8 mL CH4/g VSremoved). However, when comparing SMP obtained with the distinct types of glycerol, a higher value was observed with addition of PG, not with PTG, as expected (as confirmed by statistical analysis). The low SMP obtained with the addition of 1% (v/v) pretreated glycerol (PTG) to WAS (second step; Table 3) probably relates to the chloride precipitation method, which may have contributed to some residual elements with inhibitory effects (e.g., Ag+). The initial concentrations of chloride in the tests with 1% (v/v) crude glycerol and pretreated glycerol presented values well below the inhibitory concentrations of 0.4 and 0.2 g Cl−/L, respectively. This result indicates that in concentrations ≤ 1% (v/v), the dilution of crude glycerol in the sludge is sufficient to reduce salinity, with no need for pretreatment.
At the end of the co-digestion of sludge with 1% (v/v) glycerol, the concentration of volatile fatty acids (VFA) increased considerably, while the alkalinity (Alk) remained mostly unchanged (Fig. 1). VFA increased, on average, from 210 to 1836 mg/L, while alkalinity ranged from 273 to 236 mg/L, indicating the production and accumulation of volatile acids. Such variation resulted in very high values of the VFA/Alk ratio and low pH values. Under the control condition (WAS), VFA decreased and alkalinity increased, resulting in a low VFA/Alk ratio and neutral pH at the end of the digestion.
Values of pH, volatile fatty acids (VFA; mg HAc/L), alkalinity (Alk; mg CaCO3/L), and VFA/alk ratios in experiments with waste activated sludge alone (WAS); mixtures of sludge and 1% (v/v) crude glycerol (CG), pretreated glycerol (PTG), or pure glycerol (PG) (a); and mixtures of sludge with 0.3, 0.5, and 0.7% (v/v) of crude glycerol (b)
Considering that the final pH values still were below those recommended for anaerobic microorganisms, it can be concluded that the added glycerol was converted to volatile acids that then accumulated and inhibited the methanogenesis. The same is true for the removal of VS, which remained very low, regardless of the type of glycerol used in the co-digestion. The VFA concentration is a good indicator of the digestion conditions, as acid accumulation means the acids are not being converted to methane (Switzenbaum et al. 1990). Baba et al. (2013) observed a drop in methane yield through the accumulation of 2000–2500 mg/L of propionic acid in the digestion of sludge with crude glycerol in a semi-continuous reactor. Silvestre et al. (2015), on the other hand, in the co-digestion of sludge with 1.6% (v/v) glycerol in a continuous reactor with an HRT of 22 days and organic load rate of 2.4 g COD/L days, reported instability in the system (pH 3.3) and reduction of up to 60% methane production for concentrations of VFA above 1000 mg/L. Similar behavior was found by Nghiem et al. (2014), who reported that, with the addition of 1% (v/v) glycerol to sewage sludge in a batch reactor, instability and accumulation of total volatile acids occurred in the system.
Another good indicator of efficient anaerobic digestion is the VFA/alkalinity ratio. Researchers recommend a ratio between 0.1 and 0.35 for a healthy digester (Switzenbaum et al. 1990). Final values of the VFA/alkalinity ratio in the assays with glycerol added, unlike the control assay (ratio = 0.2), were in the range of 7–9, well above those recommended for efficient digestion. Razaviarani and Buchanan (2015), in the co-digestion of sludge with 2.72% (v/v) glycerol, showed that a VFA/alkalinity ratio of 0.7 already caused instability in the system. As verified in the current and other studies (Fountoulakis et al. 2010; Razaviarani et al. 2013; Nghiem et al. 2014; Silvestre et al. 2015), the addition of glycerol ≥ 1% (v/v) to the sludge still confers instability to the co-digestion process.
In the co-digestion experiments of WAS with crude glycerol concentrations below 1% (third step; Table 3), volatile acids were consumed as they were produced and thus did not accumulate. Such behavior led to VFA/alkalinity ratios of 0.2 to 0.3 (Fig. 1), values within the optimum range for anaerobic digestion. This allowed buffering and favored methanogenic activity, so much so that the final pH values were around 7.4 (Fig. 1). The maintenance of pH at values favorable to the activity of the anaerobic microorganisms (between 7 and 8, Weiland 2010) facilitated the production of methane, with values of specific methane production higher than the values obtained in the control.
Concentrations of glycerol below 1% (v/v) allowed the maintenance of pH values suitable for anaerobic digestion, with higher VS removals (between 16 and 21%) and higher methane production. Final volumes of methane with addition of glycerol were 31.7 to 63.8% higher than those in the control. Different from the co-digestion of sludge with 1% (v/v) crude glycerol, in the tests with lower concentrations of CG, there was a smaller increase in VFA (between 67 and 710 mg/L) and a considerable increase in total alkalinity (on average of 2398 mg/L) at the end of the digestion (Fig. 1).
The best co-digestion condition of sludge with glycerol was with addition of 0.5% (v/v) glycerol. The statistical analysis showed that, with the exception of VS removal, all the variables of this condition presented higher values in comparison with the other conditions (Table 3). The value of SMP as a function of VS applied and the percentage of methane in the biogas for this condition were 78 mL CH4/g VSapplied and 84.1%, respectively. However, for the condition with sludge only (WAS), without addition of glycerol in the same assay, an SMP of 45 mL CH4/g VSapplied and 61.9% of CH4 in the biogas were obtained.
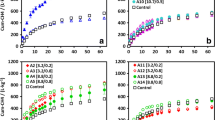
Figure 2 presents a summary of the main parameters evaluated in the co-digestion with lower percentages of crude glycerol added to the sludge, referring to the second and third steps of this study. Higher SMP is clearly observed with the reduction of the glycerol concentration to values ≤ 1% in the mixture with sludge, which allowed better buffering and pH values that were more adequate for digestion. The same happened with the VS removal, which increased with the reduction of the percentage of glycerol in the mixture with sludge.
However, the SMP values obtained in the present study (45 mL CH4/g VSapplied for WAS and 62–78 mL CH4/g VSapplied for mixtures of sludge and glycerol < 1% v/v) are well below the values obtained for the digestion of pure sludge in other studies conducted at bench scale (195 to 243 mL CH4/g VSapplied) (Huiliñir et al. 2017; Thorin et al. 2017) or for mixtures of sludge and glycerol with concentrations ≤ 1% (v/v). Razaviarani et al. (2013), for example, obtained SMP values of 300 and 380 mL CH4 /g VSapplied with 100% sludge and with a mixture of sludge and 1.1% (v/v) glycerol in a continuous reactor, respectively. Physical-chemical properties of both glycerol and sludge, as well as digestion conditions (reactor volume and configuration, operating regime, inoculum, temperature, HRT, agitation, etc.), may justify this difference in SMP values (Khalid et al. 2011).
Despite the increase in SMP for mixtures with glycerol addition between 0.3 and 0.7% (v/v), compared to the control (WSA), the VS removal was still lower, with a maximum value of 21.3% for 0.3% (v/v) CG versus 32.5% for the control. These values are in accordance with those reported by Athanasoulia et al. (2014), who found a VS reduction of 36% for the control and 24% for the mixture of sludge and 3% (v/v) glycerol, in a continuous stirred-tank reactor. The reason that VS reduction was greater in the control than in the mixtures of sludge and glycerol may be the greater availability of carbon from glycerol, which contributed to the maintenance of still-active biomass and reduced the stabilization of VS (Fountoulakis et al. 2010).
The increase of SMP in the co-digestion of sludge with glycerol below 1% (v/v) is due in large part to the high biodegradability of glycerol, which reached 68–86% removal in the experiments. Other authors reported similar results for the co-digestion of sludge with glycerol. Silvestre et al. (2015) and Jensen et al. (2014) reported 100% biodegradability with 1 and 2% (v/v) glycerol, respectively. The results obtained for chemical oxygen demand (COD) removal indicate the contribution of glycerol to methane production, considering that the soluble fraction is mostly glycerol. COD removal efficiencies of 75, 78, and 67% were obtained with addition of 0.3, 0.5, and 0.7% (v/v) glycerol, respectively.
Nghiem et al. (2014), in evaluating the co-digestion of sludge with low percentages of glycerol (0.25 and 0.5% v/v) in a batch reactor, indicated the best condition was 0.25% v/v glycerol. However, with 0.5% v/v, the authors obtained an SMP of 0.33 m3 CH4 /L glycerol applied, a higher value than the one obtained in the present study for the same concentration of glycerol added to the sludge (0.12 m3 CH4 /L glycerol applied). In the study by Nghiem et al. (2014), a comparison of the methane volumes produced by 0.5% (v/v) glycerol and the control (sludge only) at 30 days of digestion indicated values 1.85 times higher with addition of glycerol. A similar comparison can be made in our study, with a 1.63-fold higher volume of methane with 0.5% (v/v) glycerol added than with the sludge only for the same digestion period.
According to Holm-Nielsen et al. (2008), in the co-digestion of sludge and glycerol, concentrations of 5 to 7 g glycerol /L favor anaerobic digestion, because under this condition VFA do not accumulate. However, in this same study, when the concentration of glycerol was higher, organic overload occurred, which was reflected as an increase in the VFA concentration and a lower methane production rate during fermentation. In the present work, the buffering of the system was verified as well as the increase in methane production in relation to the control under all conditions tested with addition of glycerol between 0.3 and 0.7% (v/v), which are equivalent to concentrations between 3.8 and 8.8 g glycerol/L.
The best condition, according to the results obtained (Table 3, Fig. 2), for co-digestion with WAS is addition of 0.5% (v/v) or 6.3 g/L of crude glycerol. Considering the characteristics of the sludge and crude glycerol employed, this value is equivalent to 4.7 g/L of glycerol and approximately 0.6 g glycerol/g VS applied. Under this condition, the SMP as a function of VS applied was 1.7 times greater than that in the control (with sludge only).
Conclusion
High percentages of crude or pure glycerol, in the range of 5 to 20% (v/v), caused a marked drop in pH and inhibition of methane production. Reduction of the salinity of crude glycerol, either by dilution (to 1% v/v) or by pretreatment, also resulted in accumulation of VFA, pH drop, and inhibition of methane production. The rapid degradation of glycerol followed by accumulation of VFA had a more pronounced inhibitory effect than the salinity of the added glycerol. The addition of crude glycerol to the sludge in the range of 0.3 to 0.7% (v/v) led to the maintenance of pH and stabilization of the digestion system. For the evaluated system (mesophilic batch digestion without agitation), the best condition for the co-digestion of waste activated sludge would be addition of 0.6 g glycerol/g VS applied. Under this condition, there was a 1.7-fold increase in specific methane production in relation to the control (pure sludge).
References
Alves MM, Pereira MA, Sousa DZ, Cavaleiro AJ, Picavet M, Smidt H, Stams AJM (2009) Minireview waste lipids to energy: how to optimize methane production from long-chain fatty acids (LCFA). Microbiol Biotechnol 2:538–550. https://doi.org/10.1111/j.1751-7915.2009.00100.x
Athanasoulia E, Melidis P, Aivasidis A (2014) Co-digestion of sewage sludge and crude glycerol from biodiesel production. Renew Energy 62:73–78. https://doi.org/10.1016/j.renene.2013.06.040
Baba Y, Tada C, Watanabe R, Fukuda Y, Chida N, Nakai Y (2013) Anaerobic digestion of crude glycerol from biodiesel manufacturing using a large-scale pilot plant: methane production and application of digested sludge as fertilizer. Bioresour Technol 140:342–348. https://doi.org/10.1016/j.biortech.2013.04.020
Barber WPF (2012) Influence of changing drivers on realising the value of sewage sludge as a resource. Water Pract Technol 7(12):1–12. https://doi.org/10.2166/wpt.2012.067
Bhatti ZI, Furukawa K, Fujita M (1993) Treatment performance and microbial structure of a granular consortium handling methanolic waste. J Ferment Bioeng 76:218–223. https://doi.org/10.1016/0922-338X(93)90012-W
Bondioli P, Della Bella L (2005) An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur J Lipid Sci Technol 107:153–157. https://doi.org/10.1002/ejlt.200401054
Brisolara KF, Qi Y (2015) Biosolids and sludge management. Water Environ Res 87:1147–1166. https://doi.org/10.2175/106143015X14338845155507
Castrillón L, Fernández-Nava Y, Ormaechea P, Marañón E (2013) Methane production from cattle manure supplemented with crude glycerin from the biodiesel industry in CSTR and IBR. Bioresour Technol 127:312–317. https://doi.org/10.1016/j.biortech.2012.09.080
Chiu SLH, Lo IMC (2016) Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ Sci Pollut Res 23:24435–24450. https://doi.org/10.1007/s11356-016-7159-2
Cho S, Kim T, Woo HM, Kim Y, Lee J, Um Y (2015) High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol Biofuels 8(146):146. https://doi.org/10.1186/s13068-015-0336-6
Chuah LF, Yusup S, Aziz ARA, Klemeš JJ, Bokhari A, Abdullah MZ (2016) Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Techn Environ Policy 18:473–482. https://doi.org/10.1007/s10098-015-1022-x
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39. https://doi.org/10.1016/j.biotechadv.2008.07.006
Demirbas A (2017) Tomorrow’s biofuels: goals and hopes. Energ Sources Part A 39:673–679. https://doi.org/10.1080/15567036.2016.1252815
Dilallo R, Albertson OR (1961) Volatile acids by direct titration. J Water Pollut Control Fed 23:356–365 http://www.jstor.org/stable/25034391
Dills SS, Apperson A, Schmidt MR, Saier Jr MH (1980) Carbohydrate transport in bacteria. Microbiol Rev 44:385–418
Fountoulakis MS, Petousi I, Manios T (2010) Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manag 30:1849–1853. https://doi.org/10.1016/j.wasman.2010.04.011
Greenberg AE, Clesceri LS, Eaton AD (2005) Standard methods for the examination of water and wastewater. APHA, AWWA, WPCF, New York
Holm-Nielsen JB, Lomborg CJ, Oleskowicz-Popiel P, Esbensen KH (2008) On-line near infrared monitoring of glycerol-boosted anaerobic digestion processes: evaluation of process analytical technologies. Biotechnol Bioeng 99:302–313. https://doi.org/10.1002/bit.21571
Hu S, Luo X, Wan C, Li Y (2012) Characterization of crude glycerol from biodiesel plants. J Agric Food Chem 60:5915–5921. https://doi.org/10.1021/jf3008629
Huiliñir C, Pinto-Villegas P, Castillo A, Montalvo S, Guerrero L (2017) Biochemical methane potential from sewage sludge: effect of an aerobic pretreatment and fly ash addition as source of trace elements. Waste Manag 64:140–148. https://doi.org/10.1016/j.wasman.2017.03.023
Jenicek P, Kutil J, Benes O, Todt V, Zabranska J, Dohanyos M (2013) Energy self-sufficient sewage wastewater treatment plants: is optimized anaerobic sludge digestion the key. Water Sci Technol 68:1739–1744. https://doi.org/10.2166/wst.2013.423
Jensen PD, Astals S, Lu Y, Devadas M, Batstone DJ (2014) Anaerobic codigestion of sewage sludge and glycerol, focusing on process kinetics, microbial dynamics and sludge dewaterability. Water Res 67:355–366. https://doi.org/10.1016/j.watres.2014.09.024
Kacprzak M, Neczaj E, Fijałkowski K, Grobelak A, Grosser A, Worwag M, Rorat A, Brattebo H, Almås Å, Singh BR (2017) Sewage sludge disposal strategies for sustainable development. Environ Res 156:39–46. https://doi.org/10.1016/j.envres.2017.03.010
Karthikeyan OP, Visvanathan C (2013) Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Environ Sci BioTechnol 12:257–284. https://doi.org/10.1007/s11157-012-9304-9
Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L (2011) The anaerobic digestion of solid organic waste. Waste Manag 31:1737–1744. https://doi.org/10.1016/j.wasman.2011.03.021
Larsen H (1962) Halophilism. In: Gunsalus IC, Stanier RY (eds) The bacteria, vol 1. Academic Press, New York, pp 297–342
Liu F, Fang B (2007) Optimization of bio-hydrogen production from biodiesel wastes by Klebsiella pneumoniae. Biotechnol J 2:374–380. https://doi.org/10.1002/biot.200600102
López JÁS, Santos MÁM, Pérez AFC, Martín AM (2009) Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour Technol 100:5609–5615. https://doi.org/10.1016/j.biortech.2009.06.017
Luo X, Ge X, Cui S, Li Y (2016) Value-added processing of crude glycerol into chemicals and polymers. Bioresour Technol 215:144–154. https://doi.org/10.1016/j.biortech.2016.03.042
Ma J, van Wambeke M, Carballa M, Verstraete W (2008) Improvement of the anaerobic treatment of potato processing wastewater in a UASB reactor by co-digestion with glycerol. Biotechnol Lett 30:861–867. https://doi.org/10.1007/s10529-007-9617-x
Maragkaki AE, Fountoulakis M, Gypakis A, Kyriakou A, Lasaridi K, Manios T (2017) Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag 59:362–370. https://doi.org/10.1016/j.wasman.2016.10.043
Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Ener Rev 36:412–427. https://doi.org/10.1016/j.rser.2014.04.039
Mininni G, Laera G, Bertanza G, Canato M, Sbrilli A (2015) Mass and energy balances of sludge processing in reference and upgraded wastewater treatment plants. Environ Sci Pollut Res 22:7203–7215. https://doi.org/10.1007/s11356-014-4013-2
Nghiem LD, Nguyen TT, Manassa P, Fitzgerald SK, Dawson M, Vierboom S (2014) Co-digestion of sewage sludge and crude glycerol for on-demand biogas production. Int Biodeterior Biodegrad 95:160–166 http://ro.uow.edu.au/eispapers/2600
Nitayavardhana S, Khanal SK (2011) Biodiesel-derived crude glycerol bioconversion to animal feed: a sustainable option for a biodiesel refinery. Bioresour Technol 102:5808–5814. https://doi.org/10.1016/j.biortech.2011.02.058
Padula AD, Santos MS, Ferreira L, Borenstein D (2012) The emergence of the biodiesel industry in Brazil: current figures and future prospects. Energy Policy 44:395–405. https://doi.org/10.1016/j.enpol.2012.02.003
Razaviarani V, Buchanan ID (2015) Anaerobic co-digestion of biodiesel waste glycerin with municipal wastewater sludge: microbial community structure dynamics and reactor performance. Bioresour Technol 182:8–17. https://doi.org/10.1016/j.biortech.2015.01.095
Razaviarani V, Buchanan ID, Malik S, Katalambula H (2013) Pilot scale anaerobic co-digestion of municipal wastewater sludge with biodiesel waste glycerin. Bioresour Technol 133:206–212. https://doi.org/10.1016/j.biortech.2013.01.101
Riffat R, Krongthamchat K (2006) Specific methanogenic activity of halophilic and mixed cultures in saline wastewater. Int J Environ Sci Technol 2:291–299. https://doi.org/10.1007/BF03325889
Ripley LE, Boyle WC, Converse JC (1986) Improved alkalimetric monitoring for anaerobic digestor of high-strength wastes. J Water Pollut Control Fed 58:406–411 http://www.jstor.org/stable/25042933
Rivaldi JD, Sarrouh BF, da Silva SS (2009) Development of biotechnological processes using glycerol from biodiesel production. In: Mendez-Vilas A (ed) Current research topics in applied microbiology and microbial biotechnology, 1st edn. World Scientific, Singapore, pp 429–433. https://doi.org/10.1142/9789812837554_0089
Rivero M, Solera R, Perez M (2014) Anaerobic mesophilic co-digestion of sewage sludge with glycerol: enhanced biohydrogen production. Int J Hydrogen Energy 39:2481–2488. https://doi.org/10.1016/j.ijhidene.2013.12.006
Robra S, da Cruz RS, de Oliveira AM, Neto JA, Santos JV (2010) Generation of biogas using crude glycerin from biodiesel production as a supplement to cattle slurry. Biomass Bioenergy 34:1330–1335. https://doi.org/10.1016/j.biombioe.2010.04.021
Shen Y, Linville JL, Urgun-Demirtas M, Mintz MM, Snyder SW (2015) An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towards energy-neutral WWTPs. Renew Sust Energ Rev 50:346–362. https://doi.org/10.1016/j.rser.2015.04.129
Silvestre G, Fernández B, Bonmatí A (2015) Addition of crude glycerine as strategy to balance the C/N ratio on sewage sludge thermophilic and mesophilic anaerobic co-digestion. Bioresour Technol 193:377–385. https://doi.org/10.1016/j.biortech.2015.06.098
Svensson M, Baxter D (2016) IEA bioenergy task 37—country reports summary 2015. IEA Bioenergy, Netherlands
Switzenbaum MS, Giraldo-Gomez E, Hickey RF (1990) Monitoring of the anaerobic methane fermentation process. Enzym Microb Technol 12:722–730. https://doi.org/10.1016/0141-0229(90)90142-D
Thompson JC, He BB (2006) Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agric 22:261–265. https://doi.org/10.13031/2013.20272
Thorin E, Olsson J, Schwede S, Nehrenheim E (2017) Biogas from co-digestion of sewage sludge and microalgae. Energy Procedia 105:1037–1042. https://doi.org/10.1016/j.egypro.2017.03.449
Vallero MVG, Hulshoff Pol LW, Lettinga G, Lens PNL (2003) Effect of NaCl on thermophilic (55 °C) methanol degradation in sulfate reducing granular sludge reactors. Water Res 37:2269–2280. https://doi.org/10.1016/S0043-1354(03)00024-1
Viana MB, Freitas AV, Leitão RC, Pinto GAS, Santaella ST (2012) Anaerobic digestion of crude glycerol: a review. Environ Technol Rev 1:37–41. https://doi.org/10.1080/09593330.2012.692723
Vijayaraghavan K, Ramanujam TK (1999) Effect of chloride and condensable tannin in anaerobic degradation of tannery wastewaters. Bioprocess Eng 20:499–503. https://doi.org/10.1007/s004490050621
Wang H, Brown SL, Magesan GN, Slade AH, Quintern M, Clinton PW, Payn TW (2008) Technological options for the management of biosolids. Environ Sci Pollut Res Int 15:308–317. https://doi.org/10.1007/s11356-008-0012-5
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. https://doi.org/10.1007/s00253-009-2246-7
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5:13. https://doi.org/10.1186/1754-6834-5-13
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–219. https://doi.org/10.1016/j.copbio.2007.05.002
Funding
This work was supported by project funds from the Brazilian Innovation Agency - FINEP, Research National Council (CNPq), and Carlos Chagas Filho Research Foundation - Rio de Janeiro State (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
dos Santos Ferreira, J., Volschan, I. & Cammarota, M.C. Co-digestion of sewage sludge with crude or pretreated glycerol to increase biogas production. Environ Sci Pollut Res 25, 21811–21821 (2018). https://doi.org/10.1007/s11356-018-2260-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2260-3