Abstract
Physical and chemical properties of biodiesel are influenced by the structural features of fatty acid, such as with saturated, monounsaturated and polyunsaturated fatty acids. In this study, seven non-edible oils have been selected, which include waste cooking oil derived palm olein, Calophyllum inophyllum, jatropha oil, castor oil, rubber seed oil, kapok seed oil and karanja oil. The critical parameters, e.g. cetane number (CN), iodine value (IV) and oxidation stability (OS) of biodiesel were correlated with the degree of unsaturated (DU) fatty acid, whereas the cold filter plugging point (CFPP) was correlated with the long chain saturated factor (LCSF). To meet the minimum EU requirement of EN 14214 of the critical parameter, the DU value of the CN was ≤133.5, IV ≤123.2 and OS ≤98.9. The LCSF values satisfied the Spanish regional standard—RD 61/2006 in summer (0 °C) at ≤8.4 and winter (−10 °C) at ≤0.1 of the CFPP. Based on the composition of the saturated, monounsaturated and polyunsaturated fatty acids, a triangular chart for the biodiesel property prediction was developed. This can then be used as a reference for non-edible oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of fuel worldwide for transport, production and manufacturing industries, power plants, aircraft, aviation and naval transportation is growing (Delavari et al. 2015). There has been an accelerating exploitation of the current oil and gas reserve, which has brought about an increase of emissions and pollution. One possible solution is the extended exploitation of biodiesel as an alternative fuel. Biodiesel has a high flash point and lower volatile compounds. It is easy to use and is clean and safe for handling compared with petroleum diesel. Biodiesel contributes to environmental protection as it is biodegradable, renewable and non-toxic and reduces sulphur oxide emission and also greenhouse gas, which will reduce global warming problems (Chuah et al. 2015a). The sulphur content of biodiesel at 0.0018 wt% is about 28 fold lower than diesel fuel at 0.0500 wt% (Ghobadian et al. 2009). Biodiesel is one of the alternative renewable fuels, which can be produced from various sources, e.g. edible oil (Brondani et al. 2015), non-edible oil (Singh et al. 2015), animal fat (Bankovic-Ilic et al. 2014) and algae (O’Connell et al. 2013).
About 95 % of the world’s biodiesel production is derived from edible oils (Tan et al. 2011). However, it is strongly opposed by some non-government organisations worldwide due to its negative impact as it competes with food for resources. Consumption of edible oil in biodiesel production has led to the price of edible oil and biodiesel to increase to the levels 1.5 to 2 fold higher than the diesel fuel (Maddikeri et al. 2012). The raw material cost is the major contribution (75–88 %) of the total cost of biodiesel (Ghayal et al. 2013). However, intensification technology, such as hydrodynamic cavitation (Chuah et al. 2015b), ultrasound and microwave (Bulatov and Klemeš. 2011) plays an important role on biodiesel production by improving the biodiesel yield, biodiesel quality, chemicals, energy and processing time (Ghayal et al. 2013). Biodiesel is not a new invention, but scale up and the industrial production cost of biodiesel are challenging for research.
The availability of the non-edible plant oil is dependent on the regional geography, native soil, climate and vegetation (native or exotic) (Sanjid et al. 2013). India, Philippines, Thailand and China have been intensively using their local non-edible plants, e.g. jatropha for biodiesel production. Around 60 % of the world’s castor oil production is produced by India (Bankovic-Ilic et al. 2012). Malaysia is diversifying its biodiesel feedstock towards local non-edible plant oil, such as Calophyllum inophyllum—bintangor laut/nyamplung/penaga laut, Jatropha curcas (Jatropha), Ricinus communis (Castor), Hevea brasiliensis (Rubber), Ceiba pentandra (Kapok), Pongamia pinnata (Karanja) and waste from palm oil processing. Malaysia is the second largest producer of palm oil in the world. The oil seed content (wt%) from non-edible plant oil can vary significantly. Nyamplung has 65 wt% oil content, jatropha between 43 and 59 wt%, castor varies between 45 and 50 wt%, rubber varies between 40 and 60 wt%, kapok varies between 24 and 40 wt% and karanja varies between 25 and 50 wt% (Atabani et al. 2013). These are generally higher than edible oils, e.g. soybean between 15 and 20 wt%, sunflower between 25 and 35 wt% and rapeseed between 38 and 46 wt% (Atabani et al. 2012). The oil yield from non-edible plant oil also varies. Nyamplung oil yield is 4680 kg oil/ha/y, jatropha at 1590 kg oil/ha/y, castor at 1188 kg/ha/y, rubber at 50 kg oil/ha/year and karanja at 900–9000 kg oil/ha/y (Atabani et al. 2013). This compares to edible oils, such as soybean of 446 kg oil/ha/year, sunflower of 952 kg oil/ha/year and rapeseed of 1190 kg oil/ha/year (Atabani et al. 2012). The local non-edible plant oils, e.g. nyamplung, jatropha, castor, rubber, kapok and karanja have drawn the attention of the Malaysia government and researchers to consider them as biodiesel feedstock, which can be supplied at a viable quantity based on the large scale of cropped lands (Abedin et al. 2014).
These non-edible plants could bring benefits, such as utilisation of low fertility land, restoration of degraded land and contribution towards eliminating the food crisis. For this reason, non-edible oils become more attractive and promising alternate feedstock for biodiesel production (Chuah et al. 2015c). Waste cooking oil (WCO) can also be categorised as non-edible oil, around 29 Mt/year of WCO is produced and about 4.1 kg is generated per person/y worldwide (Maddikeri et al. 2012). WCO produced in Canada is 0.14 Mt/y, in EU countries is 0.85 Mt/year and the United Kingdom is 0.2 Mt/y (Chhetri et al. 2008). The Malaysian population is reaching 30 million and around 0.12 Mt/y of WCO is generated. Most of the cooking oil derived from palm olein is widely used in Malaysia as it has good resistance to oxidation at high temperatures during frying (Chuah et al. 2015a).
Minimising the carbon footprint (Lam et al. 2010) of biodiesel needs to be taken into account so that it is an advantage to utilise it in local regions rather than export to other regions. It is more environmental friendly, cleaner and more efficient at reducing the energy loss and distribution cost (Wendy Ng et al. 2014). In order to make a viable biodiesel at a commercial scale, it is important to look at non-edible oil which benefits from a low price. WCO is even cheaper—five times on average lower than refined cooking oil (RCO). Biodiesel derived from non-edible oil can be a very promising alternative feedstock, which could reduce the production cost and global emission management cost as a lower pollutant emission. However, based on the diesel fuel demand and available amount of non-edible oil in the world, it still may not able to replace diesel fuel completely, but it could contribute to reduce the dependency on diesel fuel based petroleum.
Disposal of WCO is a major problem in the world, due to polluting rivers, drainage choking (Gopal et al. 2014), odour, vermin and wildlife problems (Kalam et al. 2011). WCO has also been used as an additive to the feeding mixture for domestic animals, but it has been prohibited by EU since 2002 because it could bring harmful substances back into the food chain through meat production (Cvengros and Cvengrosova 2004). In addition, WCO for cooking can cause cancer if reused and recycled, due to its toxic contents produced when the oil is oxidised (Yaakob et al. 2013). Utilising WCO for biodiesel production is offering a solution for a serious disposal problem, feeding mixture for domestic animals, and reusing and recycling. Biodiesel properties are dependent on the oil properties (Kulkarni and Dalai 2006), oil refinement (e.g. pretreatment step), transesterification process and the quality of phases in the purification step (Ramos et al. 2009). Biodiesel properties derived from different non-edible oils are important and require extensive experimental testing, which is costly and time consuming. The properties of biodiesel can be determined by the fatty acid composition in the raw materials (Wang et al. 2012), with degree of unsaturated (DU) and long chain saturated factor (LCSF) contributing to the properties of biodiesel (Ramos et al. 2009). The fatty acid compositions of the raw materials do not change during the transesterification process and its compositions are very important to estimate some critical parameters of biodiesel, such as oxidation stability (OS), cetane number (CN), iodine value (IV) and cold filter plugging point (CFPP). A lack of studies about the influence of the fatty acid composition in the biodiesel quality especially the critical parameter was reported by Ramos et al. (2009). However, the problem was raised again several years later by Wang et al. (2012). Ramos et al. (2009) had made the correlations and predictions based on the edible oil derived from palm, olive, almond, corn and rape, whereas Wang et al. (2012) reported the native plant oil from China which included Pistacia chinensis, Xanthoceras sorbifolia and Comus wilsoniana. Ramos et al. (2009) did not correlate the oxidation stability and DU, whereas the Wang et al. (2012) made a correlation between OS and DU, but the regression value is weak, e.g. 0.6421. However, the correlation of OS and DU was investigated in this study. The OS value of all the selected oil reported in Wang et al. (2012) should be reconfirmed again as it may be influenced by human error, equipment deviation and storage condition of biodiesel. The specific reason needs to be further studied. Consequently, the main purpose of this work is to study the influence of fatty acids content in non-edible oil for biodiesel properties. The fatty acid compositions have been measured following the EN 14103 standard. Knowing the percentage of monosaturated, polysaturated and unsaturated fatty acids in non-edible oil helps predict some critical parameters of biodiesel. It also helps to reduce the cost and processing time during selection of non-edible oil.
Materials and methods
Materials
WCO was purchased from a local source—UTP Cafeteria (Seri Iskandar, Perak) at an average price of RM 0.50/L (approximate USD 0.16 in 2015). WCO originated from the RCO, which is derived from the palm olein (Brand: Alif). WCO has passed through 3–5 times cycle of heating between 180 and 200 °C and cooling at about 25–40 °C for frying. The suspended particles in WCO were removed by filtration through a normal sieve. Under agitation, the filtered WCO was heated between 105 and 110 °C for 1 h in order to remove any water. WCO was then allowed to cool down at room temperature and then stored in a container for further processing. The nyamplung oil—Calophyllum inophyllum (CI)—seed was collected at the vicinity of Kuala Kangsar, Perak, Malaysia and further processed viz. peeling, drying, extraction and degumming extraction to obtain oil before the esterification and transesterification processes. The jatropha oil (JO)—Jatropha curcas, rubber seed oil (RSO)—Hevea brasiliensis and kapok seed oil (KSO)—Ceiba pentandra were provided by Kinetics Chemical (M) Sdn. Bhd. Malaysia, whereas the castor oil (CO)—Ricinus communis—was purchased from Benua Sains Sdn. Bhd. Malaysia.
The anhydrous methanol, potassium hydroxide pellets, sulphuric acid, sodium sulphate anhydrous, toluene, 2-propanol, phenolphthalein, acetic acid (glacial), cyclo-hexane, Wijs solution, potassium iodide, sodium thiosulphate pentahydrate, starch, chloroform, ethanol, hydrochloric acid and acetone were procured from Merck. The hydranal—Coulomat AG was supplied by Fluka. A standard mixture of 37 fatty acid methyl ester is the external standard mixture with different weight percentages ranging from 2 to 4 wt%. The nonadecanoic acid methyl ester internal standard was purchased from Sigma-Aldrich. All chemicals were analytical reagent grade.
Analysis
The characteristics of the selected non-edible oil and biodiesel were analysed according to the ASTM, EN, DIN and AOCS standards. The acid, peroxide, iodine and saponification values were determined by a titration method, which included AOCS-Cd 3d-63, AOCS-Ja 8-87, AOCS-Cd 1d-92 and AOCS-Cd 3b-76. The heating value of the oil was determined using a C5000 IKA (Werke, Germany), following ASTM D 4568, whereas the density and kinematic viscosity were measured using DMA 4500 M and Lovis 2000M of Anton Paar following ASTM D 4502 and DIN 12058. The C30 Coulometric KF Titrino (Mettler Toledo) was used to determine the moisture content of the oil and biodiesel following ASTM D 2709. The cetane number of the biodiesel was analysed using a Shatox Octane meter (SX200) following ASTM D 613. The FPP 5Gs, ISL, (by PAC) was used to determine the CFPP. The CFPP was a test which involved cooling the biodiesel at a specified rate and pumping it under a vacuum through a wire mesh filter screen. CFPP was then defined as the lowest temperature at which 20 mL of the sample safely passed through the filter within 60 s following the ASTM D 6371. The CLA 5, Petrotest complying with ASTM D 93, was used to determine the flash point. The 873 Biodiesel Rancimat (Metrohm) was used to determine the OS of the sample oil following EN 14112. The fatty acid compositions of the oils were determined by converting the fatty acid of the glycerides into methyl esters of the corresponding fatty acid using GC-FID (Ghayal et al. 2013). Methyl ester peaks were identified by comparing the retention times of the reference external standard. All of the experiments were conducted in three replicates and the reported values were the averages of the individual runs and the inaccuracy percentages were less than 2 % of the average value.
DU parameter was obtained from Eq. (1), taking into account the amount of monounsaturated and polyunsaturated fatty acid (wt%) present in the oil (Ramos et al. 2009).
LCSF was calculated from the composition of the saturated fatty acid and their corresponding melting points (MP n ) by Eq. (2) (Wang et al. 2012).
Results and discussions
Characterisation of feedstock oils
The comparison characterisation of non-edible oil is presented in Table 1. The acid value is an important parameter to indicate the quality, age and purity degree of oil during processing and storage (Cho et al. 2013). The oil which contained acid values >2 mg KOH/g required two processing steps, e.g. acid esterification followed by alkaline transesterification. A high acid value results in soap formation during the transesterification process; thus, it makes difficulties in the separation step and reduces the biodiesel yield. The results revealed that the acid value of WCO was 2.04 mg KOH/g and CO was 2.22 mg KOH/g, which were slightly higher than the 2 mg KOH/g of the set point. Considering the chemical, energy and processing time, the WCO and CO were considered under a single step rather than two processing steps, whereas the CI of 42.15 mg KOH/g, JO of 21.34 mg KOH/g, RSO of 83.51 mg KOH/g and KSO of 11.80 mg KOH/g underwent two processing steps. The densities of CI, CO and KSO were both 0.91 g/cm3 and they marginally increased by 1.1 % compared to WCO, JO and RSO—0.92 g/cm3 at 20 °C. The density of the oils was slightly lower than water density, e.g. 0.99821 g/cm3 at 20 °C. Viscosity is a measure of oil resistance to flow (Sivaramakrishnan and Ravikumar. 2012). The viscosity of oil decreases with increases in temperature and low viscosity induces the oil to flow easily. The average viscosity of WCO, CI, JO, RSO and KO was 39.23 mm2/s, approximately eightfold lower than CO—315.70 mm2/s at the temperature of 40 °C. The viscosities of WCO—51.04 mm2/s—and CI—44.24 mm2/s were slightly higher than reported by Yaakob et al. (2013), in which the WCO was 42.00 mm2/s, and Ong et al. (2014) with CI of 43.96 mm2/s. A high viscosity of oil (>100 mm2/s at 40 °C) may influence the methyl ester conversion efficiency since it limits the good mixing of the reactant (Meng et al. 2008). The oil’s viscosity should be reduced to ≤5 mm2/s at 40 °C, which will meet the EN 14214 standard after the transesterification process in order for it to be suitable for engine applications without any modification.
Saponification is a process by which the fatty acids in the glycerides of oil are hydrolysed by an alkali (Toscano et al. 2012). The saponification value is the amount of alkali (mg) required to saponify one gram of oil. This value is useful for a comparative study of fatty acid chain lengths (saturated) in oil (Jesikha 2012). The values of WCO of 205 mg KOH/g, CI of 201 mg KOH/g, JO of 208 mg KOH/g and RSO of 200 mg KOH/g were higher than CO and KSO which were 179 and 194 mg KOH/g. The results revealed that the higher the saturated fatty acid, the higher chain length and the higher saponification.
Moisture is a substance which is commonly well mixed with oil (Belewu et al. 2010). The moisture content of JO (0.24 wt%), CO (0.36 wt%), RSO (0.38 wt%) and KSO (0.25 wt%) was about 6.8 fold on average lower than the CI of 2.11 wt% due to the extraction method—press (low water content) and soxhlet (high water content) extraction. The low water content of WCO makes the oil not easy to be oxidised due to the limitation of supporting bacteria growth (Umaru and Aberuagba 2012). The water content of WCO was low, although it had gone through multiple cycles of frying food. However, the water content in the oil can be removed using a preheating treatment before the transesterification process (Yaakob et al. 2013).
Saturated, monounsaturated and polyunsaturated values of feedstock oils
They are three main types of fatty acids in the oils: saturated (C n :0), monounsaturated (C n :1) and polyunsaturated (C n :2,3). They were derived from the fatty acid composition of the selected non-edible oils as illustrated in Table 2.
Cetane number of biodiesels
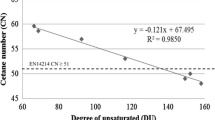
CN is the ability of the fuel to ignite quickly after being injected and a higher value indicates a better ignition quality of the fuel (Abbasi and Diwekar 2014). Biodiesel has higher CN compared to diesel fuel. The CN is one of the important critical parameters in the selection of raw materials. The minimum EU requirement of CN in biodiesel is ≤51. The biodiesel properties of waste cooking oil methyl ester (WCOME), Calophyllum inophyllum methyl ester (CIME), jatropha oil methyl ester (JOME), castor oil methyl ester (COME), kapok seed oil methyl ester (KSOME) and karanja oil methyl ester (KOME) complied with the standard, whereas rubber seed oil methyl ester’s CN value was 49 which was slightly lower than the EU standard (Table 3). Figure 1 shows the correlation between the CN and DU. CN decreased with an increase in DU and it indicated that the more unsaturation present in the oil, the less CN (Dermibas 2005). Equation (3) was developed in this study as
with a good R 2 e.g. 0.9573, in which DU of ≤133.5 qualified the oils to satisfy the EU standard. Similar findings were also reported by Ramos et al. (2009) where CN = −0.1353 (DU) + 69.506 and Wang et al. (2012) with CN = −0.1209 (DU) + 65.0958. A low CN has been associated with more than one double bond, e.g. linoleic (C18:2) and linolenic (C18:3) acid. However, the equations prescribed by Ramos et al. (2009) with DU to be ≤136.8 and Wang et al. (2012) with DU to be ≤116.6 were slightly different compared to this study.
Iodine value of biodiesels
IV is to determine the number of double bonds (unsaturated) present in the oil (Rocha et al. 2014). It is defined as the number of grams of iodine (I 2) capable of reacting with 100 g of sample oil. Islam et al. (2013) reported that the more unsaturated the fatty acid in the raw material, the higher the iodine value. The IV of biodiesel was correlated with unsaturated fatty acid. This IV value greatly influences the OS and the deposit formation in diesel engine injectors (Wang et al. 2012). Altun (2014) reported that the lowest IV of biodiesel leads to the highest CN and consequently reduces nitrogen oxides emissions. The minimum requirement of IV is 120 g I 2/100 g and 140 g I 2/100 g based on EN 14214 and Spanish regional standards.
The biodiesel derived from WCO, CI, JO, CO, KSO and KO complied with the standards, whereas RSO with 146 g I 2/100 g was 22 % higher value than the EN standard (Table 3). This high IV could be attributed to less percentage of saturated fatty acid, such as palmitic (C16:0) and stearic (C18:0) acids reported by Wang et al. (2012). The correlation between IV and DU is presented in Fig. 2 and followed Eq. (4) with
with R 2 of 0.9433. From Eq. (4), DU ≤123.2 and ≤142.0 qualified the oils to comply with the EU and Spanish regional standards. Although the equations prescribed by Ramos et al. (2009) with IV = 0.8365 (DU) + 5.6506 and Wang et al. (2012) with IV = 0.6683 (DU) + 25.0364 were different, the DU level meets with the EU standard of ≤136.7 and ≤142.1 and Spanish regional standard of ≤160.6 and ≤172.0.
Cold filter plugging point of biodiesels
CFPP is the most important critical parameter of biodiesel for low temperature applications (Wang et al. 2012). The CFPP temperature is defined as the temperature at which the fuel has crystalised, solidified or gelled enough to plug the fuel filter (Lown et al. 2014). Although the CFPP requirement is not on the list of EU standard specifications, each country can specify certain temperature limits for different season of a year depending on the local climate conditions (Knothe 2006). A limit of CFPP has been imposed in Spain called the Spanish regional standard, e.g. in summer (0 °C) and winter (−10 °C). However, Malaysia, Singapore, Thailand, Indonesia, Myanmar and other tropical countries do not have these problems. Table 3 shows the biodiesel CFPP derived from different non-edible oils. The biodiesel derived from WCO, CI, JO and KSO has poor low temperature flow properties which are 9, 1, 1 and 3 °C. It could be attributed to the precipitation of the palmitic (C16:0) and strearic (C18:0) acids which clogged the fuel filter when the liquid biodiesel was cold and a similar reason was also reported by Wang et al. (2012). However, Na-Ranong and Kitchaiya (2014) reported that the compound causing the precipitation was due to the presence of steryl glucosides. Low-temperature properties depend on the saturated fatty acid composition. The effect of the unsaturated fatty acid can be considered negligible because it acts as a solvent (Lopes et al. 2008). Moser (2014) reported that fatty acid did not affect the cold flow properties in low blending mixture which was ≤B5, unless the biodiesel contained a high percentage of saturated fatty acid methyl ester (≥C16). Therefore, LCSF was correlated with CFPP.
The correlation is presented in Fig. 3 and followed Eq. (5) with
having a high R 2 of 0.9152. The LCSF ≤8.4 can make the oils comply to the Spanish regional standard in the summer season (0 °C), whereas with ≤0.1, it qualifies the oils to meet the winter −10 °C requirement. However, the equations prescribed by Ramos et al. (2009) (CFPP = 8.9243 (LCSF) − 19.325) and Wang et al. (2012) (CFPP = 1.7556 (LCSF) − 14.772) were different, but the LCSF level to meet the standard was almost the same, which was ≤2.2 and ≤8.4 in summer and ≤1.0 and ≤2.7 in winter.
Oxidation stability of biodiesels
Biodiesel is also susceptible to oxidation which can induce ester polymerisation and form insoluble gums which are able to cause fuel filters to be clogged (Jain and Sharma 2014). One of the critical parameters affecting the use of biodiesel is OS because the presence of unsaturated fatty acid in biodiesel can make it susceptible to oxidation (Rawat et al. 2014). During storage, the OS of the biodiesel is very important because it yields products that degrade biodiesel quality and consequently affect the engine performance. Accurate measurement, prediction and control of the oxidative stability of biodiesel from different raw materials are challenging in biodiesel research (Pullen and Saeed 2014). The minimum requirement of OS set by the EU standard for biodiesel is 6 h. It is well known that most of the biodiesel derived from many common raw materials is very hard to meet the minimum requirement of EN 14214 after the transesterification (Wang et al. 2012). It has been noted that the parent oil has significantly higher oxidative stability than the corresponding methyl ester due to a loss of naturally occurring antioxidants during the transesterification reaction. A similar observation was reported by Rashid et al. (2014). Fernandes et al. (2014) also stated that the fatty acid composition with a high percentage of unsaturated fatty acids could lead to poor OS, unless antioxidants are added to the biodiesel (Rashid et al. 2014). The OS of the biodiesel may be influenced by many factors, such as the presence of air, heat, metal, traces, peroxides, light and structural features of the compound themselves especially the presence of double bonds (Bajpai and Tyagi 2006). Table 3 shows the OS of biodiesel derived from different non-edible oils. The biodiesel derived from WCO, JO, CO and KO satisfied the EN 14214 standard, whereas the CI (5.2 h), RSO (3.3 h) and KSO (4.7 h) were about 13, 45 and 22 % below the standard. For this study, the OS was correlated with DU. The correlation results are shown in Fig. 4. Equation (6) shows that the correlation between OS and DU was
with R 2 of 0.8313. From Eq. (6), with DU ≤98.9, it can qualify the oils to meet the EU standard. Ramos et al. (2009) did not report the correlation between OS and fatty acid composition. Wang et al. (2012) have developed a correlation between OS and DU with OS = −0.0384 (DU) + 7.770, which R 2 of 0.6421. From this equation prescribed by Wang et al. (2012), DU of ≤46.1 qualifies the oils to meet the EU standard. An obvious difference between this equation and the DU value may be influenced by human error, equipment deviation, storage condition, and the presence of air, heat and light. The specific reason needs to be further studied.
Prediction chart of fatty acid composition on biodiesel properties
Prediction of the biodiesel properties derived from different non-edible oils is important to avoid extensive experimental testing, which is costly due to expensive equipment and it is time consuming. Selection of the non-edible oil is dependent on the critical parameters of the biodiesel, such as CN, IV, CFPP and OS. Dmytryshyn et al. (2004) stated that the OS is influenced by the linolenic acid (C18:3). Wang et al. (2012) documented that most of the raw materials are difficult to meet the minimum requirements of OS, which could be attributed to the high unsaturated fatty acid. The biodiesel properties of the selected non-edible oils have been illustrated in Table 3. The OS cannot be integrated in the triangular chart, as in Fig. 5. In addition, the OS of the biodiesel may be influenced by many factors, such as the presence of air, heat, metal, traces, peroxides, light and structural features (Wang et al. 2012). The purpose of this chart is to group similar biodiesel properties derived from the non-edible oils. From the analysis of the data integrated in the triangular chart, the area which satisfies the limit of CN and IV exists at the far end of the polyunsaturated angular point. This is a light grey area, whereas the area that exists at the far end of the saturated angular point is marked as the darkest grey area and satisfies the CFPP minimum requirement of EN 14214. If both areas are overlapping (dark grey area) (Fig. 5), it proved that CO and KO complied with the CN, IV and CFPP minimum requirement of the EU and Spanish regional standards.
Conclusions
The biodiesel properties, such as CN, IV and OS derived from the selected non-edible oils were correlated with the DU, whereas the CFPP was influenced by LCSF. Decreasing the unsaturated fatty acid tends to give a high CN, whereas IV is the converse. The biodiesel properties conform to EN 14214, when the DU values of CN, IV and OS were ≤133.5, ≤123.2 and ≤98.9. The LCSF values satisfied the Spanish regional standard—RD 61/2006 in summer (0 °C) at ≤8.4 and winter (−10 °C) at ≤0.1 of the CFPP. The triangular chart (Fig. 5) was developed to predict the critical parameters except for OS. The area which satisfied the limit of CN and IV is the light grey area, e.g. WCO, CI, JO and KSO, whereas the darkest grey area, e.g. RSO met the CFPP minimum requirement of EN14214. If both areas are overlapping (dark grey area), only the CO and KO met the CN, IV and CFPP minimum requirement of the EU standard.
References
Abbasi S, Diwekar UM (2014) Characterization and stochastic modeling of uncertainties in the biodiesel production. Clean Technol Environ Policy 16:79–94
Abedin MJ, Masjuki HH, Kalam MA, Sanjid A, Rahman SMA (2014) Performance, emissions, and heat losses of palm and jatropha biodiesel blends in a diesel engine. Ind Crop Prod 59:96–104
Altun S (2014) Effect of the degree of unsaturation of biodiesel fuels on the exhaust emissions of a diesel power generator. Fuel 117:450–457
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013) Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew Sust Energy Rev 18:211–245
Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sust Energy Rev 16:2070–2093
Bajpai D, Tyagi VK (2006) Biodiesel: source, production, composition, properties and its benefits. J Oleo Sci 55:487–502
Banković-Ilić IB, Stamenkovic OS, Veljkovic VB (2012) Biodiesel production from non-edible plant oils. Renew Sust Energy Rev 16:3621–3647
Banković-Ilić IB, Stojković IJ, Stamenković OS, Veljkovic VB, Hung YT (2014) Waste animal fats as feedstocks for biodiesel production. Renew Sust Energy Rev 32:238–254
Belewu MA, Adekola FA, Adebayo GB, Ameen OM, Muhammad NO, Olaniyan AM, Adekola OF, Musa AK (2010) Physico-chemical characteristic of oil and biodiesel from Nigerian and Indian, Jatropha curcas seeds. Int J Biol Chem Sci 4(2):524–529
Brondani M, Hoffmann R, Mayer FD, Kleinert JS (2015) Environment and energy analysis of biodiesel production in Rio Grande do Sul, Brazil. Clean Technol Environ Policy 17:129–143
Bulatov I, Klemeš JJ (2011) Clean fuel technologies and clean and reliable energy: a summary. Clean Technol Environ Policy 13:543–546
Chhetri AB, Watts KC, Islam MR (2008) Waste cooking oil as an alternate feedstock for biodiesel production. Energies 1:3–18
Cho YJ, Kim TE, Gil B (2013) Correlation between refractive index of vegetable oils measured with surface plasmon resonance and acid values determined with the AOCS official method. LWT 53(2):517–521
Chuah LF, Abd Aziz AR, Yusup S, Bokhari A, Klemeš JJ, Abdullah MZ (2015a) Performance and emission of diesel engine fuelled by waste cooking oil methyl ester derived from palm olein using hydrodynamic cavitation. Clean Technol Environ Policy. doi:10.1007/s10098-015-0957-2
Chuah LF, Yusup S, Abd Aziz AR, Bokhari A, Abdullah MZ (2015b) Cleaner production of methyl ester using waste cooking oil derived from palm olein using a hydrodynamic cavitation reactor. J Clean Prod. doi:10.1016/j.jclepro.2015.06.112
Chuah LF, Yusup S, Abd Aziz AR, Bokhari A, Klemeš JJ, Abdullah MZ (2015c) Intensification of biodiesel synthesis from waste cooking oil (Palm Olein) in a hydrodynamic cavitation reactor: effect of operating parameters on methyl ester conversion. Chem Eng Process 95:235–240
Cvengros J, Cvengrosova Z (2004) Used frying oils and fats and their utilization in the production of methyl esters of higher fatty acids. Biomass Bioenergy 27:173–181
Delavari A, Halek F, Amini M (2015) Continuous biodiesel production in a helicoidal reactor using ultrasound-assisted transesterification reaction of waste cooking oil. Clean Technol Environ Policy 17:273–279
Dermibas A (2005) Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog Energy Combust Sci 31:466–487
Dmytryshyn SL, Dalai AK, Chaudhari ST, Mishra HK, Reaney MJ (2004) Synthesis and characterization of vegetable oil derived esters: evaluation for their diesel additive properties. Bioresour Technol 92:55–64
Dwivedi G, Sharma MP (2015) Application of Box-Behnken design in optimization of biodiesel yield from Pongamia oil and its stability analysis. Fuel 145:256–262
Fernandes AMAP, Eberlin MN, Silva PRM, Silva SR, Cunha VS, Daroda RJ, Alberici RM (2014) Unsaturation levels in biodiesel via easy ambient sonic-spray ionization mass spectrometry. Fuel 128:99–103
Ghayal D, Pandit AB, Rathod VK (2013) Optimization of biodiesel production in a hydrodynamic cavitation reactor using used frying oil. Ultrason Sonochem 20:322–328
Ghobadian B, Rahimi H, Nikbakht AM, Najafi G, Yusaf TF (2009) Diesel engine performance and exhaust emission analysis using waste cooking biodiesel fuel with an artificial neutral network. Renew Energy 34:976–982
Gopal KN, Pal A, Sharma S, Samanchi C, Sathyanarayanan K, Elango T (2014) Investigation of emission and combustion characteristics of a CI engine fueled with waste cooking oil methyl ester and diesel blends. Alex Eng J 53:281–287
Islam MA, Ayoko GA, Brown R, Stuart D, Heimann K (2013) Influence of fatty acid structure on fuel properties of algae derived biodiesel. Procedia Eng 56:591–596
Jain S, Sharma MP (2014) Effect of metal contents on oxidation stability of biodiesel/diesel blends. Fuel 116:14–18
Jesikha M (2012) Fatty acid methyl esters characteristic and esterification of some vegerable oils for production of biodiesel. Res Inventy 1(12):50–53
Kalam MA, Masjuki HH, Jayed MH, Liaquat AM (2011) Emission and performance characteristics of an indirect ignition diesel engine fuelled with waste cooking oil. Energy 36:397–402
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833
Kulkarni MG, Dalai AK (2006) Waste cooking oil an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Lam HL, Varbonov P, Klemeš JJ (2010) Minimising carbon footprint of regional biomass supply chains. Resour Conserv Recycl 54:303–309
Lopes JCS, Boros L, Krahenbuhl MA, Meirelles AJA, Daridon JL, Pauly J, Marrucho IM, Coutinho JAP (2008) Prediction of cloud points of biodiesel. Energy Fuels 22:747–752
Lown AL, Peereboom L, Mueller SA, Anderson JE, Miller DJ, Lira CT (2014) Cold flow properties for blends of biofuels with diesel and jet fuels. Fuel 117:544–551
Maddikeri GL, Pandit AB, Gogate PR (2012) Intensification approaches for biodiesel synthesis from waste cooking oil: a review. Ind Eng Chem Res 51(45):14610–14628
Meng X, Chen G, Wang Y (2008) Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process Technol 89:851–857
Moser BR (2014) Impact of fatty ester composition on low temperature properties of biodiesel–petroleum diesel blends. Fuel 115:500–506
Na-Ranong D, Kitchaiya P (2014) Precipitation above cloud point in palm oil based biodiesel during production and storage. Fuel 122:287–298
O’Connell D, Savelski M, Stewart Slater C (2013) Life cycle assessment of dewatering routes for algae derived biodiesel processes. Clean Technol Environ Policy 15:567–577
Ong HC, Masjuki HH, Mahila TMI, Silitonga AS, Chong WT, Leong KY (2014) Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers Manag 81:30–40
Pullen J, Saeed K (2014) Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process Technol 125:223–235
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Rashid U, Knothe G, Yunus R, Evangelista RL (2014) Kapok oil methyl esters. Biomass Bioenergy 66:419–425
Rawat DS, Joshi G, Lamba BY, Tiwari AK, Mallick S (2014) Impact of additives on storage stability of Karanja (Pongamia Pinnata) biodiesel blends with conventional diesel sold at retail outlets. Fuel 120:30–37
Rocha EGdA, Follegatti-Romero LA, Duvoisin S Jr, Aznar M (2014) Liquid–liquid equilibria for ternary systems containing ethylic palm oil biodiesel + ethanol + glycerol/water: experimental data at 298.15 K and 323.15 K and thermodynamic modelling. Fuel 128:356–365
Sanjid A, Masjuki HH, Kalam MA, Ashrafur Rahman SM, Abedin MJ, Palash SM (2013) Impact of palm, mustard, waste cooking oil and Calophyllum inophyllum biofuels on performance and emission of CI engine. Renew Sustain Energy Rev 27:664–682
Singh D, Ganesh A, Mahajani S (2015) Heterogeneous catalysis for biodiesel synthesis and valorization of glycerol. Clean Technol Environ Policy 17:1103–1110
Sivaramakrishnan K, Ravikumar P (2012) Determination of cetane number of biodiesel and it’s influence on physical properties. J Eng Appl Sci 7(2):205–211
Tan K, Lee K, Mohamed A (2011) Potential of waste palm cooking oil for catalyst-free biodiesel production. Energy 36:2085–2088
Toscano G, Riva G, Pedretti EF, Duca D (2012) Vegetable oil and fat viscosity forecast models based on iodine number and saponification number. Biomass Bioenergy 46:511–516
Umaru M, Aberuagba F (2012) Characteristics of a typical Nigerian Jatropha curcas oil Seeds for biodiesel production. Res J Chem Sci 2(10):7–12
Wang LB, Yu HY, He XH, Liu RY (2012) Influence of fatty acid composition of woody biodiesel plants on the fuel properties. J Fuel Chem Technol 40(4):397–404
Wendy Ng PQ, Lam HL, Varbanov PS, Klemeš JJ (2014) Waste-to-energy (WTE) network synthesis for municipal solid waste (MSW). Energy Convers Manag 85:866–874
Yaakob Z, Mohammad M, Alherbawi M, Alam Z (2013) Overview of the production of biodiesel from waste cooking oil. Renew Sust Energy Rev 18:184–193
Acknowledgments
The authors would like to thank the Universiti Teknologi PETRONAS for the Tuition Fee Assistantship (TFA) Scheme, Public Service Department of Malaysia, Marine Department Malaysia, MyRA Grant (No. 0153AB-J19) and Hungarian Project TÁMOP-4.2.2.B-15/1/KONV-2015-0004 “A Pannon Egyetem tudományos műhelyeinek támogatása”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chuah, L.F., Yusup, S., Aziz, A.R.A. et al. Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Techn Environ Policy 18, 473–482 (2016). https://doi.org/10.1007/s10098-015-1022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-1022-x